Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health
Abstract
1. Introduction
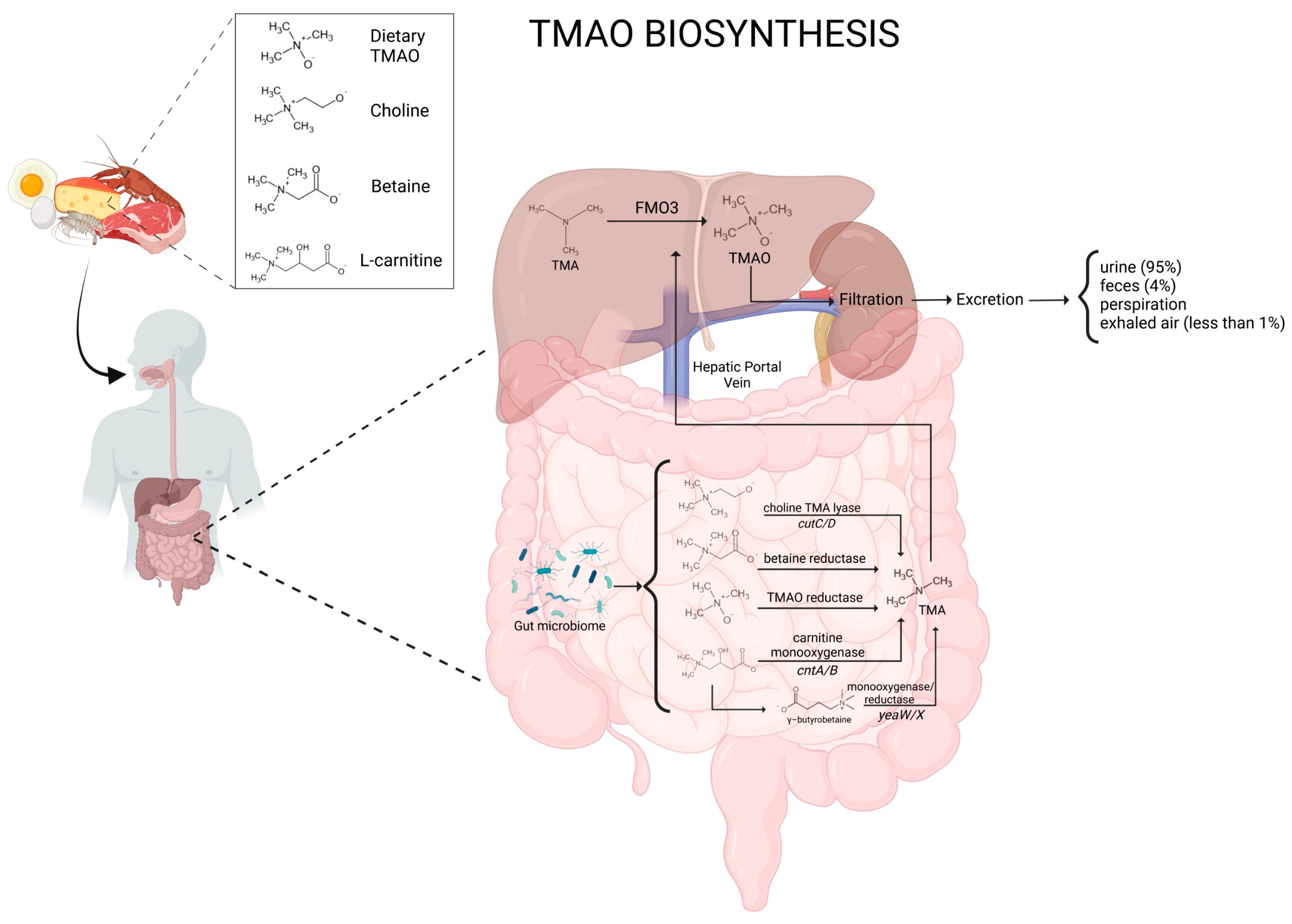
1.1. Biosynthesis of TMAO
1.2. TMAO Metabolism
1.3. Physiological Functions of TMAO
2. TMAO’s Cellular Effect
2.1. Arteriosclerosis
2.2. Inflammation
2.3. Endothelial Progenitor Cells
2.4. Monocyte–Macrophage Axis
2.5. Platelets
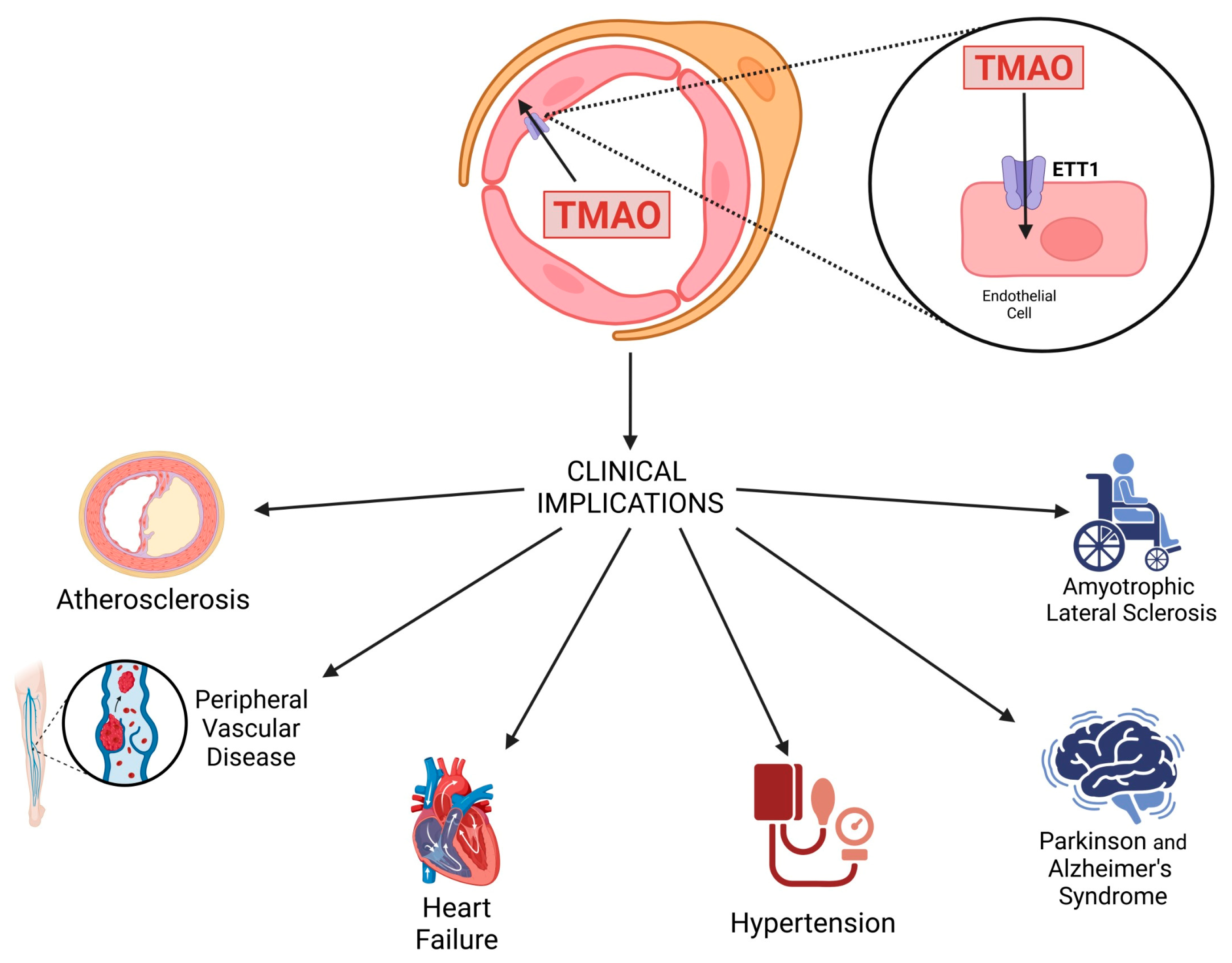
3. Clinical Implications of TMAO
3.1. Atherosclerosis
3.2. Heart Failure
3.3. Peripheral Vascular Disease
3.4. Hypertension
4. Involvement of TMAO in Neurodegenerative Diseases
4.1. Alzheimer’s Disease
4.2. Parkinson’s Disease
4.3. Amyotrophic Lateral Sclerosis
5. Current Strategies to Manage TMAO Levels
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Razavi, A.C.; Potts, K.S.; Kelly, T.N.; Bazzano, L.A. Sex, gut microbiome, and cardiovascular disease risk. Biol. Sex Differ. 2019, 10, 29. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and trimethylamine N-oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Yang, G.; Wang, P.; Xu, C. The potential role of mitochondria in the microbiota-gut-brain axis: Implications for brain health. Pharmacol. Res. 2024, 209, 107434. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Kreft, S. Gut Microbiota and the Metabolism of Phytoestrogens. Rev. Bras. Farmacogn. 2020, 30, 145–154. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-oxide: The good, the bad and the unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediators Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio 2015, 6, e02481-14. [Google Scholar] [CrossRef]
- Martínez-del Campo, A.; Bodea, S.; Hamer, H.A.; Marks, J.A.; Haiser, H.J.; Turnbaugh, P.J.; Balskusa, E.P. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. MBio 2015, 6, e00042-15. [Google Scholar] [CrossRef]
- Rath, S.; Rud, T.; Pieper, D.H.; Vital, M. Potential TMA-Producing Bacteria Are Ubiquitously Found in Mammalia. Front. Microbiol. 2020, 10, 2966. [Google Scholar] [CrossRef]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D.; et al. γ-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef]
- Wu, W.-K.; Lo, Y.-L.; Chiu, J.-Y.; Hsu, C.-L.; Lo, I.-H.; Panyod, S.; Liao, Y.-C.; Chiu, T.H.T.; Yang, Y.-T.; Kuo, H.-C.; et al. Gut microbes with the gbu genes determine TMAO production from L-carnitine intake and serve as a biomarker for precision nutrition. Gut Microbes 2025, 17, 2446374. [Google Scholar] [CrossRef]
- Zhen, J.; Zhou, Z.; He, M.; Han, H.X.; Lv, E.H.; Wen, P.B.; Liu, X.; Wang, Y.T.; Cai, X.C.; Tian, J.Q.; et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front. Endocrinol. 2023, 14, 1085041. [Google Scholar] [CrossRef]
- Ilyas, A.; Wijayasinghe, Y.S.; Khan, I.; El Samaloty, N.M.; Adnan, M.; Dar, T.A.; Poddar, N.K.; Singh, L.R.; Sharma, H.; Khan, S. Implications of trimethylamine N-oxide (TMAO) and Betaine in Human Health: Beyond Being Osmoprotective Compounds. Front. Mol. Biosci. 2022, 9, 964624. [Google Scholar] [CrossRef] [PubMed]
- Andreesen, J.R. Glycine metabolism in anaerobes. Antonie Van Leeuwenhoek 1994, 66, 223–237. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Jin, S.; Lv, J.; Li, M.; Feng, N. The gut microbiota derived metabolite trimethylamine N-oxide: Its important role in cancer and other diseases. Biomed. Pharmacother. 2024, 177, 117031. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-Oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Perez-Paramo, Y.X.; Chen, G.; Ashmore, J.H.; Watson, C.J.W.; Nasrin, S.; Adams-Haduch, J.; Wang, R.; Gao, Y.T.; Koh, W.P.; Yuan, J.M.; et al. Nicotine-N’-oxidation by flavin monooxygenase enzymes. Cancer Epidemiol. Biomark. Prev. 2019, 28, 311–320. [Google Scholar] [CrossRef]
- Ma, S.R.; Tong, Q.; Lin, Y.; Pan, L.B.; Fu, J.; Peng, R.; Zhang, X.F.; Zhao, Z.X.; Li, Y.; Yu, J.B.; et al. Berberine treats atherosclerosis via a vitamine-like effect down-regulating Choline-TMA-TMAO production pathway in gut microbiota. Signal Transduct. Target. Ther. 2022, 7, 207. [Google Scholar] [CrossRef]
- Hartiala, J.; Bennett, B.J.; Tang, W.H.W.; Wang, Z.; Stewart, A.F.R.; Roberts, R.; McPherson, R.; Lusis, A.J.; Hazen, S.L.; Allayee, H. Comparative genome-wide association studies in mice and humans for trimethylamine N-Oxide, a proatherogenic metabolite of choline and L-carnitine. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1307–1313. [Google Scholar] [CrossRef]
- Uno, Y.; Makiguchi, M.; Ushirozako, G.; Tsukiyama-Kohara, K.; Shimizu, M.; Yamazaki, H. Molecular and functional characterization of flavin-containing monooxygenases (FMO1–6) in tree shrews. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 277, 109835. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.K.; Adman, E.T.; Rettie, A.E. Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria. Arch. Biochem. Biophys. 2007, 464, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Teng, Y.; Xie, W.; Huang, L.; Wu, J.; Wang, H.; Xie, S. Effect of three oral pathogens on the TMA-TMAO metabolic pathway. Front. Cell. Infect. Microbiol. 2024, 14, 1413787. [Google Scholar] [CrossRef]
- Saaoud, F.; Liu, L.; Xu, K.; Cueto, R.; Shao, Y.; Lu, Y.; Sun, Y.; Snyder, N.W.; Wu, S.; Yang, L.; et al. Aorta- and liver-generated TMAO enhances trained immunity for increased inflammation via ER stress/mitochondrial ROS/glycolysis pathways. JCI Insight 2023, 8, 18–20. [Google Scholar] [CrossRef]
- Nasralla, M.; Laurent, H.; Baker, D.L.; Ries, M.E.; Dougan, L. A study of the interaction between TMAO and urea in water using NMR spectroscopy. Phys. Chem. Chem. Phys. 2022, 24, 21216–21222. [Google Scholar] [CrossRef]
- Lidbury, I.; Murrell, J.C.; Chen, Y. Trimethylamine N-oxide metabolism by abundant marine heterotrophic bacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 2710–2715. [Google Scholar] [CrossRef]
- Laurent, H.; Youngs, T.G.A.; Headen, T.F.; Soper, A.K.; Dougan, L. The ability of trimethylamine N-oxide to resist pressure induced perturbations to water structure. Commun. Chem. 2022, 5, 116. [Google Scholar] [CrossRef]
- Rani, A.; Jayaraj, A.; Jayaram, B.; Pannuru, V. Trimethylamine-N-oxide switches from stabilizing nature: A mechanistic outlook through experimental techniques and molecular dynamics simulation. Sci. Rep. 2016, 6, 23656. [Google Scholar] [CrossRef]
- Jeyarajah, E.J.; Li, X.S.; Jia, X.; Weeks, T.L. Circulating trimethylamine N-oxide levels following fish or seafood consumption. Eur. J. Nutr. 2023, 61, 2357–2364. [Google Scholar] [CrossRef]
- Shanmugham, M.; Bellanger, S.; Leo, C.H. Gut-Derived Metabolite, Trimethylamine-N-oxide (TMAO) in Cardio-Metabolic Diseases: Detection, Mechanism, and Potential Therapeutics. Pharmaceuticals 2023, 16, 504. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Jiang, H. Gut microbiota in atherosclerosis: Focus on trimethylamine N-oxide. Apmis 2020, 128, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. J. Cell. Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Caradonna, E.; Nemni, R.; Bifone, A.; Gandolfo, P.; Costantino, L.; Giordano, L.; Mormone, E.; Macula, A.; Cuomo, M.; Difruscolo, R.; et al. The Brain–Gut Axis, an Important Player in Alzheimer and Parkinson Disease: A Narrative Review. J. Clin. Med. 2024, 13, 4130. [Google Scholar] [CrossRef]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Mirji, G.; Worth, A.; Bhat, S.A.; El Sayed, M.; Kannan, T.; Goldman, A.R.; Tang, H.; Liu, Q.; Auslander, N.; Dang, C.V.; et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci. Immunol. 2023, 7, eabn0704. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Q.Q.; Li, L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genom. 2015, 16, S4. [Google Scholar] [CrossRef]
- Rittig, S.T.; Gaal, L.; Schubert, M.R.; Kneuer, J.M.; Kokot, K.E.; Hauke, J.; Okun, J.G.; Laufs, U.; Boeckel, J.-N. Vascular Impairment by Trimethylamine N-Oxide (TMAO) Uptake and the First Description of a TMAO Transporter in Endothelial Cells. Circulation 2021, 144, A10579. [Google Scholar] [CrossRef]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Chen, M.L.; Zhu, X.H.; Ran, L.; Lang, H.D.; Yi, L.; Mi, M.T. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Guarente, L. The SirT3 Divining Rod Points to Oxidative Stress. Mol. Cell 2011, 42, 561–568. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Armstrong, E.J.; Larson, C.J.; Brass, E.P. Pathogenesis of the Limb Manifestations and Exercise Limitations in Peripheral Artery Disease. Circ. Res. 2015, 116, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, S.; Zhang, B.; Liu, J. SIRT3 as a potential therapeutic target for heart failure. Pharmacol. Res. 2021, 165, 105432. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid. Redox Signal. 2018, 28, 711–732. [Google Scholar] [CrossRef]
- Ke, Y.; Li, D.; Zhao, M.; Liu, C.; Liu, J.; Zeng, A.; Shi, X.; Cheng, S.; Pan, B.; Zheng, L.; et al. Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic. Biol. Med. 2018, 116, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Geng, J.; Yang, C.; Wang, B.; Zhang, X.; Hu, T.; Gu, Y.; Li, J. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed. Pharmacother. 2018, 97, 941–947. [Google Scholar] [CrossRef]
- Febbraio, M.; Silverstein, R.L. CD36: Implications in cardiovascular disease. Int. J. Biochem. Cell Biol. 2007, 39, 2012–2030. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–65. [Google Scholar] [CrossRef]
- Xiong, X.; Zhou, J.; Fu, Q.; Xu, X.; Wei, S.; Yang, S.; Chen, B. The associations between TMAO-related metabolites and blood lipids and the potential impact of rosuvastatin therapy. Lipids Health Dis. 2022, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Oktaviono, Y.H.; Dyah Lamara, A.; Saputra, P.B.T.; Arnindita, J.N.; Pasahari, D.; Saputra, M.E.; Suasti, N.M.A. The roles of trimethylamine-N-oxide in atherosclerosis and its potential therapeutic aspect: A literature review. Biomol. Biomed. 2023, 23, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Helsley, R.N.; Brown, A.L.; Buffa, J.A.; Choucair, I.; Nemet, I.; Gogonea, C.B.; Gogonea, V.; Wang, Z.; Garcia-Garcia, J.C.; et al. Small molecule inhibition of gut microbial choline trimethylamine lyase activity alters host cholesterol and bile acid metabolism. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1474–H1486. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and reverse cholesterol transport: Basic mechanisms and their roles in vascular health and disease. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Li, X.; Geng, J.; Zhao, J.; Ni, Q.; Zhao, C.; Zheng, Y.; Chen, X.; Wang, L. Trimethylamine N-oxide exacerbates cardiac fibrosis via activating the NLRP3 inflammasome. Front. Physiol. 2019, 10, 866. [Google Scholar] [CrossRef]
- Lei, D.; Yu, W.; Liu, Y.; Jiang, Y.; Li, X.; Lv, J.; Li, Y. Trimethylamine N-Oxide (TMAO) Inducing Endothelial Injury: UPLC-MS/MS-Based Quantification and the Activation of Cathepsin B-Mediated NLRP3 Inflammasome. Molecules 2023, 28, 3817. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R.; Al-Arawe, N.; Hinterseher, I. The Role of the Gut Microbiome and Trimethylamine Oxide in Atherosclerosis and Age-Related Disease. Int. J. Mol. Sci. 2023, 24, 2399. [Google Scholar] [CrossRef]
- Perner, C.; Krüger, E. Endoplasmic Reticulum Stress and Its Role in Homeostasis and Immunity of Central and Peripheral Neurons. Front. Immunol. 2022, 13, 859703. [Google Scholar] [CrossRef]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019, 30, 1141–1151.e5. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pinky, P.D.; Steinke, I.; Bloemer, J.; Ramesh, S.; Kariharan, T.; Rella, R.T.; Bhattacharya, S.; Dhanasekaran, M.; Suppiramaniam, V.; et al. Gut Metabolite TMAO Induces Synaptic Plasticity Deficits by Promoting Endoplasmic Reticulum Stress. Front. Mol. Neurosci. 2020, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, E.; Pascale, A.; Marchesi, N.; Ferrero, P.; Senni, M.; Govoni, S.; Gronda, E.; Vanoli, E. Novel approaches to the post-myocardial infarction/heart failure neural remodeling. Heart Fail. Rev. 2014, 19, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, S.; Zhou, X.; Huang, B.; Zhou, L.; Li, X.; Meng, G.; Yuan, S.; Wang, Y.; Wang, Z.; et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int. J. Cardiol. 2017, 248, 286–293. [Google Scholar] [CrossRef]
- Wang, P.; Mi, Y.; Yu, H.; Teng, X.; Jin, S.; Xiao, L.; Xue, H.; Tian, D.; Guo, Q.; Wu, Y. Trimethylamine-N-oxide aggravated the sympathetic excitation in D-galactose induced aging rats by down-regulating P2Y12 receptor in microglia. Biomed. Pharmacother. 2024, 174, 116549. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Li, B.; Yang, P. Prognosis evaluation of universal acute coronary syndrome: The interplay between SYNTAX score and ApoB/ApoA1. BMC Cardiovasc. Disord. 2020, 20, 293. [Google Scholar] [CrossRef]
- Lai, N.Y.; Mills, K.; Chiu, I.M. Sensory neuron regulation of gastrointestinal inflammation and bacterial host defence. J. Intern. Med. 2017, 282, 5–23. [Google Scholar] [CrossRef]
- Sen, S.; McDonald, S.P.; Coates, P.T.H.; Bonder, C.S. Endothelial progenitor cells: Novel biomarker and promising cell therapy for cardiovascular disease. Clin. Sci. 2011, 120, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Li, Q.; Ghasemzadeh, N.; Eapen, D.J.; Moss, L.D.; Janjua, A.U.; Manocha, P.; Al Kassem, H.; Veledar, E.; Samady, H.; et al. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ. Res. 2015, 116, 289–297. [Google Scholar] [CrossRef]
- Maltais, S.; Perrault, L.P.; Ly, H.Q. The bone marrow-cardiac axis: Role of endothelial progenitor cells in heart failure. Eur. J. Cardio-Thorac. Surg. 2011, 39, 368–374. [Google Scholar] [CrossRef]
- Rigato, M.; Avogaro, A.; Fadini, G.P. Levels of Circulating Progenitor Cells, Cardiovascular Outcomes and Death: A Meta-Analysis of Prospective Observational Studies. Circ. Res. 2016, 118, 1930–1939. [Google Scholar] [CrossRef]
- Chou, R.H.; Chen, C.Y.; Chen, I.C.; Huang, H.L.; Lu, Y.W.; Kuo, C.S.; Chang, C.C.; Huang, P.H.; Chen, J.W.; Lin, S.J. Trimethylamine N-Oxide, Circulating Endothelial Progenitor Cells, and Endothelial Function in Patients with Stable Angina. Sci. Rep. 2019, 9, 4249. [Google Scholar] [CrossRef]
- Syu, J.N.; Lin, H.Y.; Huang, T.Y.; Lee, D.Y.; Chiang, E.P.I.; Tang, F.Y. Docosahexaenoic Acid Alleviates Trimethylamine-N-oxide-mediated Impairment of Neovascularization in Human Endothelial Progenitor Cells. Nutrients 2023, 15, 2190. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Y.; Tu, J.; Sun, J.; Dong, B.; Wang, Z.; Zhou, J.; Chen, L.; Tao, J.; Chen, J. TMAO-Activated Hepatocyte-Derived Exosomes Impair Angiogenesis via Repressing CXCR4. Front. Cell Dev. Biol. 2022, 9, 804049. [Google Scholar] [CrossRef]
- Williams, H.; Mack, C.; Baraz, R.; Marimuthu, R.; Naralashetty, S.; Li, S.; Medbury, H. Monocyte Differentiation and Heterogeneity: Inter-Subset and Interindividual Differences. Int. J. Mol. Sci. 2023, 24, 8757. [Google Scholar] [CrossRef]
- Park, M.D.; Silvin, A.; Ginhoux, F.; Merad, M. Macrophages in health and disease. Cell 2022, 185, 4259–4279. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Li, X.S.; Liman, T.G.; Bledau, N.; Schmidt, D.; Zimmermann, F.; Kränkel, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; et al. Gut microbiota-dependent TMAO predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arter. Thromb. Vasc. Biol. 2018, 38, 2225–2235. [Google Scholar] [CrossRef]
- Hakhamaneshi, M.S.; Abdolahi, A.; Vahabzadeh, Z.; Abdi, M.; Andalibi, P. Toll-like receptor 4: A macrophage cell surface receptor is activated by trimethylamine-n-oxide. Cell J. 2021, 23, 516–522. [Google Scholar] [CrossRef]
- Rogacev, K.S.; Cremers, B.; Zawada, A.M.; Seiler, S.; Binder, N.; Ege, P.; Große-Dunker, G.; Heisel, I.; Hornof, F.; Jeken, J.; et al. CD14++CD16+ monocytes independently predict cardiovascular events: A cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol. 2012, 60, 1512–1520. [Google Scholar] [CrossRef]
- Wang, B.Y.; Qiu, J.; Lian, J.F.; Yang, X.; Zhou, J.Q. Gut Metabolite Trimethylamine-N-Oxide in Atherosclerosis: From Mechanism to Therapy. Front. Cardiovasc. Med. 2021, 8, 723886. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Malek, M.; Wawrzyniak, A.M.; Koch, P.; Lüchtenborg, C.; Hessenberger, M.; Sachsenheimer, T.; Jang, W.; Brügger, B.; Haucke, V. Inositol triphosphate-triggered calcium release blocks lipid exchange at endoplasmic reticulum-Golgi contact sites. Nat. Commun. 2021, 12, 2673. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Qiu, X.; Liu, Y.; Yuan, C.; Yang, X. Trimethylamine N-oxide promotes tissue factor expression and activity in vascular endothelial cells: A new link between trimethylamine N-oxide and atherosclerotic thrombosis. Thromb. Res. 2019, 177, 110–116. [Google Scholar] [CrossRef]
- Ge, P.X.; Tai, T.; Jiang, L.P.; Ji, J.Z.; Mi, Q.Y.; Zhu, T.; Li, Y.F.; Xie, H.G. Choline and trimethylamine N-oxide impair metabolic activation of and platelet response to clopidogrel through activation of the NOX/ROS/Nrf2/CES1 pathway. J. Thromb. Haemost. 2023, 21, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Duan, H.; Tao, C.; Niu, S.; Hu, Y.; Duan, R.; Shen, A.; Sun, Y.; Sun, W. TMAO Promotes NLRP3 Inflammasome Activation of Microglia Aggravating Neurological Injury in Ischemic Stroke Through FTO/IGF2BP2. J. Inflamm. Res. 2023, 16, 3699–3714. [Google Scholar] [CrossRef] [PubMed]
- Boini, K.M.; Hussain, T.; Li, P.L.; Koka, S.S. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell. Physiol. Biochem. 2017, 44, 152–162. [Google Scholar] [CrossRef]
- Ma, G.H.; Pan, B.; Chen, Y.; Guo, C.X.; Zhao, M.M.; Zheng, L.M.; Chen, B.X. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017, 37, BSR20160244. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.F.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κb. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights Into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, J.; Liu, C.; Zhou, P.; Sheng, Z.; Li, J.; Chen, R.; Song, L.; Zhao, H.; Xu, B.; et al. Association Between Plasma Trimethylamine N-oxide and Neoatherosclerosis in Patients With Very Late Stent Thrombosis. Can. J. Cardiol. 2020, 36, 1252–1260. [Google Scholar] [CrossRef]
- Li, J.; Sheng, Z.; Tan, Y.; Zhou, P.; Liu, C.; Zhao, H.; Song, L.; Zhou, J.; Chen, R.; Chen, Y.; et al. Association of plasma trimethylamine N-Oxide level with healed culprit plaques examined by optical coherence tomography in patients with ST-Segment elevation myocardial infarction. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 145–152. [Google Scholar] [CrossRef]
- Pescari, D.; Mihuta, M.S.; Bena, A.; Stoian, D. Independent Predictors of Circulating Trimethylamine N-Oxide (TMAO) and Resistin Levels in Subjects with Obesity: Associations with Carotid Intima-Media Thickness and Metabolic Parameters. Nutrients 2025, 17, 798. [Google Scholar] [CrossRef]
- Bao, M.; Li, H.; Li, J. Circulating trimethylamine N-oxide is correlated with high coronary artery atherosclerotic burden in individuals with newly diagnosed coronary heart disease. BMC Cardiovasc. Disord. 2024, 24, 265. [Google Scholar] [CrossRef]
- Farhangi, M.A. Gut microbiota-dependent trimethylamine N-oxide and all-cause mortality: Findings from an updated systematic review and meta-analysis. Nutrition 2020, 78, 110856. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef]
- Papandreou, C.; Bulló, M.; Hernández-Alonso, P.; Ruiz-Canela, M.; Li, J.; Guasch-Ferre, M.; Toledo, E.; Clish, C.; Corella, D.; Estruch, R.; et al. Choline Metabolism and Risk of Atrial Fibrillation and Heart Failure in the PREDIMED Study. Clin. Chem. 2021, 67, 288–297. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Li, X.S.; Wu, Y.; Wang, Z.; Khaw, K.T.; Wareham, N.J.; Nieuwdorp, M.; Boekholdt, S.M.; Hazen, S.L. Plasma trimethylamine N-oxide (TMAO) levels predict future risk of coronary artery disease in apparently healthy individuals in the EPIC-Norfolk prospective population study. Am. Heart J. 2021, 236, 80–86. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; DiDonato, J.A.; Sun, Q.; Rimm, E.B.; Hu, F.B.; Rexrode, K.M.; Manson, J.A.E.; Qi, L. Long-Term Changes in Gut Microbial Metabolite Trimethylamine N-Oxide and Coronary Heart Disease Risk. J. Am. Coll. Cardiol. 2020, 75, 763–772. [Google Scholar] [CrossRef]
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Räber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gutmicrobiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017, 38, 814–824. [Google Scholar] [CrossRef]
- Senthong, V.; Wang, Z.; Li, X.S.; Fan, Y.; Wu, Y.; Tang, W.H.W.; Hazen, S.L. Intestinal microbiota-generated metabolite Trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: The contributory role of intestinal microbiota in a COURAGE-like patient cohort. J. Am. Heart Assoc. 2016, 5, e002816. [Google Scholar] [CrossRef] [PubMed]
- Oakley, C.I.; Vallejo, J.A.; Wang, D.; Gray, M.A.; Tiede-Lewis, L.M.; Shawgo, T.; Daon, E.; Zorn, G.; Stubbs, J.R.; Wacker, M.J. Trimethylamine-N-oxide acutely increases cardiac muscle contractility. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1272–H1282. [Google Scholar] [CrossRef]
- Li, W.; Huang, A.; Zhu, H.; Liu, X.; Huang, X.; Huang, Y.; Cai, X.; Lu, J.; Huang, Y. Gut microbiota-derived trimethylamine N-oxide is associated with poor prognosis in patients with heart failure. Med. J. Aust. 2020, 213, 374–379. [Google Scholar] [CrossRef]
- Suzuki, T.; Heaney, L.M.; Bhandari, S.S.; Jones, D.J.L.; Ng, L.L. Trimethylamine N-oxide and prognosis in acute heart failure. Heart 2016, 102, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, Z.; Cui, J.; Li, D.; Lu, J.; Cui, X.; Xie, L.; Wu, Y.; Lin, Q.; Li, Y. Trimethylamine N-Oxide in Heart Failure: A Meta-Analysis of Prognostic Value. Front. Cardiovasc. Med. 2022, 9, 817396. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Fan, Y.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic Value of Elevated Levels of Intestinal Microbe Generated TMAO in patients with heart failure: Refining the gut hypothesis. Am. J. Coll. Cardiol. 2014, 64, 1908–1914. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Shrestha, K.; Borowski, A.G.; Wu, Y.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J. Card. Fail. 2015, 21, 91–96. [Google Scholar] [CrossRef]
- Trøseid, M.; Ueland, T.; Hov, J.R.; Svardal, A.; Gregersen, I.; Dahl, C.P.; Aakhus, S.; Gude, E.; Bjørndal, B.; Halvorsen, B.; et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015, 277, 717–726. [Google Scholar] [CrossRef]
- Suzuki, T.; Yazaki, Y.; Voors, A.A.; Jones, D.J.L.; Chan, D.C.S.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Hillege, H.L.; et al. Association with outcomes and response to treatment of trimethylamine N-oxide in heart failure: Results from BIOSTAT-CHF. Eur. J. Heart Fail. 2019, 21, 877–886. [Google Scholar] [CrossRef]
- Kapelios, C.J.; Malliaras, K.; Kaldara, E.; Vakrou, S.; Nanas, J.N. Loop diuretics for chronic heart failure: A foe in disguise of a friend? Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 54–63. [Google Scholar] [CrossRef]
- Li, D.Y.; Wang, Z.; Jia, X.; Yan, D.; Shih, D.M.; Hazen, S.L.; Lusis, A.J.; Tang, W.H.W. Loop Diuretics Inhibit Renal Excretion of Trimethylamine N-Oxide. JACC Basic to Transl. Sci. 2021, 6, 103–115. [Google Scholar] [CrossRef]
- Li, T.; Chen, Y.; Gua, C.; Li, X. Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front. Physiol. 2017, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Senthong, V.; Wang, Z.; Fan, Y.; Wu, Y.; Hazen, S.L.; Tang, W.H.W. Trimethylamine N-oxide and mortality risk in patients with peripheral artery disease. J. Am. Heart Assoc. 2016, 5, e004237. [Google Scholar] [CrossRef] [PubMed]
- Amato, B.; Novellino, E.; Morlando, D.; Vanoli, C.; Vanoli, E.; Ferrara, F.; Difruscolo, R.; Goffredo, V.M.; Compagna, R.; Tenore, G.C.; et al. Benefits of Taurisolo in Diabetic Patients with Peripheral Artery Disease. J. Cardiovasc. Dev. Dis. 2024, 11, 174. [Google Scholar] [CrossRef]
- Roncal, C.; Martínez-Aguilar, E.; Orbe, J.; Ravassa, S.; Fernandez-Montero, A.; Saenz-Pipaon, G.; Ugarte, A.; Estella-Hermoso de Mendoza, A.; Rodriguez, J.A.; Fernández-Alonso, S.; et al. Trimethylamine-N-Oxide (TMAO) Predicts Cardiovascular Mortality in Peripheral Artery Disease. Sci. Rep. 2019, 9, 15580. [Google Scholar] [CrossRef]
- Chen, L.; Jin, Y.; Wang, N.; Yuan, M.; Lin, T.; Lu, W.; Wang, T. Trimethylamine N-oxide impairs perfusion recovery after hindlimb ischemia. Biochem. Biophys. Res. Commun. 2020, 530, 95–99. [Google Scholar] [CrossRef]
- Liang, H.; Yu, A.; Wang, Z.; Zhang, N.; Wang, Q.; Gao, H.; Gao, J.; Wang, X.; Wang, H. Atherosclerotic patients with diabetes mellitus may break through the threshold of healthy TMAO levels formed by long-term statins therapy. Heliyon 2023, 9, e13657. [Google Scholar] [CrossRef]
- Querio, G.; Antoniotti, S.; Geddo, F.; Levi, R.; Gallo, M.P. Modulation of Endothelial Function by TMAO, a Gut Microbiota-Derived Metabolite. Int. J. Mol. Sci. 2023, 24, 5806. [Google Scholar] [CrossRef]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.; et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II–induced hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef]
- Han, J.M.; Guo, L.; Chen, X.H.; Xie, Q.; Song, X.Y.; Ma, Y.L. Relationship between trimethylamine N-oxide and the risk of hypertension in patients with cardiovascular disease: A meta-analysis and dose-response relationship analysis. Medicine 2024, 103, e36784. [Google Scholar] [CrossRef]
- Nie, J.; Xie, L.; Zhao, B.X.; Li, Y.; Qiu, B.; Zhu, F.; Li, G.F.; He, M.; Wang, Y.; Wang, B.; et al. Serum trimethylamine N-oxide concentration is positively associated with first stroke in hypertensive patients. Stroke 2018, 49, 2021–2028. [Google Scholar] [CrossRef]
- Danne, O. Trimethylamine N-oxide and ACE inhibitors: Fighting a new enemy with an established weapon? Biomarkers 2018, 23, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, Q.Q. Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst. Biol. 2016, 10, 63. [Google Scholar] [CrossRef]
- Vernetti, L.; Gough, A.; Baetz, N.; Blutt, S.; Broughman, J.R.; Brown, J.A.; Foulke-Abel, J.; Hasan, N.; In, J.; Kelly, E.; et al. Functional Coupling of Human Microphysiology Systems: Intestine, Liver, Kidney Proximal Tubule, Blood-Brain Barrier and Skeletal Muscle. Sci. Rep. 2017, 7, 42296. [Google Scholar] [CrossRef]
- Del Rio, D.; Zimetti, F.; Caffarra, P.; Tassotti, M.; Bernini, F.; Brighenti, F.; Zini, A.; Zanotti, I. The gut microbial metabolite trimethylamine-N-oxide is present in human cerebrospinal fluid. Nutrients 2017, 9, 1053. [Google Scholar] [CrossRef]
- Hoyles, L.; Jiménez-Pranteda, M.L.; Chilloux, J.; Brial, F.; Myridakis, A.; Aranias, T.; Magnan, C.; Gibson, G.R.; Sanderson, J.D.; Nicholson, J.K.; et al. Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome 2018, 6, 73. [Google Scholar] [CrossRef]
- Levine, Z.A.; Larini, L.; LaPointe, N.E.; Feinstein, S.C.; Shea, J.E. Regulation and aggregation of intrinsically disordered peptides. Proc. Natl. Acad. Sci. USA 2015, 112, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, B. Research progress on the association between Parkinson’s disease and dystonia. Clin. Med. China 2024, 40, 312–316. [Google Scholar] [CrossRef]
- Jackson, R.J.; Rose, J.; Tulloch, J.; Henstridge, C.; Smith, C.; Spires-Jones, T.L. Clusterin accumulates in synapses in Alzheimer’s disease and is increased in apolipoprotein E4 carriers. Brain Commun. 2019, 1, fcz003. [Google Scholar] [CrossRef]
- Scaramozzino, F.; Peterson, D.W.; Farmer, P.; Gerig, J.T.; Graves, D.J.; Lew, J. TMAO promotes fibrillization and microtubule assembly activity in the C-terminal repeat region of tau. Biochemistry 2006, 45, 3684–3691. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Brunt, V.E.; LaRocca, T.J.; Bazzoni, A.E.; Sapinsley, Z.J.; Miyamoto-Ditmon, J.; Gioscia-Ryan, R.A.; Neilson, A.P.; Link, C.D.; Seals, D.R. The gut microbiome–derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience 2021, 43, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Zhou, L.; Wu, Z. The microbiota-gut-brain axis: A potential target in the small-molecule compounds and gene therapeutic strategies for Parkinson’s disease. Neurol. Sci. 2025, 46, 561–578. [Google Scholar]
- Palumbo, L.; Carinci, M.; Guarino, A.; Asth, L.; Zucchini, S.; Missiroli, S.; Rimessi, A.; Pinton, P.; Giorgi, C. The NLRP3 Inflammasome in Neurodegenerative Disorders: Insights from Epileptic Models. Biomedicines 2023, 11, 2825. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in parkinson’s disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Ritson, M.; Wheeler-Jones, C.P.D.; Stolp, H.B. Endothelial dysfunction in neurodegenerative disease: Is endothelial inflammation an overlooked druggable target? J. Neuroimmunol. 2024, 391, 578363. [Google Scholar] [CrossRef] [PubMed]
- Roodveldt, C.; Bernardino, L.; Oztop-Cakmak, O.; Dragic, M.; Fladmark, K.E.; Ertan, S.; Busra, A.; Pita, C.; Ciglar, L.; Garraux, G.; et al. The immune system in Parkinson’s disease: What we know so far. Brain 2024, 147, 3306–3324. [Google Scholar] [CrossRef]
- Uversky, V.N.; Li, J.; Fink, A.L. Trimethylamine-N-oxide-induced folding of K-synuclein. FEBS. Lett 2001, 509, 31–35. [Google Scholar]
- Chen, S.J.; Kuo, C.H.; Kuo, H.C.; Chen, C.C.; Wu, W.K.; Liou, J.M.; Wu, M.S.; Lin, C.H. The Gut Metabolite Trimethylamine N-oxide Is Associated With Parkinson’s Disease Severity and Progression. Mov. Disord. 2020, 35, 2115–2116. [Google Scholar] [CrossRef]
- Voigt, R.M.; Wang, Z.; Brown, J.M.; Engen, P.A.; Naqib, A.; Goetz, C.G.; Hall, D.A.; Metman, L.V.; Shaikh, M.; Forsyth, C.B.; et al. Gut microbial metabolites in Parkinson’s disease: Association with lifestyle, disease characteristics, and treatment status. Neurobiol. Dis. 2022, 170, 105780. [Google Scholar] [CrossRef]
- Lee, A.; Arachchige, B.J.; Reed, S.; Henderson, R.; Aylward, J.; McCombe, P.A. Plasma from some patients with amyotrophic lateral sclerosis exhibits elevated formaldehyde levels. J. Neurol. Sci. 2020, 409, 116589. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Y.; Zhao, M.; Zheng, L.; Fan, D. Changes in the concentrations of trimethylamine N-oxide (TMAO) and its precursors in patients with amyotrophic lateral sclerosis. Sci. Rep. 2020, 10, 15198. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Zhang, H.; Xiang, Q.; Shen, L.; Guo, X.; Zhai, C.; Hu, H. Targeting Trimethylamine N-Oxide: A New Therapeutic Strategy for Alleviating Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 864600. [Google Scholar] [CrossRef]
- Busby, M.G.; Fischer, L.; Da Costa, K.A.; Thompson, D.; Mar, M.H.; Zeisel, S.H. Choline- and betaine-defined diets for use in clinical research and for the management of trimethylaminuria. J. Am. Diet. Assoc. 2004, 104, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Wiese, G.N.; Biruete, A.; Moorthi, R.N.; Moe, S.M.; Lindemann, S.R.; Hill Gallant, K.M. Plant-Based Diets, the Gut Microbiota, and Trimethylamine N-Oxide Production in Chronic Kidney Disease: Therapeutic Potential and Methodological Considerations. J. Ren. Nutr. 2021, 31, 121–131. [Google Scholar] [CrossRef]
- Argyridou, S.; Davies, M.J.; Biddle, G.J.H.; Bernieh, D.; Suzuki, T.; Dawkins, N.P.; Rowlands, A.V.; Khunti, K.; Smith, A.C.; Yates, T. Evaluation of an 8-Week Vegan Diet on Plasma Trimethylamine-N-Oxide and Postchallenge Glucose in Adults with Dysglycemia or Obesity. J. Nutr. 2021, 151, 1844–1853. [Google Scholar] [CrossRef]
- Griffin, L.E.; Djuric, Z.; Angiletta, C.J.; Mitchell, C.M.; Baugh, M.E.; Davy, K.P.; Neilson, A.P. A Mediterranean diet does not alter plasma trimethylamine: N-oxide concentrations in healthy adults at risk for colon cancer. Food Funct. 2019, 10, 2138–2147. [Google Scholar] [CrossRef]
- Qiu, L.; Tao, X.; Xiong, H.; Yu, J.; Wei, H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018, 9, 4299–4309. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, M.; Liu, Y.; Xu, M.; Shi, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Bifidobacterium breve and Bifidobacterium longum Attenuate Choline-Induced Plasma Trimethylamine N-Oxide Production by Modulating Gut Microbiota in Mice. Nutrients 2022, 14, 1222. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Z.; Lv, Y.; Tong, L.; Liu, T.; Yi, H.; Zhou, X.; Yu, Z.; Tian, X.; Cui, Q.; et al. Reduction of intestinal trimethylamine by probiotics ameliorated lipid metabolic disorders associated with atherosclerosis. Nutrition 2020, 79–80, 110941. [Google Scholar] [CrossRef]
- Boutagy, N.E.; Neilson, A.P.; Osterberg, K.L.; Smithson, A.T.; Englund, T.R.; Davy, B.M.; Hulver, M.W.; Davy, K.P. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity 2015, 23, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Latif, F.; Mubbashir, A.; Khan, M.S.; Shaikh, Z.; Memon, A.; Alvares, J.; Azhar, A.; Jain, H.; Ahmed, R.; Kanagala, S.G. Trimethylamine N-oxide in cardiovascular disease: Pathophysiology and the potential role of statins. Life Sci. 2025, 361, 123304. [Google Scholar] [CrossRef]
- Nolan, J.A.; Skuse, P.; Govindarajan, K.; Patterson, E.; Konstantinidou, N.; Casey, P.G.; MacSharry, J.; Shanahan, F.; Stanton, C.; Hill, C.; et al. The influence of rosuvastatin on the gastrointestinal microbiota and host gene expression profiles. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G488–G497. [Google Scholar] [CrossRef]
- Larabi, A.B.; Masson, H.L.P.; Bäumler, A.J. Bile acids as modulators of gut microbiota composition and function. Gut Microbes 2023, 15, 2172671. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Zhou, H.; Zhou, X.; Xia, Y.; Dong, X.; Zhong, W.; Tang, S.; Wang, L.; Wen, S.; et al. Gut microbiome associates with lipid-lowering effect of rosuvastatin in Vivo. Front. Microbiol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Kummen, M.; Solberg, O.G.; Storm-Larsen, C.; Holm, K.; Ragnarsson, A.; Trøseid, M.; Vestad, B.; Skårdal, R.; Yndestad, A.; Ueland, T.; et al. Rosuvastatin alters the genetic composition of the human gut microbiome. Sci. Rep. 2020, 10, 5397. [Google Scholar] [CrossRef]
- Li, D.Y.; Li, X.S.; Chaikijurajai, T.; Li, L.; Wang, Z.; Hazen, S.L.; Tang, W.H.W. Relation of Statin Use to Gut Microbial Trimethylamine N-Oxide and Cardiovascular Risk. Am. J. Cardiol. 2022, 178, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Schefold, J.C.; Filippatos, G.; Hasenfuss, G.; Anker, S.D.; Von Haehling, S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat. Rev. Nephrol. 2016, 12, 610–623. [Google Scholar] [CrossRef]
- Adel, F.W.; Chen, H.H. Trimethylamine-N-Oxide: The Link Between Loop Diuretics and Cardiovascular Outcomes? JACC Basic Transl. Sci. 2021, 6, 116–118. [Google Scholar] [CrossRef]
- Thomas, M.C.; Tikellis, C.; Burns, W.C.; Thallas, V.; Forbes, J.M.; Cao, Z.; Osicka, T.M.; Russo, L.M.; Jerums, G.; Ghabrial, H.; et al. Reduced tubular cation transport in diabetes: Prevented by ACE inhibition. Kidney Int. 2003, 63, 2152–2161. [Google Scholar] [CrossRef]
- Teft, W.A.; Morse, B.L.; Leake, B.F.; Wilson, A.; Mansell, S.E.; Hegele, R.A.; Ho, R.H.; Kim, R.B. Identification and Characterization of Trimethylamine-N-oxide Uptake and Efflux Transporters. Mol. Pharm. 2017, 14, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Konop, M.; Radkowski, M.; Grochowska, M.; Perlejewski, K.; Samborowska, E.; Ufnal, M. Enalapril decreases rat plasma concentration of TMAO, gut bacteria-derived cardiovascular marker. Biomarkers 2018, 23, 380–385. [Google Scholar] [CrossRef]
- Kalagi, N.A.; Abbott, K.A.; Alburikan, K.A.; Alkofide, H.A.; Stojanovski, E.; Garg, M.L. Modulation of Circulating Trimethylamine N-Oxide Concentrations by Dietary Supplements and Pharmacological Agents: A Systematic Review. Adv. Nutr. 2019, 10, 876–887. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio 2016, 7, e02210-15. [Google Scholar] [CrossRef]
- Sulaiman, M.; Matta, M.J.; Sunderesan, N.R.; Gupta, M.P.; Periasamy, M.; Gupta, M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H833–H843. [Google Scholar] [CrossRef]
- Terao, J. Potential Role of Quercetin Glycosides as Anti-Atherosclerotic Food-Derived Factors for Human Health. Antioxidants 2023, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of grape pomace polyphenolic extract (Taurisolo®) in reducing tmao serum levels in humans: Preliminary results from a randomized, placebo-controlled, cross-over study. Nutrients 2019, 11, 139. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Hassan, S.T.S.; Tenore, G.C.; Novellino, E. Effect of grape pomace polyphenols with or without pectin on TMAO serum levels assessed by LC/MS-based assay: A preliminary clinical study on overweight/obese subjects. Front. Pharmacol. 2019, 10, 575. [Google Scholar] [CrossRef]
- Martelli, A.; Flori, L.; Gorica, E.; Piragine, E.; Saviano, A.; Annunziata, G.; Di Minno, M.N.D.; Ciampaglia, R.; Calcaterra, I.; Maione, F.; et al. Vascular effects of the polyphenolic nutraceutical supplement taurisolo®: Focus on the protection of the endothelial function. Nutrients 2021, 13, 1540. [Google Scholar] [CrossRef]
- Lapi, D.; Stornaiuolo, M.; Sabatino, L.; Sommella, E.; Tenore, G.; Daglia, M.; Scuri, R.; Di Maro, M.; Colantuoni, A.; Novellino, E. The Pomace Extract Taurisolo Protects Rat Brain From Ischemia-Reperfusion Injury. Front. Cell. Neurosci. 2020, 14, 3. [Google Scholar] [CrossRef]
| Cell Type | TMAO Effect | Cellular Mechanism | Interaction Site | Reference |
|---|---|---|---|---|
| Monocytes | Increased pro-inflammatory monocyte levels (CD14++CD16+) | Not fully understood | - | [76] |
| Macrophages | Induction of endoplasmic reticulum (ER) stress | Activation of Toll-like receptor 4 (TLR4) | ER, TLR4 | [77] |
| M1 macrophage polarization | Activation of NLRP3 inflammasome | NLRP3 | [84,85] | |
| Foam cell formation | Increased oxidized LDL cholesterol uptake and reduced cholesterol efflux | - | [50] | |
| Platelets | Increased platelet aggregation | Altered Ca2+ signaling | - | [80] |
| Endothelial Progenitor Cells (EPCs) | Reduced EPC number and function | Inactivation of Akt/eNOS and MAPK/ERK signaling pathways | Akt/eNOS, MAPK/ERK | [71,72] |
| Increased inflammation and oxidative stress | Increased microRNA-221 (miR-221) expression | miR-221 | [72] | |
| Impaired neovascularization | - | - | [60,86] | |
| Endothelial Cells | Endothelial dysfunction | Increased expression of adhesion molecules such as VCAM-1 | VCAM-1 | [87] |
| Increased production of reactive oxygen species (ROS) | - | [41,87] | ||
| Increased production of pro-inflammatory cytokines | NF-κB | [76,87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caradonna, E.; Abate, F.; Schiano, E.; Paparella, F.; Ferrara, F.; Vanoli, E.; Difruscolo, R.; Goffredo, V.M.; Amato, B.; Setacci, C.; et al. Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health. Metabolites 2025, 15, 220. https://doi.org/10.3390/metabo15040220
Caradonna E, Abate F, Schiano E, Paparella F, Ferrara F, Vanoli E, Difruscolo R, Goffredo VM, Amato B, Setacci C, et al. Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health. Metabolites. 2025; 15(4):220. https://doi.org/10.3390/metabo15040220
Chicago/Turabian StyleCaradonna, Eugenio, Federico Abate, Elisabetta Schiano, Francesca Paparella, Fulvio Ferrara, Emilio Vanoli, Rossana Difruscolo, Vito Maria Goffredo, Bruno Amato, Carlo Setacci, and et al. 2025. "Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health" Metabolites 15, no. 4: 220. https://doi.org/10.3390/metabo15040220
APA StyleCaradonna, E., Abate, F., Schiano, E., Paparella, F., Ferrara, F., Vanoli, E., Difruscolo, R., Goffredo, V. M., Amato, B., Setacci, C., Setacci, F., & Novellino, E. (2025). Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health. Metabolites, 15(4), 220. https://doi.org/10.3390/metabo15040220








