Skin Brightening Efficacy of Exosomes Derived from Human Adipose Tissue-Derived Stem/Stromal Cells: A Prospective, Split-Face, Randomized Placebo-Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Characterization of ASC-Exosomes
2.2. Cell-Based Assay
2.3. Preparation of the ASC-Exosome-Containing Formulation
2.4. Clinical Evalution
2.5. Statistical Analysis
3. Results
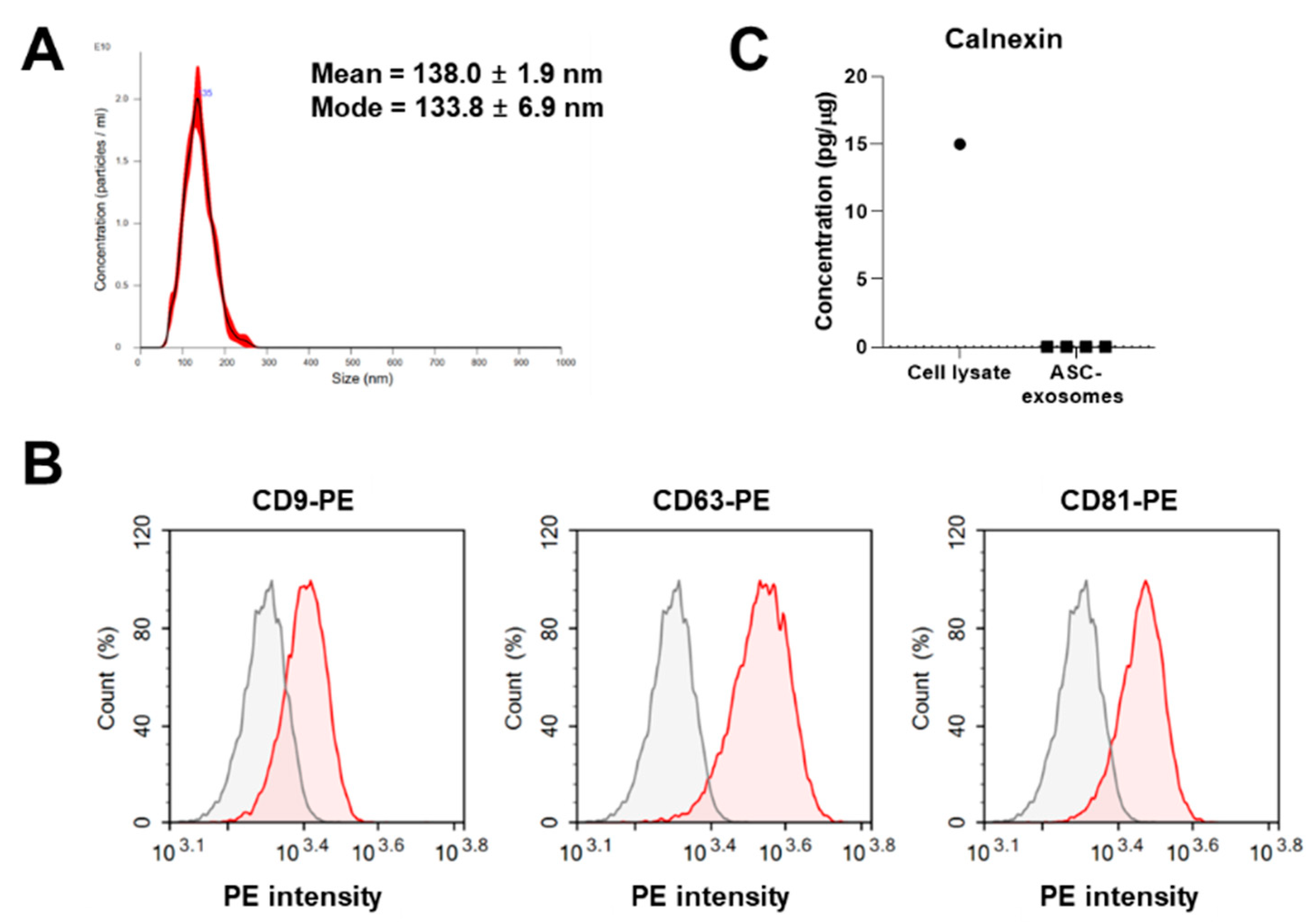
3.1. Isolation and Characterization of ASC-Exosomes
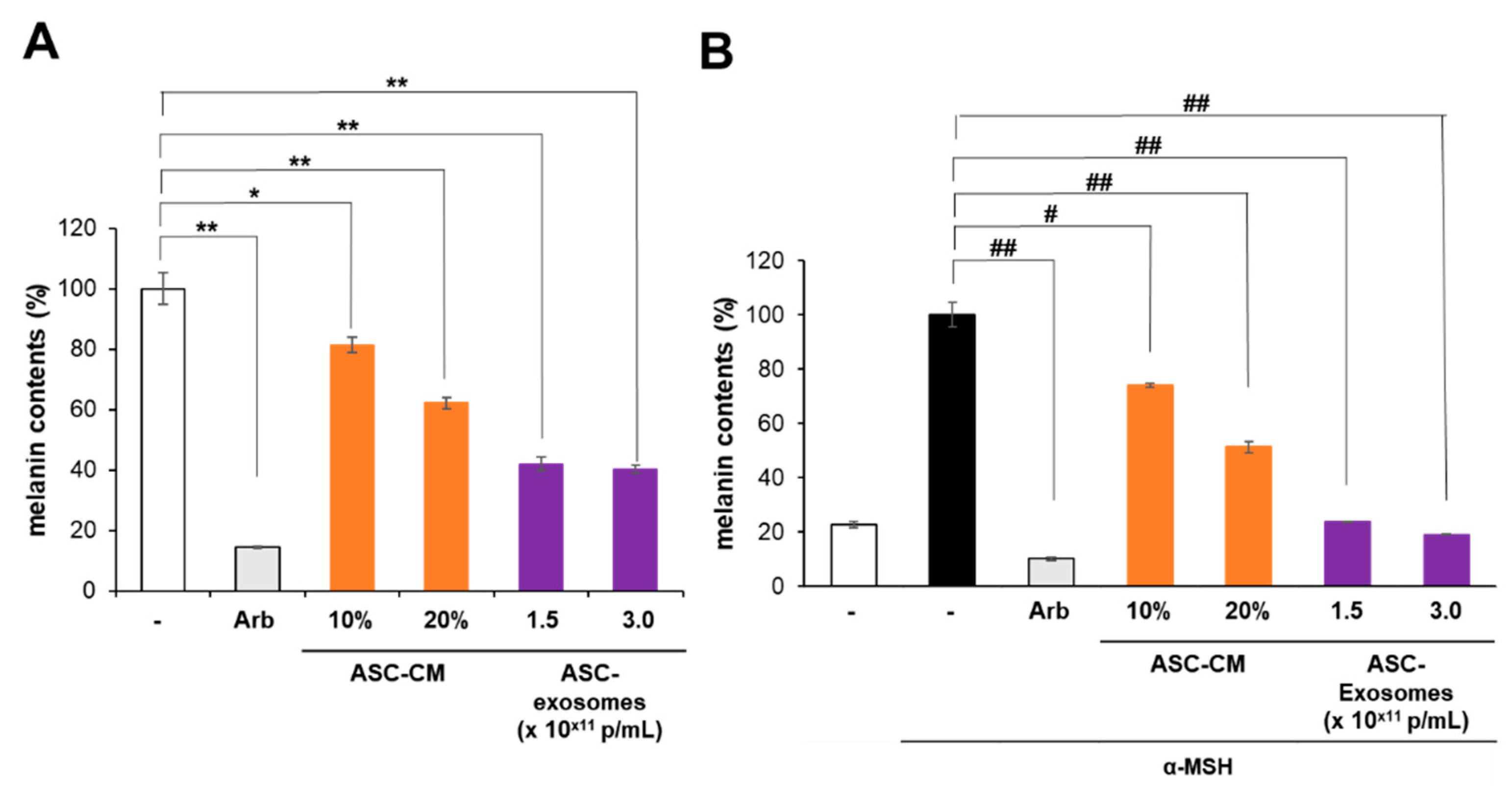
3.2. Antipigmentation Effect of ASC-Exosomes In Vitro
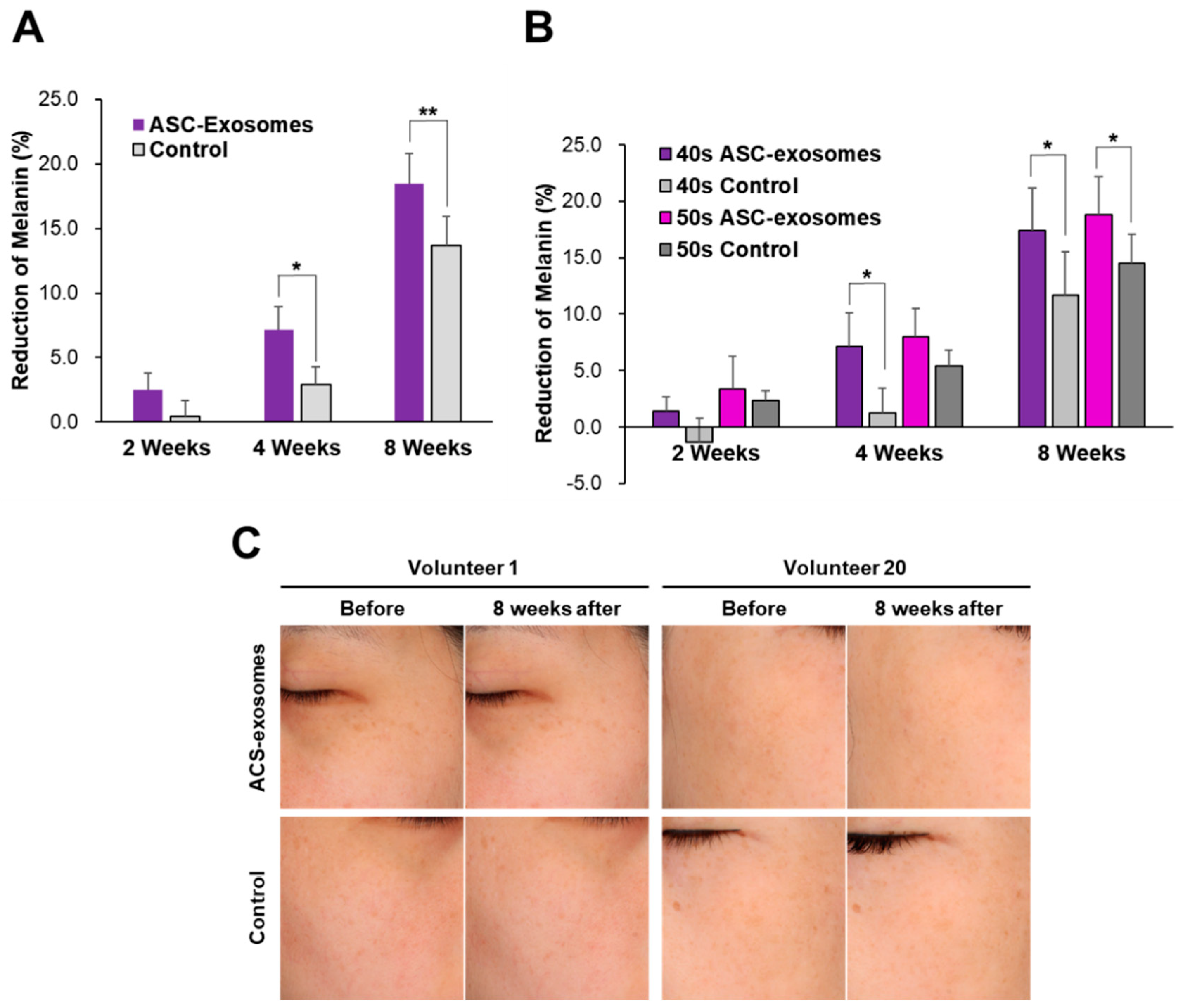
3.3. Skin Brightening Efficacy of ASC-Exosomes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Berlitz, S.J.; De Villa, D.; Inácio, L.A.M.; Davies, S.; Zatta, K.C.; Guterres, S.S.; Külkamp-Guerreiro, C. Azelaic acid-loaded nanoemulsion with hyaluronic acid—A new strategy to treat hyperpigmentary skin disorders. Drug Dev. Ind. Pharm. 2019, 45, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Arora, P.; Garg, K.V. Cosmeceuticals for hyperpigmentation: What is available? J. Cutan. Aesthet. Surg. 2013, 6, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Kim, H.-k.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Park, J.-H.; Park, H.-S.; Choi, K.-A.; Seol, K.-C.; Oh, S.-I.; Kang, S.; Hong, S. Neural stem cells inhibit melanin production by activation of Wnt inhibitors. J. Dermatol. Sci. 2013, 72, 274–283. [Google Scholar] [CrossRef]
- Kim, D.-W.; Jeon, B.-J.; Hwang, N.-H.; Kim, M.-S.; Park, S.-H.; Dhong, E.-S.; Yoon, E.-S.; Lee, B.-I. Adipose-derived stem cells inhibit epidermal melanocytes through an interleukin-6-mediated mechanism. Plasti. Reconstr. Surg. 2014, 134, 470–480. [Google Scholar] [CrossRef]
- Kim, E.S.; Jeon, H.B.; Lim, H.; Shin, J.H.; Park, S.J.; Jo, Y.K.; Oh, W.; Yang, Y.S.; Cho, D.-H.; Kim, J.-Y. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells inhibits melanogenesis by promoting proteasomal degradation of MITF. PLoS ONE 2015, 10, e0128078. [Google Scholar] [CrossRef]
- Wang, X.; Shu, X.; Huo, W.; Zou, L.; Li, L. Efficacy of protein extracts from medium of adipose-derived stem cells via microneedles on Asian skin. J. Cosmet. Laser Ther. 2018, 20, 237–244. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Yi, Y.W.; Lee, J.H.; Kim, S.-Y.; Pack, C.-G.; Ha, D.H.; Park, S.R.; Youn, J.; Cho, B.S. Advances in analysis of biodistribution of exosomes by molecular imaging. Int. J. Mol. Sci. 2020, 21, 665. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-O.; Ha, D.H.; Kim, J.O.; Crumrine, D.A.; Meyer, J.M.; Wakefield, J.S.; Lee, Y.; Kim, B.; Kim, S.; Kim, H.-K.; et al. Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells 2020, 9, 680. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Kim, S.-D.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Jung, J.Y.; Lee, J.H.; Park, S.R.; Youn, J.; et al. Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul. Toxicol. Pharmacol. 2020, 115, 104686. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.H.; Yang, S.H.; Lee, J.; Park, B.C.; Park, K.Y.; Jung, J.Y.; Bae, Y.; Park, G.-H. Combination treatment with human adipose tissue stem cell-derived exosomes and fractional CO2 laser for acne scars: A 12-week prospective, double-blind, randomized, split-face study. ACTA Derm. Venereol. 2020, 100, adv00310. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.L.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guéré, C.; André, N.; Vié, K.; van Niel, G.; Raposo, G. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Hachiya, A.; Murase, D.; Tanabe, H.; Kasamatsu, S.; Takahashi, Y.; Moriwaki, S.; Hase, T. Quantitative changes in the secretion of exosomes from keratinocytes homeostatically regulate skin pigmentation in a paracrine manner. J. Dermatol. 2006, 28, 269–276. [Google Scholar] [CrossRef]
- Lee, J.H.; Ha, D.H.; Go, H.-k.; Youn, J.; Kim, H.-k.; Jin, R.C.; Miller, R.B.; Kim, D.-h.; Cho, B.S.; Yi, Y.W. Reproducible large-scale isolation of exosomes derived from adipose tissue-derived mesenchymal stem/stromal cells and their application in acute kidney injury. Int. J. Mol. Sci. 2020, 21, 4774. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, H.J.; Lee, H.J.; Lee, K.; Hong, D.; Lim, H.; Cho, K.; Jung, N.; Yi, Y.W. Application of a non-hazardous vital dye for cell counting with automated cell counters. Anal. Biochem. 2016, 492, 8–12. [Google Scholar] [CrossRef]
- Grand View Research, Skin Lighting Products Market Size worth $13.7 Billion by 2025. Available online: https://www.grandviewresearch.com/press-release/global-skin-lightening-products-market (accessed on 19 August 2020).
- Pillaiyer, T.; Manickam, M.; Jung, S.-H. Recent development of signaling pathways inhibitors of melanogenesis. Cell Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef]
- Bonaventure, J.; Domingues, M.J.; Larue, L. Cellular and molecular mechanisms controlling the migration of melanocytes and melanoma cells. Pigment Cell Melanoma Res. 2013, 26, 316–325. [Google Scholar] [CrossRef]
- Jimenez-Cervantes, C.; Garcia-Borron, J.C.; Valverde, P.; Solano, F.; Lozano, J.A. Tyrosinase isoenzymes in mammalian melanocytes: 1. biochemical characterization of two melanosomal tyrosinases from B16 mouse melanoma. Eur. J. Biochem. 1993, 217, 549–556. [Google Scholar] [CrossRef]
- Ng, K.W. Penetration enhancement of topical formulations. Pharmaceutics 2018, 10, 51. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Yoo, S.M.; Park, H.H.; Lim, H.J.; Kim, Y.-L.; Lee, S.; Seo, K.-W.; Kang, K.-S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. [Google Scholar] [CrossRef]
- Elkhoury, K.; Kocak, P.; Kang, A.; Arab-Tehrany, E.; Ward, J.E.; Shin, S.R. Engineering smart targeting nanovesicles and their combination with hydrogels for controlled drug delivery. Pharmaceutics 2020, 12, 849. [Google Scholar] [CrossRef]
- Yang, G.; Chen, Q.; Wen, D.; Chen, Z.; Wang, J.; Chen, G.; Wang, Z.; Zhang, X.; Zhang, Y.; Hu, Q.; et al. A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano 2019, 13, 4354–4360. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Cores, J.; Huang, K.; Su, T.; Dinh, P.-Y.; Cheng, K. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ASC Nano 2019, 13, 11273–11282. [Google Scholar] [CrossRef]
- Yi, Y.W.; Cho, B.S. Exosome Kit and Method for Enhancing Transdermal Permeation of Exosomes by Using Same. Patent WO2019088545A1, 9 May 2019. [Google Scholar]
- Belhadj, Z.; He, B.; Deng, H.; Song, S.; Zhang, H.; Wang, X.; Dai, W.; Zhang, Q. A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J. Extracell. Vesicles 2020, 9, 1806444. [Google Scholar] [CrossRef]
- Oldernborg, P.-A.; Zheleznyak, A.; Fang, Y.-F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Barkal, A.A.; Weiskopf, K.; Kao, K.S.; Gordon, S.R.; Rosental, B.; Yiu, Y.Y.; Geroge, B.M.; Markovic, M.; Ring, N.G.; Tsai, J.M.; et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat. Immunol. 2017, 19, 76–84. [Google Scholar] [CrossRef]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Kristiansen, G.; Winzer, K.-J.; Mayordomo, E.; Bellach, J.; Schluns, K.; Denkert, C.; Dahl, E.; Pilarsky, C.; Altevogt, P.; Guski, H.; et al. CD24 expression is a new prognostic marker in breast cancer. Clin. Cancer Res. 2003, 9, 4906–4913. [Google Scholar]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.-C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018, 3, e99263. [Google Scholar] [CrossRef]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 21, 4997–5000. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal protein, RNA and lipids. Nuc. Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef]
- Simpson, R.J.; Kalra, H.; Mathivanan, S. ExoCarta as a resource for exosomal research. J. Extracell. Vesicles 2012, 1, 18374. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kim, S.-Y.; Moon, S.-J.; Chung, J.-H.; Kim, K.-H.; Cho, K.-H.; Park, K.-C. Ceramide inhibits cell proliferation through Atk/PKB inactivation and decreases melanin synthesis in Mel-Ab cells. Pigment Cell Res. 2001, 14, 110–115. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, S.-Y.; Chung, J.-H.; Kim, K.-H.; Eun, H.-C.; Park, K.-C. Delayed ERK activation by ceramide reduced melanin synthesis in human melanocytes. Cell. Signal. 2002, 14, 779–875. [Google Scholar] [CrossRef]
- Kim, D.-S.; Hwang, E.-S.; Lee, J.-E.; Kim, S.-Y.; Kwon, S.-B.; Park, K.-C. Sphigosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J. Cell Sci. 2003, 116, 1699–1706. [Google Scholar] [CrossRef]
- Bemis, L.T.; Chen, R.; Amato, C.M.; Classen, E.H.; Robinson, S.E.; Coffey, D.G.; Erickson, P.F.; Shellman, Y.G.; Robinson, W.A. MicroRNA-137 targets microphthalmia-associated transcription factor in melanoma cell lines. Cancer Res. 2008, 68, 1362–1368. [Google Scholar] [CrossRef]
- Rambow, F.; Bechadergue, A.; Saintigny, G.; Morizot, F.; Mahe, C.; Larue, L. miR-330-5p targets tyrosinase and induces depigmentation. J. Invest. Dermatol. 2014, 134, 2846–2849. [Google Scholar] [CrossRef]
- Dynoodt, P.; Mestdagh, P.; Van Peer, G.; Vandesompele, J.; Goossens, K.; Peelman, L.J.; Geusens, B.; Speeckaert, R.M.; Lambert, J.L.W.; Van Gele, M.J.L. Identification of miR-145 as a key regulator of the pigmentary process. J. Invest. Dermatol. 2013, 133, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Moellmann, G.; Kuklinska, E.; Bomirski, A.; Pawelek, J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, L-tyrosine and L-dopa. J. Cell Sci. 1988, 89, 287–296. [Google Scholar] [PubMed]
- Wolnicka-Glubisz, A.; Nogal, K.; Zadlo, A.; Plonka, P.M. Curcumin does not switch melanin synthesis towards pheomelanin in B16F10 cells. Arch. Dermatol. Res. 2015, 307, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Plensdorf, S.; Livieratos, M.; Dada, N. Pigmentation disorders: Diagnosis and management. Am. Fam. Physician. 2017, 96, 797–804. [Google Scholar] [PubMed]
- Kwon, S.H.; Na, J.-I.; Choi, J.-Y.; Park, K.-C. Melasma: Updates and perspectives. Exp. Dermatol. 2019, 28, 704–708. [Google Scholar] [CrossRef] [PubMed]

| Age | Volunteer #1 | ASC-Exosomes | Placebo Control | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Weeks | 2 Weeks | 4 Weeks | 8 Weeks | 0 Weeks | 2 Weeks | 4 Weeks | 8 Weeks | ||
| 30s | 1 | 207.7 | 198.5 | 200.0 | 186.5 | 170.8 | 165.7 | 169.2 | 147.0 |
| 2 | 195.5 | 195.4 | 195.4 | 170.6 | 193.5 | 193.5 | 191.0 | 175.1 | |
| 40s | 3 | 185.9 | 185.9 | 185.0 | 165.4 | 185.6 | 186.0 | 188.0 | 172.8 |
| 4 | 211.1 | 207.7 | 198.4 | 177.6 | 209.1 | 205.1 | 196.8 | 178.0 | |
| 5 | 180.0 | 183.1 | 180.2 | 159.9 | 187.9 | 188.0 | 187.1 | 166.2 | |
| 6 | 214.4 | 211.0 | 209.6 | 210.1 | 220.4 | 239.0 | 234.6 | 234.2 | |
| 7 | 210.5 | 203.4 | 193.9 | 180.9 | 225.5 | 223.0 | 215.5 | 217.1 | |
| 8 | 200.4 | 206.4 | 199.4 | 204.7 | 197.0 | 207.1 | 200.9 | 199.9 | |
| 9 | 168.5 | 168.4 | 168.6 | 163.3 | 186.6 | 183.4 | 186.6 | 180.5 | |
| 10 | 190.5 | 190.1 | 190.1 | 165.5 | 204.0 | 202.0 | 204.6 | 180.4 | |
| 11 | 175.6 | 174.9 | 174.6 | 159.8 | 158.8 | 159.1 | 159.0 | 144.8 | |
| 12 | 195.8 | 186.6 | 184.9 | 187.0 | 190.0 | 190.1 | 183.5 | 183.0 | |
| 13 | 212.6 | 212.5 | 181.9 | 180.1 | 223.4 | 220.0 | 217.7 | 202.9 | |
| 50s | 14 | 155.0 | 148.0 | 146.1 | 140.6 | 155.4 | 155.2 | 143.0 | 140.6 |
| 15 | 184.7 | 184.5 | 184.4 | 160.0 | 202.9 | 196.4 | 196.0 | 187.8 | |
| 16 | 229.9 | 229.7 | 221.0 | 216.0 | 213.4 | 213.3 | 210.4 | 200.8 | |
| 17 | 121.9 | 126.1 | 123.4 | 108.1 | 130.8 | 130.4 | 130.4 | 124.8 | |
| 18 | 232.0 | 211.9 | 219.4 | 194.5 | 204.6 | 199.8 | 203.0 | 176.2 | |
| 19 | 170.7 | 170.4 | 149.6 | 147.0 | 187.1 | 187.2 | 180.6 | 166.0 | |
| 20 | 170.0 | 161.8 | 164.6 | 163.3 | 180.8 | 176.7 | 171.8 | 171.1 | |
| 21 | 130.7 | 135.3 | 122.3 | 115.0 | 123.4 | 120.6 | 120.0 | 115.4 | |
| Mean | 187.78 | 185.31 | 180.61 | 169.33 | 188.14 | 187.70 | 185.22 | 174.50 | |
| SD | 28.88 | 26.67 | 27.22 | 27.43 | 27.73 | 29.16 | 28.70 | 28.95 | |
| SEM | 6.30 | 5.82 | 5.94 | 5.99 | 6.05 | 6.36 | 6.26 | 6.32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, B.S.; Lee, J.; Won, Y.; Duncan, D.I.; Jin, R.C.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Park, B.C.; et al. Skin Brightening Efficacy of Exosomes Derived from Human Adipose Tissue-Derived Stem/Stromal Cells: A Prospective, Split-Face, Randomized Placebo-Controlled Study. Cosmetics 2020, 7, 90. https://doi.org/10.3390/cosmetics7040090
Cho BS, Lee J, Won Y, Duncan DI, Jin RC, Lee J, Kwon HH, Park G-H, Yang SH, Park BC, et al. Skin Brightening Efficacy of Exosomes Derived from Human Adipose Tissue-Derived Stem/Stromal Cells: A Prospective, Split-Face, Randomized Placebo-Controlled Study. Cosmetics. 2020; 7(4):90. https://doi.org/10.3390/cosmetics7040090
Chicago/Turabian StyleCho, Byong Seung, Jinah Lee, Yujin Won, Diane I. Duncan, Richard C. Jin, Joon Lee, Hyuck Hoon Kwon, Gyeong-Hun Park, Steven Hoseong Yang, Byung Cheol Park, and et al. 2020. "Skin Brightening Efficacy of Exosomes Derived from Human Adipose Tissue-Derived Stem/Stromal Cells: A Prospective, Split-Face, Randomized Placebo-Controlled Study" Cosmetics 7, no. 4: 90. https://doi.org/10.3390/cosmetics7040090
APA StyleCho, B. S., Lee, J., Won, Y., Duncan, D. I., Jin, R. C., Lee, J., Kwon, H. H., Park, G.-H., Yang, S. H., Park, B. C., Park, K. Y., Youn, J., Chae, J., Jung, M., & Yi, Y. W. (2020). Skin Brightening Efficacy of Exosomes Derived from Human Adipose Tissue-Derived Stem/Stromal Cells: A Prospective, Split-Face, Randomized Placebo-Controlled Study. Cosmetics, 7(4), 90. https://doi.org/10.3390/cosmetics7040090





