Valorizing Agro-Industrial By-Products for Sustainable Cultivation of Chlorella sorokiniana: Enhancing Biomass, Lipid Accumulation, Metabolites, and Antimicrobial Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Supplement Preparation and Pretereatment of Biowaste Residues
2.2. DNS Assay
2.3. Growth of Microalgae Strain
2.4. Preparation of Extracts with Green Organic Solvents
2.5. Antimicrobial Tests
2.5.1. Agar Well Diffusion Method
2.5.2. Broth Dilution Method
2.6. Characterization of Extracts Using GC-MS
2.7. Analysis of the Lipid Profiles of the Extracts
2.8. Statistical Analysis
3. Results and Discussion
3.1. Pretreatment Efficacy and Sugar Releasee
3.2. Microalgae Growth Performance
3.2.1. Twenty-Four-Well Plate Experiments
3.2.2. Flask Cultures
3.2.3. pH Variation
3.3. Green Extraction Yields (Lipophilic Compounds)
3.4. Antimicrobial Activity Assessed by the Agar Well Diffusion Method
3.5. Minimum Inhibitory Concentrations
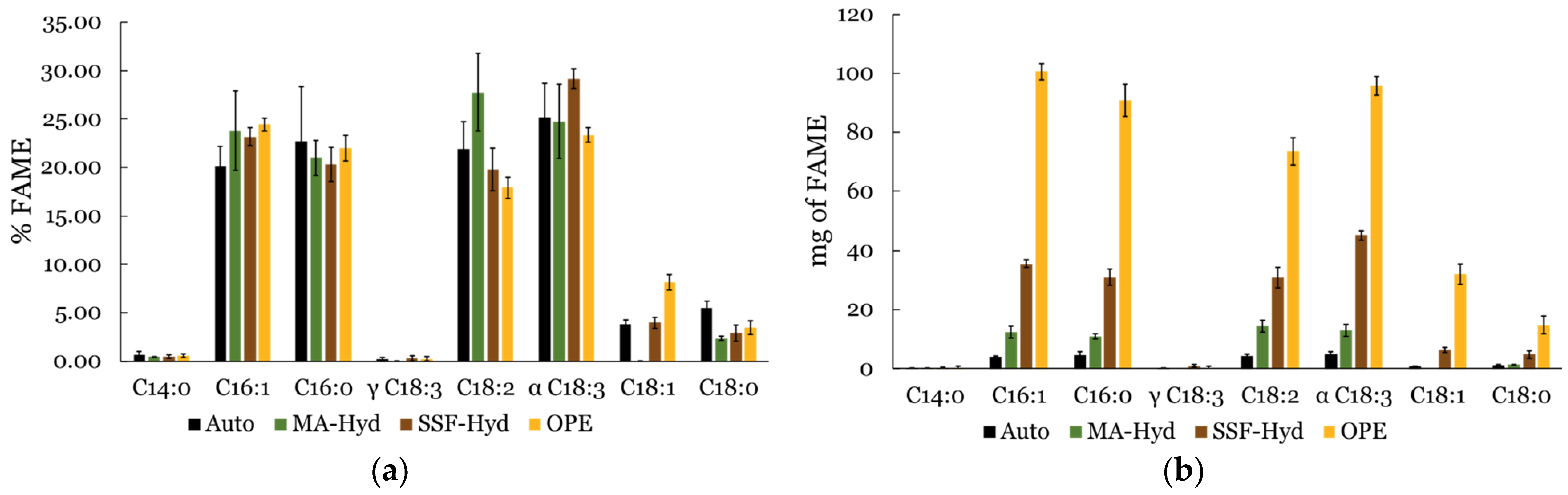
3.6. Fatty Acid Methyl Ester (FAME) Profile Analysis
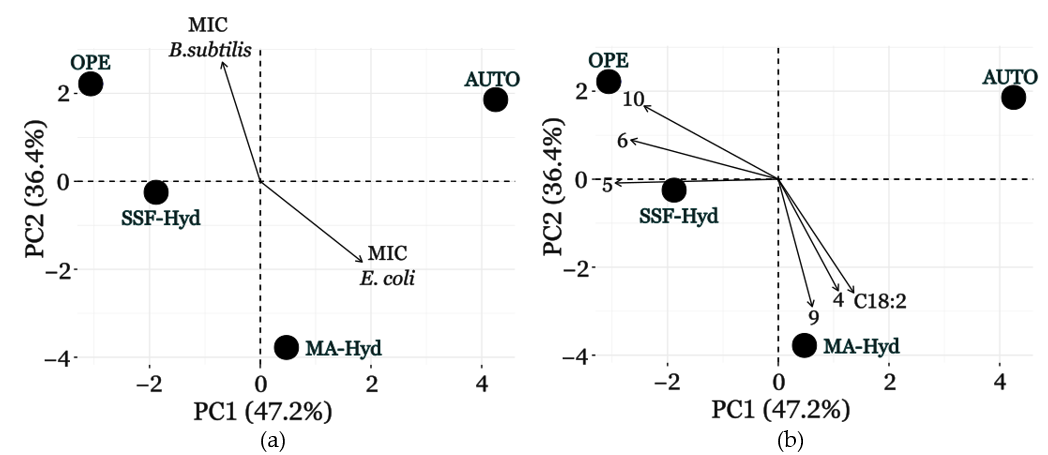
3.7. Lipid Profile, GC-MS Analysis, and Antimicrobial Activity Correlation Analyzed via PCA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huesemann, M.; Chavis, A.; Edmundson, S.; Rye, D.; Hobbs, S.; Sun, N.; Wigmosta, M. Climate-Simulated Raceway Pond Culturing: Quantifying the Maximum Achievable Annual Biomass Productivity of Chlorella sorokiniana in the Contiguous USA. J. Appl. Phycol. 2018, 30, 287–298. [Google Scholar] [CrossRef]
- Melo, J.M.; Telles, T.S.; Ribeiro, M.R.; de Carvalho Junior, O.; Andrade, D.S. Chlorella sorokiniana as Bioremediator of Wastewater: Nutrient Removal, Biomass Production, and Potential Profit. Bioresour. Technol. Rep. 2022, 17, 100933. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.-E.; Cho, Y.B.; Hwang, S.J. Growth Rate, Organic Carbon and Nutrient Removal Rates of Chlorella sorokiniana in Autotrophic, Heterotrophic and Mixotrophic Conditions. Bioresour. Technol. 2013, 144, 8–13. [Google Scholar] [CrossRef] [PubMed]
- León-Vaz, A.; León, R.; Díaz-Santos, E.; Vigara, J.; Raposo, S. Using Agro-Industrial Wastes for Mixotrophic Growth and Lipids Production by the Green Microalga Chlorella sorokiniana. New Biotechnol. 2019, 51, 31–38. [Google Scholar] [CrossRef]
- Cecchin, M.; Benfatto, S.; Griggio, F.; Mori, A.; Cazzaniga, S.; Vitulo, N.; Delledonne, M.; Ballottari, M. Molecular Basis of Autotrophic vs Mixotrophic Growth in Chlorella sorokiniana. Sci. Rep. 2018, 8, 6465. [Google Scholar] [CrossRef]
- Dragone, G. Challenges and Opportunities to Increase Economic Feasibility and Sustainability of Mixotrophic Cultivation of Green Microalgae of the Genus Chlorella. Renew. Sustain. Energy Rev. 2022, 160, 112284. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Bashan, Y.; Bashan, Y.; Bashan, Y. Microalgal Heterotrophic and Mixotrophic Culturing for Bio-Refining: From Metabolic Routes to Techno-Economics. In Algal Biorefineries: Volume 2: Products and Refinery Design; Springer: Cham, Switzerland, 2015; pp. 61–131. [Google Scholar] [CrossRef]
- Domínguez Gámez, M.; Iván Romo-Sáenz, C.; Gonzalez Ochoa, G.; Garcia, A.; Domínguez-Gámez, M.; Romo-Sáenz, C.I.; Gomez-Flores, R.; González-Ochoa, G.; García-Romero, A.; Orozco-Flores, A.A.; et al. In Vitro Antitumor, Antioxidant, and Hemolytic Activities of Chlorella sorokiniana Methanol Extracts and Collective Fractions. Appl. Sci. 2024, 14, 9613. [Google Scholar] [CrossRef]
- Fang, Y.; Cai, Y.; Zhang, Q.; Ruan, R.; Zhou, T. Research Status and Prospects for Bioactive Compounds of Chlorella Species: Composition, Extraction, Production, and Biosynthesis Pathways. Process Saf. Environ. Prot. 2024, 191, 345–359. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Odjadjare, E.C.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chlorella sorokiniana and Chlorella minutissima Exhibit Antioxidant Potentials, Inhibit Cholinesterases and Modulate Disaggregation of β-Amyloid Fibrils. Electron. J. Biotechnol. 2019, 40, 1–9. [Google Scholar] [CrossRef]
- Shaima, A.F.; Mohd Yasin, N.H.; Ibrahim, N.; Takriff, M.S.; Gunasekaran, D.; Ismaeel, M.Y.Y. Unveiling Antimicrobial Activity of Microalgae Chlorella sorokiniana (UKM2), Chlorella sp. (UKM8) and Scenedesmus sp. (UKM9). Saudi J. Biol. Sci. 2022, 29, 1043–1052. [Google Scholar] [CrossRef]
- Cheng, C.H.; Du, T.B.; Pi, H.C.; Jang, S.M.; Lin, Y.H.; Lee, H.T. Comparative Study of Lipid Extraction from Microalgae by Organic Solvent and Supercritical CO2. Bioresour. Technol. 2011, 102, 10151–10153. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Jimenez-Lopez, C.; Fraga, M.; Lourenço-Lopes, C.; García-Oliveira, P.; Lorenzo, J.M.; Perez-Lamela, C.; Prieto, M.A.; Simal-Gandara, J. Extraction, Properties, and Applications of Bioactive Compounds Obtained from Microalgae. Curr. Pharm. Des. 2020, 26, 1929–1950. [Google Scholar] [CrossRef] [PubMed]
- Mirzapour-Kouhdasht, A.; Taghizadeh, M.S.; Niazi, A.; Garcia-Vaquero, M. Green and Environmentally Friendly Technologies to Develop Macroalgal Biorefineries. In Seaweeds and Seaweed-Derived Compounds; Springer: Cham, Switzerland, 2024; pp. 19–50. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green Solvents for Sustainable Organic Synthesis: State of the Art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- Tundo, P.; Aricò, F.; Rosamilia, A.E.; Grego, S.; Rossi, L. Dimethyl Carbonate: Green Solvent and Ambident Reagent. In Green Chemical Reactions; Springer: Dordrecht, The Netherlands, 2008; pp. 213–232. [Google Scholar] [CrossRef]
- Pyo, S.H.; Park, J.H.; Chang, T.S.; Hatti-Kaul, R. Dimethyl Carbonate as a Green Chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Albert, R.E. Carcinogen Risk Assessment in the U.S. Environmental Protection Agency. Crit. Rev. Toxicol. 1994, 24, 75–85. [Google Scholar] [CrossRef]
- Van Wassenhove, L.N.; Lebreton, B.; Lorenz, T. The REACH Directive and Its Impact on the European Chemical Industry: A Critical Review. INSEAD 2008, Available at SSRN: https://ssrn.com/abstract=1259658 , Working Paper No. 53. [Google Scholar] [CrossRef]
- Ezejiofor, T.I.N.; Enebaku, U.E.; Ogueke, C. Waste to Wealth—Value Recovery from Agro-Food Processing Wastes Using Biotechnology: A Review. Biotechnol. J. Int. 2014, 4, 418–481. [Google Scholar] [CrossRef]
- Ayala, J.R.; Montero, G.; Coronado, M.A.; García, C.; Curiel-Alvarez, M.A.; León, J.A.; Sagaste, C.A.; Montes, D.G. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules 2021, 26, 1348. [Google Scholar] [CrossRef]
- Park, W.K.; Moon, M.; Kwak, M.S.; Jeon, S.; Choi, G.G.; Yang, J.W.; Lee, B. Use of Orange Peel Extract for Mixotrophic Cultivation of Chlorella Vulgaris: Increased Production of Biomass and FAMEs. Bioresour. Technol. 2014, 171, 343–349. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; Orrego-Alzate, C.E.; Acosta-Medina, C.D.; Cardona-Alzate, C.A. Integral Use of Orange Peel Waste through the Biorefinery Concept: An Experimental, Technical, Energy, and Economic Assessment. Biomass Convers. Biorefin. 2021, 11, 645–659. [Google Scholar] [CrossRef]
- Ángel Siles López, J.; Li, Q.; Thompson, I.P. Biorefinery of Waste Orange Peel. Crit. Rev. Biotechnol. 2010, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Paini, J.; Midolo, G.; Valenti, F.; Ottolina, G. One-Pot Combined Hydrodistillation of Industrial Orange Peel Waste for Essential Oils and Pectin Recovery: A Multi-Objective Optimization Study. Sustainability 2025, 17, 293. [Google Scholar] [CrossRef]
- Michalak, I.; Messyasz, B. Concise Review of Cladophora spp.: Macroalgae of Commercial Interest. J. Appl. Phycol. 2020, 33, 133–166. [Google Scholar] [CrossRef]
- Kousar, A.; Khan, H.A.; Farid, S.; Zhao, Q.; Zeb, I. Recent Advances on Environmentally Sustainable Valorization of Spent Mushroom Substrate: A Review. Biofuels Bioprod. Biorefin. 2024, 18, 639–651. [Google Scholar] [CrossRef]
- Wood, I.P.; Elliston, A.; Ryden, P.; Bancroft, I.; Roberts, I.N.; Waldron, K.W. Rapid Quantification of Reducing Sugars in Biomass Hydrolysates: Improving the Speed and Precision of the Dinitrosalicylic Acid Assay. Biomass Bioenergy 2012, 44, 117–121. [Google Scholar] [CrossRef]
- Kirrolia, A.; Bishnoi, N.; Singh, R. Effect of Shaking, Incubation Temperature, Salinity and Media Composition on Growth Traits of Green Microalgae Chlorococcum sp. J. Algal Biomass Util. 2012, 3, 46–53. [Google Scholar]
- Valgas, C.; de Souza, S.M.; Smânia, E.F.A.; Smânia, A., Jr. Screening Methods to Determine Antibacterial Activity of Natural Products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R Software Package for Dose-Response Studies: The Concept and Data Analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]
- Rubin, J.E. Antimicrobial Susceptibility Testing Methods and Interpretation of Results. In Antimicrobial Therapy in Veterinary Medicine; Wiley: Hoboken, NJ, USA, 2013; pp. 11–20. [Google Scholar]
- Kumar, V.; Muthuraj, M.; Palabhanvi, B.; Ghoshal, A.K.; Das, D. Evaluation and Optimization of Two Stage Sequential in Situ Transesterification Process for Fatty Acid Methyl Ester Quantification from Microalgae. Renew. Energy 2014, 68, 560–569. [Google Scholar] [CrossRef]
- Lee, M. Data Analysis with RStudio: An Easygoing Introduction by Franz Kronthaler and Silke Zöllner Berlin, Germany: Springer-Verlag GmbH, DE, 2021. pp. 131. Biometrics 2021, 77, 1502–1503. [Google Scholar] [CrossRef]
- Tallarida, R.J.; Murray, R.B. Duncan Multiple Range Test. In Manual of Pharmacologic Calculations; Springer: New York, NY, USA, 1987; pp. 125–127. [Google Scholar]
- Cuevas, A.; Febrero, M.; Fraiman, R. An Anova Test for Functional Data. Comput. Stat. Data Anal. 2004, 47, 111–122. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Niu, Y.; Yang, J.; Zhou, J.; Yuan, Y. Metabolic Profiling Reveals Growth Related FAME Productivity and Quality of Chlorella sorokiniana with Different Inoculum Sizes. Biotechnol. Bioeng. 2012, 109, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gargouri, M.; Feng, J.; Park, J.J.; Gao, D.; Miao, C.; Dong, T.; Gang, D.R.; Chen, S. Regulation of Starch and Lipid Accumulation in a Microalga Chlorella sorokiniana. Bioresour. Technol. 2015, 180, 250–257. [Google Scholar] [CrossRef]
- Marchello, A.E.; dos Santos, A.C.; Lombardi, A.T.; de Souza, C.W.O.; Montanhim, G.C. Physiological and Ecological Aspects of Chlorella sorokiniana (Trebouxiophyceae) Under Photoautotrophic and Mixotrophic Conditions. Microb. Ecol. 2018, 76, 791–800. [Google Scholar] [CrossRef]
- Proietti Tocca, G.; Agostino, V.; Menin, B.; Tommasi, T.; Fino, D.; Di Caprio, F. Mixotrophic and Heterotrophic Growth of Microalgae Using Acetate from Different Production Processes. Rev. Environ. Sci. Bio/Technol. 2024, 23, 93–132. [Google Scholar] [CrossRef]
- Alcántara, C.; Fernández, C.; García-Encina, P.A.; Muñoz, R. Mixotrophic Metabolism of Chlorella sorokiniana and Algal-Bacterial Consortia under Extended Dark-Light Periods and Nutrient Starvation. Appl. Microbiol. Biotechnol. 2015, 99, 2393–2404. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, M.; Liu, B.; Wen, F.; Yang, Z.; Liu, J. Transcriptome and Metabolome Profiling of a Novel Isolate Chlorella sorokiniana G32 (Chlorophyta) Displaying Enhanced Starch Accumulation at High Growth Rate under Mixotrophic Condition. Front. Microbiol. 2022, 12, 760307. [Google Scholar] [CrossRef]
- Park, J.E.; Zhang, S.; Han, T.H.; Hwang, S.J. The Contribution Ratio of Autotrophic and Heterotrophic Metabolism during a Mixotrophic Culture of Chlorella sorokiniana. Int. J. Environ. Res. Public 2021, 18, 1353. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; Ahn, C.Y.; Oh, H.M. Comparison of Several Methods for Effective Lipid Extraction from Microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; Carvalho Junior, R.N. de From Waste to Sustainable Industry: How Can Agro-Industrial Wastes Help in the Development of New Products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Impact of Microalgae Characteristics on Their Conversion to Biofuel. Part II: Focus on Biomethane Production. Biofuels Bioprod. Biorefining 2012, 6, 205–218. [Google Scholar] [CrossRef]
- Samorì, C.; Basaglia, M.; Casella, S.; Favaro, L.; Galletti, P.; Giorgini, L.; Marchi, D.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Dimethyl Carbonate and Switchable Anionic Surfactants: Two Effective Tools for the Extraction of Polyhydroxyalkanoates from Microbial Biomass. Green Chem. 2015, 17, 1047–1056. [Google Scholar] [CrossRef]
- Righi, S.; Baioli, F.; Samorì, C.; Galletti, P.; Tagliavini, E.; Stramigioli, C.; Tugnoli, A.; Fantke, P. A Life Cycle Assessment of Poly-Hydroxybutyrate Extraction from Microbial Biomass Using Dimethyl Carbonate. J. Clean. Prod. 2017, 168, 692–707. [Google Scholar] [CrossRef]
- Abreu, A.P.; Morais, R.C.; Teixeira, J.A.; Nunes, J. A Comparison between Microalgal Autotrophic Growth and Metabolite Accumulation with Heterotrophic, Mixotrophic and Photoheterotrophic Cultivation Modes. Renew. Sustain. Energy Rev. 2022, 159, 112247. [Google Scholar] [CrossRef]
- Chea, J.D.; Christon, A.; Pierce, V.; Reilly, J.H.; Russ, M.; Savelski, M.; Slater, C.S.; Yenkie, K.M. Framework for Solvent Recovery, Reuse, and Recycling in Industries. Comput. Aided Chem. Eng. 2019, 47, 199–204. [Google Scholar] [CrossRef]
- Ilieva, Y.; Zaharieva, M.M.; Najdenski, H.; Kroumov, A.D. Antimicrobial Activity of Arthrospira (Former Spirulina) and Dunaliella Related to Recognized Antimicrobial Bioactive Compounds. Int. J. Mol. Sci. 2024, 25, 5548. [Google Scholar] [CrossRef]
- Mkhize, S.S.; Cedric Simelane, M.B.; Mongalo, I.N.; Pooe, O.J. The Effect of Supplementing Mushroom Growing Substrates on the Bioactive Compounds, Antimicrobial Activity, and Antioxidant Activity of Pleurotus Ostreatus. Biochem. Res. Int. 2022, 2022, 9436614. [Google Scholar] [CrossRef]
- Kingston, A.W.; Subramanian, C.; Rock, C.O.; Helmann, J.D. A ΣW-Dependent Stress Response in Bacillus Subtilis That Reduces Membrane Fluidity. Mol. Microbiol. 2011, 81, 69–79. [Google Scholar] [CrossRef]
- Sanchez-Salas, J.L.; Santiago-Lara, M.L.; Setlow, B.; Sussman, M.D.; Setlow, P. Properties of Bacillus Megaterium and Bacillus Subtilis Mutants Which Lack the Protease That Degrades Small, Acid-Soluble Proteins during Spore Germination. J. Bacteriol. 1992, 174, 807. [Google Scholar] [CrossRef]
- Stirk, W.A.; van Staden, J. Bioprospecting for Bioactive Compounds in Microalgae: Antimicrobial Compounds. Biotechnol. Adv. 2022, 59, 107977. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.H.; Abdullah, F.O. Microwave-Assisted Extraction and Phytochemical Profile of Nonea Pulmonarioides and Its Antifungal, Antibacterial, and Antioxidant Activities. J. Food Qual. 2022, 2022, 5135880. [Google Scholar] [CrossRef]
- Pejin, B.; Savic, A.; Sokovic, M.; Glamoclija, J.; Ciric, A.; Nikolic, M.; Radotic, K.; Mojovic, M. Further in Vitro Evaluation of Antiradical and Antimicrobial Activities of Phytol. Nat. Prod. Res. 2014, 28, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Woźniak, K.; Los, R.; Głowniak, K.; Malm, A. Antimicrobial Activity of Fatty Acids from Fruits of Peucedanum cervaria and P. alsaticum. Chem. Biodivers. 2010, 7, 2748–2754. [Google Scholar] [CrossRef]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and Medium-Chain Fatty Acids Exhibit Antimicrobial Activity for Oral Microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
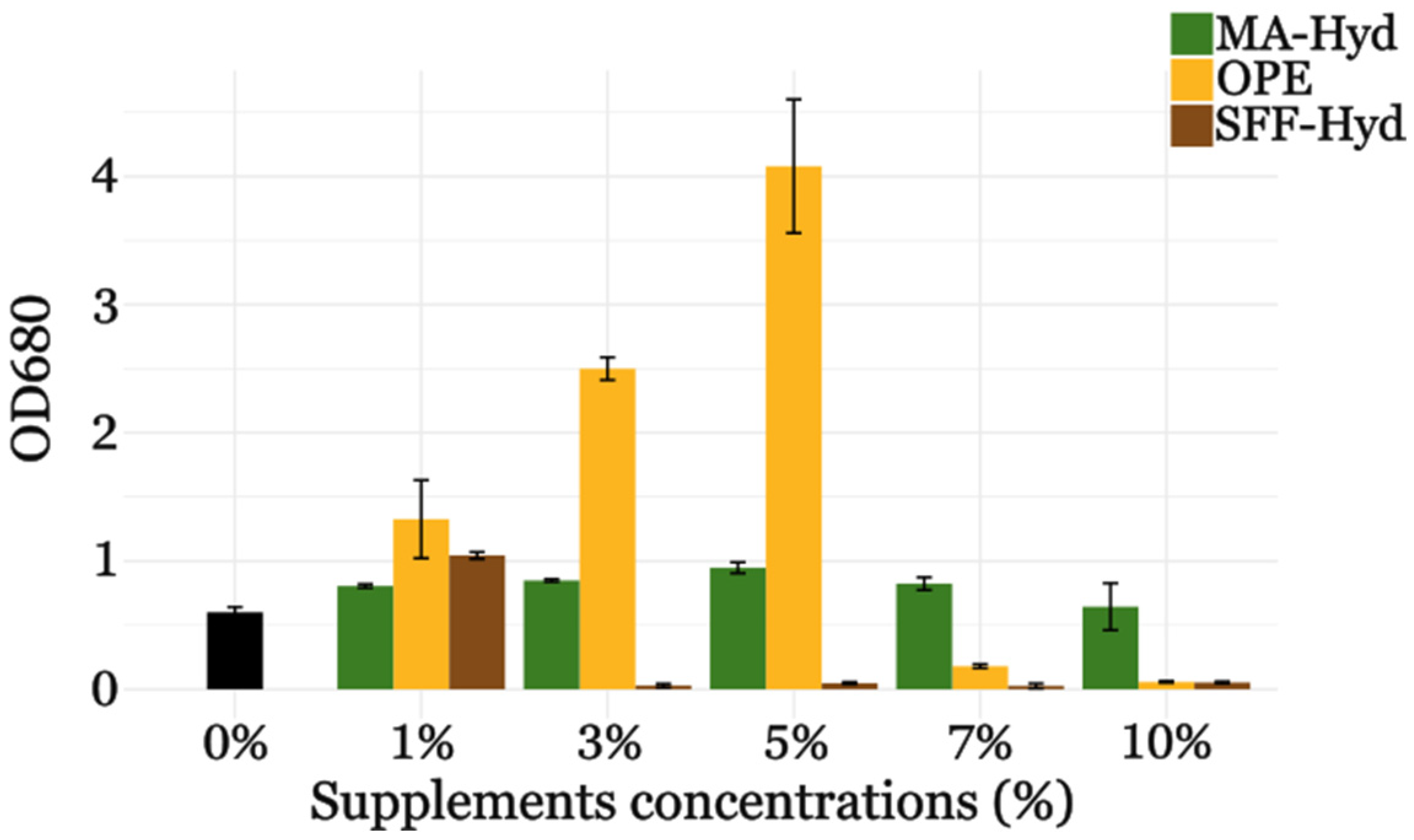
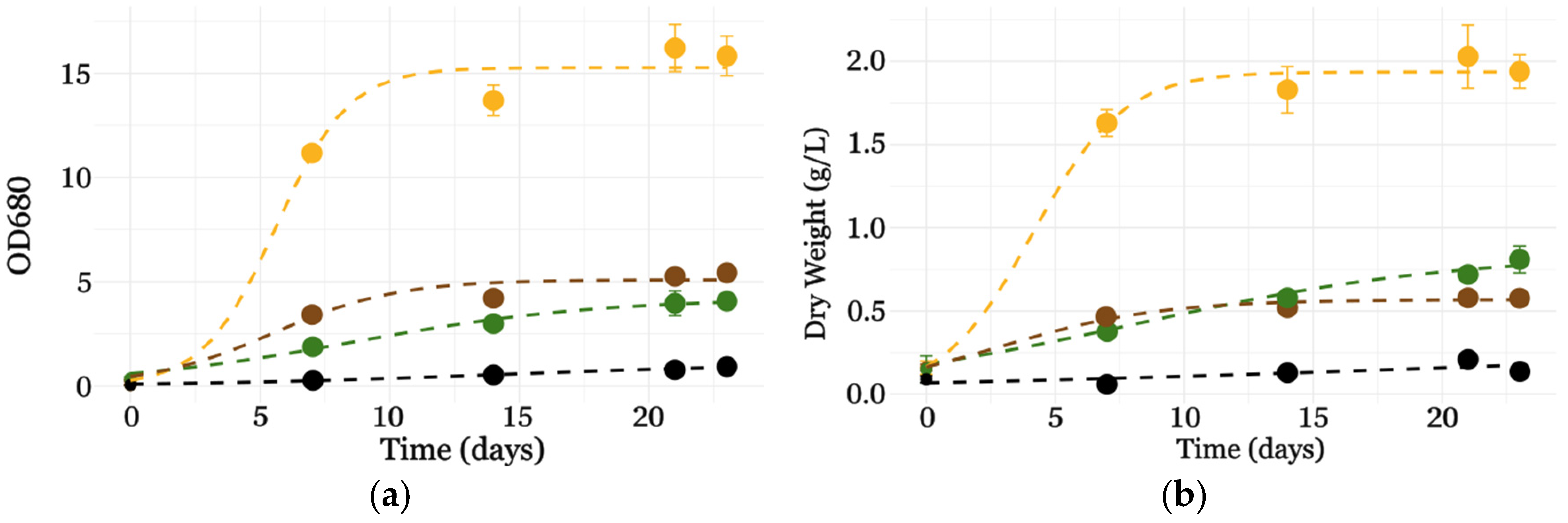


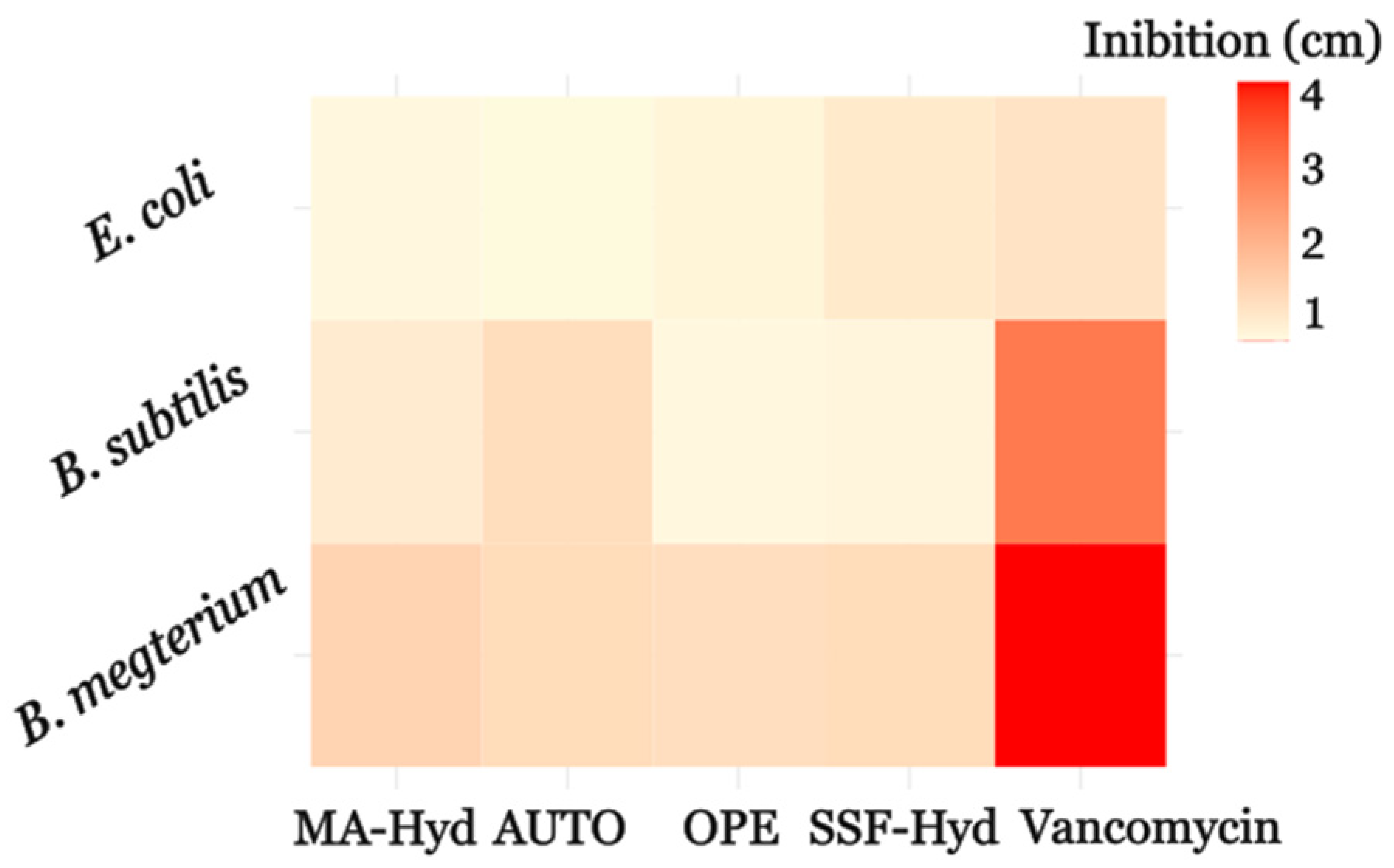
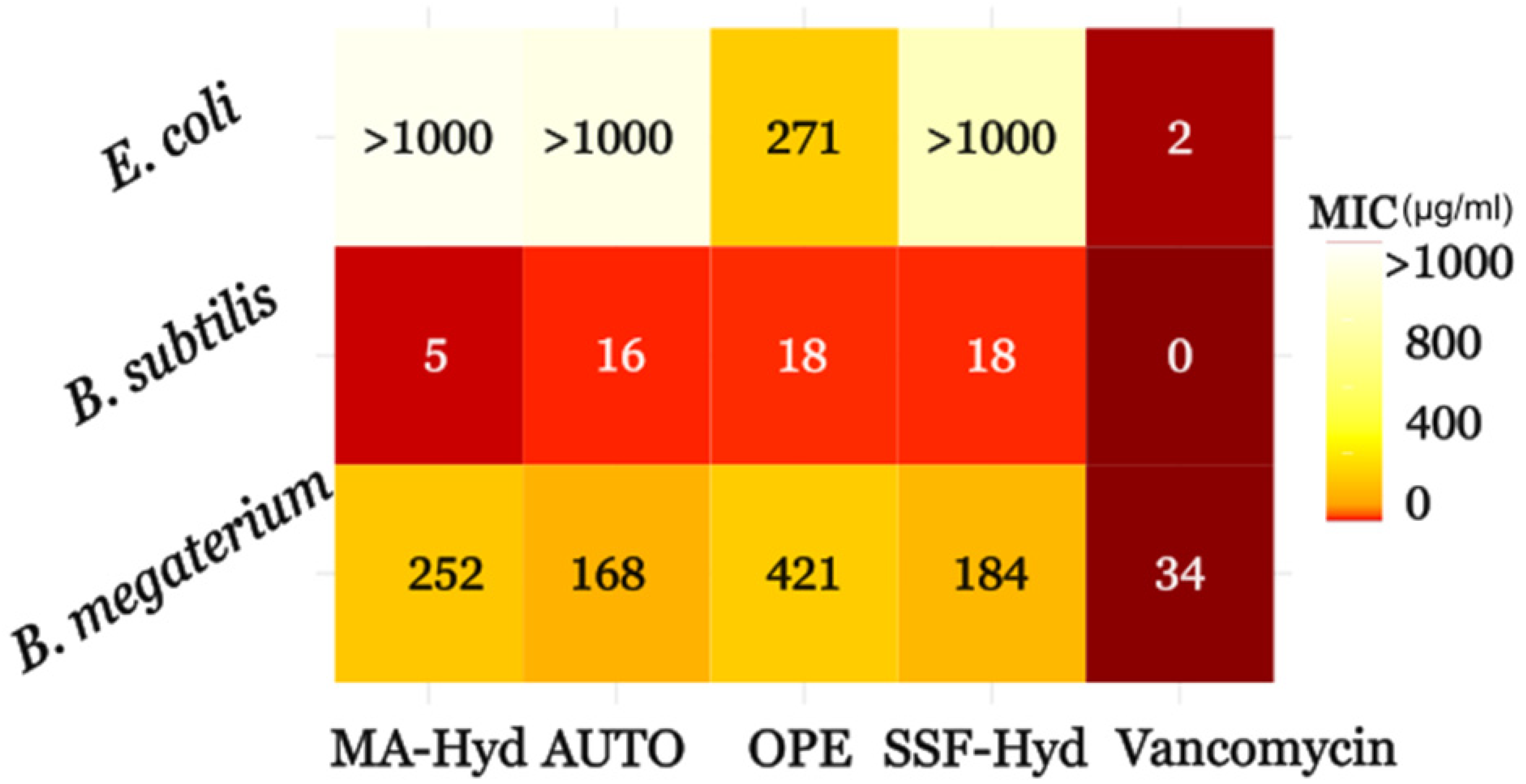
| Supplement | Biomass 1 mg/mL (DW) | Relative Biomass Growth |
|---|---|---|
| AUTO | 0.14 ± 0.04 | 1 |
| OPE | 1.94 ± 0.10 | 13.86 |
| SSF-Hyd | 0.58 ± 0.01 | 4.14 |
| MA-Hyd | 0.81 ± 0.08 | 5.79 |
| Supplements | Saturated FAs 1 (%) | Unsaturated FAs 1 (%) |
|---|---|---|
| AUTO | 28.8 ± 5.6 | 71.2 ± 4.9 |
| MA-Hyd | 23.7 ± 1.8 | 76.3 ± 6.8 |
| SSF-Hyd | 23.6 ± 1.9 | 76.4 ± 2.7 |
| OPE | 26.0 ± 1.5 | 74.0 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lio, E.; Esposito, C.; Paini, J.; Gandolfi, S.; Secundo, F.; Ottolina, G. Valorizing Agro-Industrial By-Products for Sustainable Cultivation of Chlorella sorokiniana: Enhancing Biomass, Lipid Accumulation, Metabolites, and Antimicrobial Potential. Metabolites 2025, 15, 212. https://doi.org/10.3390/metabo15030212
Lio E, Esposito C, Paini J, Gandolfi S, Secundo F, Ottolina G. Valorizing Agro-Industrial By-Products for Sustainable Cultivation of Chlorella sorokiniana: Enhancing Biomass, Lipid Accumulation, Metabolites, and Antimicrobial Potential. Metabolites. 2025; 15(3):212. https://doi.org/10.3390/metabo15030212
Chicago/Turabian StyleLio, Elia, Carlo Esposito, Jacopo Paini, Stefano Gandolfi, Francesco Secundo, and Gianluca Ottolina. 2025. "Valorizing Agro-Industrial By-Products for Sustainable Cultivation of Chlorella sorokiniana: Enhancing Biomass, Lipid Accumulation, Metabolites, and Antimicrobial Potential" Metabolites 15, no. 3: 212. https://doi.org/10.3390/metabo15030212
APA StyleLio, E., Esposito, C., Paini, J., Gandolfi, S., Secundo, F., & Ottolina, G. (2025). Valorizing Agro-Industrial By-Products for Sustainable Cultivation of Chlorella sorokiniana: Enhancing Biomass, Lipid Accumulation, Metabolites, and Antimicrobial Potential. Metabolites, 15(3), 212. https://doi.org/10.3390/metabo15030212








