Clinical Evaluation of Topical Lotion Containing 2-Aza-8-Oxohypoxanthine on Skin Barrier Function against Water Loss
Abstract
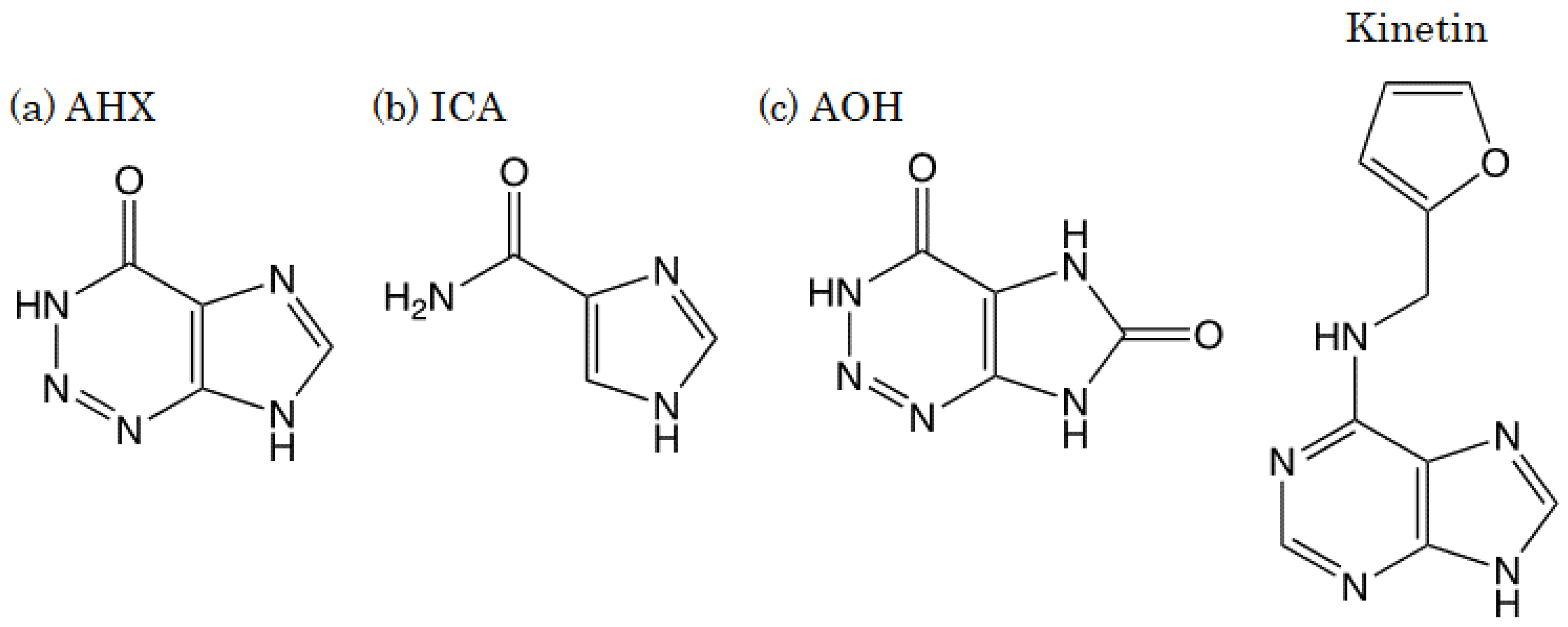
:1. Introduction
2. Materials and Methods
2.1. Preparation of Test Substance
2.2. Clinical Trial
2.2.1. Selection of Subjects
- (1)
- Women in their 40 s or 50 s at the time of consent;
- (2)
- Those with wrinkles equivalent to wrinkle grade 1 to 3 at the corner of the right and left eyes;
- (3)
- Those who could give written consent based on their own free will before the start of the study;
- (4)
- Those who could give consent to the use of only the test sample in the designated area.
2.2.2. Clinical Trial Study
2.2.3. Evaluation and Statistical Analysis
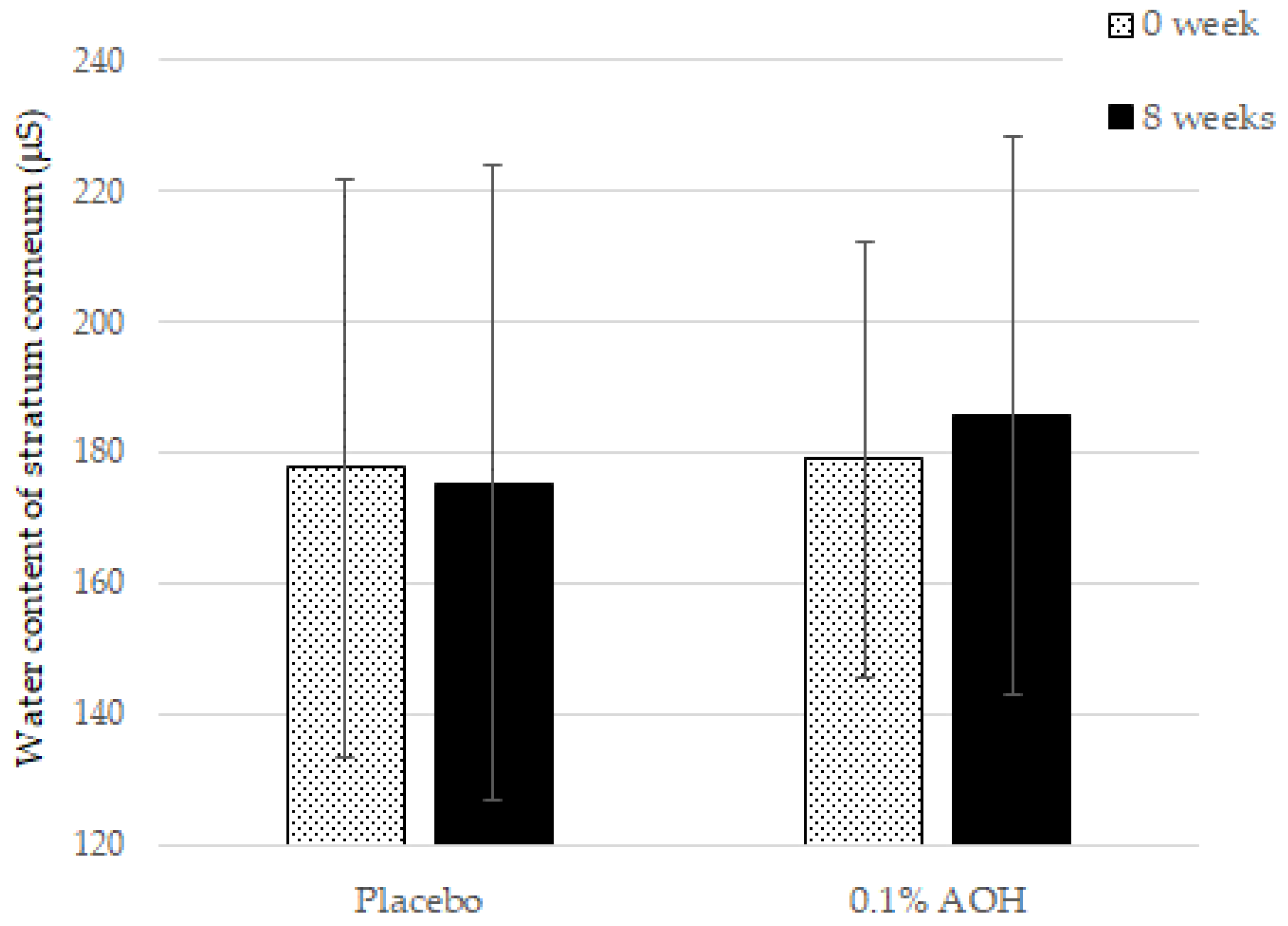
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramsbottom, J. Rate of growth of fungus rings. Nature 1926, 117, 158–159. [Google Scholar] [CrossRef]
- Smith, J.D. Fungi and turf diseases. J. Sports Turf. Res. Inst. 1957, 10, 324–352. [Google Scholar]
- Shantz, H.L.; Piemeisel, R.L. Fungus fairy rings in Eastern Colorado and their effect on vegetation. J. Agric. Res. 1917, 11, 191–245. [Google Scholar]
- Choi, J.-H.; Fushimi, K.; Abe, N.; Tanaka, H.; Maeda, S.; Morita, A.; Hara, M.; Motohashi, R.; Matsunaga, J.; Eguchi, Y.; et al. Disclosure of the "fairy" of fairy-ring-forming fungus Lepista sordida. Chembiochem 2010, 11, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Abe, N.; Tanaka, H.; Fushimi, K.; Nishina, Y.; Morita, A.; Kiriiwa, Y.; Motohashi, R.; Hashizume, D.; Koshino, H.; et al. Plant-growth regulator, Imidazole-4-Carboxamide, produced by the fairy ring forming fungus Lepista sordida. J. Agric. Food Chem. 2010, 58, 9956–9959. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Ohnishi, T.; Yamakawa, Y.; Takeda, S.; Sekiguchi, S.; Maruyama, W.; Yamashita, K.; Suzuki, T.; Morita, A.; Ikka, T.; et al. The source of “fairy rings”: 2-Azahypoxanthine and its metabolite found in a novel purine metabolic pathway in plants. Angew. Chem. Int. Ed. 2014, 53, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Mitchinson, A. Fairy chemicals. Nature 2014, 505, 298. [Google Scholar] [CrossRef]
- Asai, T.; Choi, J.-H.; Ikka, T.; Fushimi, K.; Abe, N.; Tanaka, H.; Yamakawa, Y.; Kobori, H.; Kiriiwa, Y.; Motohashi, R.; et al. Effect of 2-Azahypoxanthine (AHX) produced by the fairy-ring-forming fungus on the growth and the grain yield of rice. Jpn. Agric. Res. Q. JARQ 2015, 49, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Tobina, H.; Choi, J.-H.; Asai, T.; Kiriiwa, Y.; Asakawa, T.; Kan, T.; Morita, A.; Kawagishi, H. 2-Azahypoxanthine and imidazole-4-carboxamide produced by the fairy-ring-forming fungus increase wheat yield. Field Crop. Res. 2014, 162, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Kawagishi, H. Fairy chemicals—A candidate for a new family of plant hormones and possibility of practical use in agriculture. Biosci. Biotechnol. Biochem. 2018, 82, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H. Are fairy chemicals a new family of plant hormones? Proc. Jpn. Acad. Ser. B 2019, 95, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baran, R.; Maibach, H.I. Textbook of Cosmetic Dermatology, 5th ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 113–116. [Google Scholar]
- Aoshima, H.; Hyodo, S.; Ibuki, R.; Wu, J.; Choi, J.-H.; Kawagishi, H. Safety evaluation of 2-aza-8-oxohypoxanthine based on in vitro and human patch tests. Fundam. Toxicol. Sci. 2020, 7, 207–214. [Google Scholar] [CrossRef]
- Aoshima, H.; Matsumoto, T.; Ibuki, R.; Kawagishi, H. Safety evaluation of 2-aza-8-oxohypoxanthine by in vitro skin sensitization and human tests. Fundum. Toxicol. Sci. 2021, submitted. [Google Scholar]
- Aoshima, H.; Ito, M.; Ibuki, R.; Kawagishi, H. The potential of 2-aza-8-Oxohypoxanthine as a cosmetic ingredient. Cosmetics 2021, 8, 60. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kikuchi, A.; Pumkaeo, P.; Hirai, H.; Tokuyama, S.; Kawagishi, H. Bioconversion of AHX to AOH by resting cells of Burkholderia contaminans CH-1. Biosci. Biotechnol. Biochem. 2016, 80, 2045–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contet-Audonneau, J.L.; Jeanmaire, C.; Pauly, G. A histological study of human wrinkle structures: Comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br. J. Dermatol. 1999, 140, 1038–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoshima, H.; Ibuki, R.; Ito, M.; Kawagishi, H. Clinical Evaluation of Topical Lotion Containing 2-Aza-8-Oxohypoxanthine on Skin Barrier Function against Water Loss. Cosmetics 2021, 8, 83. https://doi.org/10.3390/cosmetics8030083
Aoshima H, Ibuki R, Ito M, Kawagishi H. Clinical Evaluation of Topical Lotion Containing 2-Aza-8-Oxohypoxanthine on Skin Barrier Function against Water Loss. Cosmetics. 2021; 8(3):83. https://doi.org/10.3390/cosmetics8030083
Chicago/Turabian StyleAoshima, Hisae, Rinta Ibuki, Masayuki Ito, and Hirokazu Kawagishi. 2021. "Clinical Evaluation of Topical Lotion Containing 2-Aza-8-Oxohypoxanthine on Skin Barrier Function against Water Loss" Cosmetics 8, no. 3: 83. https://doi.org/10.3390/cosmetics8030083
APA StyleAoshima, H., Ibuki, R., Ito, M., & Kawagishi, H. (2021). Clinical Evaluation of Topical Lotion Containing 2-Aza-8-Oxohypoxanthine on Skin Barrier Function against Water Loss. Cosmetics, 8(3), 83. https://doi.org/10.3390/cosmetics8030083





