Flavonoids Profile, Taxonomic Data, History of Cosmetic Uses, Anti-Oxidant and Anti-Aging Potential of Alpinia galanga (L.) Willd
Abstract
1. Introduction
2. The Taxonomic Description of Alpinia galanga (L.) Willd
- −
- Maranta galanga L., Sp. Pl. ed. 2: 3 (1762).
- −
- Galanga major Garsault in Figure Pl. Méd.: t. 16 a (1764).
- −
- Heritiera alba Retz. in Observ. Bot. 6: 17 (1791).
- −
- Alpinia alba (Retz.) Roscoe in Trans. Linn. Soc. London 8: 346 (1807).
- −
- Zingiber galangal (L.) Stokes in Bot. Mat. Med. 1: 72 (1812).
- −
- Alpinia viridiflora Griff., Not. Pl. Asiat. 3: 423 (1851).
- −
- Alpinia rheedii Wight, Icon. pl. Ind. orient. 6: 19, t. 2026 (1853).
- −
- Alpinia zingiberina Hook.f. in Bot. Mag.: t. 6944 (1887).
- −
- Alpinia bifida Warb. in Bot. Jahrb. Syst. 13: 275 (1891).
- −
- Languas galanga (L.) Stuntz in Bull. Bur. Pl. Industr. U.S.D.A. 261: 21 (1912).
3. Flavonoids Profile of A. galanga
4. Cosmetic Uses (Past to Present)
5. Potential Biological Activities: Antioxidant and Anti-Aging Activities of Flavonoids from A. galanga for Cosmetic Application
6. Future Perspectives and Research Directions
- −
- To evaluate the type and amount of phytochemical compounds of A. galanga from various populations that grow in different habitats that may be affected by different geographic and environmental factors.
- −
- To compare the flavonoid phytochemical profiles as well as the potential biological activities—for example, antioxidant, anti-aging and anti-wrinkle activities for cosmetic application—of A. galanga and its closely related species within the same genus such as A. officinarum Hance, A. zerumbet (Pers.) B. L. Burtt and R. M. Sm. and A. chinensis (Retz.) Roscoe, in order to increase the potential alternative choices of the flavonoid-rich bioactive compounds for various cosmetic proposals.
- −
- To analyze the most promising part of A. galanga that contains high-quality and a large quantity of the target flavonoid phytochemicals, so as to decrease the cost of cosmetic/cosmeceutical product development.
- −
- To apply the new innovative extraction techniques—for example, ultrasonic-assisted extraction (UAE), UAE combined with the macroporous resin adsorption, or other green chemistry techniques—to increase the yield of extraction of A. galanga’s flavonoids.
- −
- To investigate the mechanism of interesting biological activities, especially the anti-aging and antioxidant activities of the known and promising flavonoids from A. galanga using both in-vitro and in-cellulo assays that will enhance the efficacy of cosmetic and/or cosmeceutical products.
- −
- To examine the inhibition potential of flavonoid-rich extracts/compounds from A. galanga on major aging factors—e.g., enzymes such as tyrosinase, elastase, hyaluronidase, etc. This may be helpful to provide a new powerful anti-aging molecule for cosmeceutical products.
- −
- To investigate the safety of the potential extract and/or pure flavonoid phytochemical compounds from A. galanga in cosmetic/cosmeceutical products, in order to confirm its safety and to provide the customer’s confidence.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jirovetz, L.; Buchbauer, G.; Shafi, M.P.; Leela, N.K. Analysis of the essential oils of the leaves, stems, rhizomes and roots of the medicinal plant Alpinia galanga from southern India. Acta Pharm. 2003, 53, 73–81. [Google Scholar]
- Mahae, N.; Chaiseri, S. Antioxidant activities and antioxidative components in extracts of Alpinia galanga (L.) Sw. Kasetsart J. Nat. Sci. 2009, 43, 358–369. [Google Scholar]
- Chudiwal, A.K.; Jain, D.P.; Somani, R.S. Alpinia galanga Willd—An overview on phyto-pharmacological properties. Indian J. Nat. Prod. Resour. 2010, 1, 143–149. [Google Scholar]
- Jain, A.P.; Pawar, R.S.; Lodhi, S.; Singhai, A.K. Immunomodulatory and anti-oxidant potential of Alpinia galanga Linn. rhizomes. Pharmacogn. Commun. 2012, 2, 30–37. [Google Scholar] [CrossRef]
- Divakaran, S.A.; Hema, P.S.; Nair, M.S.; Nair, C.K.K. Antioxidant capacity and radioprotective properties of the flavonoids galangin and kaempferide isolated from Alpinia galanga L. (Alpinia galanga) against radiation induced cellular DNA damage. Int. J. Radiat. Res. 2013, 11, 81–89. [Google Scholar]
- Bian, M.-Q.; Wang, H.Q.; Kang, J.; Chen, R.-Y.; Yang, Y.-F.; Wu, H.-Z. Flavonoids from the seeds of Alpinia galanga Willd. Acta Pharmacol. Sin. 2014, 49, 359–362. [Google Scholar]
- Chouni, A.; Paul, S. A Review on Phytochemical and Pharmacological Potential of Alpinia galanga. Pharmacogn. J. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Mickymaray, S.; Al Aboody, M.S. In Vitro Antioxidant and Bactericidal Efficacy of 15 Common Spices: Novel Therapeutics for Urinary Tract Infections? Medicina 2019, 55, 289. [Google Scholar] [CrossRef]
- Wu, T.L.; Larsen, K. Alpinia galanga. In Flora of China; Wu, Z.-Y., Raven, P.H., Eds.; Missouri Botanical Garden Press: St. Louis, MO, USA, 2000; pp. 322–377. [Google Scholar]
- Kress, W.J.; Liu, A.-Z.; Newman, M.; Li, Q.-J. The molecular phylogeny of Alpinia (Alpinia galanga): A complex and polyphyletic genus of gingers. Am. J. Bot. 2005, 92, 167–178. [Google Scholar] [CrossRef]
- Larsen, K. Distribution patterns and diversity centres of Alpinia galanga in SE Asia. Biol. Skr. 2005, 55, 219–228. [Google Scholar]
- Larsen, K.; Larsen, S.S. Gingers of Thailand; Queen Sirikit Botanic Garden: Chiang Mai, Thailand, 2006. [Google Scholar]
- Schumman, K. Zingiberaceae; Engler, A., Ed.; Leipzig: Leipzig, Germany, 1904. [Google Scholar]
- Smith, R.M. Alpinia (Alpinia galanga): A Proposed New Infrageneric Classification. Edinb. J. Bot. 1990, 47, 1–75. [Google Scholar] [CrossRef]
- Larsen, K. Annotated key to the genera of Alpinia galanga of Thailand. Nat. Hist. Bull. Siam Soc. 1980, 28, 151–169. [Google Scholar]
- Larsen, K.; Ibrahim, H.; Khaw, S.H.; Saw, L.G. Gingers of Peninsular Malaysia and Singapore; Natural History Publications (Borneo): Kinabalu, Malaysia, 1999. [Google Scholar]
- Nair, A.G.R.; Gunasegaran, R. Chemical investigation of certain South Indian plants. Indian J. Chem. 1982, 21B, 979–980. [Google Scholar]
- Jaju, S.B.; Indurwade, N.H.; Sakarkar, D.M.; Fuloria, N.K.; Ali, M.D.; Das, S.; Basu, S.P. Galangoflavonoid isolated from rhizome of Alpinia galanga (L) Sw (Zingiberaceae). Trop. J. Pharm. Res. 2009, 8, 545–550. [Google Scholar] [CrossRef]
- Buckingham, J.; Rajit, V.; Munasinghe, N. Dictionary of Flavonoids with CD-ROM; CRC Press: Boca Raton, FL, USA, 2015; pp. 927–928, 943–944. [Google Scholar]
- Drouet, S.; Garros, L.; Hano, C.; Tungmunnithum, D.; Renouard, S.; Hagège, D.; Maunit, B.; Lainé, E. A Critical View of Different Botanical, Molecular, and Chemical Techniques Used in Authentication of Plant Materials for Cosmetic Applications. Cosmetics 2018, 5, 30. [Google Scholar] [CrossRef]
- Nazir, M.; Tungmunnithum, D.; Bose, S.; Drouet, S.; Garros, L.; Giglioli-Guivarc’H, N.; Abbasi, B.H.; Hano, C. Differential Production of Phenylpropanoid Metabolites in Callus Cultures of Ocimum basilicum L. with Distinct In Vitro Antioxidant Activities and In Vivo Protective Effects against UV stress. J. Agric. Food Chem. 2019, 67, 1847–1859. [Google Scholar] [CrossRef]
- Drouet, S.; Leclerc, E.A.; Garros, L.; Tungmunnithum, D.; Kabra, A.; Abbasi, B.H.; Lainé, É.; Hano, C. A Green Ultrasound-Assisted Extraction Optimization of the Natural Antioxidant and Anti-Aging Flavonolignans from Milk Thistle Silybum marianum (L.) Gaertn. Fruits for Cosmetic Applications. Antioxidants 2019, 8, 304. [Google Scholar] [CrossRef]
- Khurshid, R.; Ullah, M.A.; Tungmunnithum, D.; Drouet, S.; Shah, M.; Zaeem, A.; Hameed, S.; Hano, C.; Abbasi, B.H. Lights triggered differential accumulation of antioxidant and antidiabetic secondary metabolites in callus culture of Eclipta alba L. PLoS ONE 2020, 15, e0233963. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in Antioxidant Flavonoids of Stamen Extracts from Nymphaea lotus L. Using Ultrasonic-Assisted Extraction and Macroporous Resin Adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Renouard, S.; Drouet, S.; Blondeau, J.P.; Hano, C. A Critical Cross-Species Comparison of Pollen from Nelumbo nucifera Gaertn. vs. Nymphaea lotus L. for Authentication of Thai Medicinal Herbal Tea. Plants 2020, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- Ahlina, F.N.; Nugraheni, N.; Salsabila, I.A.; Haryanti, S.; Da’i, M.; Meiyanto, E. Revealing the Reversal Effect of Galangal (Alpinia galanga L.) Extract Against Oxidative Stress in Metastatic Breast Cancer Cells and Normal Fibroblast Cells Intended as a Co-Chemotherapeutic and Anti-Ageing Agent. Asian Pac. J. Cancer Prev. 2020, 21, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.Y.; Chen, J.Y.; Weng, Y.S.; Aneja, R.; Chen, C.J.; Huang, C.Y.; Kuo, W.W. Galangin suppresses H2O2-induced aging in human dermal fibroblasts. Environ. Toxicol. 2017, 32, 2419–2427. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.-S.; Tao, W.; Miao, Q.-B.; Lu, S.-C.; Zhu, Y.-B. Galangin Dampens Mice Lipopolysaccharide-Induced Acute Lung Injury. Inflammation 2014, 37, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Kicuntod, J.; Khuntawee, W.; Wolschann, P.; Pongsawasdi, P.; Chavasiri, W.; Kungwan, N.; Rungrotmongkol, T. Inclusion complexation of pinostrobin with various cyclodextrin derivatives. J. Mol. Graph. Model. 2016, 63, 91–98. [Google Scholar] [CrossRef]
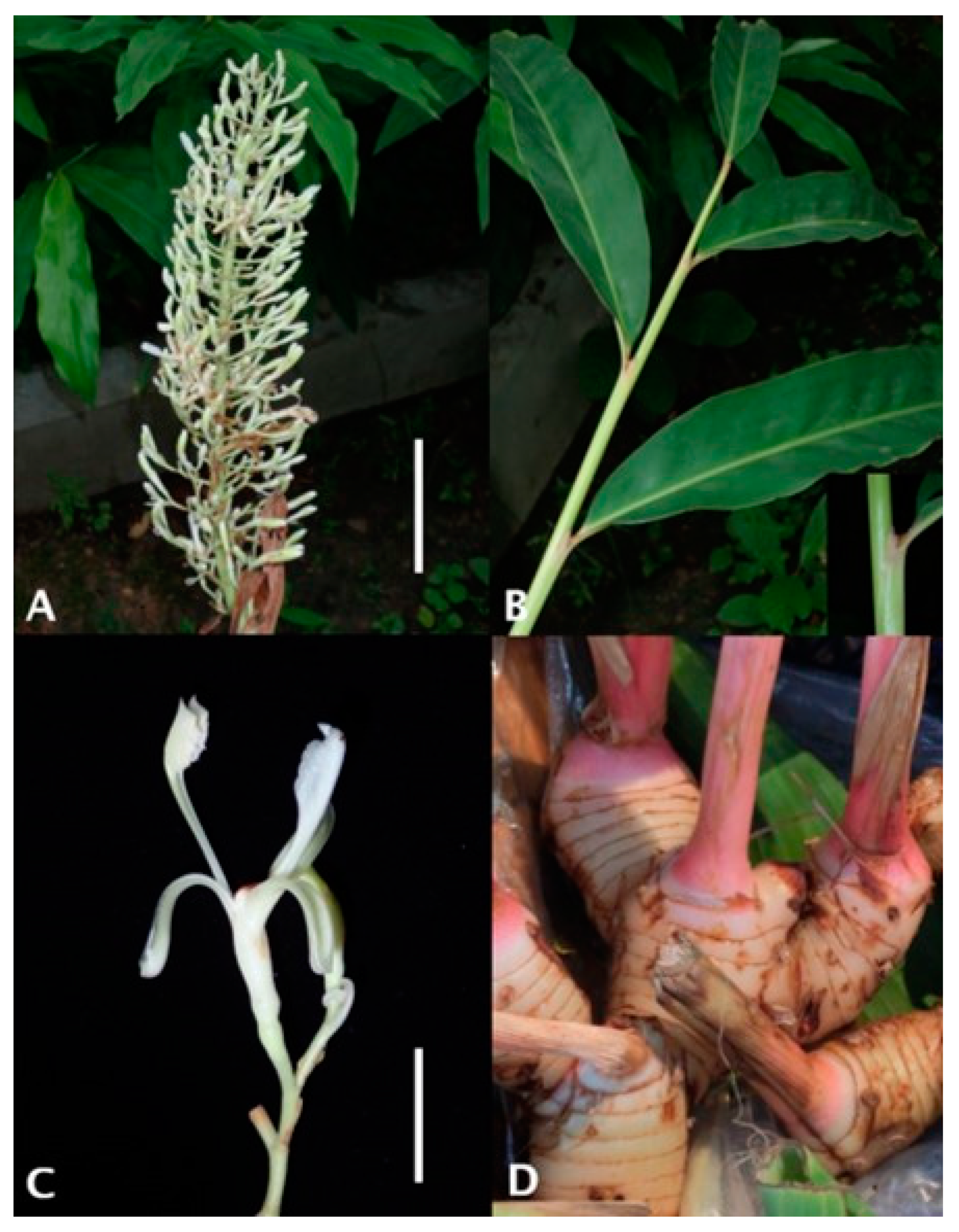
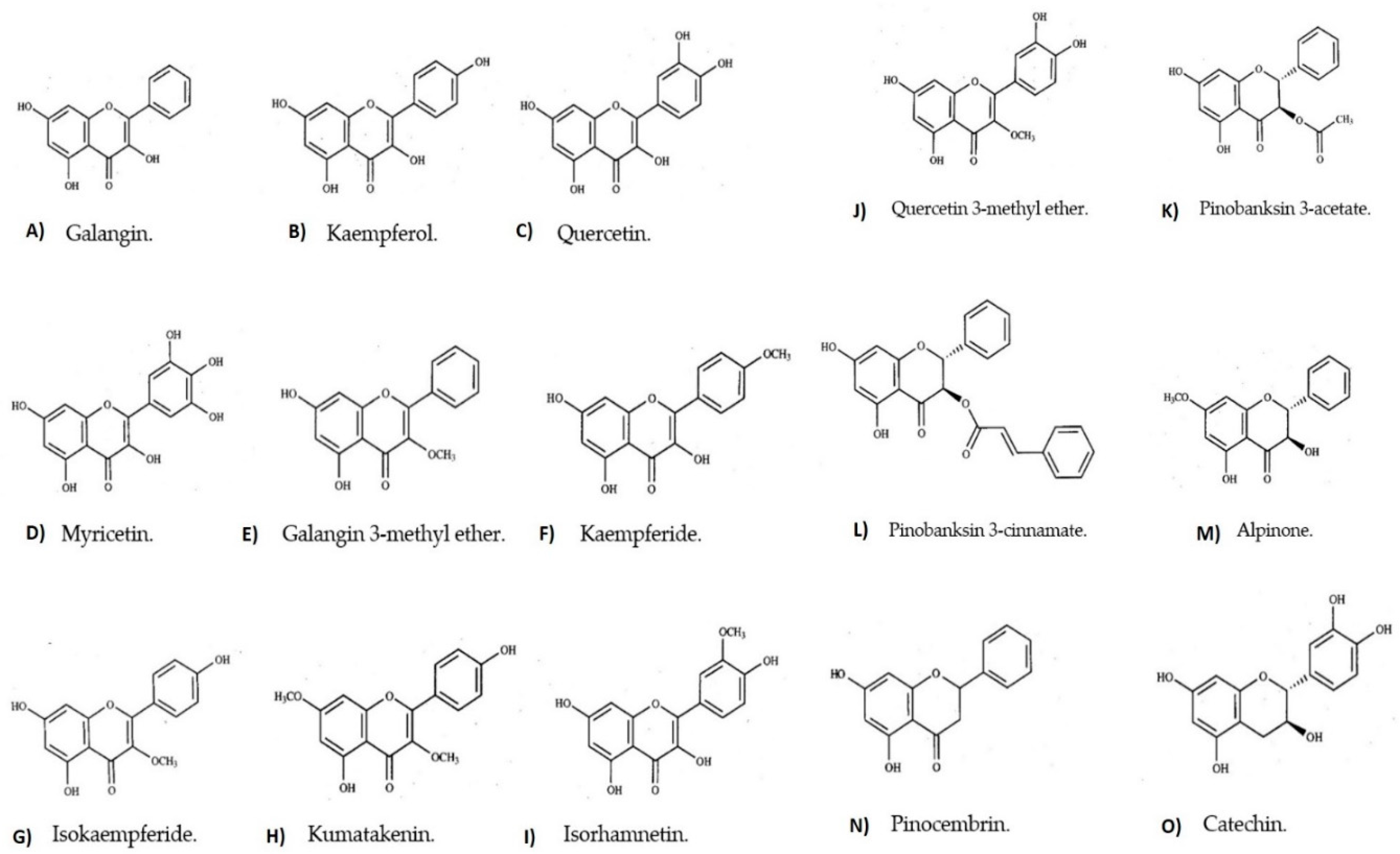
| Dihydroflavonol | (2R,3R)-Alpinone (sd) [6], Pinobanksin 3-acetate, (2R,3R)-Pinobanksin 3-cinnamate, (2R,3S)-Pinobanksin 3-cinnamate (sd) [6] |
| Flavan 3-ol | Catechin (rz) [2] |
| Flavanone | Pinocembrin (sd) [6] |
| Flavonol | Galangoflavonoside? [19], Galangin (rz, rt) [3,4,5,6,7,18], Galangin 3-methyl ether (rt, sd) [6,18], Kaempferide (rz) [4,5,7], Isokaempferide, Kumatakenin (sd) [6], Myricetin [2], Kaempferol (rz) [3,4,7], Isorhamnetin, Quercetin 3-methyl ether (rz), Quercetin (rz) [4,7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungmunnithum, D.; Tanaka, N.; Uehara, A.; Iwashina, T. Flavonoids Profile, Taxonomic Data, History of Cosmetic Uses, Anti-Oxidant and Anti-Aging Potential of Alpinia galanga (L.) Willd. Cosmetics 2020, 7, 89. https://doi.org/10.3390/cosmetics7040089
Tungmunnithum D, Tanaka N, Uehara A, Iwashina T. Flavonoids Profile, Taxonomic Data, History of Cosmetic Uses, Anti-Oxidant and Anti-Aging Potential of Alpinia galanga (L.) Willd. Cosmetics. 2020; 7(4):89. https://doi.org/10.3390/cosmetics7040089
Chicago/Turabian StyleTungmunnithum, Duangjai, Nobuyuki Tanaka, Ayumi Uehara, and Tsukasa Iwashina. 2020. "Flavonoids Profile, Taxonomic Data, History of Cosmetic Uses, Anti-Oxidant and Anti-Aging Potential of Alpinia galanga (L.) Willd" Cosmetics 7, no. 4: 89. https://doi.org/10.3390/cosmetics7040089
APA StyleTungmunnithum, D., Tanaka, N., Uehara, A., & Iwashina, T. (2020). Flavonoids Profile, Taxonomic Data, History of Cosmetic Uses, Anti-Oxidant and Anti-Aging Potential of Alpinia galanga (L.) Willd. Cosmetics, 7(4), 89. https://doi.org/10.3390/cosmetics7040089







