Natural Flavones and Flavonols: Relationships among Antioxidant Activity, Glycation, and Metalloproteinase Inhibition
Abstract
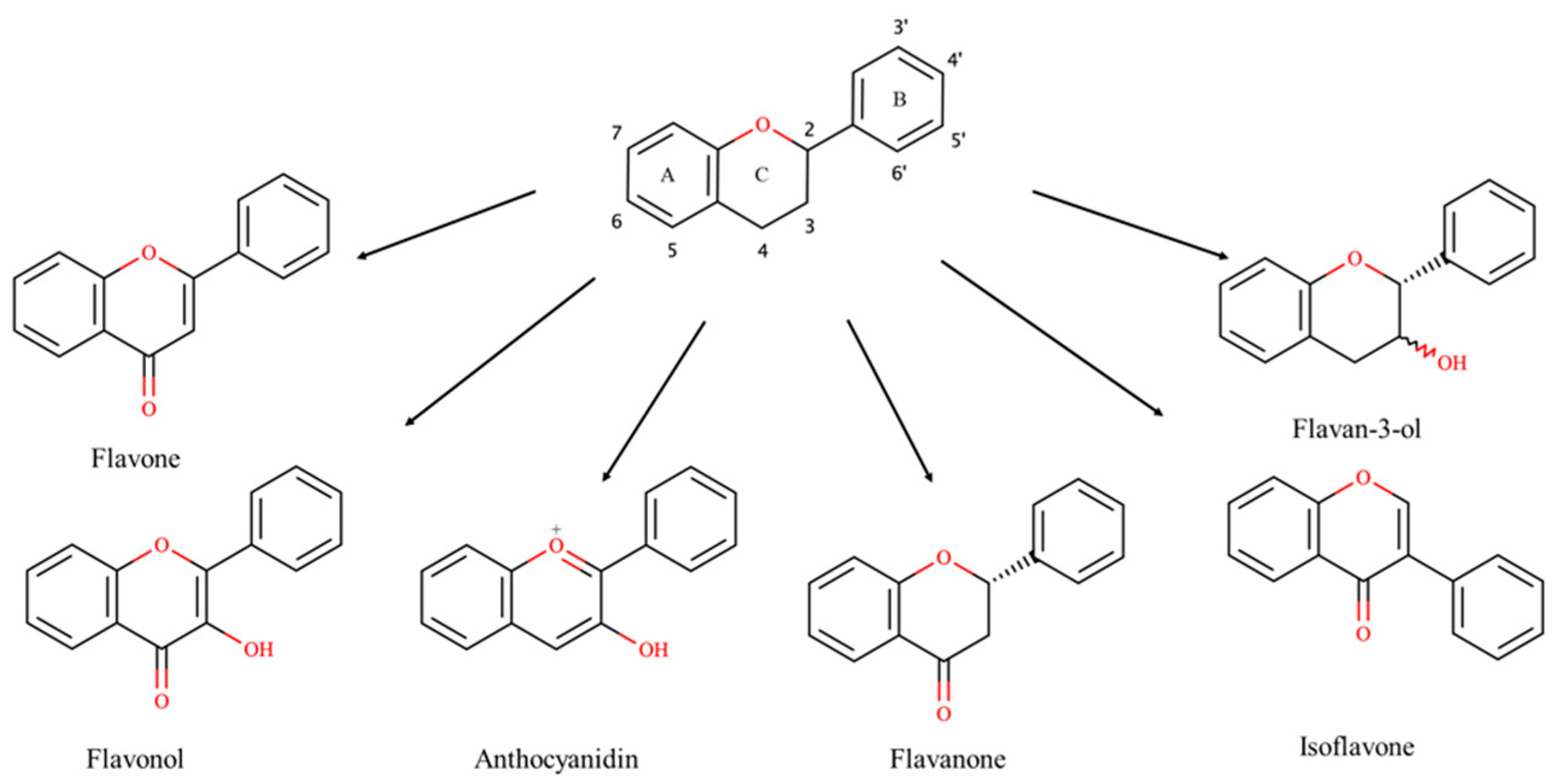
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oxygen Radical Absorbance Capacity (Orac) Assay
2.3. NO Scavenger Assay
2.4. Anti-glycation Activity
2.5. MMP Inhibition Assay
2.6. QSAR Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, C.S.; Park, S.; Kim, J. The role of glycation in the pathogenesis of aging and its prevention through herbal products and physical exercise. J. Exerc. Nutr. Biochem. 2017, 21, 55–61. [Google Scholar] [CrossRef]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosisin diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Percik, R.; Stumvoll, M. Obesity and cancer. Exp. Clin. Endocr. Diab. 2009, 117, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, P.J.; Carrington, A.L.; Frost, G.S.; Boulton, A.J. Neurovascular disease, antioxidants and glycation in diabetes. Diabetes Metab. Res. 2002, 18, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A.M. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs metalloproteinases inhibition. Crit. Rev. Food Sci. 2018, 58, 893–904. [Google Scholar] [CrossRef]
- Sparvero, L.J.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; Amoscato, A.A.; Zeh, H.J.; et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 2009, 7, 1–17. [Google Scholar] [CrossRef]
- Panico, A.M.; Vicini, P.; Geronikaki, A.; Incerti, M.; Cardile, V.; Crascì, L.; Messina, R.; Ronsisvalle, S. Heteroarylimino-4-thiazolidinonesas inhibitors of cartilage degradation. Bioorgan. Chem. 2011, 39, 48–52. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and their inhibitors: Potential for the development of new therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef]
- Levin, M.; Udi, Y.; Solomonov, I.; Sagi, I. Next generation matrix metalloproteinase inhibitors—Novel strategies bring new prospects. Biochim. Biophys. Acta 2017, 1864, 1927–1939. [Google Scholar] [CrossRef]
- Fields, G.B. The rebirth of matrix metalloproteinase inhibitors: Moving beyond the dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef]
- Lauro, M.R.; Crascì, L.; Carbone, C.; Aquino, R.P.; Panico, A.M.; Puglisi, G. Encapsulation of a citrus by-product extract: Development, characterization and stability studies of a nutraceutical with antioxidant and metalloproteinases inhibitory activity. LWT Food Sci Technol. 2015, 62, 169–176. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Galano, A. Free radicals induced oxidative stress at a molecular level: The current status, challenges and perspectives of computational chemistry based protocols. J. Mex. Chem. Soc. 2015, 59, 231–262. [Google Scholar] [CrossRef]
- Trouillas, P.; Marsal, P.; Siri, D.; Lazzaroni, R.; Duroux, J.L. A DFT study of the reactivity of OH groups in quercetin and taxifolin antioxidants: The specificity of the 3-OH site. Food Chem. 2006, 97, 679–688. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Leopoldini, M.; Pitarch, I.P.; Russo, N.; Toscano, M. Structure, conformation, and electronic properties of apigenin, luteolin, and taxifolin antioxidants. A first principle theoretical study. J. Phys. Chem. A 2004, 108, 92–96. [Google Scholar] [CrossRef]
- Leopoldini, M.; Chiodo, S.G.; Russo, N.; Toscano, M. Detailed investigation of the OH radical quenching by natural antioxidant caffeic acid studied by quantum mechanical models. J. Chem. Theory Comput. 2011, 7, 4218–4233. [Google Scholar] [CrossRef]
- Moalin, M.; Van Strijdonck, G.P.; Beckers, M.; Hagemen, G.; Borm, P.; Bast, A.; Haenen, G.R. A planar conformation and the hydroxyl groups in the B and C rings play a pivotal role in the antioxidant capacity of quercetin and quercetin derivatives. Molecules 2011, 16, 9636–9650. [Google Scholar] [CrossRef]
- Jeong, J.M.; Choi, C.H.; Kang, S.K.; Lee, I.H.; Lee, J.Y.; Jung, H. Antioxidant and chemosensitizing effects of flavonoids with hydroxy and/or methoxy groups and structure-activity relationship. J. Pharm. Pharm. Sci. 2007, 10, 537–546. [Google Scholar] [CrossRef]
- Treml, J.; Smejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. F 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Huang, L.; Zhang, C.; Zhang, Y. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Rafat Husain, S.; Cillard, J.; Cillard, P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Eto, M.; Masuoka, C.; Yamasaski, T.; Harano, K.; Ono, M. Molecular orbital analysis of antioxidative activity of phenolics from Tessaria integrifolia and Piper elongatum. Food Sci. Technol. Res. 2008, 14, 415–420. [Google Scholar] [CrossRef][Green Version]
- Wu, C.H.; Yen, G.C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subratty, A.H. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 2013, 6, 561–569. [Google Scholar] [CrossRef]
- Sim, G.S.; Lee, B.C.; Cho, H.S.; Lee, J.W.; Kim, J.H.; Lee, D.H.; Kim, J.H.; Pyo, H.B.; Moon, D.C.; Oh, K.W.; et al. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch. Pharm. Res. 2007, 30, 290–298. [Google Scholar] [CrossRef]
- Ende, C.; Gebhardt, R. Inhibition of matrix metalloproteinase-2 and -9 activities by selected flavonoids. Planta Med. 2004, 70, 1006–1008. [Google Scholar] [CrossRef]
- Crascì, L.; Basile, L.; Panico, A.; Puglia, C.; Bonina, F.P.; Basile, P.M.; Rizza, L.; Guccione, S. Correlating in vitro target-oriented screening and docking: Inhibition of matrix metalloproteinases activities by flavonoids. Planta Med. 2017, 83, 901–911. [Google Scholar] [CrossRef]
- Montenegro, L.; Panico, A.; Santagati, L.; Siciliano, E.A.; Intagliata, S.; Modica, M. Solid lipid nanoparticles loading idebenone ester with pyroglutamic acid: In vitro antioxidant activity and in vivo topical efficacy. Nanomaterials 2019, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Crascì, L.; Cardile, V.; Longhitano, G.; Nanfitò, F.; Panico, A. Anti-degenerative effect of Apigenin, Luteolin and Quercetin on human keratinocyte and chondrocyte cultures: SAR evaluation. Drug Res. 2018, 68, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Derbré, S.; Gatto, J.; Pelleray, A.; Coulon, L.; Séraphin, D.; Richomme, P. Automating a 96-well microtiter plate assay for identification of AGEs inhibitors or inducers: Application to the screening of a small natural compounds library. Anal. Bioanal. Chem. 2010, 398, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Rafter, J.; Jenner, A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005, 81, 268S–276S. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Ramasamym, R.; Schmidtm, A.M. Mechanisms of disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 285–293. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Winsz-Szczotka, K.; Klimek, K.; Olczyk, K. Plasma biomarkers of oxidative and AGE-mediated damage of proteins and glycosaminoglycans during healthy ageing: A possible association with ECM metabolism. Mech. Ageing Dev. 2012, 133, 538–548. [Google Scholar] [CrossRef]
- Nagai, R.; Murray, D.B.; Metz, T.O.; Baynes, J.W. Chelation: A fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 2012, 61, 549–559. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q) SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Kameda, K.; Matsunaga, T.; Abe, N.; Hanada, H.; Ishizaka, H.; Ono, H.; Saitoh, M.; Fukui, K.; Fukuda, I.; Osanai, T.; et al. Correlation of oxidative stress with activity of matrix metalloproteinase in patients with coronary artery disease. Eur. Heart J. 2003, 24, 2180–2185. [Google Scholar] [CrossRef]
- Oh, S.H.; Lim, S.C. A rapid and transient ROS generation by cadmium triggers apoptosis via caspase-dependent pathway in HepG2 cells and this is inhibited through N-acetylcysteine-mediated catalase upregulation. Toxicol. Appl. Pharmacol. 2006, 212, 212–223. [Google Scholar] [CrossRef]
- Kar, S.; Subbaram, S.; Carrico, P.M.; Melendez, J.A. Redox-control of matrix metalloproteinase-1: A critical link between free radicals, matrix remodeling and degenerative disease. Respir. Physiol. Neurobiol. 2010, 174, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Aljada, A.; Ghanim, H.; Mohanty, P.; Syed, T.; Bandyopadhyay, A.; Dandona, P. Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase in mononuclear cells, and in plasma tissue factor and matrix metalloproteinase concentrations. Am. J. Clin. Nutr. 2004, 80, 51–57. [Google Scholar] [CrossRef]
- Amić, D.; Davidović-Amić, D.; Beslo, D.; Rastija, V.; Lucić, B.; Trinajstić, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef]
- Takano-Ishikawa, Y.; Goto, M.; Yamaki, K. Structure–activity relations of inhibitory effects of various flavonoids on lipopolysaccharide-induced prostaglandin E 2 production in rat peritoneal macrophages: Comparison between subclasses of flavonoids. Phytomedicine 2006, 13, 310–317. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S. Recent advances in topical delivery of flavonoids: A review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- Montenegro, L. Lipid-based nanoparticles as carriers for dermal delivery of antioxidants. Curr. Drug Metab. 2017, 18, 469–480. [Google Scholar] [CrossRef]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to improve resveratrol systemic and topical bioavailability: An update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.; Isacchi, B.; Righeschi, C.; Guccione, C.; Bergonzi, M. Flavonoids loaded in nanocarriers: An opportunity to increase oral bioavailability and bioefficacy. Food Nutr. Sci. 2014, 5, 1212–1227. [Google Scholar] [CrossRef]
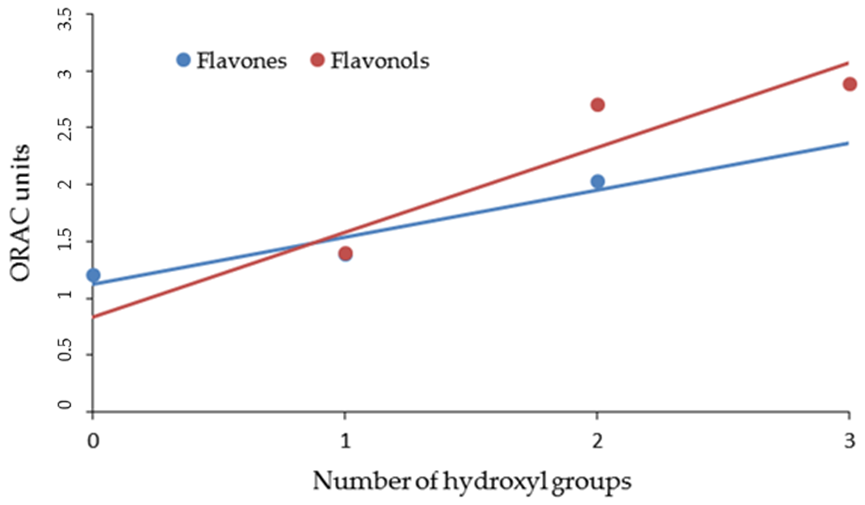

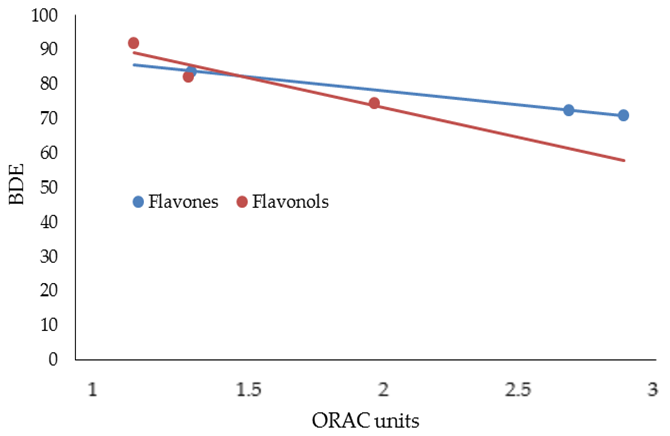
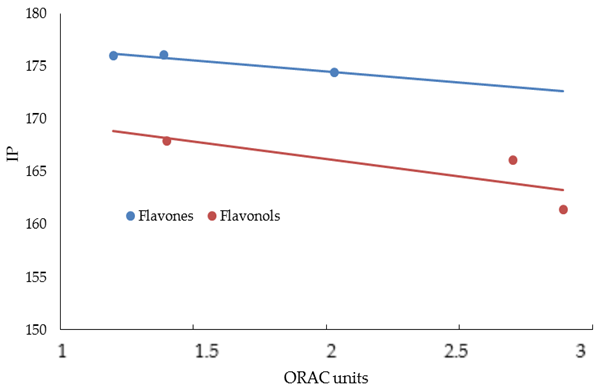
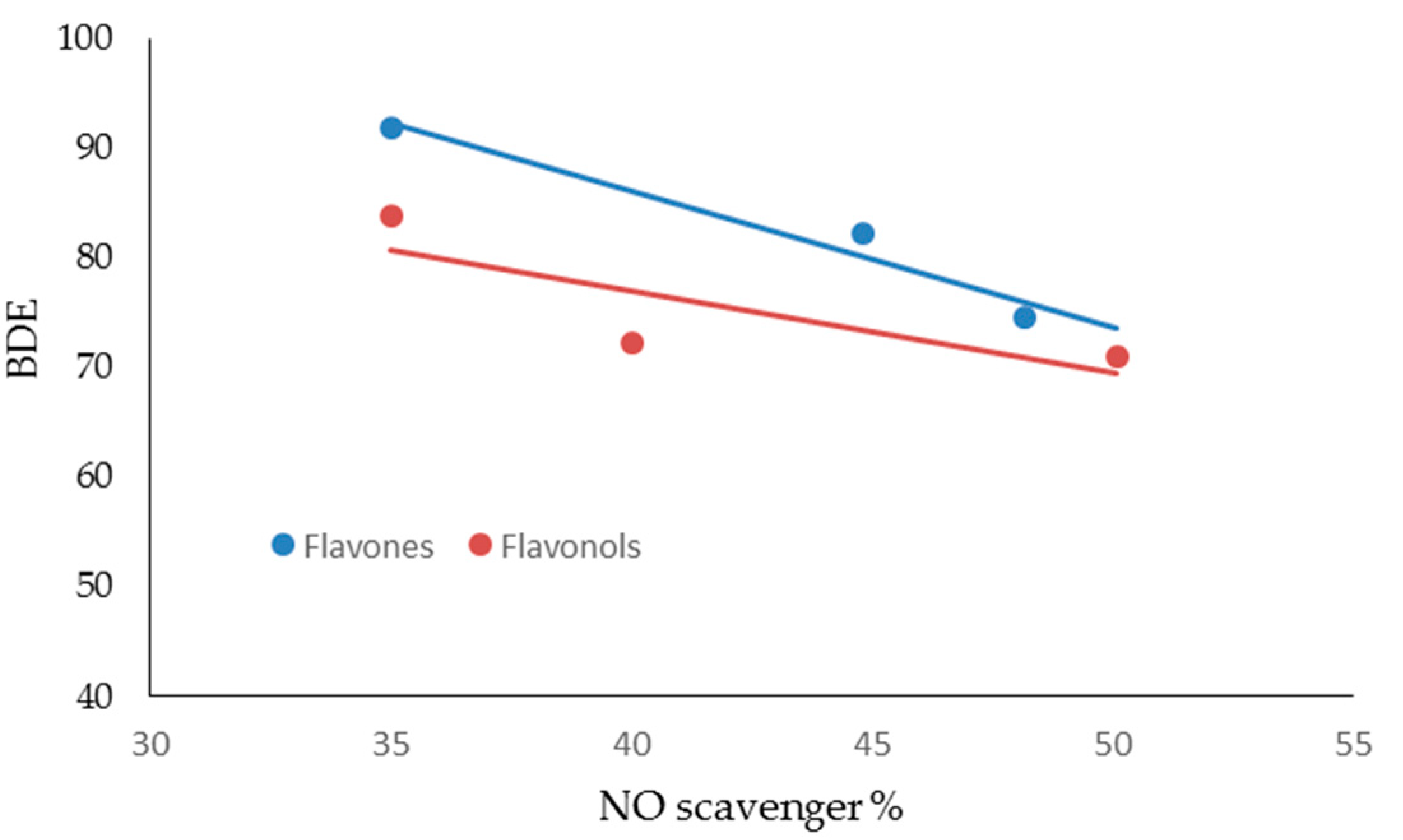
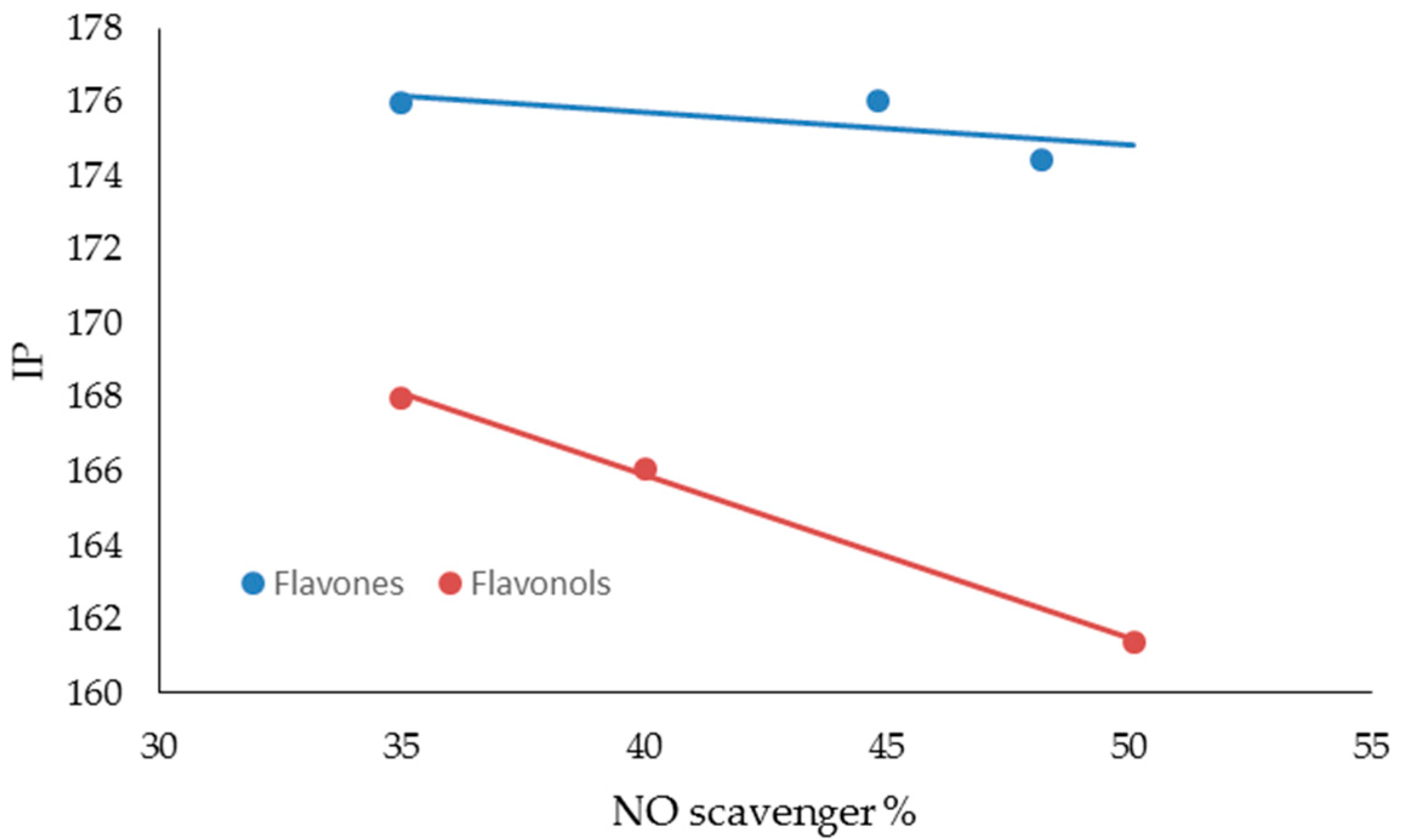

| FLAVONES | ORAC Units (µM) | NO Scavenger (%) | AGEs Inhibition (%) | MMP-1 (IC50) | MMP-13 (IC50) | MMP-2 (IC50) | MMP-9 (IC50) |
|---|---|---|---|---|---|---|---|
| LUTEOLIN | 2.03 ± 0.11 | 48.19 ± 0.18 | 60.04 ± 0.10 | 3.0 ± 0.11 | 1.0 ± 0.14 | 20.0 ±0.13 | 22.0 ± 0.23 |
| APIGENIN | 1.39 ± 0.13 | 44.81 ± 0.26 | 59.10 ± 0.12 | 6.0 ± 0.13 | 3.0 ± 0.12 | 16.0 ± 0.11 | 18.0 ± 0.15 |
| CHRYSIN | 1.20 ± 0.10 | 35.00 ± 0.28 | 45.08 ± 0.10 | 7.0 ± 0.22 | 10.0 ± 0.24 | 33.0 ± 0.18 | 31.0 ± 0.17 |
| FLAVONOLS | ORAC Units (µM) | NO Scavenger (%) | AGEs inhibition (%) | MMP-1 (IC50) | MMP-13 (IC50) | MMP-2 (IC50) | MMP-9 (IC50) |
|---|---|---|---|---|---|---|---|
| MIRYCETIN | 2.89 ± 0.09 | 50.10 ± 0.18 | 58.04 ± 0.11 | 3.0 ± 0.11 | 2.00 ± 0.13 | 17.00 ± 0.23 | 19.00 ± 0.31 |
| QUERCETIN | 2.70 ± 0.10 | 40.01 ± 0.09 | 52.04 ± 0.12 | 1.0 ± 0.16 | 3.00 ± 0.17 | 12.00 ± 0.19 | 22.00 ± 0.16 |
| KAEMPFEROL | 1.40 ± 0.11 | 35.00 ± 0.26 | 38.02 ± 0.17 | 4.0 ± 0.14 | 2.00 ± 0.15 | 24.00 ± 0.21 | 27.00 ± 0.23 |
| FLAVONES | BDE (Kcal·mol−1) | IP (Kcal·mol−1) | TPSA | Log P |
|---|---|---|---|---|
| LUTEOLIN | 74.54 | 174.44 | 107.22 | 2.26 |
| APIGENIN | 82.20 | 176.05 | 86.99 | 2.20 |
| CHRYSIN | 91.85 | 176.00 | 66.76 | 2.50 |
| FLAVONOLS | BDE (Kcal·mol−1) | IP (Kcal·mol−1) | TPSA | Log P |
|---|---|---|---|---|
| MIRYCETIN | 71.08 | 161.40 | 147.68 | 1.76 |
| QUERCETIN | 72.35 | 166.08 | 127.45 | 2.03 |
| KAEMPFEROL | 83.80 | 167.99 | 107.22 | 2.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronsisvalle, S.; Panarello, F.; Longhitano, G.; Siciliano, E.A.; Montenegro, L.; Panico, A. Natural Flavones and Flavonols: Relationships among Antioxidant Activity, Glycation, and Metalloproteinase Inhibition. Cosmetics 2020, 7, 71. https://doi.org/10.3390/cosmetics7030071
Ronsisvalle S, Panarello F, Longhitano G, Siciliano EA, Montenegro L, Panico A. Natural Flavones and Flavonols: Relationships among Antioxidant Activity, Glycation, and Metalloproteinase Inhibition. Cosmetics. 2020; 7(3):71. https://doi.org/10.3390/cosmetics7030071
Chicago/Turabian StyleRonsisvalle, Simone, Federica Panarello, Giusy Longhitano, Edy Angela Siciliano, Lucia Montenegro, and Annamaria Panico. 2020. "Natural Flavones and Flavonols: Relationships among Antioxidant Activity, Glycation, and Metalloproteinase Inhibition" Cosmetics 7, no. 3: 71. https://doi.org/10.3390/cosmetics7030071
APA StyleRonsisvalle, S., Panarello, F., Longhitano, G., Siciliano, E. A., Montenegro, L., & Panico, A. (2020). Natural Flavones and Flavonols: Relationships among Antioxidant Activity, Glycation, and Metalloproteinase Inhibition. Cosmetics, 7(3), 71. https://doi.org/10.3390/cosmetics7030071






