Diet-Induced Proteomic and Metabolomic Signatures in Chronic Kidney Disease: A Precision Nutrition Approach
Abstract
1. Introduction
2. Molecular Signatures Associated with the Intake of Protein-Rich Foods and Their Connection to CKD
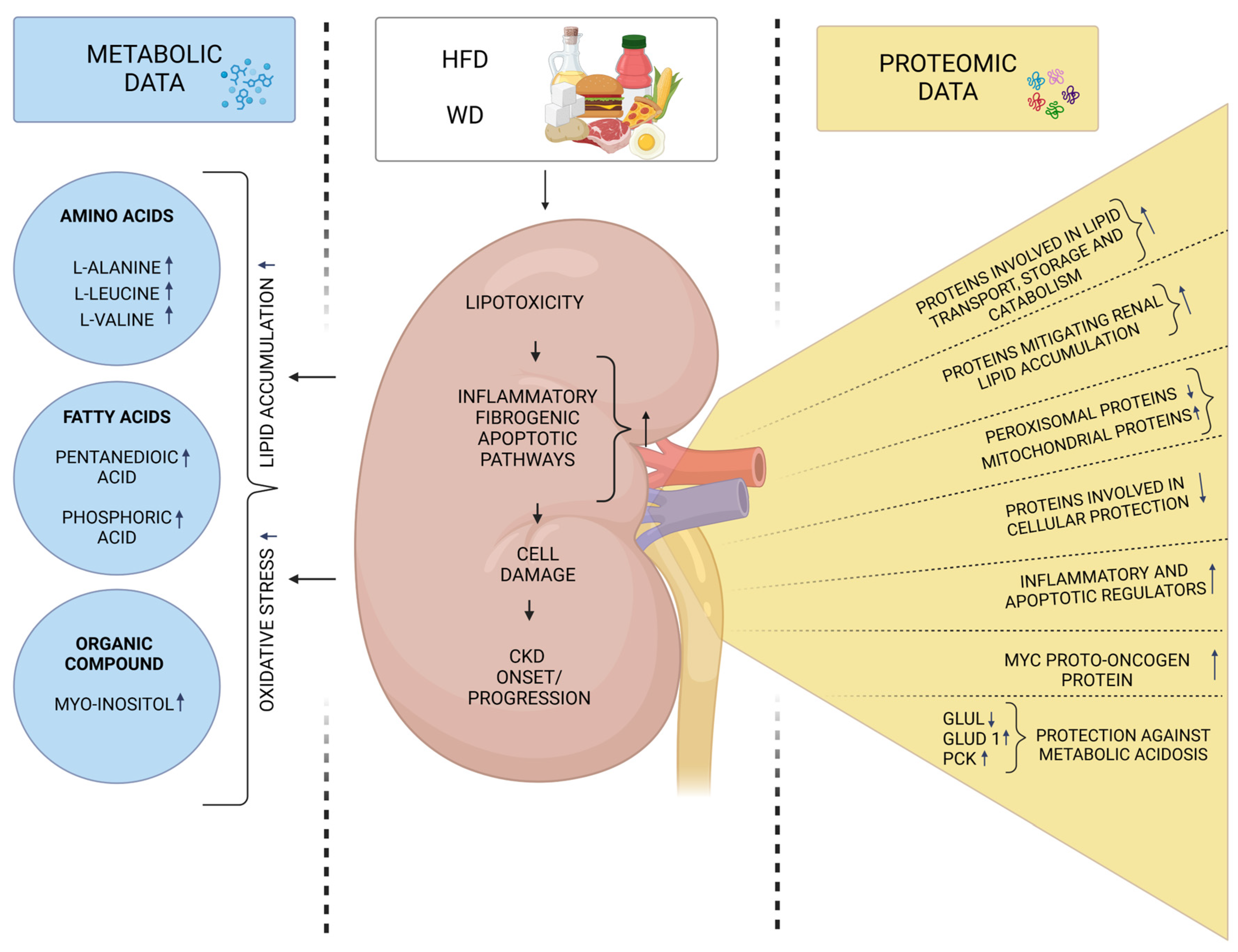
3. Molecular Signatures Associated with the Intake of High-Fat Diets and Their Connection to CKD
4. Molecular Signatures Associated with the Intake of Pre-, Pro-, and Synbiotics and Their Connection to CKD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Onopiuk, A.; Tokarzewicz, A.; Gorodkiewicz, E. Cystatin C: A kidney function biomarker. Advan. Clin. Chem. 2015, 68, 57–69. [Google Scholar] [CrossRef]
- Ogobuiro, I.; Tuma, F. Physiology, Renal; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Van Beusecum, J.; Inscho, E.W. Regulation of renal function and blood pressure control by P2 purinoceptors in the kidney. Curr. Opin. Pharmacol. 2015, 21, 82–88. [Google Scholar] [CrossRef]
- Sahay, M.; Kalra, S.; Bandgar, T. Renal endocrinology: The new frontier. Indian J. Endocrinol. Metab. 2012, 16, 154–155. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Giannese, D.; Panichi, V.; Cupisti, A. Mediterranean dietary pattern adjusted for CKD patients: The MedRen diet. Nutrients 2023, 15, 1256. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Grams, M.E.; Coresh, J.; Rebholz, C.M. Plant-based diets and incident CKD and kidney function. Clin. J. Am. Soc. Nephrol. 2019, 14, 682–691. [Google Scholar] [CrossRef]
- Hariharan, D.; Vellanki, K.; Kramer, H. The Western diet and chronic kidney disease. Curr. Hypertens. Rep. 2015, 17, 16. [Google Scholar] [CrossRef]
- Teixeira, D.E.; Peruchetti, D.B.; Souza, M.C.; das Graças Henriques, M.G.; Pinheiro, A.A.S.; Caruso-Neves, C. A high salt diet induces tubular damage associated with a pro-inflammatory and pro-fibrotic response in a hypertension-independent manner. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165907. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, H.; Zhuo, H.; Yu, C.; Liu, Y. High fat diet induces kidney injury via stimulating Wnt/β-catenin signaling. Front. Med. 2022, 9, 851618. [Google Scholar] [CrossRef]
- van Westing, A.C.; Küpers, L.K.; Geleijnse, J.M. Diet and kidney function: A literature review. Curr. Hypertens. Rep. 2020, 22, 14. [Google Scholar] [CrossRef]
- Joshi, S.; Kalantar-Zadeh, K.; Chauveau, P.; Carrero, J.J. Risks and benefits of different dietary patterns in CKD. Am. J. Kidney Dis. 2023, 81, 352–360. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Martinez, J.A.; Milagro, F.I. Holistic integration of omics tools for precision nutrition in health and disease. Nutrients 2022, 14, 4074. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.E.; Song, R.J.; Xu, X.; Gerszten, R.E.; Ngo, D.; Clish, C.B.; Corlin, L.; Ma, J.; Xanthakis, V.; Jacques, P.F. Proteomic and metabolomic correlates of healthy dietary patterns: The Framingham Heart Study. Nutrients 2020, 12, 1476. [Google Scholar] [CrossRef] [PubMed]
- Dubin, R.F.; Rhee, E.P. Proteomics and metabolomics in kidney disease, including insights into etiology, treatment, and prevention. Clin. J. Am. Soc. Nephrol. 2020, 15, 404. [Google Scholar] [CrossRef] [PubMed]
- Al-Amrani, S.; Al-Jabri, Z.; Al-Zaabi, A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and applications in human medicine. World J. Biol. Chem. 2021, 12, 57. [Google Scholar] [CrossRef]
- Fuchs, D.; Winkelmann, I.; Johnson, I.T.; Mariman, E.; Wenzel, U.; Daniel, H. Proteomics in nutrition research: Principles, technologies and applications. Br. J. Nutr. 2005, 94, 302–314. [Google Scholar] [CrossRef]
- Chen, Y.; Michalak, M.; Agellon, L.B. Importance of nutrients and nutrient metabolism on human health. Yale J. Biol. Med. 2018, 91, 95. [Google Scholar]
- Ganesh, V.; Hettiarachchy, N.S. Nutriproteomics: A promising tool to link diet and diseases in nutritional research. BBA-Proteins Proteom. 2012, 1824, 1107–1117. [Google Scholar] [CrossRef]
- Gong, H.; Zhong, H.; Cheng, L.; Li, L.-P.; Zhang, D.-K. Post-translational protein lactylation modification in health and diseases: A double-edged sword. J. Transl. Med. 2024, 22, 41. [Google Scholar] [CrossRef]
- Lobel, L.; Cao, Y.G.; Fenn, K.; Glickman, J.N.; Garrett, W.S. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science 2020, 369, 1518. [Google Scholar] [CrossRef]
- Turi, K.N.; Romick-Rosendale, L.; Ryckman, K.K.; Hartert, T.V. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. J. Allergy Clin. Immunol. 2018, 141, 1191–1201. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Bhupathiraju, S.N.; Hu, F.B. Use of metabolomics in improving assessment of dietary intake. Clin. Chem. 2018, 64, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Chen, J.; Kim, H.; Wong, K.E.; Steffen, L.M.; Yu, B.; Boerwinkle, E.; Levey, A.S.; Grams, M.E.; Rhee, E.P. Serum Metabolomic Markers of Protein-Rich Foods and Incident CKD: Results from the Atherosclerosis Risk in Communities Study. Kidney Med. 2024, 6, 100793. [Google Scholar] [CrossRef] [PubMed]
- Hotea, I.; Sirbu, C.; Plotuna, A.-M.; Tîrziu, E.; Badea, C.; Berbecea, A.; Dragomirescu, M.; Radulov, I. Integrating (nutri-) metabolomics into the one health tendency—The key for personalized medicine advancement. Metabolites 2023, 13, 800. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.-J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The effects of high-protein diets on kidney health and longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef]
- Marckmann, P.; Osther, P.; Pedersen, A.N.; Jespersen, B. High-protein diets and renal health. J. Ren. Nutr. 2015, 25, 1–5. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Appel, L.J.; Anderson, C.A.M.; Miller Iii, E.R. Effect of a high-protein diet on kidney function in healthy adults: Results from the OmniHeart trial. Am. J. Kidney Dis. 2013, 61, 547–554. [Google Scholar] [CrossRef]
- Sällström, J.; Carlström, M.; Olerud, J.; Fredholm, B.B.; Kouzmine, M.; Sandler, S.; Persson, A.E.G. High-protein-induced glomerular hyperfiltration is independent of the tubuloglomerular feedback mechanism and nitric oxide synthases. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1263–R1268. [Google Scholar] [CrossRef]
- Jiao, A.; Zhao, Y.; Chu, L.; Yang, Y.; Jin, Z. A review on animal and plant proteins in regulating diabetic kidney disease: Mechanism of action and future perspectives. J. Funct. Foods 2024, 119, 106353. [Google Scholar] [CrossRef]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The role of the anabolic properties of plant-versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef]
- Molina, P.; Gavela, E.; Vizcaíno, B.; Huarte, E.; Carrero, J.J. Optimizing diet to slow CKD progression. Front. Med. 2021, 8, 654250. [Google Scholar] [CrossRef]
- Ren, X.; Chen, J.; Abraham, A.G.; Xu, Y.; Siewe, A.; Warady, B.A.; Kimmel, P.L.; Vasan, R.S.; Rhee, E.P.; Furth, S.L. Plasma metabolomics of dietary intake of protein-rich foods and kidney disease progression in children. J. Ren. Nutr. 2024, 34, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Zheng, Z.; Grams, M.E.; Appel, L.J.; Sarnak, M.J.; Inker, L.A.; Levey, A.S.; Coresh, J. Serum metabolites associated with dietary protein intake: Results from the Modification of Diet in Renal Disease (MDRD) randomized clinical trial. Am. J. Clin. Nutr. 2019, 109, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Flis, Z.; Molik, E. Importance of bioactive substances in sheep’s milk in human health. Int. J. Mol. Sci. 2021, 22, 4364. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Liu, J.; Wang, X.; Liu, X.; Jiang, L.; Jiang, Y.; Ma, Y.; Wang, J.; Yuan, H.; An, X. Multi-omics analysis of kidney tissue metabolome and proteome reveals the protective effect of sheep milk against adenine-induced chronic kidney disease in mice. Food Funct. 2024, 15, 7046–7062. [Google Scholar] [CrossRef]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory links between high fat diets and diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Gancheva, S.; Jelenik, T.; Alvarez-Hernandez, E.; Roden, M. Interorgan metabolic crosstalk in human insulin resistance. Physiol. Rev. 2018, 98, 1371–1415. [Google Scholar] [CrossRef]
- Ruan, X.Z.; Varghese, Z.; Moorhead, J.F. An update on the lipid nephrotoxicity hypothesis. Nat. Rev. Nephrol. 2009, 5, 713–721. [Google Scholar]
- D’Agati, V.D.; Chagnac, A.; De Vries, A.P.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef]
- De Vries, A.P.J.; Ruggenenti, P.; Ruan, X.Z.; Praga, M.; Cruzado, J.M.; Bajema, I.M.; D D’Agati, V.; Lamb, H.J.; Barlovic, D.P.; Hojs, R. Fatty kidney: Emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014, 2, 417–426. [Google Scholar] [CrossRef]
- Aguila, M.B.; Mandarim-de-Lacerda, C.A. Effects of chronic high fat diets on renal function and cortical structure in rats. Exp. Toxicol. Pathol. 2003, 55, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Agborbesong, E.; Li, X. The role of mitochondria in acute kidney injury and chronic kidney disease and its therapeutic potential. Int. J. Mol. Sci. 2021, 22, 11253. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Maffioli, E.; Vianello, E.; Nonnis, S.; Grassi Scalvini, F.; Spatola, L.; Roccabianca, P.; Tedeschi, G.; Corsi Romanelli, M.M. A wide-proteome analysis to identify molecular pathways involved in kidney response to high-fat diet in mice. Int. J. Mol. Sci. 2022, 23, 3809. [Google Scholar] [CrossRef]
- Wypych, A.; Ożgo, M.; Bernaciak, M.; Herosimczyk, A.; Barszcz, M.; Gawin, K.; Ciechanowicz, A.K.; Kucia, M.; Pierzchała, M.; Poławska, E. Effect of feeding high fat diets differing in fatty acid composition on oxidative stress markers and protein expression profiles in mouse kidney. J. Anim. Feed Sci. 2024, 33, 170–184. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Oe, Y.; Kim, Y.C.; Kanoo, S.; Goodluck, H.A.; Lopez, N.; Diedrich, J.; Pinto, A.M.; Evensen, K.G.; Currais, A.J.M.; Maher, P. Western diet exacerbates a murine model of Balkan nephropathy. Am. J. Physiol. Renal Physiol. 2025, 328, F15–F28. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, Y.; Guo, Y.; Xue, X.; Zhao, S.; Geng, C.; Li, Y.; Yang, R.; Gan, Y.; Li, H. The impact of high-glucose or high-fat diets on the metabolomic profiling of mice. Front. Nutr. 2023, 10, 1171806. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Li, J.; Zhao, Y.; Chen, X. Metabonomic profiling reveals difference in altered metabolic pathways between chronic kidney disease and high-fat-induced insulin resistance in rats. Kidney Blood Press. Res. 2018, 43, 1199–1211. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G., Jr.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics-a review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, S.; Haghighatdoost, F. The effects of prebiotic, probiotic, and synbiotic supplementation on blood parameters of renal function: A systematic review and meta-analysis of clinical trials. Nutrition 2018, 51, 104–113. [Google Scholar] [CrossRef]
- Rossi, M.; Klein, K.; Johnson, D.W.; Campbell, K.L. Pre-, pro-, and synbiotics: Do they have a role in reducing uremic toxins? A systematic review and meta-analysis. Int. J. Nephrol. 2012, 2012, 673631. [Google Scholar] [CrossRef]
- Huang, Y.H.; Xin, W.; Xiong, J.C.; Yao, M.Y.; Zhang, B.; Zhao, J.H. The Intestinal Microbiota and Metabolites in the Gut-Kidney-Heart Axis of Chronic Kidney Disease. Front. Pharmacol. 2022, 13, 837500. [Google Scholar] [CrossRef]
- Zheng, H.J.; Guo, J.; Wang, Q.H.; Wang, L.S.; Wang, Y.H.; Zhang, F.; Huang, W.J.; Zhang, W.T.; Liu, W.J.; Wang, Y.X. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598. [Google Scholar] [CrossRef]
- Firouzi, S.; Mohd-Yusof, B.-N.; Majid, H.-A.; Ismail, A.; Kamaruddin, N.-A. Effect of microbial cell preparation on renal profile and liver function among type 2 diabetics: A randomized controlled trial. BMC Complement. Altern. Med. 2015, 15, 433. [Google Scholar] [CrossRef]
- Zybailov, B.L.; Glazko, G.V.; Rahmatallah, Y.; Andreyev, D.S.; McElroy, T.; Karaduta, O.; Byrum, S.D.; Orr, L.; Tackett, A.J.; Mackintosh, S.G. Metaproteomics reveals potential mechanisms by which dietary resistant starch supplementation attenuates chronic kidney disease progression in rats. PLoS ONE 2019, 14, e0199274. [Google Scholar] [CrossRef]
- Sohn, M.B.; Gao, B.; Kendrick, C.; Srivastava, A.; Isakova, T.; Gassman, J.J.; Fried, L.F.; Wolf, M.; Cheung, A.K.; Raphael, K.L. Targeting Gut Microbiome with Prebiotic in Patients with CKD: The TarGut-CKD Study. Kidney Int. Rep. 2024, 9, 671–685. [Google Scholar] [CrossRef]

| Sample Type | Study Design | Type of Analysis/Analysis Platform | Major Findings | Clinical Implications | Reference |
|---|---|---|---|---|---|
| Serum | A prospective cohort study of 3726 middle-aged participants with atherosclerosis risk in communities without CKD at baseline. Study examined the impact of six protein-rich foods (fish, nuts, legumes, red and processed meat, eggs, and poultry) on serum metabolites over 1 year. Associations were analyzed using multivariable linear regression and meta-analyzed with fixed-effects models, adjusting for key demographic and lifestyle factors. | Untargeted metabolomic analysis/ GC-MS LC-MS | Thirty significant associations were found between protein-rich foods and serum metabolites (fish, n = 8; nuts, n = 5; legumes, n = 0; red and processed meat, n = 5; eggs, n = 3; and poultry, n = 9). Metabolites improved the discrimination of high protein intake beyond covariates. Fish consumption was positively linked to 1-docosahexaenoylglycerophosphocholine (22:6n3), which was inversely associated with incident CKD. | These findings support using metabolomic markers to improve dietary assessment and kidney disease prevention. The link between fish consumption, 1-docosahexaenoylglycerophosphocholine (22:6n3), and lower CKD risk reinforces guidelines recommending a Mediterranean diet for CKD patients. | Bernard et al. [23] |
| Plasma | A prospective cohort study of 484 cases of chronic kidney disease in children participants. Linear regression examined associations between dietary intake of total, animal, and plant protein, as well as chicken, dairy, nuts and beans, red and processed meat, fish, and eggs. Cox models assessed the link between protein-related metabolites and CKD progression, adjusting for demographic and clinical covariates. | Untargeted metabolomic analysis/ LC-MS | Sixty metabolites were linked to dietary protein intake, with ten also associated with CKD progression (animal protein: 1, dairy: 7, red and processed meat: 2, nuts and beans: 1). These included amino acids, lipids, nucleotides, and other compounds. Notably, GPE (P-16:0/18:1), linked to red and processed meat, was associated with an 88% higher CKD risk, while 3- ureidopropionate showed a 48% lower risk. | Distinct metabolic properties and biomarkers of dietary protein sources suggest that metabolites form animal proteins, especially red and processed meat, may negatively impact kidney health in children. This highlights the need for protein source consideration in pediatric dietary guidelines. | Ren et al. [32] |
| Serum | A randomized clinical trial examined dietary protein restriction in CKD patients (age 18–70). Participants with moderate CKD (n = 585) followed either a moderate– or low-protein diet, while those with severe CKD (n = 255) followed a low- or very-low diet. Multivariable linear regression was used to analyze differences in log-transformed metabolite levels based on randomly assigned dietary protein intervention groups. | Untargeted metabolomic analysis/ RP-UHPLC/MS | 130 metabolites differed significantly between participants on a low-protein vs. moderate-protein diet, and 32 metabolites between those on a very-low-protein diet vs. low-protein diet; 11 metabolites were consistently associated with protein intake across both studies: 3-methylhistidine, N-acetyl-3-methylhistidine, xanthurenate, isovalerylcarnitine, creatine, kynurenate, 1-(1-enyl-palmitoyl)-2-arachidonoyl-GPE (P-16:0/20:4), 1-(1-enyl-stearoyl)-2-arachidonoyl-GPE (P-18:0/20:4), 1-(1-enyl-palmitoyl)-2-arachidonoyl-GPC (P-16:0/20:4), sulfate, and γ-glutamylalanine. | Metabolomic blood biomarkers offer a valuable tool for assessing protein intake, aiding dietary interventions for CKD management. Given the challenges of long-term dietary modifications, metabolite measurements can help monitor adherence in clinical trials and guide targeted nutrition counseling for improved patient outcomes. | Rebholz et al. [33] |
| Kidney tissue | A study on mice with adenine-induced chronic kidney disease divided into three groups (n = 10 per group): (1) control group—fed a standard diet, (2) model group—fed a diet containing 0.2% adenine; and (3) sheep milk group—fed a diet with 0.2% adenine supplemented with 1.25 mL of sheep milk fed for four weeks. | Proteomic analysis/SDS-PAGE and LC-MS/MS Non-targeted metabolomic analysis/LC-MS/MS | Proteomic analysis revealed decresed expression of fibrosis-associated proteins, including VCAM1 and collagen, suggesting a role in slowing CKD progression. Metabolomic profiling showed decresed TMAO levels, a biomarker of kidney damage. Integrated data confirmed the down-regulation of the JAK1/STAT3/HIF-1α signaling pathway, contributing to the delay in renal injury. | These findings suggest that sheep milk may protect against kidney damage in CKD by inhibiting fibrosis and inflammation through the JAK1/STAT3/HIF-1α pathway. Its anti-inflammatory, antifibrotic, and anti-apoptotic properties make it a potential adjunct for CKD management. However, further research is needed to identify the specific bioactive compounds responsible for these effects. | Wei et al. [35] |
| Sample Type | Study Design | Type of Analysis/Analysis Platform | Major Findings | Reference |
|---|---|---|---|---|
| Kidney tissue | A study on 6-week-old male C57BL/6N mice divided into two groups (n = 7 per group): (1) control group—fed a normal chow diet (10% fat), (2) HFD group—fed a high-fat-diet (60% fat) for fourteen weeks. | Proteomic analysis/nanoHPLC MS/MS | The HF diet up-regulated renal proteins involved in lipid transport, storage, and localization. The HF diet altered lipid metabolism from peroxisomes to mitochondria by down-regulating peroxisomal proteins and key components of the PPAR pathway. Increased expression of HMGCS2, CPT2, FABP4, ACAA2, ACOT2, CPT1A, and ALDH2, while BDH1 and ACADL were down-regulated. | Dozio et al. [46] |
| Kidney tissue | A study on 10-week-old male Swiss Webster mice divided into four groups (n = 6 per group): (1) STD group—fed a standard diet, (2) SFA group—fed a diet rich in saturated fatty acids (3) HR group—fed a HFD diet rich in polyunsaturated fatty acids with a linoleic to α-linolenic acid ratio of 14:1 (4) LR group—fed a HFD diet rich in polyunsaturated fatty acids with a linoleic to α-linolenic acid ratio of 5:1 for three months. | Proteomic analysis/ 2-DE MALDI-TOF MS | SFA diet affected 11 proteins: 7 up-regulated (PRDX6, PRDX1, PPIA, LDHD, ACOT2, HIBADH, and ALDH6A1) and 4 down-regulated (HSPD1, Apo-E, IDH1, and ATP5F1B). In the HR group, 7 proteins were altered: 4 down-regulated (HSPA5, PRDX6, ATP5F1B, and ALDH6A1), and 3 up-regulated (PPIA, AKR1A1, and IDH2). The LR diet altered 12 proteins: 9 up-regulated (PRDX1, P4HB, AKR1A1, ENO1, ETHE1, IDH1, ETFA, ALDH6A1, and HAAO) and 3 down-regulated (IDH1, ATP5F1B). | Wypych et al. [47] |
| Kidney tissue | A study on 5-week-old male C57BL/6J mice divided into four groups: (1) control—standard chow diet (2) WD group—high-fat (42% kcal), high-saturated fatty acids (>60% total), and high-sucrose (34%) diet for 8 weeks, (3) control + AA—standard chow with aristolochic acid (AA) every three days for 3 weeks, and (4) WD + AA—WD diet with AA every three days for 3 weeks. | Proteomic analysis/ TMT-labeled peptides were analyzed on an Orbitrap Eclipse Tribrid Mass Spectrometer | Approximately 1000 proteins were differentially expressed in the kidneys of AA-treated mice on a WD compared to controls. WD exacerbated AA-induced down-regulation of carbon metabolism pathways, including glycolysis, pyruvate, TCA, and fatty acid metabolism, indicating impaired kidney energy homeostasis. This group also showed increased immune-related proteins, suggesting kidney inflammation. The WD diet alone altered 19 proteins compared to controls, with 12 expressed in the proximal tubules. Up-regulated proteins: Arg2, Ces1d, Glud1, Pck1, Ugt2b37, and Vill. Down-regulated proteins: Acmsd, Acsm3, Car3, Glul, Hsd11b1, and Phgdh. | Oe et al. [49] |
| Kidney tissue, serum | A study on 8-week-old male C57BL/6 mice divided into three groups (n = 7 per group): (1) control group—fed a standard diet, (2) HGD group—fed high-glucose diet (75.9% carbohydrate, 14.7% protein and 9.4% fat), (3) HFD group—fed a high-fat diet (25% carbohydrate, 15% protein and 60% fat) | Untargeted metabolomic analysis/ GC-MS | The HFD diet affected 28 metabolites (AA, FA derivatives and others), with 9 increased and 1 decreased in serum, and 6 in the kidney. These metabolites are involved in many metabolic pathways related to energy, amino acid, and lipid metabolism. | Xie et al. [50] |
| Serum, liver, muscle tissue | A study on male Sprague Dawley rats. Rats underwent 5/6 nephrectomy for CKD induction or sham surgery. After two weeks, rats were fed a standard chow diet (SCD) or a high-fat diet (HFD) for 16 weeks to produce rat models for CKD-induced insulin resistance or HFD. | Untargeted metabolomic analysis/ UPLC-MS/OPLS-DA | A total of 101 metabolites in serum, 59 in liver, and 41 in muscle were associated with CKD-induced IR, while 58 in serum, 38 in liver, and 17 in muscle were linked to HFD-induced IR. CKD affected tryptophan and arginine metabolism, whereas HFD impaired lipid and purine metabolism. | Xu et al. [51] |
| Sample Type | Study Design | Type of Analysis/Analysis Platform | Major Findings | Reference |
|---|---|---|---|---|
| Cecal contents | A study on 10-week-old male Sprague Dawley rats (n = 9 per group) induced CKD with a diet containing 0.7% adenine for 2 weeks, followed by a three-week intervention with either digestible starch (amylopectin)—CKD-DS group—or indigestible starch (HAMRS2)—CKD-RS group. Both isocaloric diets contained 14.5% protein, 66.9% carbohydrate, and 18.6% fat. | Proteomic analysis/TMT—Orbitrap Fusion Tribrid mass spectrometer iBAQ HPLC | A total of 9386 unique proteins were identified, with 5834 quantified. In CKD-RS vs. CKD-DS rats, 125 proteins with reduced expression (enzymes, proteins associated with humoral immune response, epithelial–mesenchymal transition (thioredoxin, S100-A6)) while 54 increased (enzymes, immunoglobulins, annexins, ion channel proteins, and sodium pump proteins). | Zybailov et al. [60] |
| Plasma, urine | A nonrandomized, open-label, 3-phase crossover study with repeated measures. Of 17 eligible subjects, 13 completed treatment. Phases included pretreatment (weeks 1–8), p-inulin treatment (weeks 9–20, 8g p-inulin twice daily), and post-treatment (weeks 21–28). | Untargeted metabolomics analysis/ GC-MS | Urinary levels of carbohydrate metabolites, including raffinose, 1-kestose, and beta-gentiobiosis, increased. During p-inulin supplementation, urine levels of beta-sitosterol and 4-methylcatechol increased, with 4-methylcatechol, inversely correlated with p-cresol. | Sohn et al. [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabała, S.; Herosimczyk, A. Diet-Induced Proteomic and Metabolomic Signatures in Chronic Kidney Disease: A Precision Nutrition Approach. Metabolites 2025, 15, 211. https://doi.org/10.3390/metabo15030211
Cabała S, Herosimczyk A. Diet-Induced Proteomic and Metabolomic Signatures in Chronic Kidney Disease: A Precision Nutrition Approach. Metabolites. 2025; 15(3):211. https://doi.org/10.3390/metabo15030211
Chicago/Turabian StyleCabała, Sandra, and Agnieszka Herosimczyk. 2025. "Diet-Induced Proteomic and Metabolomic Signatures in Chronic Kidney Disease: A Precision Nutrition Approach" Metabolites 15, no. 3: 211. https://doi.org/10.3390/metabo15030211
APA StyleCabała, S., & Herosimczyk, A. (2025). Diet-Induced Proteomic and Metabolomic Signatures in Chronic Kidney Disease: A Precision Nutrition Approach. Metabolites, 15(3), 211. https://doi.org/10.3390/metabo15030211







