Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations
Abstract
1. Introduction
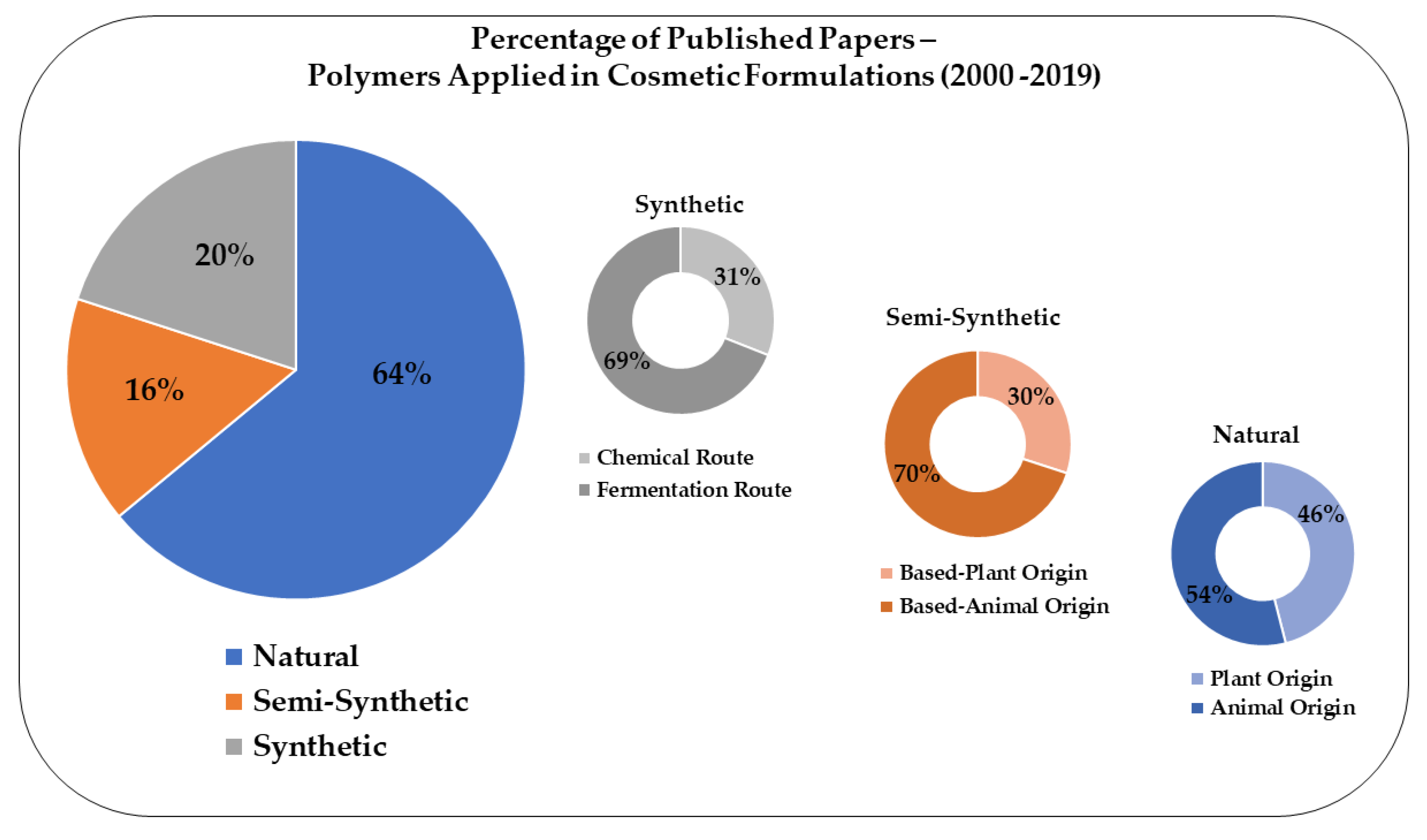
2. Methodology and Data Analysis
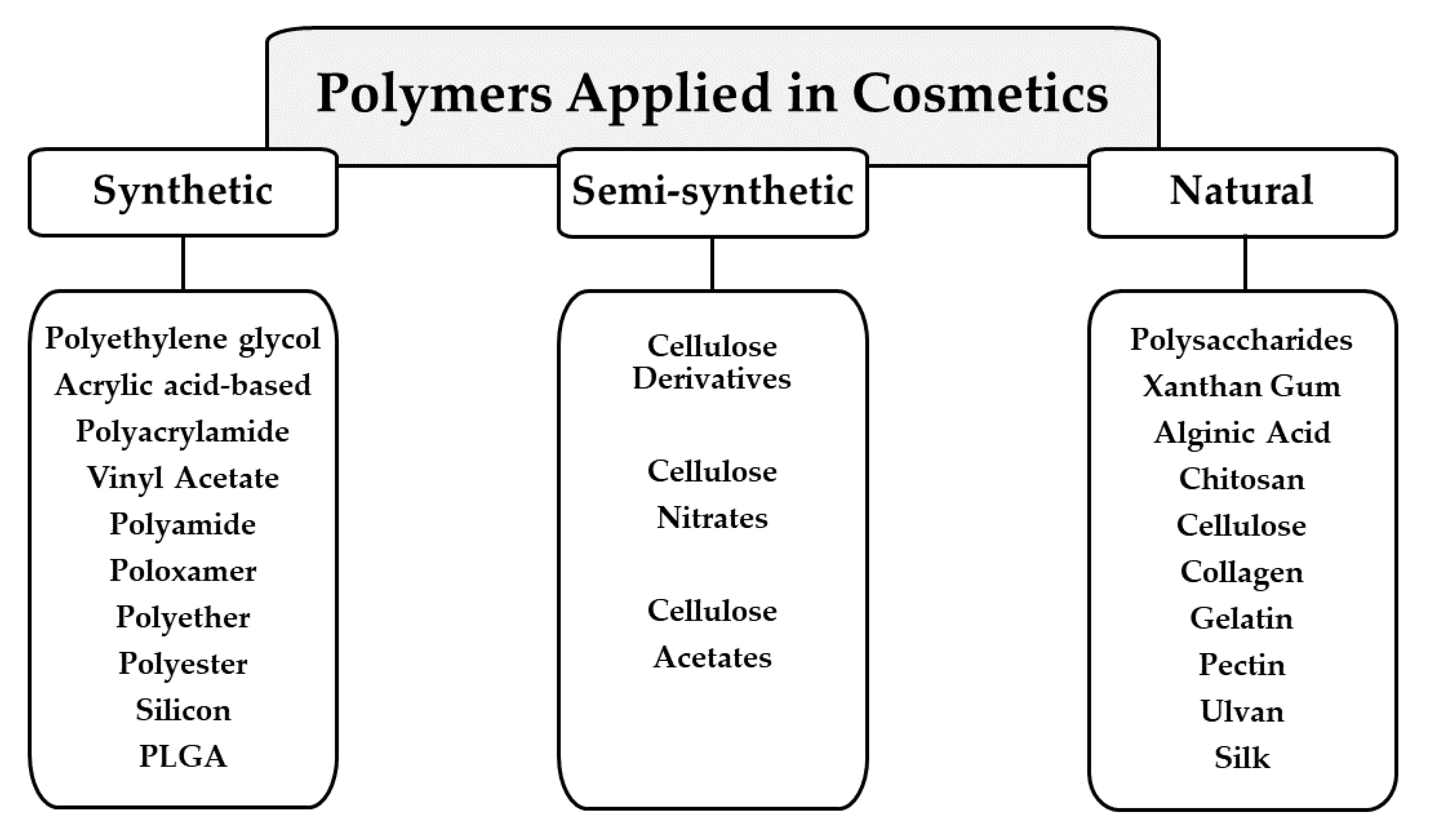
3. Polymers Applied in Cosmetic Formulations
3.1. Synthetic Polymers
3.2. Semi-Synthetic Polymers
3.3. Natural Polymers
4. Overview of Polymers Applied to Cosmetics
5. New Trends in Cosmetics
6. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allied Market Research. Available online: https://www.alliedmarketresearch.com/ (accessed on 12 August 2020).
- Cosmetics Info. Available online: https://cosmeticsinfo.org/Regulation-in-eu (accessed on 12 August 2020).
- Dias-Ferreira, J.; Fernandes, A.R.; Soriano, J.L.; Naveros, B.C.; Severino, P.; da Silva, C.F.; Souto, E.B. Chapter 13—Skin rejuvenation: Biopolymers applied to UV sunscreens and sheet masks. In Biopolymer Membranes and Films; de Moraes, M.A., da Silva, C.F., Vieira, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 309–330. [Google Scholar] [CrossRef]
- Gawade, R.P.; Chinke, S.L.; Alegaonkar, P.S. Chapter 17—Polymers in cosmetics. In Polymer Science and Innovative Applications; AlMaadeed, M.A.A., Ponnamma, D., Carignano, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 545–565. [Google Scholar] [CrossRef]
- Severino, P.; Fangueiro, J.F.; Chaud, M.V.; Cordeiro, J.; Silva, A.M.; Souto, E.B. Chapter 1—Advances in nanobiomaterials for topical administrations: New galenic and cosmetic formulations. In Nanobiomaterials in Galenic Formulations and Cosmetics; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 1–23. [Google Scholar] [CrossRef]
- Goddard, E.D.; Gruber, J.V. Principles of Polymer Science and Technology in Cosmetics and Personal Care; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Patil, A.; Sandewicz, R.W. Cosmetic science and polymer chemistry: Perfect together. In Polymers for Personal Care and Cosmetics; American Chemical Society: Washington, DC, USA, 2013; Volume 1148, pp. 13–37. [Google Scholar]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Nam, H.C.; Park, W.H. Aliphatic Polyester-Based Biodegradable Microbeads for Sustainable Cosmetics. ACS Biomater. Sci. Eng. 2020, 6, 2440–2449. [Google Scholar] [CrossRef]
- Smith, J.A.; Murphy, B.J. 24—Soft cell approach to personal care: Hydrophilic active-filled polyurethane delivery systems. In Delivery System Handbook for Personal Care and Cosmetic Products; Rosen, M.R., Ed.; William Andrew Publishing: Norwich, NY, USA, 2005; pp. 513–532. [Google Scholar] [CrossRef]
- Johnson, W., Jr.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Polyquaternium-22 and Polyquarternium-39 as Used in Cosmetics. Int. J. Toxicol. 2016, 35, 47s–53s. [Google Scholar] [CrossRef] [PubMed]
- Tafuro, G.; Costantini, A.; Baratto, G.; Busata, L.; Semenzato, A. Rheological and Textural Characterization of Acrylic Polymer Water Dispersions for Cosmetic Use. Ind. Eng. Chem. Res. 2019, 58, 23549–23558. [Google Scholar] [CrossRef]
- Lochhead, R.Y. The Role of Polymers in Cosmetics: Recent Trends; ACS Publications: Washington, DC, USA, 2007. [Google Scholar]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Savage, R. Effects of rheology modifiers on the flow curves of idealised and food suspensions. Food Hydrocoll. 2000, 14, 209–215. [Google Scholar] [CrossRef]
- Kunz, R.I.; Brancalhão, R.M.; Ribeiro, L.F.; Natali, M.R. Silkworm Sericin: Properties and Biomedical Applications. BioMed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef]
- Padamwar, M.; Pawar, A. Silk sericin and its applications: A review. J. Sci. Ind. Res. 2004, 64, 323–329. [Google Scholar]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Łęska, B.; Messyasz, B.; Schroeder, G. Application of algae biomass and algae extracts in cosmetic formulations. In Algae Biomass: Characteristics and Applications: Towards Algae-Based Products; Chojnacka, K., Wieczorek, P.P., Schroeder, G., Michalak, I., Eds.; Springer International Publishing: Cham, Germany, 2018; pp. 89–101. [Google Scholar] [CrossRef]
- Li, Q.; Hu, F.; Zhu, B.; Ni, F.; Yao, Z. Insights into ulvan lyase: Review of source, biochemical characteristics, structure and catalytic mechanism. Crit. Rev. Biotechnol. 2020, 40, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Fruijtier-Pölloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Poomanee, W.; Khunkitti, W.; Chaiyana, W.; Leelapornpisid, P. Optimization of Mangifera indica L. Kernel Extract-Loaded Nanoemulsions via Response Surface Methodology, Characterization, Stability, and Skin Permeation for Anti-Acne Cosmeceutical Application. Pharmaceutics 2020, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Germershaus, O.; Lühmann, T.; Rybak, J.C.; Ritzer, J.; Meinel, L. Application of natural and semi-synthetic polymers for the delivery of sensitive drugs. Int. Mater. Rev. 2015, 60, 101–131. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. HPMC/PVP Dissolving Microneedles: A Promising Delivery Platform to Promote Trans-Epidermal Delivery of Alpha-Arbutin for Skin Lightening. AAPS PharmSciTech 2019, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Ferritto, M.S. Polymers for personal care and cosmetics: Overview. In Polymers for Personal Care and Cosmetics; ACS Publications: Washington, DC, USA, 2013; pp. 3–11. [Google Scholar]
- Daudt, R.M.; Back, P.I.; Cardozo, N.S.M.; Marczak, L.D.F.; Külkamp-Guerreiro, I.C. Pinhão starch and coat extract as new natural cosmetic ingredients: Topical formulation stability and sensory analysis. Carbohydr. Polym. 2015, 134, 573–580. [Google Scholar] [CrossRef]
- Viyoch, J.; Patcharaworakulchai, P.; Songmek, R.; Pimsan, V.; Wittaya-Areekul, S. Formulation and development of a patch containing tamarind fruit extract by using the blended chitosan-starch as a rate-controlling matrix. Int. J. Cosmet. Sci. 2003, 25, 113–125. [Google Scholar] [CrossRef]
- Barbosa, G.P.; Debone, H.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Design and characterization of chitosan/zeolite composite films—Effect of zeolite type and zeolite dose on the film properties. Mater. Sci. Eng. C 2016, 60, 246–254. [Google Scholar] [CrossRef]
- Hissae Yassue-Cordeiro, P.; Zandonai, C.H.; Pereira Genesi, B.; Santos Lopes, P.; Sanchez-Lopez, E.; Garcia, M.L.; Camargo Fernandes-Machado, N.R.; Severino, P.; Souto, B.E.; Ferreira da Silva, C. Development of Chitosan/Silver Sulfadiazine/Zeolite Composite Films for Wound Dressing. Pharmaceutics 2019, 11, 535. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.d.C.; Santini, A.; Souto, E.B. Chapter 8—Delivery of Antimicrobials by Chitosan-Composed Therapeutic Nanostructures. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 203–222. [Google Scholar] [CrossRef]
- Andreani, T.; Kiill, C.P.; de Souza, A.L.; Fangueiro, J.F.; Fernandes, L.; Doktorovova, S.; Santos, D.L.; Garcia, M.L.; Gremiao, M.P.; Souto, E.B.; et al. Surface engineering of silica nanoparticles for oral insulin delivery: Characterization and cell toxicity studies. Colloids Surf. B Biointerfaces 2014, 123, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Ataide, J.A.; Gerios, E.F.; Cefali, L.C.; Fernandes, A.R.; Teixeira, M.D.C.; Ferreira, N.R.; Tambourgi, E.B.; Jozala, A.F.; Chaud, M.V.; Oliveira-Nascimento, L.; et al. Effect of Polysaccharide Sources on the Physicochemical Properties of Bromelain-Chitosan Nanoparticles. Polymers 2019, 11, 1681. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Fangueiro, J.F.; Smitha, J.; Cinu, T.A.; Chacko, A.J.; Premaletha, K.; Souto, E.B. Cross-linked chitosan microspheres for oral delivery of insulin: Taguchi design and in vivo testing. Colloids Surf. B Biointerfaces 2012, 92, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; da Silva, C.F.; da Silva, M.A.; Santana, M.H.A.; Souto, E.B. Chitosan Cross-Linked Pentasodium Tripolyphosphate Micro/Nanoparticles Produced by Ionotropic Gelation. Sugar Tech 2016, 18, 49–54. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater. Sci. Eng. C 2017, 77, 1349–1362. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.-Y.; Li, J.-J.; Su, X.-M.; Wu, Z.-Y.; Li, P.-F.; Lei, F.-H.; Tan, X.-C.; Shi, Z.-W. Synthesis of magnetic molecularly imprinted polymers for the selective separation and determination of metronidazole in cosmetic samples. Anal. Bioanal. Chem. 2015, 407, 3875–3880. [Google Scholar] [CrossRef]
- Popa, L.; Ghica, M.V.; Dinu-Pîrvu, C.-E. Hydrogels-Smart Materials for Biomedical Applications; IntechOpen: London, UK, 2019. [Google Scholar]
- Mohire, N.C.; Yadav, A.V. Chitosan-based polyherbal toothpaste: As novel oral hygiene product. Ind. J. Dent. Res. 2010, 21, 380. [Google Scholar] [CrossRef]
- Wassel, M.O.; Khattab, M.A. Antibacterial activity against Streptococcus mutans and inhibition of bacterial induced enamel demineralization of propolis, miswak, and chitosan nanoparticles based dental varnishes. J. Adv. Res. 2017, 8, 387–392. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.l.L.; Heras Caballero, A. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Giunchedi, P.; Juliano, C.; Gavini, E.; Cossu, M.; Sorrenti, M. Formulation and in vivo evaluation of chlorhexidine buccal tablets prepared using drug-loaded chitosan microspheres. Eur. J. Pharm. Biopharm. 2002, 53, 233–239. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. A novel biomaterial: Bacterial cellulose and its new era applications. Biotechnol. Appl. Biochem. 2014, 61, 101–110. [Google Scholar] [CrossRef]
- Iijima, M.; Hatakeyama, T.; Hatakeyama, H. Gel–sol–gel transition of kappa-carrageenan and methylcellulose binary systems studied by differential scanning calorimetry. Thermochim. Acta 2014, 596, 63–69. [Google Scholar] [CrossRef]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion Formation and Stabilization by Biomolecules: The Leading Role of Cellulose. Polymers 2019, 11, 1570. [Google Scholar] [CrossRef]
- Kumar-Sarangi, M.; Chandra-Joshi, B.; Ritchie, B. Natural bioenhancers in drug delivery: An overview. P. R. Health Sci. J. 2018, 37, 12–18. [Google Scholar]
- Murugesh Babu, K. 7—Spider silks and their applications. In Silk; Murugesh Babu, K., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 156–176. [Google Scholar] [CrossRef]
- Murugesh Babu, K. 6—Developments in the processing and applications of silk. In Silk; Murugesh Babu, K., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 140–155. [Google Scholar] [CrossRef]
- Babu, K.M. Silk: Processing, Properties and Applications; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Li, P.-H.; Lu, W.-C.; Chan, Y.-J.; Ko, W.-C.; Jung, C.-C.; Le Huynh, D.T.; Ji, Y.-X. Extraction and characterization of collagen from sea cucumber (Holothuria cinerascens) and its potential application in moisturizing cosmetics. Aquaculture 2020, 515, 734590. [Google Scholar] [CrossRef]
- Cindana Mo’o, F.R.; Wilar, G.; Devkota, H.P.; Wathoni, N. Ulvan, a Polysaccharide from Macroalga Ulva sp.: A Review of Chemistry, Biological Activities and Potential for Food and Biomedical Applications. Appl. Sci. 2020, 10, 5488. [Google Scholar] [CrossRef]
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Morelli, A.; Massironi, A.; Puppi, D.; Creti, D.; Domingo Martinez, E.; Bonistalli, C.; Fabroni, C.; Morgenni, F.; Chiellini, F. Development of ulvan-based emulsions containing flavour and fragrances for food and cosmetic applications. Flavour Fragr. J. 2019, 34, 411–425. [Google Scholar] [CrossRef]
- Hössel, P.; Dieing, R.; Nörenberg, R.; Pfau, A.; Sander, R. Conditioning polymers in today’s shampoo formulations-efficacy, mechanism and test methods. Int. J. Cosmet. Sci. 2000, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Rigoletto, R.; Koelmel, D.; Zhang, G.; Gillece, T.W.; Foltis, L.; Moore, D.J.; Qu, X.; Sun, C. The effect of various cosmetic pretreatments on protecting hair from thermal damage by hot flat ironing. J. Cosmet. Sci. 2011, 62, 265–282. [Google Scholar]
- Velasco, M.; Baby, A.; Inoue, S.; Aktagawa, D.; Dario, M. Formulações de Fixadores/Estilizantes Capilares. Cosmet. Toil. Bras. 2014, 26, 38–43. [Google Scholar]
- Abryun, E. Polímeros em produtos de cuidado pessoal. Cosmet. Toil. Bras. 2011, 23, 42–46. [Google Scholar]
- Lang, G.; Forestier, S.; Junino, A. Cosmetic Temporary Coloring Compositions Containing Protein Derivatives. U.S. Patent US5192332A, 14 October 1983. [Google Scholar]
- Kalopissis, G.; Viout, A. Hair Dyeing Compositions Containing Amphoteric Surface Active Agents. U.S. Patent US3436167A, 15 February 1962. [Google Scholar]
- Kalopissis, G. Hair Coloring Compositions Containing Organic Acid Anhydride-Dye Polymers. U.S. Patent US3797994A, 28 January 1966. [Google Scholar]
- Gupta, S.; Vyas, S.P. Carbopol/chitosan based pH triggered in situ gelling system for ocular delivery of timolol maleate. Sci. Pharm. 2010, 78, 959–976. [Google Scholar] [CrossRef]
- Flor, J.; Davolos, M.R.; Correa, M.A. Protetores Solares. Quim. Nova 2007, 30, 153–158. [Google Scholar]
- Draelos, Z.D. Cosmetics and Dermatologic Problems and Solutions; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Pereira, I.; Zielinska, A.; Ferreira, N.R.; Silva, A.M.; Souto, E.B. Optimization of linalool-loaded solid lipid nanoparticles using experimental factorial design and long-term stability studies with a new centrifugal sedimentation method. Int. J. Pharm. 2018, 549, 261–270. [Google Scholar] [CrossRef]
- Zielinska, A.; Martins-Gomes, C.; Ferreira, N.R.; Silva, A.M.; Nowak, I.; Souto, E.B. Anti-inflammatory and anti-cancer activity of citral: Optimization of citral-loaded solid lipid nanoparticles (SLN) using experimental factorial design and LUMiSizer(R). Int. J. Pharm. 2018, 553, 428–440. [Google Scholar] [CrossRef]
- Zielinska, A.; Ferreira, N.R.; Durazzo, A.; Lucarini, M.; Cicero, N.; Mamouni, S.E.; Silva, A.M.; Nowak, I.; Santini, A.; Souto, E.B. Development and Optimization of Alpha-Pinene-Loaded Solid Lipid Nanoparticles (SLN) Using Experimental Factorial Design and Dispersion Analysis. Molecules 2019, 24, 2683. [Google Scholar] [CrossRef]
- Zielińska, A.; Ferreira, N.R.; Feliczak-Guzik, A.; Nowak, I.; Souto, E.B. Loading, release profile and accelerated stability assessment of monoterpenes-loaded solid lipid nanoparticles (SLN). Pharm. Dev. Technol. 2020, 25, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Thies, C. Microencapsulation. In Encyclopedia of Polymer Science Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Szymański, P.; Markowicz, M.; Mikiciuk-Olasik, E. Adaptation of high-throughput screening in drug discovery-toxicological screening tests. Int. J. Mol. Sci. 2012, 13, 427–452. [Google Scholar] [CrossRef] [PubMed]
- Steiling, W.; Almeida, J.F.; Assaf Vandecasteele, H.; Gilpin, S.; Kawamoto, T.; O’Keeffe, L.; Pappa, G.; Rettinger, K.; Rothe, H.; Bowden, A.M. Principles for the safety evaluation of cosmetic powders. Toxicol. Lett. 2018, 297, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Lukic, M.; Jaksic, I.; Krstonosic, V.; Cekic, N.; Savic, S. A combined approach in characterization of an effective w/o hand cream: The influence of emollient on textural, sensorial and in vivo skin performance. Int. J. Cosmet. Sci. 2012, 34, 140–149. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic Ingredients as Emerging Pollutants of Environmental and Health Concern. A Mini-Review. Cosmetics 2017, 4, 11. [Google Scholar] [CrossRef]
- Kim, D.J.; Chang, S.S.; Lee, J. Anti-Aging Potential of Substance P-Based Hydrogel for Human Skin Longevity. Int. J. Mol. Sci. 2019, 20, 4453. [Google Scholar] [CrossRef]
- Zheng, S.; Liang, S.; Chen, Y.; Brook, M.A. Hyperbranched Silicone MDTQ Tack Promoters. Molecules 2019, 24, 4133. [Google Scholar] [CrossRef]
- Verschoore, M.; Nielson, M. The Rationale of Anti-Aging Cosmetic Ingredients. J. Drugs Dermatol. 2017, 16, s94. [Google Scholar]
- Timm, K.; Myant, C.; Nuguid, H.; Spikes, H.; Grunze, M. Investigation of friction and perceived skin feel after application of suspensions of various cosmetic powders. Int. J. Cosmet. Sci. 2012, 34, 458–465. [Google Scholar] [CrossRef]
- Gold, M.H.; Sadick, N.S. Optimizing outcomes with polymethylmethacrylate fillers. J. Cosmet. Dermatol. 2018, 17, 298–304. [Google Scholar] [CrossRef]
- Kang, S.H.; Moon, S.H.; Rho, B.I.; Youn, S.J.; Kim, H.S. Wedge-shaped polydioxanone threads in a folded configuration (“Solid fillers”): A treatment option for deep static wrinkles on the upper face. J. Cosmet. Derm. 2019, 18, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Gedanken, A.; Perkas, N.; Perelshtein, I.; Lipovsky, A. Imparting pharmaceutical applications to the surface of fabrics for wound and skin care by ultrasonic waves. Curr. Med. Chem. 2018, 25, 5739–5754. [Google Scholar] [CrossRef] [PubMed]
- Fornes, T.; Paul, D.R. Structure and properties of nanocomposites based on nylon-11 and-12 compared with those based on nylon-6. J. Macromol. 2004, 37, 7698–7709. [Google Scholar] [CrossRef]
- Rafiq, R.; Cai, D.; Jin, J.; Song, M. Increasing the toughness of nylon 12 by the incorporation of functionalized graphene. Carbon 2010, 48, 4309–4314. [Google Scholar] [CrossRef]
- Moussour, M.; Lavarde, M.; Pensé-Lhéritier, A.M.; Bouton, F. Sensory analysis of cosmetic powders: Personal care ingredients and emulsions. Int. J. Cosmet. Sci. 2017, 39, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.K.; Fok, L. Evidence of microbeads from personal care product contaminating the sea. Mar. Pollut. Bull. 2016, 109, 582–585. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Tataru, G.; Popa, M.; Costin, D.; Desbrieres, J. Microparticles based on natural and synthetic polymers for ophthalmic applications. J. Biomed. Mater. Res. Part A 2012, 100, 1209–1220. [Google Scholar] [CrossRef]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Garduno, M.L.; Garcia, M.L.; Calpena, A.C. Biopharmaceutical profile of hydrogels containing pranoprofen-loaded PLGA nanoparticles for skin administration: In vitro, ex vivo and in vivo characterization. Int. J. Pharm. 2016, 501, 350–361. [Google Scholar] [CrossRef]
- Araujo, J.; Vega, E.; Lopes, C.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Colloids Surf. B Biointerfaces 2009, 72, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Canadas, C.; Alvarado, H.; Calpena, A.C.; Silva, A.M.; Souto, E.B.; Garcia, M.L.; Abrego, G. In vitro, ex vivo and in vivo characterization of PLGA nanoparticles loading pranoprofen for ocular administration. Int. J. Pharm. 2016, 511, 719–727. [Google Scholar] [CrossRef]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduno-Ramirez, M.L.; Garcia, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef]
- Andreani, T.; Dias-Ferreira, J.; Fangueiro, J.F.; Souza, A.L.R.; Kiill, C.P.; Gremião, M.P.D.; García, M.L.; Silva, A.M.; Souto, E.B. Formulating octyl methoxycinnamate in hybrid lipid-silica nanoparticles: An innovative approach for UV skin protection. Heliyon 2020, 6, e03831. [Google Scholar] [CrossRef]
- Barbosa, T.C.; Nascimento, L.E.D.; Bani, C.; Almeida, T.; Nery, M.; Santos, R.S.; Menezes, L.R.O.; Zielinska, A.; Fernandes, A.R.; Cardoso, J.C.; et al. Development, Cytotoxicity and Eye Irritation Profile of a New Sunscreen Formulation Based on Benzophenone-3-poly(epsilon-caprolactone) Nanocapsules. Toxics 2019, 7, 51. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Fernandes, A.R.; Sanchez-Lopez, E.; Sousa, I.M.O.; Figueiredo, M.C.; Ruiz, A.; Foglio, M.A.; Mazzola, P.G.; Souto, E.B. Evaluation of In Vitro Solar Protection Factor (SPF), Antioxidant Activity, and Cell Viability of Mixed Vegetable Extracts from Dirmophandra mollis Benth, Ginkgo biloba L., Ruta graveolens L., and Vitis vinifera L. Plants 2019, 8, 453. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Fernandes, A.R.; Sousa, I.M.O.; Goncalves, F.; Eberlin, S.; Davila, J.L.; Jozala, A.F.; Chaud, M.V.; Sanchez-Lopez, E.; et al. Flavonoid-Enriched Plant-Extract-Loaded Emulsion: A Novel Phytocosmetic Sunscreen Formulation with Antioxidant Properties. Antioxidants 2019, 8, 443. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Design and engineering of nanogels for cancer treatment. Drug Discov. Today 2011, 16, 457–463. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.-H.; Adibkia, K. Box-Behnken experimental design for preparation and optimization of ciprofloxacin hydrochloride-loaded CaCO3 nanoparticles. J. Drug Deliv. Sci. Technol. 2015, 29, 125–131. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19. [Google Scholar]
- Khezri, K.; Saeedi, M.; Dizaj, S.M. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Ataide, J.A.; Gerios, E.F.; Mazzola, P.G.; Souto, E.B. Bromelain-loaded nanoparticles: A comprehensive review of the state of the art. Adv. Colloid Interface Sci. 2018, 254, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Muller, R.H. Cosmetic features and applications of lipid nanoparticles (SLN, NLC). Int. J. Cosmet. Sci. 2008, 30, 157–165. [Google Scholar] [CrossRef]
- Souto, E.B.; Muller, R.H.; Gohla, S. A novel approach based on lipid nanoparticles (SLN) for topical delivery of alpha-lipoic acid. J. Microencapsul. 2005, 22, 581–592. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Junyaprasert, V.B.; Souto, E.B.; Muller, R.H. Development of ascorbyl palmitate nanocrystals applying the nanosuspension technology. Int. J. Pharm. 2008, 354, 227–234. [Google Scholar] [CrossRef]
- Doktorovova, S.; Kovacevic, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef]
- Gupta, S.; Bansal, R.; Gupta, S.; Jindal, N.; Jindal, A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Ind. Dermatol. Online J. 2013, 4, 267. [Google Scholar] [CrossRef]
| Polymer | Properties | Ref. |
|---|---|---|
| Synthetic | ||
| PEG/PPG | Surface activity, humectant, | [7] |
| Dimethicone | emollience, enhanced comfort, and protection | |
| Poloxamer | Thermoreversible hydrogels, increase the viscosity in body temperature, surfactant non-ionic | [8] |
| Poly (lactic acid), Poly (ε-caprolactone) | Exfoliants microbeads, biodegradable–alternative to non-biodegradable polymers (e.g., polyethylene) | [9] |
| Polyurethanes | Film formation, elastic properties, shape memory effects, surface feel, gloss, and water resistance | [10] |
| Polyquaternarium | Conditioning, antistatic and film-forming | [11] |
| Semi-Synthetic | ||
| Nitrocellulose, Acrylate-copolymers | Film formation, extended product wear, enhanced skin protection, improved product aesthetics | [12] |
| Hydroxypropyl methylcellulose, Hydroxyethyl cellulose, Polyacrylic-acid, Polyamides | Rheological control, ease of application and enhanced product shelf life | [7] |
| Natural | ||
| Starch | Emulsifying ability, film formation, high viscosity | [13] |
| Chitosan | Moisturizing elastic film, active lip care ingredient, hydration, long-term color adhesion, antimicrobial properties, and fragrance adhesion | [14] |
| Cellulose | Rheological control, film formation, leaves the hair soft and smooth, well-hydrated, and is anti-static | [15] |
| Sericin | Improves elasticity, large capacity to absorb water, and moisturizes | [16,17] |
| Collagen | Antioxidant properties, antihypertensive activity, lipid-lowering activity, as well as reparative properties in damaged skin | [18] |
| Hyaluronic acid | Skin conditioning agents, moisturizing, skin protective, and anti-aging properties | [19] |
| Ulvan Carrageenan | Gelling properties, but it is affected by boric acid, divalent cations, and pH | [20,21,22] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations. Cosmetics 2020, 7, 75. https://doi.org/10.3390/cosmetics7040075
Alves TFR, Morsink M, Batain F, Chaud MV, Almeida T, Fernandes DA, da Silva CF, Souto EB, Severino P. Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations. Cosmetics. 2020; 7(4):75. https://doi.org/10.3390/cosmetics7040075
Chicago/Turabian StyleAlves, Thais F. R., Margreet Morsink, Fernando Batain, Marco V. Chaud, Taline Almeida, Dayane A. Fernandes, Classius F. da Silva, Eliana B. Souto, and Patricia Severino. 2020. "Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations" Cosmetics 7, no. 4: 75. https://doi.org/10.3390/cosmetics7040075
APA StyleAlves, T. F. R., Morsink, M., Batain, F., Chaud, M. V., Almeida, T., Fernandes, D. A., da Silva, C. F., Souto, E. B., & Severino, P. (2020). Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations. Cosmetics, 7(4), 75. https://doi.org/10.3390/cosmetics7040075










