Lipid Nanoparticles Based Cosmetics with Potential Application in Alleviating Skin Disorders
Abstract
:1. Introduction
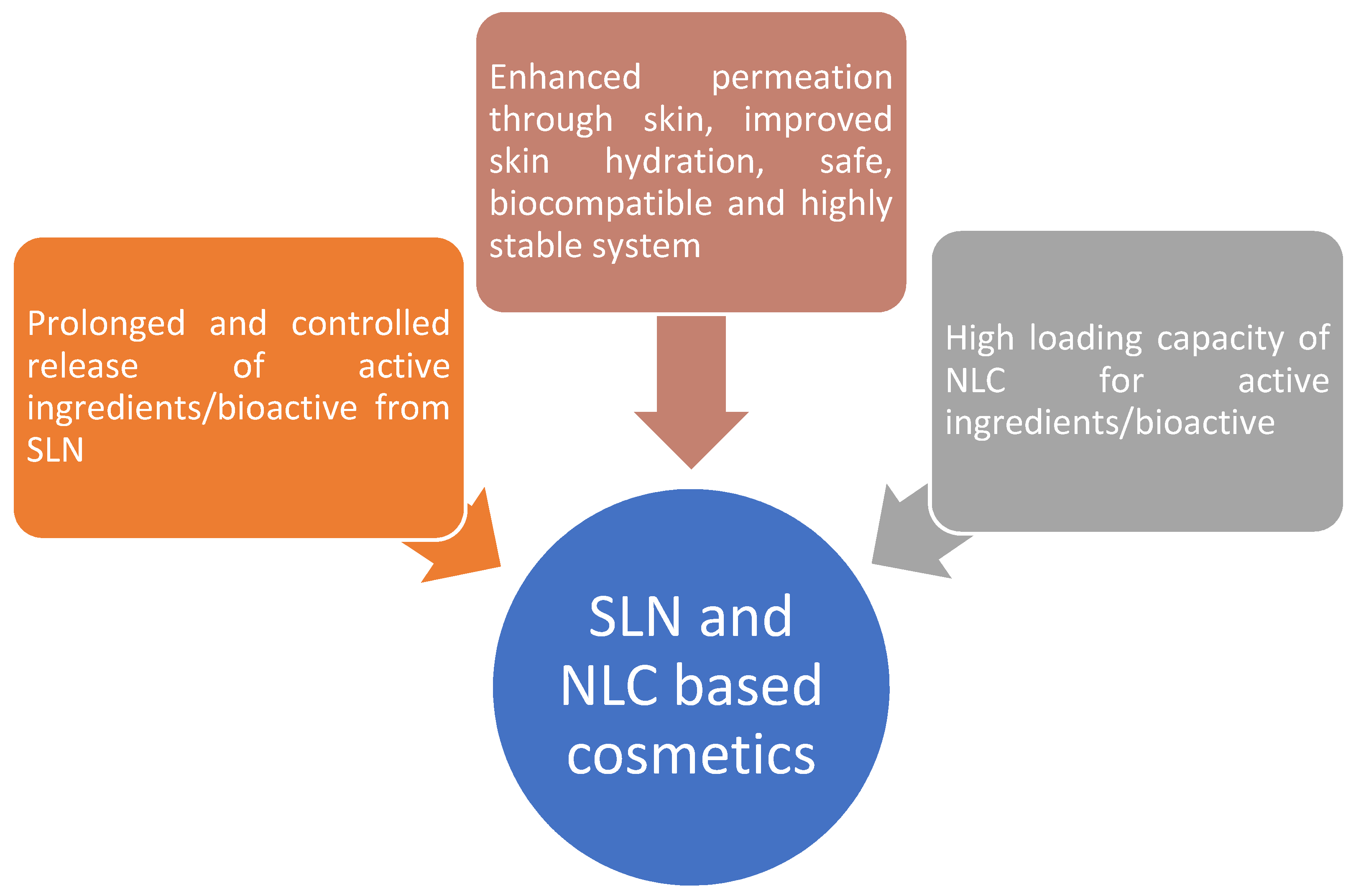
2. Lipid Nanoparticles Utilized in Formulation Design of Nanocosmetics
3. Mechanism of Percutaneous Absorption and Release of Active Ingredient from Lipid Nanoparticles Based Cosmetics
3.1. Release of Active Ingredients/Bioactive from Lipid Nanoparticles Based Cosmetic Product
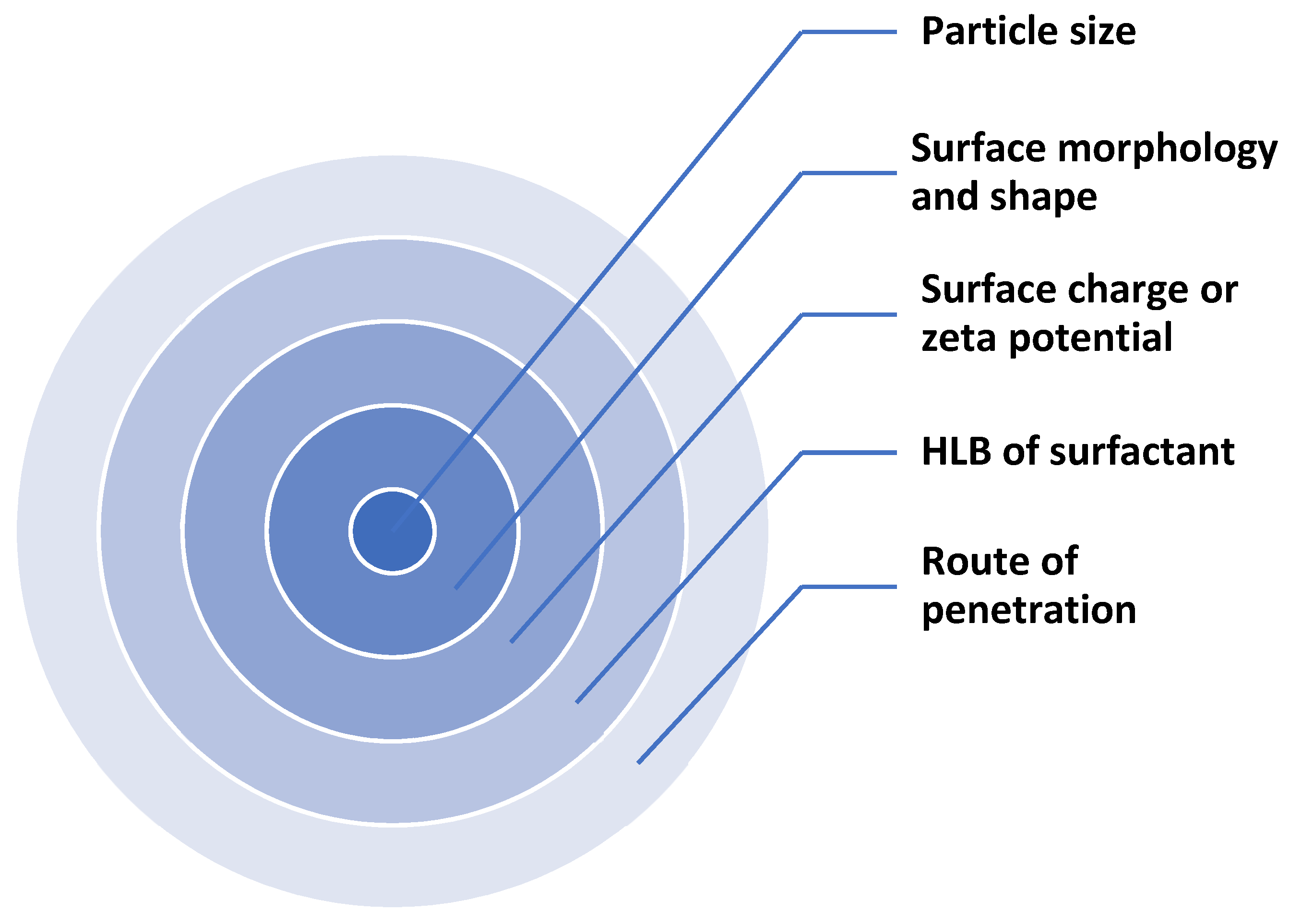
3.2. Mechanism of Absorption of Lipid Nanoparticles Based Cosmetic Products across Skin Layers
3.3. Permeation of Lipid Nanoparticles through Follicular Routes
4. Significance of Lipid Nanoparticles Based Cosmetics Utilized in Targeting Cutaneous Inflammations
4.1. Lipid Nanoparticles Based Cosmetics/Cosmeceuticals and Acne
4.2. Lipid Nanoparticles Based Cosmetics/Cosmeceuticals and Skin Mycoses
4.3. Lipid Nanoparticles Based Cosmetics/Cosmeceuticals and Psoriasis
4.4. Lipid Nanoparticles Based Cosmetics/Cosmeceuticals and Atopic Dermatitis
4.5. Other Specialized Applications of Lipid Nanoparticles Based Cosmetics/Cosmeceuticals
5. Contemporary Research Signify the Role of Lipid Nanoparticles Based Cosmetic/Cosmeceutical Product Development
6. Opportunities and Challenges of Lipid Nanoparticles Based Cosmetic Products
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dederen, J. Future trends in personal care. Chim. Oggi. 2006, 11, 10–16. [Google Scholar]
- Draelos, Z.D. Nutrition and enhancing youthful-appearing skin. Clin. Dermatol. 2010, 28, 400–408. [Google Scholar] [CrossRef]
- Rona, C.; Berardesca, E. Aging skin and food supplements: The myth and the truth. Clin. Dermatol. 2008, 26, 641–647. [Google Scholar] [CrossRef]
- Alvarez, A.M.R.; Rodríguez, M.L.G. Lipids in pharmaceutical and cosmetic preparations. Grasas Y Aceites 2000, 51, 74–96. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, A.; Nowak, I. Fatty acids in vegetable oils and their importance in cosmetic industry. CHEMIK Nauka-Technika-Rynek 2014, 68, 103–110. [Google Scholar]
- Kligman, A.M. Cosmetics a dermatologists looks to the future: Promises and problems. Dermatol. Clin. 2000, 18, 699–709. [Google Scholar] [CrossRef]
- Ahsan, H. The biomolecules of beauty: Biochemical pharmacology and immunotoxicology of cosmeceuticals. J. Immunoass. Immunochem. 2018, 40, 91–108. [Google Scholar] [CrossRef]
- Kim, S.-K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacologi-cal, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95. [Google Scholar] [CrossRef] [Green Version]
- Bialek, A.; Bialek, M.; Jelinska, M.; Tokarz, A. Fatty acid profile of new promising unconventional plant oils for cosmetic use. Int. J. Cosmet. Sci. 2016, 38, 382–388. [Google Scholar] [CrossRef]
- Bonnet, C. Lipids, a natural raw material at the heart of cosmetics innovation. OCL 2018, 25, D501. [Google Scholar] [CrossRef]
- Wissing, S.; Lippacher, A.; Müller, R. Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J. Cosmet. Sci. 2001, 52, 313–324. [Google Scholar]
- Wissing, S.A.; Muller, R.H. Cosmetic applications for solid lipid nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.-Z. Biocompatibility and Toxicity of Nanoparticles and Nanotubes. J. Nanomater. 2012, 2012, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Bansal, R.; Gupta, S.; Jindal, N.; Jindal, A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013, 4, 267. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.I.; Imam, S.S.; Ahmad, M.Z.; Vuddanda, P.R.; Alshehri, S.; Mahdi, W.A.; Ahmad, J. Recent Progress in Lipid Nanoparticles for Cancer Theranostics: Opportunity and Challenges. Pharmaceutics 2021, 13, 840. [Google Scholar] [CrossRef]
- Brugè, F.; Damiani, E.; Marcheggiani, F.; Offerta, A.; Puglia, C.; Tiano, L. A comparative study on the possible cytotoxic ef-fects of different nanostructured lipid carrier (NLC) compositions in human dermal fibroblasts. Int. J. Pharm. 2015, 495, 879–885. [Google Scholar] [CrossRef]
- Uner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharma-ceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carrier Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef] [Green Version]
- Khezri, K.; Saeedi, M.; Dizaj, S.M. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharm. 2018, 106, 1499–1505. [Google Scholar] [CrossRef]
- Patel, D.; Kumar, V.; Kesharwani, R.; Mazumdar, B. Lipid Nanoparticle a Novel Carrier for Cosmetics and Topical Prepara-tion: A Review. Inven. Rapid Cosmeceuticals 2015, 3, 1–6. [Google Scholar]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Beck, R.; Guterres, S.; Pohlmann, A. Nanocosmetics and Nanomedicines: New Approaches for Skin Care; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.-C. Lipid nanoparticles for improved topical application of drugs for skin dis-eases. Adv. Drug. Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef]
- Haftek, M.; Teillon, M.-H.; Schmitt, D. Stratum corneum, corneodesmosomes and ex vivo percutaneous penetration. Microsc. Res. Tech. 1998, 43, 242–249. [Google Scholar] [CrossRef]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef]
- Kakadia, P.; Conway, B.R. Solid Lipid Nanoparticles: A Potential Approach for Dermal Drug Delivery. Am. J. Pharmacol. Sci. 2014, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Muzykantov, V.; Muro, S. Targeting delivery of drugs in the vascular system. Int. J. Transp. Phenom. 2011, 12, 41–49. [Google Scholar] [PubMed]
- Kaur, S.; Nautyal, U.; Singh, R.; Singh, S.; Devi, A. Nanostructure Lipid Carrier (NLC): The new generation of lipid nanopar-ticles. Asian Pac. J. Health Sci. 2015, 2, 76–93. [Google Scholar]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parhi, R.; Suresh, P. Production of solid lipid nanoparticles—Drug loading and release mechanism. J. Chem. Pharm. Res. 2010, 2, 211–227. [Google Scholar]
- Hu, F.-Q.; Jiang, S.-P.; Du, Y.-Z.; Yuan, H.; Ye, Y.-Q.; Zeng, S. Preparation and characteristics of monostearin nanostructured lipid carriers. Int. J. Pharm. 2006, 314, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Castelli, F.; Puglia, C.; Sarpietro, M.G.; Rizza, L.; Bonina, F. Characterization of indomethacin-loaded lipid nanoparticles by differential scanning calorimetry. Int. J. Pharm. 2005, 304, 231–238. [Google Scholar] [CrossRef]
- Marcato, P.D.; Durán, N. New Aspects of Nanopharmaceutical Delivery Systems. J. Nanosci. Nanotechnol. 2008, 8, 2216–2229. [Google Scholar] [CrossRef] [Green Version]
- Müller, R.H.; Alexiev, U.; Sinambela, P.; Keck, C.M. Nanostructured lipid carriers (NLC): The second generation of solid lipid nanoparticles. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2016; pp. 161–185. [Google Scholar]
- Traversier, M.; Gaslondes, T.; Milesi, S.; Michel, S.; Delannay, E. Polar lipids in cosmetics: Recent trends in extraction, separation, analysis and main applications. Phytochem. Rev. 2018, 17, 1179–1210. [Google Scholar] [CrossRef]
- Lauterbach, A.; Müller-Goymann, C.C. Applications and limitations of lipid nanoparticles in dermal and transdermal drug delivery via the follicular route. Eur. J. Pharm. Biopharm. 2015, 97, 152–163. [Google Scholar] [CrossRef]
- Suter, F.; Schmid, D.; Wandrey, F.; Zülli, F. Heptapeptide-loaded solid lipid nanoparticles for cosmetic anti-aging applica-tions. Eur. J. Pharm. Biopharm. 2016, 108, 304–309. [Google Scholar] [CrossRef]
- Date, A.; Naik, B.; Nagarsenker, M. Novel Drug Delivery Systems: Potential in Improving Topical Delivery of Antiacne Agents. Ski. Pharmacol. Physiol. 2005, 19, 2–16. [Google Scholar] [CrossRef]
- Munster, U.; Nakamura, C.; Haberland, A.; Jores, K.; Mehnert, W.; Rummel, S.; Schaller, M.; Korting, H.C.; Zouboulis, C.C.; Blume-Peytavi, U.; et al. Ru58841-myristate-prodrug development for topical treatment of acne and androgenic alopecia. Pharmazie 2005, 60, 8–12. [Google Scholar]
- Kaur, I.P.; Kakkar, S. Topical delivery of antifungal agents. Expert Opin. Drug Deliv. 2010, 7, 1303–1327. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Müller, R.H. The use of SLN and NLC as topical particulate carriers for imidazole antifungal agents. Die Pharm. 2006, 61, 431–437. [Google Scholar]
- Souto, E.B.; Muller, R.H. Rheological and in vitro release behaviour of clotrimazole-containing aqueous solid lipid nanoparti-cle dispersions and commercial creams. Pharmazie 2007, 62, 505–509. [Google Scholar] [PubMed]
- Sanna, V.; Gavini, E.; Cossu, M.; Rassu, G.; Giunchedi, P. Solid lipid nanoparticles (SLN) as carriers for the topical delivery of econazole nitrate: In-vitro characterization, ex-vivo and in-vivo studies. J. Pharm. Pharmacol. 2010, 59, 1057–1064. [Google Scholar] [CrossRef]
- Passerini, N.; Gavini, E.; Albertini, B.; Rassu, G.; di Sabatino, M.; Sanna, V.; Giunchedi, P.; Rodriguez, L. Evaluation of solid lipid microparticles produced by spray congealing for topical application of econazole nitrate. J. Pharm. Pharmacol. 2009, 61, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Bhalekar, M.R.; Pokharkar, V.; Madgulkar, A.; Patil, N.; Patil, N. Preparation and evaluation of miconazole nitrate-loaded solid lipid na-noparticles for topical delivery. AAPS Pharm. Sci. Tech. 2009, 10, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Jain, S.; Khare, P.; Gulbake, A.; Bansal, D.; Jain, S.K. Design and development of solid lipid nanoparticles for topical delivery of an anti-fungal agent. Drug Deliv. 2010, 17, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Huang, Z.R.; Zhuo, R.Z.; Fang, J.Y. Combination of calcipotriol and methotrexate in nanostructured lipid carriers for topical delivery. Int. J. Nanomed. 2010, 5, 117–128. [Google Scholar]
- Katoh, N. Future perspectives in the treatment of atopic dermatitis. J. Dermatol. 2009, 36, 367–376. [Google Scholar] [CrossRef]
- Pople, P.V.; Singh, K.K. Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis. Int. J. Pharm. 2010, 398, 165–178. [Google Scholar] [CrossRef]
- Silva, A.C.; Santos, D.; Ferreira, D.C.; Souto, E.B. Minoxidil-loaded nanostructured lipid carriers (NLC): Characterization and rheo-logical behaviour of topical formulations. Pharmazie 2009, 64, 177–182. [Google Scholar] [PubMed]
- Padois, K.; Cantieni, C.; Bertholle, V.; Bardelc, C.; Pirot, F.; Falson, F. Solid lipid nanoparticles suspension versus commercial solutions for dermal deliv-ery of minoxidil. Int. J. Pharm. 2011, 416, 300–304. [Google Scholar] [PubMed]
- Arsenie, L.V.; Lacatusu, I.; Oprea, O.; Bordei, N.; Bacalum, M.; Badea, N. Azelaic acid-willow bark ex-tract-panthenol—Loaded lipid nanoparticles improve the hydration effect and antioxidant action of cosmetic formulations. Ind. Crop. Prod. 2020, 154, 112658. [Google Scholar] [CrossRef]
- Mandawgade, S.D.; Patravale, V.B. Development of SLNs from natural lipids: Application to topical delivery of tretinoin. Int. J. Pharm. 2008, 363, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, W.; Chen, H.; Ni, Q.; Xu, H.; Yang, X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int. J. Pharm. 2007, 328, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-Y.; Fang, C.-L.; Liu, C.-H.; Su, Y.-H. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640. [Google Scholar] [CrossRef]
- Agrawal, Y.; Petkar, K.C.; Sawant, K.K. Development, evaluation and clinical studies of Acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int. J. Pharm. 2010, 401, 93–102. [Google Scholar] [CrossRef]
- Lacatusu, I.; Istrati, D.; Bordei, N.; Popescu, M.; Seciu, A.M.; Panteli, L.M.; Badea, N. Synergism of plant extract and vegeta-ble oils-based lipid nanoparticless: Emerging trends in development of advanced cosmetic prototype products. Mater. Sci. Eng. C. Mater. Biol. Appl. 2020, 108, 110412. [Google Scholar] [CrossRef]
- Masiero, J.F.; Barbosa, E.J.; Macedo, L.D.O.; de Souza, A.; Yukuyama, M.N.; Arantes, G.J.; Bou-Chacra, N.A. Vegetable oils in pharmaceutical and cosmetic lipid-based nanocarriers preparations. Ind. Crop. Prod. 2021, 170, 113838. [Google Scholar] [CrossRef]

| Product Name | Active Ingredient | Manufacturer |
|---|---|---|
| NanoLipid Repair CLR | Black currant seed oil and manuka oil | Chemisches Laboratorium Dr. Kurt Richter, CLR—Berlin, Germany |
| Cutanova Cream Nano Repair Q10 | Coenzyme Q10, polypeptide, hibiscus extract, ginger extract, ketosugar | Dr. Rimpler GmbH, Wedemark, Germany |
| Intensive Serum NanoRepair Q10 | Coenzyme Q10, polypeptide, mafane extract | Dr. Rimpler GmbH, Wedemark, Germany |
| Cutanova Cream NanoVital Q10 | Coenzyme Q10, TiO2, polypeptide, ursolic acid, oleanolic acid, sunflower seed extract | Dr. Rimpler GmbH, Wedemark, Germany |
| NLC deep effect repair cream | Q10, TiO2, highly active oligo saccharides | Beate Johnen GmbH, Aschheim, Germany |
| Extra moist softener | Coenzyme Q10, -3 und -6 unsaturated fatty acids | Amorepacific Corp. Seoul, South Korea |
| Regenerations creme intensive | M. ternifolia seed oil, avocado, urea, black currant seed scholl oil | Scholl, Mannheim, Germany |
| SURMER Crème | kukuinut oil, Monoi Tiare Tahiti®, pseudopeptide, hydrolyzed wheat protein | Lancray International S.A. Paris, France |
| Olivenol Augenpflegebalsam | Senegal, Tocopheryl Acetate Olea Europaea Oil, Prunus Amygdalus Dulcis Oil, Hydrolized Milk Protein, Tocopheryl Acetate, Rhodiola rosea, Root Extract, Caffeine | Dr. Theiss Naturwaren GmbH, Homburg, Germany |
| Swiss Cellular White Illuminating Eye Essence | Glycoprotiens, Panax ginseng root extract, Equisetum Arvense extract | Laboratoires La Prairie SA, Volketswil, Zurich, Switzerland |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, J. Lipid Nanoparticles Based Cosmetics with Potential Application in Alleviating Skin Disorders. Cosmetics 2021, 8, 84. https://doi.org/10.3390/cosmetics8030084
Ahmad J. Lipid Nanoparticles Based Cosmetics with Potential Application in Alleviating Skin Disorders. Cosmetics. 2021; 8(3):84. https://doi.org/10.3390/cosmetics8030084
Chicago/Turabian StyleAhmad, Javed. 2021. "Lipid Nanoparticles Based Cosmetics with Potential Application in Alleviating Skin Disorders" Cosmetics 8, no. 3: 84. https://doi.org/10.3390/cosmetics8030084
APA StyleAhmad, J. (2021). Lipid Nanoparticles Based Cosmetics with Potential Application in Alleviating Skin Disorders. Cosmetics, 8(3), 84. https://doi.org/10.3390/cosmetics8030084






