Abstract
Background: Probiotics and their products are increasingly used in skincare in recent years. Postbiotics are defined as any substance derived through the metabolic activity of a probiotic microorganism, which exerts a direct or indirect beneficial effect on the host. The extracellular metabolites of probiotic bacteria have antimicrobial activities, protect against acne, and improve skin condition. We studied skin protective activities of the extracellular metabolite (LactoSporin) of a spore-forming probiotic Bacillus coagulans MTCC 5856 in vitro. Methods: LactoSporin was evaluated for antioxidant activity by free radical scavenging activity and reactive oxygen quenching activity in human dermal fibroblast cells. Protection of fibroblasts from UV-induced apoptosis and cell death was studied by flow cytometry and neutral red uptake assays. Enzyme inhibition assays were carried out for collagenase, Elastase, and Hyaluronidase. Gene expression studies were carried out using polymerase chain reaction. Results: LactoSporin showed antioxidant activity and was found to protect skin cells from UV-induced apoptosis and cell death. LactoSporin inhibited collagenase, elastase, and hyaluronidase activity and upregulated the expression of hyaluronan synthase, transforming growth factor and epidermal growth factor, which are associated with extracellular matrix integrity. Conclusions: These results suggest LactoSporin is a skin protective postbiotic with wide application in cosmetic formulations.
1. Introduction
The skin is the largest and complex protective organ with a network of multiple cells in a symbiotic relationship with microbial communities. The microbiome is a general term used to refer to the microbial community inhabiting the skin, mucosal cavities, and the gastrointestinal (GI) tract of the human body [1,2]. The majority of microbiota inhabiting the skin are harmless or beneficial [3]. These commensal bacteria are crucial for maintaining a healthy cutaneous environment, manage the dermal immune system to protect against pathogens, and help manage barrier injury [4,5]. Several studies have shown the involvement of skin microbiome in a wide range of molecular and cellular processes within the skin [6]. However, since the skin is continuously exposed to exogenous and endogenous factors, a disruption in the microbiome creates dysbiosis. This perturbation of the skin microbiome can cause skin diseases such as acne, eczema infections, allergies, autoimmune diseases, and aging [7,8,9,10]. Therapeutic strategies to preserve the skin microbiota are becoming increasingly popular in recent years.
Probiotics and prebiotics are extensively used to restore gut microbiome and provide a range of health benefits in recent years [11]. Normalization of intestinal microflora with probiotics and prebiotics has shown a positive effect on several skin conditions like atopic dermatitis, acne, burn injuries, and scars. They also rejuvenate the skin and improve innate skin immunity [12,13]. More recently, the chemical by-products of bacterial fermentation, such as antimicrobial peptides and fragments of dead bacterial cells, termed “Postbiotics,” have gained considerable attention for their beneficial physiological effects [14]. The concept of postbiotics has evolved since most of the beneficial effects of microbiota are mediated by their metabolites. Postbiotics are also reported to induce health benefits similar to that of probiotics, although they do not contain live organisms [15]. The Lactobacillus species (probiotics) and their metabolites are reported to improve skin hydration, elasticity, gloss, and reduce the extent of wrinkles in the skin [14,16,17]. Several human clinical studies suggest that probiotic components not only exert dermal beneficial property through the gastrointestinal route but also help via topical application [12]. The topical application of probiotics was reported to modify the skin barrier function, increase the antimicrobial properties of the skin, and protect against acne and erythema [18,19,20]. The cell-free extracts of probiotic lactic acid bacteria were found to inhibit the expression of virulence factors by opportunistic dermal pathogens in an in vitro study, while topical application of Bifidobacterium longum sp. significantly decreased inflammation in skin explants [21,22]. Although few studies using probiotic metabolites have shown a positive effect on the skin, the use of postbiotics for dermal application is still in its infancy [12].
Bacillus coagulans MTCC 5856 (LactoSpore®) is a non-pathogenic Gram-positive, endospore-forming patented probiotic strain. It is a facultative anaerobe, grows optimally at a slightly acidic pH range of 5.5 to 6.2 and a temperature of 37 °C. The strain produces L(+) lactic acid as a primary product after germination and prevents the growth of pathogenic microbes in the GI tract and has received the generally regarded as Safe (GRAS) status from USFDA [23,24]. LactoSporin is an extracellular metabolite produced by B. coagulans MTCC 5856 under specific growth conditions [25]. LactoSporin was earlier reported to have anti-microbial activity and has been evaluated in a clinical study for efficacy against acne [26]. The product was found to be safe as a cosmetic ingredient and has been successfully registered as per the Cosmetics Regulation (Regulation (EC) No 1223/2009). In the current study, LactoSporin was evaluated as an active ingredient for cosmetic application using in vitro test systems.
2. Materials and Methods
2.1. Materials
2,2-Diphenyl-1-picryl-hydrazyl (DPPH), bovine serum albumin, D-Ribose, Neutral red, Hyaluronic acid, Hyaluronidase enzyme from bovine testes, Hexadecylpyridinium chloride monohydrate, and Dichloro-dihydro-fluorescein diacetate (DCFH-DA) were procured from Sigma Aldrich. The analytical grade methanol was procured from SRL Chemicals, Mumbai (Maharashtra), India. LactoSporin®, a patented and fermented product obtained from Bacillus coagulans MTCC 5856, is a proprietary product of Sabinsa Corporation, NJ, USA. Enzchek® collagenase and Enzchek® Elastase assay kits were procured from Invitrogen, MA, USA. Annexin V-FITC apoptosis kit was procured from Biovision, Milpitas, CA, USA and SYBR Green qPCR master mix was obtained from Thermo Fischer Scientific, MA, USA.
2.2. Strains, Media, and Culture Conditions
BALB/3T3 mouse fibroblasts cells (NCCS, Pune, Maharashtra, India) were cultured in normal Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Thermo Fischer Scientific, MA, USA) supplemented with 10% fetal bovine serum and 40 µg/mL gentamycin. The human dermal fibroblasts adult (HDFa) cell line (Gibco® by Thermo Fischer Scientific, MA, USA) was maintained in fibroblast basal medium with supplement mix (PromoCell, Heidelberg, Germany). The cultures were grown in a humidified incubator at 37 °C in 5% CO2.
2.3. Preparation of LactoSporin
LactoSporin is an extracellular metabolite preparation purified from the probiotic strain B. coagulans MTCC 5856 (LactoSpore®). The bacterial culture was grown in a defined media and the broth was withdrawn after 12 h of fermentation when the pH was between 5.5 and 5.8. The fermented broth of B. coagulans MTCC 5856 culture was harvested and centrifuged to remove the cells. The filtrate was concentrated ten folds and 1.5 volumes of chilled acetone was added to remove the high molecular weight proteins and centrifuged. The supernatant was passed through an adsorbent resin and eluted with demineralized water, concentrated, and filtered through a 0.2-micron filter to get the final product, which was used for all the in vitro assays [24]. Analysis of LactoSporin revealed the presence of several small peptides and hence the product was standardized to contain not less than 10 mg/mL of protein content (1% v/v) and antimicrobial activity not less than 200 AU/mL against Micrococcus luteus.
2.4. DPPH Free Radical Scavenging Activity
The antioxidant potential of LactoSporin was estimated based on scavenging activity of the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical. The assay was carried out by following the modified method, as described earlier [27]. Briefly, 20 μL of various concentrations of LactoSporin in methanol was mixed with 180 μL of methanolic solution of DPPH (0.066 mM) in a 96-well microplate. The plates were incubated in the dark at room temperature for 30 min and the absorbance was measured at 517 nm using a Tecan-Sunrise microplate reader (Sunrise, TW, Tecan). The percentage radical scavenging activity was calculated using the formula,.
DPPH solution was used as a control and tertiary butylhydroquinone (TBHQ) solution in methanol served as a positive control. The concentration of the sample for 50% scavenging of DPPH radicals (IC50) was estimated.
2.5. Cell Viability Assay
HDFa cells were seeded at a density of 2.5 × 103 cells/well in DMEM with 10% Fetal Bovine Serum (FBS) in a 96-well plate. After overnight incubation, cells were treated with various concentrations of LactoSporin in DMEM media with 10% FBS for 72 h at 37 °C. To determine the viability of cells, MTT [3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide] solution was added to each well at a final concentration of 500 μg/mL and further incubated at 37 °C for 3 h [28]. The MTT-formazan product formed due to activity of mitochondrial dehydrogenase was dissolved in DMSO and estimated by measuring the absorbance at 570 nm in a Tecan microplate reader (Sunrise, TW, Tecan Männedorf, Switzerland).
2.6. Intracellular Reactive Oxygen Species (ROS) Scavenging Activity
Intracellular ROS by UV-A irradiation was measured using DCFH-DA [29]. DCFH-DA is a non-fluorescent lipophilic ester which penetrates the plasma membrane and gets activated by cellular esterase-mediated cleavage of acetate to form DCFH. Subsequent oxidation of DCFH in the presence of ROS results in the formation of fluorescent DCF. The increase in fluorescence intensity is proportional to the level of intracellular ROS generated. In short, HDFa cells (5 × 104 cells/well) were seeded in 96-well black microplates and allowed to grow as a monolayer for 24 h. Cells were pre-treated with different concentrations of LactoSporin in Phosphate buffered saline (PBS) for one hour and further exposed to UV-A radiation at a dose of 15 Joules/m2 for 60 min. Freshly prepared DCFH-DA reagent was added to all the wells at a concentration of 10 μg/well and incubated at 37 °C for 30 min. The fluorescence was recorded at a wavelength of 485:520 (Ex:Em) nm in BMG FluoStar Optima (Ortenberg, Germany) microplate reader. The percentage of ROS scavenging was calculated with respect to the fluorescence intensity of UV-A irradiated control cells.
2.7. Protective Effect on UV-Induced Cytotoxicity
UV protective efficacy of LactoSporin was evaluated by using the modified method of Repeto et al. [30]. Balb/3T3 mouse fibroblast cells were seeded at a density of 1 × 104 cells/well in 96-well plates and incubated for 24 h for monolayer formation. Cells were pre-treated with varying nontoxic concentrations of LactoSporin in PBS for 60 min in three individual plates. One plate was exposed to UV-A dose of 15 Joules/m2 for 60 min, the second plate was exposed to a UV-B dose of 4.6 Joule/m2 for 30 min, and the third plate was kept in the dark. The UV dose was calculated using the formula .
The cells were washed with sterile PBS and further incubated for 24 h in the presence of LactoSporin in fresh culture medium containing 5% of FBS. The cell viability was determined by neutral red (3-amino-7-dimethylamino-2-methylphenazine hydrochloride) uptake assay. In short, after specific treatments, the cells were incubated with 50 µg/mL of neutral red for three hours, washed and the dye was extracted, followed by absorbance at 540 nm (Tecan microplate reader). In another set of the same experiment, the cells were stained by 0.5% crystal violet in 20% methanol for 20 min and visualized by an inverted phase-contrast microscope (Olympus, Tokyo, Japan).
2.8. Annexin V Apoptosis Detection Assay
The protective activity of LactoSporin against UV-B-induced apoptosis was assessed by using flow cytometry [31]. The HDFa cells (2 × 105 cells) were treated with various concentrations of LactoSporin (0.5, 1, and 2%). After 24 h of pretreatment, cells were exposed to UV-B dose of 4.6 Joule/m2 for 30 min. The cells were stained with annexin V-FITC and propidium iodide according to the manufacturer’s instructions. The cells were analyzed by flow cytometry to determine cell death and the number of apoptotic cells.
2.9. Anti-Collagenase Assay
Inhibition of collagenase enzyme was estimated by using Enzchek® collagenase, gelatinase assay kit, Invitrogen, Thermo Fischer Scientific, MA, USA, as per the manufacturer’s instructions. The assay was performed using Type IV collagenase from Clostridium histolyticum with DQ™ gelatin as a substrate. Different concentrations of LactoSporin (80 µL/well) were preincubated for 10 min at room temperature with 20 µL of 12.5 µg/mL gelatin substrate. Hundred microliters of the 0.8 U/mL Collagenase enzyme solution was added, and the fluorescence intensity was measured at Em: 520 nm and Ex: 485 nm after 30 min of incubation using BMG FLUOstar Optima Microplate reader (Ortenberg, Germany).
The extent of inhibition was calculated by using the equation , where B is the fluorescence in the presence of the enzyme, BC is the fluorescence in the absence of the enzyme, T is the fluorescence of enzyme activity in the presence of LactoSporin, and TC is the fluorescence of the LactoSporin alone.
2.10. Anti-Elastase Assay
Inhibition of elastase enzyme was estimated by using Enzchek® elastase assay kit, Invitrogen, Thermo Fischer Scientific, MA, USA as per the manufacturer’s instructions. The assay was performed using DQ elastin from bovine neck ligament as a substrate. The protocol and calculations were followed as described for the collagenase assay.
2.11. Anti-Glycation Assay
Anti-glycation activity was evaluated as described earlier [32]. Briefly, 10 µL of various sample concentrations were added to 40 µL of 25 mg/mL bovine serum albumin and 50 µL of 150 mg/mL D-Ribose in a 96-well black microplate. D-ribose with buffer served as control. The plate containing the mixture was incubated for 24 h at 37 °C. The advanced glycation product was detected by measuring the fluorescence intensity at Ex/Em of 390/460 nm by using BMG FLUOstar Optima Microplate reader (Ortenberg, Germany).
2.12. Anti-Hyaluronidase Assay
The assay was performed following the method suggested by Sigma protocol with slight modifications. A volume of 50 µL hyaluronidase (20 U mL−1) in enzyme diluent (20 mM Sodium Phosphate with 77 mM Sodium Chloride and 0.01% (w/v) Bovine Serum Albumin, pH 7.0 at 37 °C) were mixed with 50 µL of different concentrations of LactoSporin diluted with enzyme diluent and incubated at 37 °C for 10 min. The reaction was then initiated by the addition of 100 µL of the substrate in the form of hyaluronic acid (0.03% in 300 mM sodium phosphate, pH 5.35 at 37 °C) solution and incubated at 37 °C for 45 min. The undigested hyaluronic acid (HA) was precipitated with 1 mL of cetylpyridinium chloride. After incubation at room temperature for 10 min, 200 μL of each sample was transferred to 96-well microplates, and the absorbance of the reaction mixture was measured at 600 nm using a microplate reader (Sunrise, TW, Tecan Männedorf, Switzerland). All solutions were prepared freshly. The absorbance in the absence of enzyme was used as a control value for maximum inhibition. The percentage of inhibition was calculated using the formula .
2.13. RNA Extraction and Quantitative RT-PCR
The modulatory effect of LactoSporin on hydrogen peroxide-induced oxidative stress in dermal fibroblasts was assessed by using the qRT-PCR method. HDFa cells were seeded at a density of 3 × 10−5 cells/well in DMEM with 10% FBS in a six-well plate. After overnight incubation, cells were pre-treated for 1 h with or without LactoSporin in DMEM media with 10% FBS. After pretreatment, cells were induced with H2O2 (125 µM) with or without LactoSporin for 1 h followed by overnight incubation in DMEM media with 5% FBS. After respective treatments, total cellular RNA was isolated using Trizol reagent® (Ambion, Life Technologies, MA, USA), according to the manufacturer’s instructions, followed by RNase-free DNase I treatment (Thermo Fisher Scientific) to remove any genomic DNA. Messenger RNA quality and concentration were analyzed spectrophotometrically (Nano Drop Lite, Thermo Fisher Scientific). One microgram of total RNA was reverse transcribed into cDNA using Revert-aid First Strand cDNA synthesis kit (Thermo Fisher Scientific), according to the manufacturer’s instructions, and stored at −80 °C until use. Quantitative real-time PCR (qRT-PCR) was performed using SYBR Green qPCR master mix fluorescent dye using Light cycler 96 (Roche Life Science) according to the manufacturer’s instructions (Thermo Scientific). The primers used for the analysis are provided in Table 1. Expression levels for all genes were normalized to β-actin gene amplification and expressed as fold change transformed by Log2 with respect to untreated control.

Table 1.
List of forward and reverse primers used in quantitative real-time PCR (qRT-PCR) study.
3. Results
3.1. Antioxidant Activity
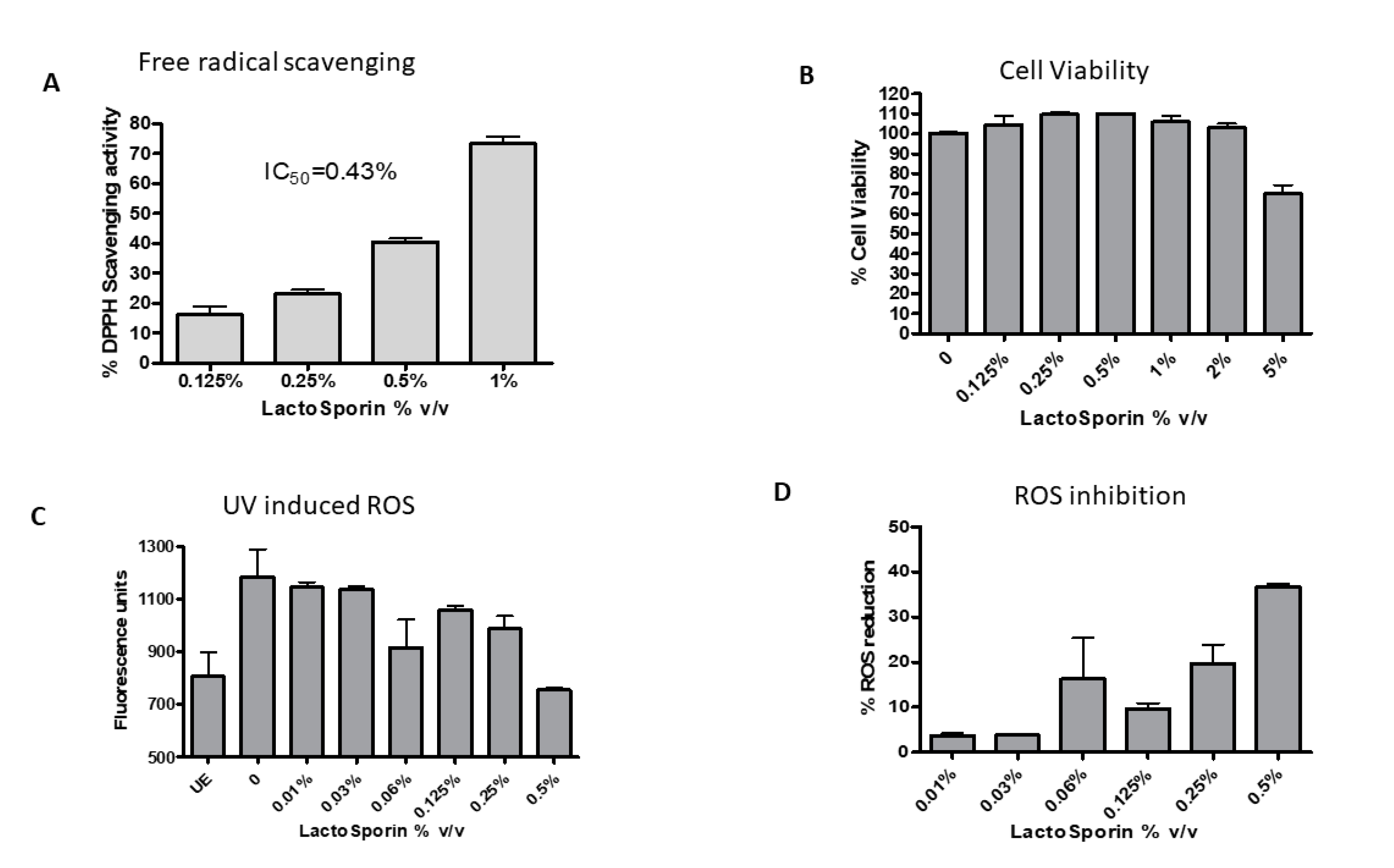
Antioxidant activity of the extracellular metabolite LactoSporin was evaluated by DPPH free radical scavenging assay and inhibition of intracellular reactive oxygen species. DPPH, a water-soluble free radical, reacts with antioxidants to dissipate the color in the reaction mixture. It measures the capacity of the extract to scavenge free radicals in the solution [33]. A dose-dependent reduction in the free radical activity was observed with 50% inhibition (IC50) at 0.43% v/v (Figure 1A). The positive control tert-Butylhydroquinone (TBHQ) showed free radical scavenging activity at an IC50 value of 2.5 μg/mL.
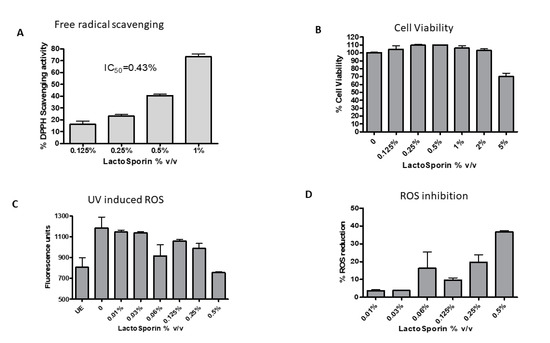
Figure 1.
Effect of various concentrations of LactoSporin on (A) 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity and (B) Cell viability at different concentrations of LactoSporin (C) Intracellular reactive oxygen species (ROS) induced by UV-A, (D) ROS scavenging activity. Data are represented as Mean ±SD, n = 3. UE: Unexposed cells.
The constant exposure of skin to environmental pollution and stress induces oxidative stress and increase in reactive oxygen species (ROS), which damage cellular proteins. The cell viability was checked with different concentrations of LactoSporin before UV exposure. The viability of the cells was not compromised at 2% LactoSporin, while at 5%, we observed a reduction. All the cell-based assays were performed at a maximum concentration of 2% v/v of LactoSporin. Exposure to UV-A-induced ROS in cells and treatment with LactoSporin resulted in a dose-dependent reduction of ROS with 36.7% scavenging activity at 0.5% v/v in UV-A-exposed fibroblasts (Figure 1B). The positive control Quercetin showed an IC50 value of 0.25 μg/mL.
3.2. UV Protection Effect
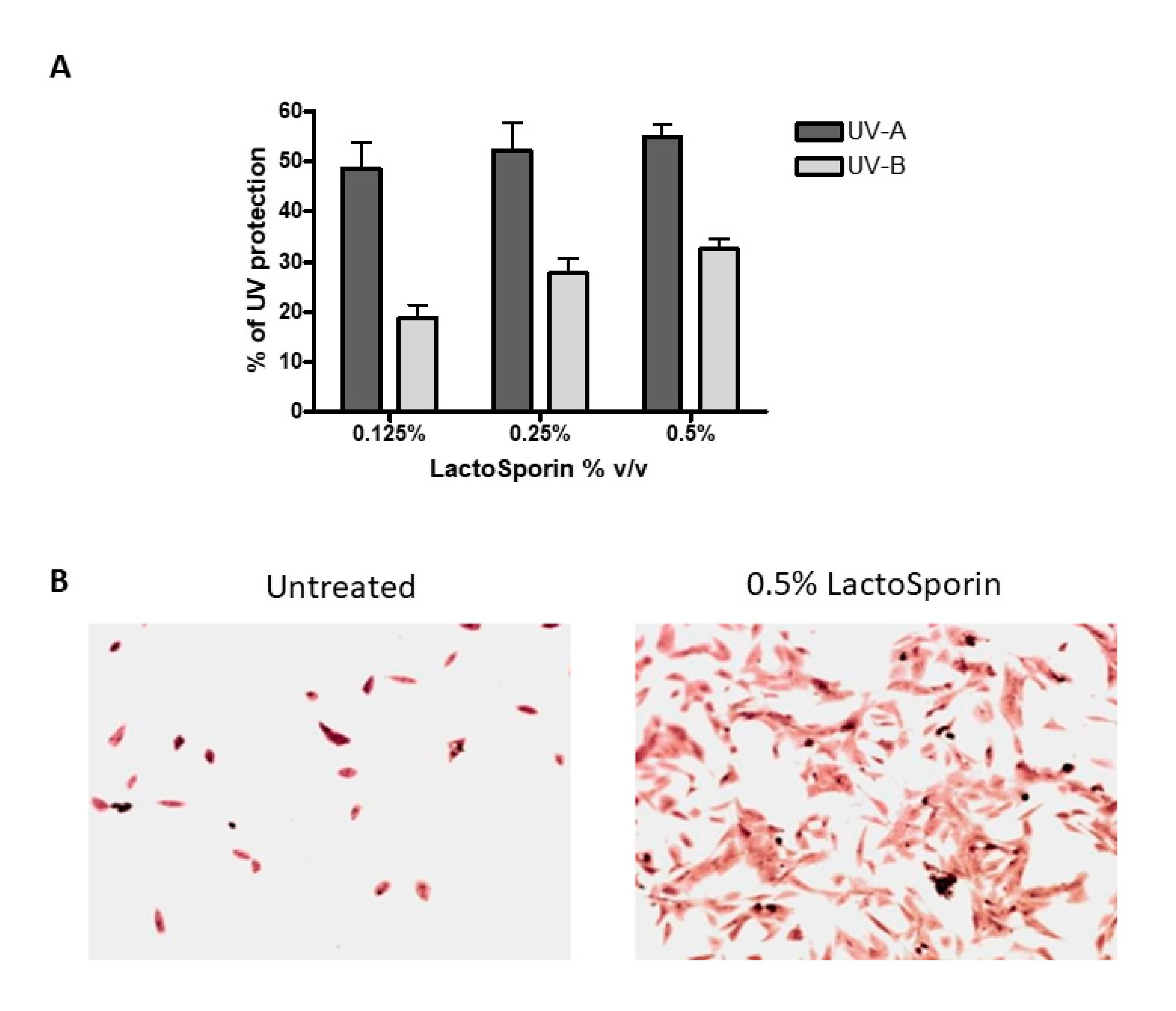
Human dermal fibroblasts were exposed to 15 J/cm2 UV-A and 4.6 J/cm2 UV-B radiation resulting in cell death. Treatment with LactoSporin at varying concentrations before UV exposure resulted in 48.6–54.9% protection from UV-A radiation and 18.7–32.46% against UV-B exposure, respectively (Figure 2A). At higher concentration of 2% and 1%, protection was not observed. The Crystal violet staining of the HDFa cells exposed to UV-B radiation showed intact cells with intact morphological features upon LactoSporin treatment. In contrast, the loss of membrane integrity and alterations in morphological features were observed in UV-B-exposed untreated cells, thus showing the potency of LactoSporin at low concentration in inducing UV protection (Figure 2B).
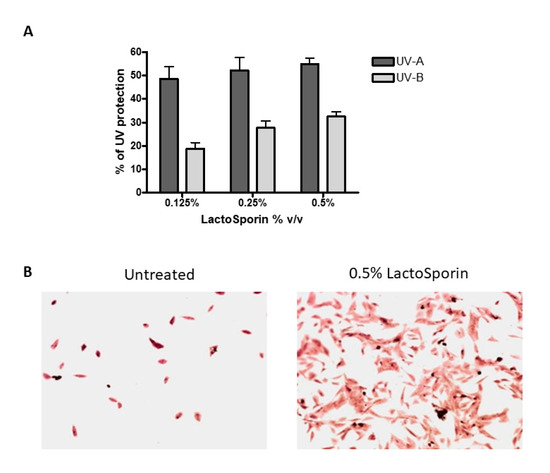
Figure 2.
(A). Percentage protection of LactoSporin against UV-A- and UV-B-induced cell death, (B). UV-B irradiated BALB/3T3 cells, treated with 0.5% LactoSporin, stained with Crystal violet (20×). Data are represented as Mean±SD, n = 3.
3.3. Apoptosis Assay
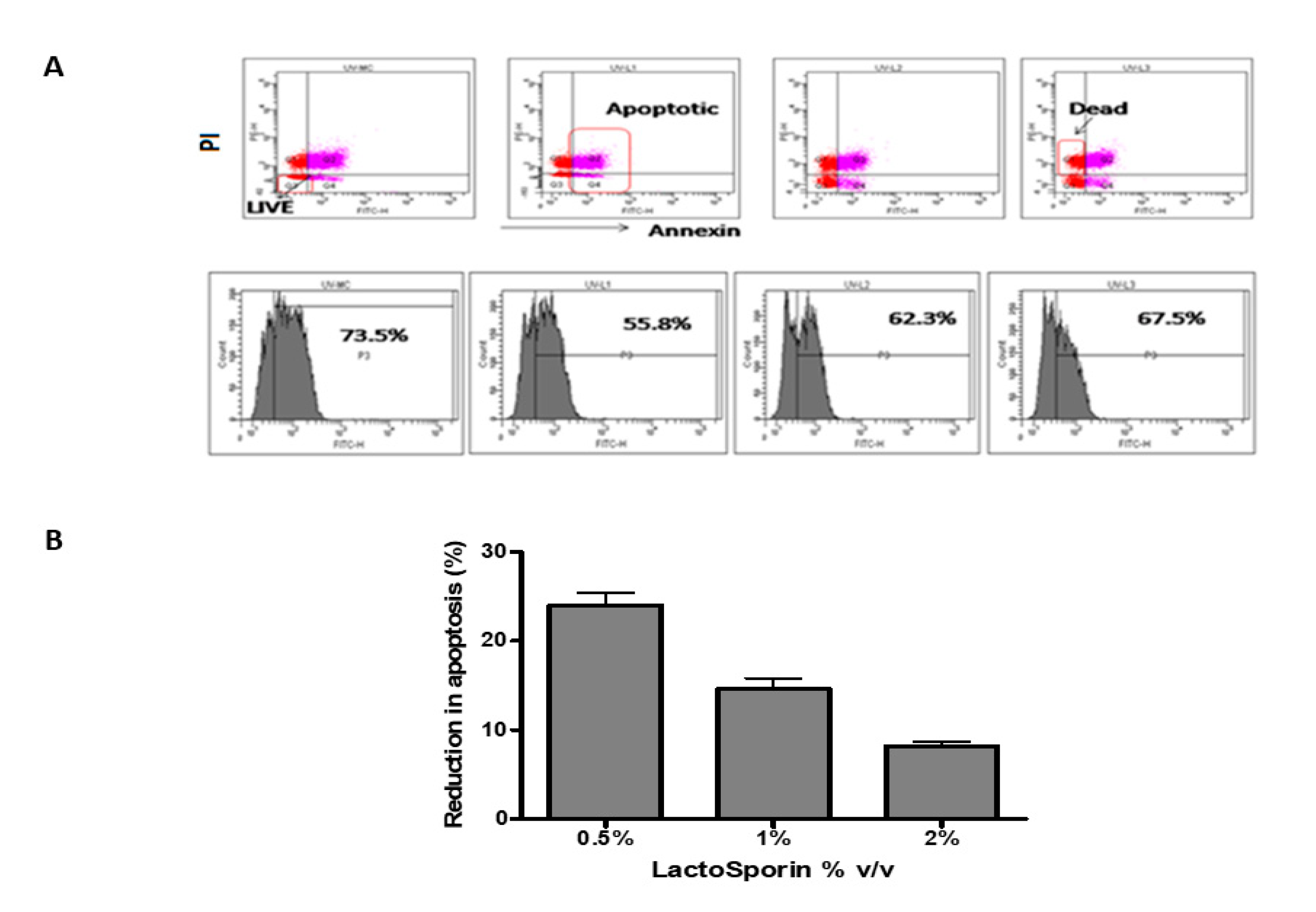
The apoptosis of the cells was assessed by staining the cells with annexin V and while dead cells were stained by propidium iodide [31]. The fluorescent cells were assessed using a flow cytometer. The fibroblast cells exposed to UV-B radiation showed 73.5% positive for annexin, while it reduced to 55.8%, 62.3%, and 67.5% at 0.5%, 1%, and 2%, respectively, LactoSporin pretreatment (Figure 3A). LactoSporin at 0.5% lowered apoptosis by 23.7%, whereas at 1% and 2% of LactoSporin, the reduction was 14.8% and 7.7%, respectively (Figure 3B). These results in concurrence with the UV protection wherein higher concentrations were not very effective. Thus, from the above results, 0.5% was found to be optimum in protecting the dermal fibroblastic cells compared with higher concentrations.
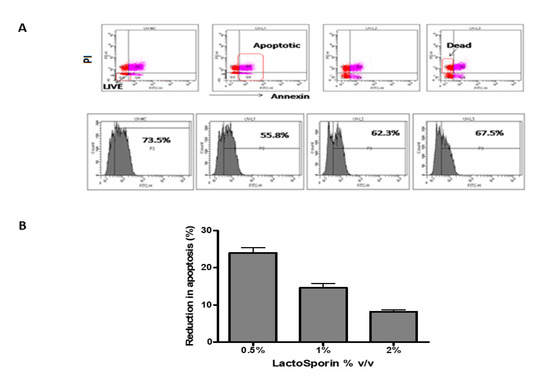
Figure 3.
Protective effect of LactoSporin on UV-B-induced apoptosis. (A). Dot plot and histogram of UV-B exposed cells treated with LactoSporin, (B) Percentage reduction of apoptotic cells after LactoSporin treatment. Results are expressed as Mean ± SD (n = 3).
3.4. Anti-Collagenase Activity
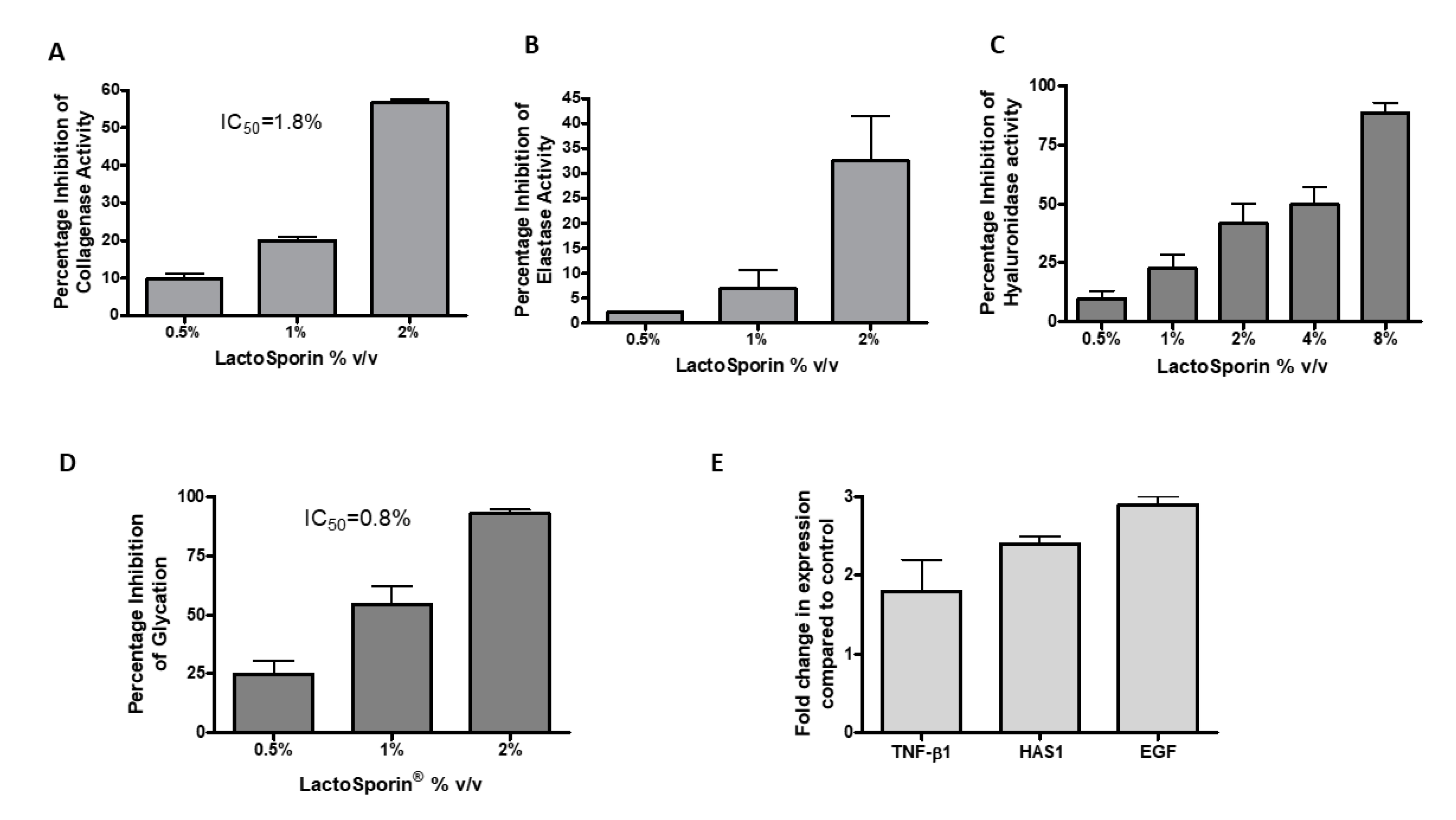
Aging is associated with an increased collagenase activity, resulting in the depletion of collagen in the skin [34]. The anti-collagenase activity of LactoSporin was assessed by the percentage inhibition of collagenase activity at different concentrations (0.5–2% v/v). LactoSporin showed a dose-dependent inhibition of collagenase activity in vitro with a 56.61% reduction in activity at 2% v/v. The IC50 of LactoSporin was calculated to be 1.8% v/v (Figure 4A). 1,10-Phenanthroline, monohydrate provided in the kit showed 99% inhibition at 4 mM.
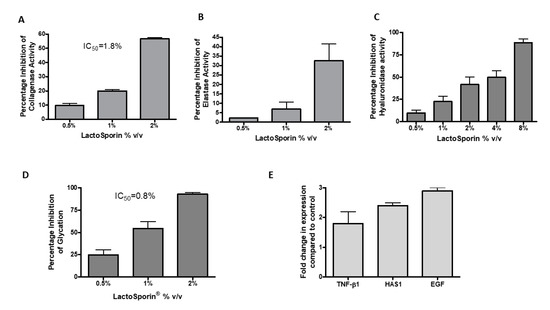
Figure 4.
Effect of various concentrations of LactoSporin in preserving the extracellular membrane layer. (A) Percentage inhibition of collagenase activity; (B) Percentage inhibition of elastase activity; (C) Hyaluronidase enzyme inhibition; (D) Percentage inhibition of advanced glycation end products (AGEs) and (E) Gene expression study of transforming growth factor (TGF)-β, hyaluronic acid synthase (HAS1), and epidermal growth factor (EGF). Data are represented as Mean ± SD, n = 3.
3.5. Anti-Elastase Activity
Skin fibroblast elastase plays a crucial role in wrinkle formation through the degeneration of elastic fiber [35]. LactoSporin showed moderate activity in inhibiting the activity of Elastase with 32.5% inhibition at 2% v/v (Figure 4B). N-Methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone provided in the kit showed 99% inhibition at 0.025 mM.
3.6. Anti-Hyaluronidase Assay
Hyaluronidase is the proteolytic enzyme on the dermis responsible for hyaluronan/ Hyaluronic acid (HA) degradation on the extracellular matrix [36]. HA is responsible for skin moisture retention [37]. In the current study, hyaluronidase inhibition was studied by using bovine serum albumin. Dose-dependent reduction in hyaluronidase activity was observed with an IC50 value of 2.95% v/v (Figure 4C). Tannic acid was used as a positive control with an IC50 value of 194.7 μg/mL.
3.7. Anti-Glycation Effect
Glycation, the process of undesired cross-linking of collagen proteins with sugar molecules, impairs the collagen fibers function by making the skin hard and aged [38]. The anti-glycation effect of LactoSporin was studied by using ribose sugar and bovine serum albumin. Treatment with LactoSporin at 0.5%, 1%, and 2% v/v concentration showed a dose-dependent inhibition of Advanced Glycation End Products (AGEs) by 24.95%, 54.55%, and 93.06%, respectively. The IC50 was calculated to be 0.8% v/v of LactoSporin (Figure 4D). The positive control Arbutin inhibited the formation of AGE at an IC50 value of 10 mM.
3.8. Gene Expression Studies
LactoSporin was evaluated for its effect on the gene expression of transforming growth factor (TGF-β), hyaluronic acid synthase (HAS1), and epidermal growth factor (EGF) in human dermal fibroblasts. Oxidative stress is known to induce senescence or accelerated aging. We used the oxidative senescence model to check the expression of genes related to aging [39,40]. In the study, treatment with 0.25% LactoSporin showed significant enhancement of the TGF-β, HAS, and EGF by 2.14, 1.8, and 2.94-fold, respectively, in human dermal fibroblasts (Figure 4E). The fold increase in the measured genes indicated the potentiality and capability of LactoSporin to maintain the extracellular matrix integrity.
4. Discussion
In the present study, we explored the in vitro skin protective activity of the partially purified extracellular metabolite (LactoSporin) from the probiotic bacterial strain B. coagulans MTCC 5856. The extracellular metabolite was a powerful antioxidant with reactive oxygen quenching activity. It showed protection against UV-induced cellular damage and inhibited collagenase activity, suggesting its potential use in preventing accelerated aging. Earlier studies demonstrated the antimicrobial effect of this preparation against a panel of skin microbial pathogens. In a recent clinical study, LactoSporin-based formulation showed a reduction in acne severity [26]. Further, the product was found to be clinically safe and well-tolerated in the human patch test and the clinical study.
Extracellular metabolites are produced by microorganisms as a by-product of metabolic activity in a specific environment [41]. The culture supernatants of Lactobacillus acidophilus and L. casei have shown anti-oxidant and anti-inflammatory effects [42]. Few of these metabolites include fatty acids, exopolysaccharides, biosurfactants, enzymes, peptides, and vitamins, which are slowly replacing conventional chemicals in various cosmetics products [43]. Bacterial metabolites, cell wall fragments, and even dead bacteria have been shown to elicit immune responses on the skin and improve the skin’s barrier function. Cell-free extract of the probiotic lactic acid bacteria was found to exert antimicrobial and immunomodulatory activities, suggesting the beneficial effects of probiotic metabolites [22]. Topical application of the cell lysate from Bifidobacterium longum on human skin explants was shown to decrease vasodilation, edema, Tumor necrosis factor (TNF)-alpha release, and mast cell degranulation [21]. In a recent study, tryptophan metabolite from a skin microbiota was found to reduce skin inflammation in atopic dermatitis patients [44]. Since metabolites have better stability than live bacteria and are easy to formulate, they are better suited for topical applications. Possible mechanisms of action of these metabolites were reported to be mediated by hyaluronic acid production, generation of ceramides, improvement of the skin barrier, antimicrobial activity, and improvement in the innate immune defense of host cells [12].
Antioxidants are one of the active ingredients used in topical skincare products. An imbalance between ROS production and antioxidant defense gives rise to oxidative stress, which causes progressive damage to cellular structures, resulting in accelerated aging [45]. Further, increasing the number of free radicals can initiate wrinkling, photoaging, elastosis, drying, and skin pigmentation. Topical antioxidants are reported to terminate the chain reactions by removing free radical intermediates and inhibiting other oxidation reactions [46]. Many cosmetic formulations contain various combinations of plant extracts, providing natural antioxidants like polyphenols, flavonoids, stilbenes, and terpenes [46]. LactoSporin was found to reduce ROS as well as scavenge free radicals in vitro, which form the basic requirement for a topical skincare product.
UV radiations consisting of UV-A and UV-B are the major extrinsic factors that cause sun-induced skin damage [47]. UV-B being shorter in wavelength with higher power can easily penetrate deeper into the tissues of the skin, causing structural damage to the DNA and inducing apoptosis. UV-A radiations are mostly responsible for ROS generation, such as superoxide anion, hydrogen peroxide, and singlet oxygen, causing ROS-mediated inflammatory skin damage [48]. In the present study, LactoSporin was able to protect the skin cells from UV-induced cell death and apoptosis. LactoSporin showed UV protection and reduced apoptosis at lower concentrations, but at higher nontoxic concentrations, we did not observe a protective effect. Many natural products follow a hermetic dose response [49]. These natural cosmetics, which follow the hermetic pathways, have been reported to be useful as antioxidant, anti-inflammatory, and sunblock agents [50]. These results, along with its ROS quenching activity, suggest that the product may be used to prevent sunburns.
Skin aging is characterized by the loss of elastin and collagen fiber network, leading to wrinkling. Collagen, the most abundant component of human connective tissue, is reported to be always under UV stress [51]. Photodegradation of collagen results in skin wrinkles, loss of skin structural integrity, and may even lead to cancer [52,53]. During the process of aging, collagen production decreases due to increases in collagenase activity. The breakdown of collagen causes a defective dermis and wrinkle formation [54]. Glycation in skin due to the undesired cross-linking of collagen proteins with sugar molecules eventually impairs the function of collagen fibers, leading to skin hardening and aging [38]. EGF is the critical factor that stimulates collagen production and supports cell renewal through the interaction between keratinocytes and fibroblasts [55]. LactoSporin was able to inhibit the activity of collagenase and suppress glycation while increasing EGF expression, suggesting its potential to prevent premature aging by multiple mechanisms. In a similar study, the exopolysaccharides isolated from sixty Lactobacilli strains showed up to 100% anti-collagenase activity and up to 87% anti-elastase activity [56].
One of the most critical indicators of skin barrier function is skin hydration, which is also a cause of aging [57]. Skin moisture and elasticity are controlled by elastin fibers and HA [58]. HA in extracellular matrix molecules performs various functions, including hydration, lubrication of joints, and tissue repair regulation by managing the activation of inflammatory cells [59]. In a clinical study, topical application of 0.1% HA for 60 days showed significant improvement in skin hydration and elasticity [60]. HA is synthesized by the enzymes HAS and degraded by hyaluronidase into fragments of various sizes [37]. The expression of HAS is upregulated by transforming growth factor TGF-β1 in the dermis and epidermis [61]. LactoSporin inhibits the hyaluronidase activity, thereby inhibiting HA fragmentation. Increased expression of TGF-β, and HAS1 in skin fibroblasts, suggests that LactoSporin could prevent skin moisture loss and maintain skin firmness.
5. Conclusions
The results of the present study suggest the potential of LactoSporin in improving skin health by divergent mechanisms. LactoSporin has also been shown to have antimicrobial properties and reduce acne severity in a clinical study [26]. By reducing oxidative stress and preserving extracellular matrix proteins, LactoSporin may reduce the appearance of wrinkles and sagging of skin. Future clinical studies with formulations containing LactoSporin may further substantiate the results of these preliminary observations.
Author Contributions
Conceptualization, S.M. and M.M.; methodology, L.M. and L.L.; production, S.A.; validation, L.M. and L.L.; formal analysis, L.L.; resources, M.M.; writing—original draft preparation, L.L. and L.M.; writing—review and editing, S.M., K.N., S.A., and M.M.; project administration, S.M.; All authors have read and agreed to the published version of the manuscript.
Funding
The project was funded by Sami Labs Limited and Sabinsa Corporation. The project did not receive any external funding.
Acknowledgments
The authors thank all the members of the Bioresearch group, who were part of the project. The technical assistance by Shwetha N is gratefully acknowledged.
Conflicts of Interest
All the authors are affiliated with Sami Labs Limited or Sabinsa Corporation.
Patents
Muhammed. Majeed; Kalyanam. Nagabhushanam; Sivakumar. Arumugam; Furqan. Ali. Method of producing partially purified extracellular metabolite products from Bacillus coagulans and biological applications thereof. U.S. Patent No.: US 9.596,861 B2, 21 March 2017.
References
- Young, V.B. The role of the microbiome in human health and disease: An introduction for clinicians. BMJ 2017, 356, j831. [Google Scholar] [CrossRef] [PubMed]
- Paetzold, B.; Willis, J.R.; Pereira de Lima, J.; Knödlseder, N.; Brüggemann, H.; Quist, S.R.; Gabaldón, T.; Güell, M. Skin microbiome modulation induced by probiotic solutions. Microbiome 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.L.; Nakatsuji, T. Microbial symbiosis with the innate immune defense system of the skin. J. Investig. Dermatol. 2011, 131, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.-A.; Gearty, S.V.; Pauli, M.L.; Nosbaum, A.; Gratz, I.K.; Otto, M.; Moon, J.J.; Liese, J. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 2015, 43, 1011–1021. [Google Scholar]
- Patra, V.; Gallais Serezal, I.; Wolf, P. Potential of Skin Microbiome, Pro- and/or Pre-Biotics to Affect Local Cutaneous Responses to UV Exposure. Nutrients 2020, 12, 1795. [Google Scholar] [CrossRef]
- Dreno, B.; Araviiskaia, E.; Berardesca, E.; Gontijo, G.; Sanchez Viera, M.; Xiang, L.F.; Martin, R.; Bieber, T. Microbiome in healthy skin, update for dermatologists. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2038–2047. [Google Scholar] [CrossRef]
- Baviera, G.; Leoni, M.C.; Capra, L.; Cipriani, F.; Longo, G.; Maiello, N.; Ricci, G.; Galli, E. Microbiota in healthy skin and in atopic eczema. Biomed Res. Int. 2014, 2014, 436921. [Google Scholar] [CrossRef]
- Prescott, S.L.; Larcombe, D.L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Etten, E.V.; Horwitz, P.; Kozyrskyj, A.; et al. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [CrossRef]
- Floch, M.H. Probiotics and Prebiotics. Gastroenterol. Hepatol. 2014, 10, 680–681. [Google Scholar]
- Lew, L.C.; Liong, M.T. Bioactives from probiotics for dermal health: Functions and benefits. J. Appl. Microbiol. 2013, 114, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Baquerizo Nole, K.L.; Yim, E.; Keri, J.E. Probiotics and prebiotics in dermatology. J. Am. Acad. Dermatol. 2014, 71, 814–821. [Google Scholar] [CrossRef]
- Cicenia, A.; Scirocco, A.; Carabotti, M.; Pallotta, L.; Marignani, M.; Severi, C. Postbiotic activities of lactobacilli-derived factors. J. Clin. Gastroenterol. 2014, 48 (Suppl. 1), S18–S22. [Google Scholar] [CrossRef]
- Zolkiewicz, J.; Marzec, A.; Ruszczynski, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Aoki, R.; Sasaki, K.; Suzuki, C.; Mizumachi, K. Oral intake of heat-killed cells of Lactococcus lactis strain H61 promotes skin health in women. J. Nutr. Sci. 2012, 1, e18. [Google Scholar] [CrossRef]
- Lee, D.E.; Huh, C.S.; Ra, J.; Choi, I.D.; Jeong, J.W.; Kim, S.H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.H.; et al. Clinical Evidence of Effects of Lactobacillus plantarum HY7714 on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef]
- Di Marzio, L.; Cinque, B.; Cupelli, F.; De Simone, C.; Cifone, M.G.; Giuliani, M. Increase of skin-ceramide levels in aged subjects following a short-term topical application of bacterial sphingomyelinase from Streptococcus thermophilus. Int. J. Immunopathol. Pharm. 2008, 21, 137–143. [Google Scholar] [CrossRef]
- Kang, B.S.; Seo, J.G.; Lee, G.S.; Kim, J.H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; Rhee, J.H.; Chung, M.J.; et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef]
- Muizzuddin, N.; Maher, W.; Sullivan, M.; Schnittger, S.; Mammone, T. Physiological effect of a probiotic on skin. J. Cosmet. Sci. 2012, 63, 385–395. [Google Scholar]
- Guéniche, A.; Bastien, P.; Ovigne, J.M.; Kermici, M.; Courchay, G.; Chevalier, V.; Breton, L.; Castiel-Higounenc, I. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp. Dermatol. 2010, 19, e1–e8. [Google Scholar] [CrossRef]
- Iordache, F.; Iordache, C.; Chifiriuc, M.C.; Bleotu, C.; Pavel, M.; Smarandache, D.; Sasarman, E.; Laza, V.; Bucu, M.; Dracea, O.; et al. Antimicrobial and immunomodulatory activity of some probiotic fractions with potential clinical application. Arch. Zootech. 2008, 11, 41–51. [Google Scholar]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Arumugam, S.; Pande, A.; Majeed, S.; Ali, F. A Double-Blind, Placebo-Controlled, Parallel Study Evaluating the Safety of Bacillus coagulans MTCC 5856 in Healthy Individuals. J. Clin. Toxicol. 2016, 6, 283. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Eshuis-de Ruiter, T.; Booij-Veurink, J.; de Vries, Y.P.; Ali, F. Evaluation of genetic and phenotypic consistency of Bacillus coagulans MTCC 5856: A commercial probiotic strain. World J. Microbiol. Biotechnol. 2016, 32, 60. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Ali, F. Method of producing partially purified extracellular metabolite products from Bacillus coagulans and biological applications thereof. U.S. Patent No. 9,596,861, 2017. [Google Scholar]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Mundkur, L.; Rajalakshmi, H.R.; Shah, K.; Beede, K. Novel Topical Application of a Postbiotic, LactoSporin®, in Mild to Moderate Acne: A Randomized, Comparative Clinical Study to Evaluate its Efficacy, Tolerability and Safety. Cosmetics 2020, 7, 70. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Hirano, H.; Tabuchi, Y.; Kondo, T.; Zhao, Q.L.; Ogawa, R.; Cui, Z.G.; Feril, L.B., Jr.; Kanayama, S. Analysis of gene expression in apoptosis of human lymphoma U937 cells induced by heat shock and the effects of alpha-phenyl N-tert-butylnitrone (PBN) and its derivatives. Apoptosis 2005, 10, 331–340. [Google Scholar] [CrossRef]
- Sero, L.; Sanguinet, L.; Blanchard, P.; Dang, B.T.; Morel, S.; Richomme, P.; Seraphin, D.; Derbre, S. Tuning a 96-well microtiter plate fluorescence-based assay to identify AGE inhibitors in crude plant extracts. Molecules 2013, 18, 14320–14339. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.M.; Araújo, M.E.M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; Maroni, P.; Ozer, N.; Zingg, J.M.; Azzi, A. Age-dependent increase of collagenase expression can be reduced by alpha-tocopherol via protein kinase C inhibition. Free Radic. Biol. Med. 1999, 27, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Moriwaki, S.; Suzuki, Y.; Takema, Y.; Imokawa, G. The role of elastases secreted by fibroblasts in wrinkle formation: Implication through selective inhibition of elastase activity. Photochem. Photobiol. 2001, 74, 283–290. [Google Scholar] [CrossRef]
- Tu, P.T.; Tawata, S. Anti-Oxidant, Anti-Aging, and Anti-Melanogenic Properties of the Essential Oils from Two Varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Chen, J.H.; Ozanne, S.E.; Hales, C.N. Methods of cellular senescence induction using oxidative stress. Methods Mol. Biol. (Cliftonn. J.) 2007, 371, 179–189. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Mishra, A.; Kumar, L.M.; Prakash, N.S.; Prabhu, S.; Ramakrishnan, S. Protective Effects of Triphala on Dermal Fibroblasts and Human Keratinocytes. PLoS ONE 2016, 11, e0145921. [Google Scholar] [CrossRef]
- Pinu, F.R.; Villas-Boas, S.G. Extracellular Microbial Metabolomics: The State of the Art. Metabolites 2017, 7, 43. [Google Scholar]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid. Based Complementary Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.L.; Rajput, M.; Oza, T.; Trivedi, U.; Sanghvi, G. Eminence of Microbial Products in Cosmetic Industry. Nat. Prod. Bioprospect. 2019, 9, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Luo, Y.; Zhu, Z.; Zhou, Y.; Sun, L.; Gao, J.; Sun, J.; Wang, G.; Yao, X.; Li, W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J. Allergy Clin. Immunol. 2019, 143, 2108–2119.e2112. [Google Scholar] [CrossRef] [PubMed]
- Bogdan Allemann, I.; Baumann, L. Antioxidants used in skin care formulations. Ski. Ther. Lett. 2008, 13, 5–9. [Google Scholar]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Poljsak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pr. 2012, 2012, 135206. [Google Scholar] [CrossRef]
- Sanches Silveira, J.E.; Myaki Pedroso, D.M. UV light and skin aging. Rev. Environ. Health 2014, 29, 243–254. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.-S.H. Natural anti-aging skincare: Role and potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef]
- Jariashvili, K.; Madhan, B.; Brodsky, B.; Kuchava, A.; Namicheishvili, L.; Metreveli, N. UV damage of collagen: Insights from model collagen peptides. Biopolymers 2012, 97, 189–198. [Google Scholar] [CrossRef]
- Pugliese, P.T. Physiology of the Skin II: An Expanded Scientific Guide for the Skin Care Professional; Allured Publishing Corporation: Carol Stream, IL, USA, 2001. [Google Scholar]
- Varani, J.; Spearman, D.; Perone, P.; Fligiel, S.E.; Datta, S.C.; Wang, Z.Q.; Shao, Y.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am. J. Pathol. 2001, 158, 931–942. [Google Scholar] [CrossRef]
- Shaheda, S.A.; Duraivel, S.; Niharika, R.; Anusha, P.; Qudusiya, S. A review on natural bioactive compounds as potential anti-wrinkle agents. World J. Pharm. Pharm. Sci. 2014, 3, 528–544. [Google Scholar]
- Aldag, C.; Nogueira Teixeira, D.; Leventhal, P.S. Skin rejuvenation using cosmetic products containing growth factors, cytokines, and matrikines: A review of the literature. Clin. Cosmet. Investig. Dermatol. 2016, 9, 411–419. [Google Scholar] [CrossRef]
- Shirzad, M.; Hamedi, J.; Motevaseli, E.; Modarressi, M.H. Anti-elastase and anti-collagenase potential of Lactobacilli exopolysaccharides on human fibroblast. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1051–1061. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the Aging Skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef]
- Park, J.I.; Lee, J.E.; Shin, H.J.; Song, S.; Lee, W.K.; Hwang, J.S. Oral Administration of Glycine and Leucine Dipeptides Improves Skin Hydration and Elasticity in UVB-Irradiated Hairless Mice. Biomol. Ther. 2017, 25, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Göllner, I.; Voss, W.; von Hehn, U.; Kammerer, S. Ingestion of an Oral Hyaluronan Solution Improves Skin Hydration, Wrinkle Reduction, Elasticity, and Skin Roughness: Results of a Clinical Study. J. Evid. Based Complementary Altern. Med. 2017, 22, 816–823. [Google Scholar] [CrossRef]
- Pavicic, T.; Gauglitz, G.G.; Lersch, P.; Schwach-Abdellaoui, K.; Malle, B.; Korting, H.C.; Farwick, M. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J. Drugs Dermatol. 2011, 10, 990–1000. [Google Scholar] [PubMed]
- Averbeck, M.; Gebhardt, C.A.; Voigt, S.; Beilharz, S.; Anderegg, U.; Termeer, C.C.; Sleeman, J.P.; Simon, J.C. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J. Investig. Dermatol. 2007, 127, 687–697. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).