Abstract
Rosacea is a chronic inflammatory skin disease mainly affecting the facial skin. Our aim was to determine the appearance of pro- and anti- inflammatory cytokines in rosacea-affected facial tissue. Materials and Methods: Rosacea tissue were obtained from eight patients (aged 35 to 50 years). The control group (CG) included four facial skin samples (49 to 70 years). Routine staining and immunohistochemistry for IL-1, IL-10, LL-37, HBD-2, and HBD-4 proceeded. Results: Inflammation was observed in all the rosacea samples. A statistically significant difference was seen between epithelial HBD-2 positive cells in comparison to the control. There was a strong positive correlation between HBD-4 in the epithelium and HBD-4 in the connective tissue, IL-10 in the epithelium and IL-1 in the connective tissue, and IL-1 in the epithelium and IL-10 in the connective tissue. Conclusion: Increased levels of IL-10 and decreased levels of IL-1 show the balance between anti- and pro-inflammatory tissue responses. A significant amount of HBD-2 in the epithelium proves its important role in the local immune response of rosacea-affected tissue. The last effect seems to be intensified by the elevated level of LL-37 in the epithelium.
1. Introduction
Rosacea is a common chronic inflammatory disease affecting the facial skin, with an estimated geographic prevalence variation from 1%–22% [1,2]. Four rosacea subtypes have been recognized, with the most commonly observed features being transient and persistent facial flushing, telangiectasia, inflammatory papules and pustules, and the hyperplasia of the connective tissue [3].
Although rosacea etiology and pathophysiology are poorly understood, a wide spectrum of trigger factors of rosacea, such as physical (UV, temperature), biological (microbiota, food), and endogenous (genetic, stress) stimuli [4], takes place in the manifestation of the clinical features of rosacea and complicates the understanding of the etiology of the disease. Recent findings indicate that trigger factors stimulate the development of innate and adaptive immune system dysregulation that can lead to rosacea initiation and aggravation and also lead to a release of various mediators from keratinocytes, endothelial cells, mast cells, macrophages, and T helper type 1 and Th17 cells [5]. These mediators cause the unusually long-term relapsing inflammatory reaction in the facial skin. Therefore, pro-inflammatory and anti-inflammatory mediators play the main role in the development of rosacea.
Immunological reactions in rosacea-affected tissues involve the secretion of some inflammatory mediators, including IL-1, IL-10, defensin-2, defensin-4, and cathelicidin (LL-37). Interleukin-1 (IL-1) is a pleiotropic cytokine and the primary mediator of cutaneous inflammation; that is why the deregulation of the IL-1 system has been suggested to play one of the main roles in inflammatory skin diseases [6,7]. All the biological effects of IL-1 are mediated by two IL-1 receptor ligands, IL-1α and IL-1β [6]. Keratinocytes produce IL-1α, which is associated with mechanisms of microbial invasion, inflammation, immunological reactions, and tissue injury in the skin [8,9]. Trigger factor-induced IL-1α production in the epithelium activates a chain of sequential reactions associated with epidermal steam cell proliferation stimulated by the IL-1α-dependent increased activity of dermal fibroblasts [10]. IL-1β is primarily produced by monocytes and macrophages, but a limited amount is also produced by human keratinocytes in inflammatory conditions [7]. Recent studies show that distinct cytokines (for example, IL-1β) can also trigger beta-defensin expression [11].
Interleukin-10 (IL-10) is a key player as an anti-inflammatory cytokine and it is a negative regulator of immune responses. Monocytes, regulatory T lymphocytes, and B cells, which have an important place in immune response, are the major source of IL-10 in human subjects [12]. Relevant studies have indicated that IL-10 inhibits the phase-specific infiltration of neutrophils and macrophages and cytokine overexpression in the inflammatory response of cutaneous wound healing [13]. The suppressor properties of IL-10 are expressed as a reduction in the activity of several inflammatory components and a wide range of cells, including CD4+ and CD8+ T cells, B cells, antigen-presenting cells (APCs), and natural killer (NK) cells [14].
Human β-defensins 2 encoded by the DEFB4 gene are small cationic antimicrobial polypeptides of innate immune system which can act as a barrier against the majority of pathogens [15,16]. These antimicrobial polypeptides are highly expressed at the surface of epithelial barriers, but their expression can also be induced in immune cells [17]. HBD-2 is the first human defence that is produced following the stimulation of epithelial cells by different pathogen-associated molecular patterns, such as bacterial lipoprotein, LPS, and other TLR agonists and by distinct cytokines such as IFN-γ and IL-1β that can also promote beta-defensin expression [11,18]. Evidence that HBD-2 stimulates keratinocyte migration and proliferation, stimulates cytokine or chemokine production, and initiates the process of tissue repair expands their role as immune regulators and enhances the importance of these mediators in human innate immunity [19]. Human beta defensin 2 plays one of the main roles in the development of rosacea according to its wide spectrum of activating factors and effects on keratinocytes.
HBD-4 are small, multifunctional cationic peptides which are expressed in certain epithelia and neutrophils [20,21]. HBD-4 reportedly has antimicrobial activity, especially against Pseudomonas aeruginosa, and is not normally expressed in unaffected skin [22]. Furthermore, the gene expression of HBD-4 in keratinocytes is upregulated by TNF-α, IL-1β, Ca2+, and phorbol 12-myristate 13-acetate (PMA) [23].
LL-37, the sole human cathelicidin, is an antimicrobial peptide that is able to act as an anti- and pro-inflammatory factor [24,25]. Cathelicidins are able to differentiate normal cells, abnormal cells, and pathogens; they directly and selectively destroy the membranes of various microbes and cancer cells, but at the same time normal cells are not damaged [25]. LL-37 are produced by many different cells, such as macrophages, neutrophils, and keratinocytes [26]. Mast cells are one of the primary sources of cathelicidin LL-37, and their (mast cells) number is increased in the dermis of rosacea patients [27]. The activation of CAMP gene and secretion of cathelicidin mRNA in keratinocytes is strongly induced by the activation of the vitamin D pathway (UV as a trigger factor), and this could explain why rosacea occurs mainly in the facial skin [28]. Overall, the higher cathelicidin expression in rosacea supports the hypothesis that an abnormal inflammatory response of the innate immune system has an important place in the pathophysiology of rosacea.
The aim of this work was to compare different pro- and anti-inflammatory mediator activities in rosacea-affected facial skin and healthy tissue.
2. Materials and Methods
2.1. Material Characteristics of Subjects
This study was approved by the Ethics Committee for Clinical Research of Medicine and Pharmaceutical Products at Pauls Stradins Clinical University Hospital Development Foundation in Latvia (Nr.190107 – 1L/z). All of the patients gave their informed consent to participate in the study after the nature of the study had been fully explained. Rosacea patients underwent a biopsy of the nasolabial region of the face under local anesthesia. A special “punch” method was used for skin biopsies. The pieces of biopsy tissue were approximately 2–3 mm2 (square millimeters), containing epidermis and dermis, including cells of these layers. The study was conducted at the Institute of Anatomy and Anthropology, Latvia. The facial skin material was obtained from 8 rosacea patients, with 3 males in the age from 32–36 years and 5 females in the age from 35 to 50 years. Four control specimens were obtained from patients with variable age (from 49 to 70) and gender (2 males, 2 females) during facial plastic surgery. Tissues were not associated with inflammation, rosacea, or any other pathology.
2.2. Immunohistochemical Analysis
The tissue specimens were fixed in a mixture of 2% formaldehyde and 0.2% picric acid in 0.1 M phosphate buffer (pH 7.2). Afterwards, they were rinsed in Tyrode buffer containing 10% saccharose for 12 h then embedded into paraffin and cut into 3 µm thin sections. These sections were stained with hematoxylin and eosin for routine morphological evaluation. Biotin-Streptavidin biochemical method was used for immunohistochemistry (IMH) to detect: LL-37 (orb88370, working dilution 1:100, Biorbyt Limited, Cambridge, UK); HBD-4 (ab14419, working dilution 1:200, Abcam, San Francisco, California); HBD-2 (sc-20798, working dilution 1:100, Santa Cruz Biotechnology, Inc., Dallas, Texas, United States); IL-1 (orb308737,working dilution 1:100, Biorbyt Limited, Cambridge, UK); IL-10 (orb100193, working dilution 1:400, Biorbyt Limited, Cambridge, UK).
The stained slides were analyzed by light microscopy using nonparametric evaluation. The results were evaluated by grading the appearance of the positively stained cells in the visual field [29]. The designation was as follows: 0, no positive structures in the visual field; 0/+, occasional positive structures in the visual field; +, a few positive structures; +/++, a few to a moderate number of positive structures in the visual field; ++, a moderate number of positive structures in the visual field; ++/+++, moderate to numerous positive structures in the visual field; +++, numerous positive structures with the visual field; +++/++++, numerous to abundant positive structures in the visual field; ++++, abundant positive structures in the visual field.
For visual illustration, a Leica DM500RB digital camera and Microsoft Photo editor (version 19051.16210.0) were used.
2.3. Statistic Analysis
The statistical data processing was performed with IBM SPSS (Statistical Package for the Social Sciences) version 25.0. We used the non-parametric Mann–Whitney U test to compare the control group versus the patient group and Spearman’s rank correlation coefficient (ρ), where ρ = 0–0.3 was assumed as a very weak correlation, ρ = 0.3–0.5 was assumed as a weak correlation, ρ = 0.5–0.7 was assumed as a moderate correlation, ρ = 0.7–0.9 was assumed as a strong correlation, and ρ = 0.9–1 was assumed as a very strong correlation. The significance level for all tests was selected as a p-value < 0.05 (5%).
3. Results
The rosacea-affected tissue material contained stratified squamosa epithelium and submucosal connective tissue in all samples. Routine staining showed the presence of intraepithelial lymphocytes and subepithelial inflammation with lymphocytes and macrophages, with signs of hyalinization in a few of the cases (Figure 1).
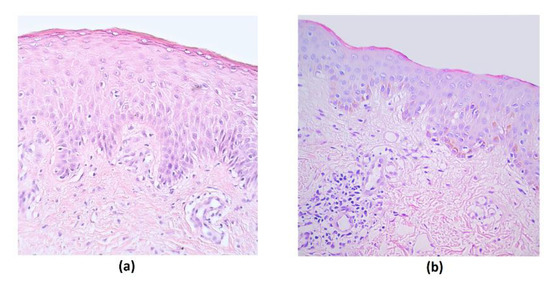
Figure 1.
Routine stained micrographs of rosacea-affected facial skin tissues. (a) Note intraepithelial lymphocytes in a 44-year-old female. Hematoxylin and eosin, X 200; (b) inflammation in the subepithelial connective tissue in a 35-year-old female. Hematoxylin and eosin, X 200.
IL–1α was present in a moderate number of epithelial cells and in a few subepithelial connective tissue cells of rosacea-affected samples. The IL-1α distribution both in the epithelium and subepithelium showed only occasional positive cells in the control group (Table 1, Figure 2a,b). In rosacea-affected tissue, the number of IL-10-containing epitheliocytes greatly exceed the number of immunoreactive cells in the epithelium of the control group (a few positive structures). Positive cells of IL-10 were more observed in the connective tissue of the affected group (a few to moderate) than in the connective tissue of the control group (few) (Table 1, Figure 2c,d). A moderate number of HBD-2 immunoreactive positive structures appeared in the epithelium and subepithelium of the rosacea-affected group. A few to a moderate number of HBD-2-containing structures were observed in the epithelium of the control group, while no positive structures were detected in the connective tissues (Table 1, Figure 2e,f).

Table 1.
Relative number of cytokines, defensins, and cathelicidin-positive structures in the rosacea-affected facial epithelium and subepithelium.
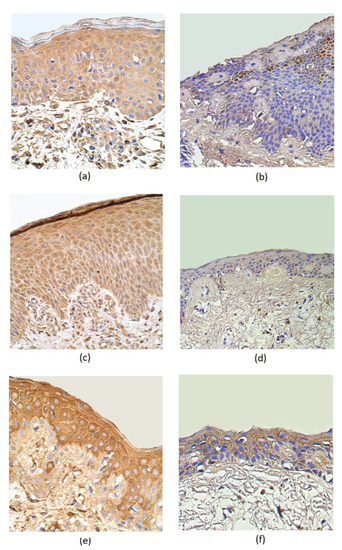
Figure 2.
Immunohistochemical micrographs in rosacea-affected tissues and in control samples. (a) Moderate number of IL-1α-positive epitheliocytes and subepithelial cells in a 45-year-old female. IL-1α IMH, X 400; (b) occasional positive structures in epithelium and subepithelial connective tissue of control group in a 49-year-old male. IL-1α IMH, X 400; (c) moderate to numerous IL-10-containing epithelial cells and moderate immunoreactive cells in the connective tissue of a 44-year-old female. IL-10 IMH, X 250; (d) a few positive IL-10 structures in the epithelium and connective tissue of a 66-year-old male. IL-10 IMH, X 200; (e) moderate number of HBD-2-marked immunoreactive positive structures appearing in the epithelium and few to moderate in the subepithelium of a 35-year-old female. HBD-2 IMH, X 400; (f) moderate HBD-2-positive cells in the epithelium and a few positive structures in the connective tissue of a 66-year-old male. HBD-2 IMH, X 400.
The HBD-4 distribution in the epithelium showed a moderate number of immunoreactive cells in patient samples, while the control group showed only a few factor positive structures. Few HBD-4 marked structures were seen in the connective tissue of the affected group and no positive structures in the connective tissue of the control group (Table 1, Figure 3).
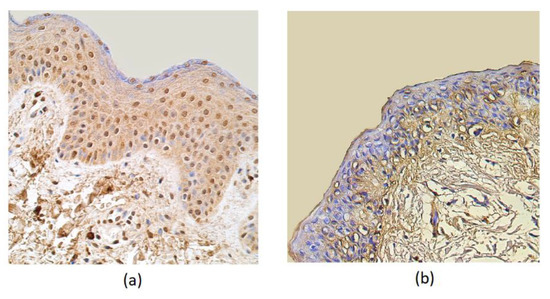
Figure 3.
Immunohistochemical micrographs in rosacea-affected tissues and in control samples. (a) Note moderate HBD-4-containing epitheliocytes and in the subepithelial connective tissue of a 32-year-old rosacea-affected male. HBD-4 IMH, X 250; (b) only a few HBD-4-positive structures were displayed in the epithelium and no positive-stained structures in the connective tissue cells of 60-year-old control subject. HBD-4 IMH, X 200.
Moderate to numerous cathelicidin-positive epithelial cells were detected in the patient samples. In the epithelium and connective tissue of the control group and in the subepithelium of affected tissue, a few positive structures of cathelicidin were detected (Table 1, Figure 4a,b).
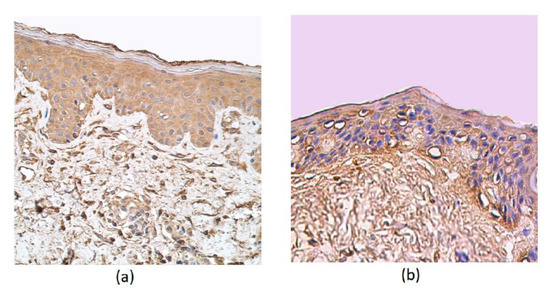
Figure 4.
Immunohistochemical micrographs in rosacea-affected tissues and in control samples. (a) Note the moderate LL-37-containing epithelial cells and only connective tissue cells in a 35-year-old female. LL-37 IMH, X 250; (b) a few positive structures in the epithelium and connective tissue of the control group of a 66-year-old male. LL-37 IMH, X 200.
A statistically significant difference between the marker distribution in the rosacea-affected tissue and the control group was noticed with HBD-2 in the epithelium (Table 2).

Table 2.
Mann–Whitney U test revealing statistically significant difference between rosacea-affected subjects and the control group.
A correlation analysis of the rosacea-affected facial skin samples demonstrate a strong positive correlation between HBD-4 in the connective tissue and HBD-4 in the epithelium. Furthermore, a strong positive correlation was detected in the rosacea-affected tissue between IL-1 in the epithelium and IL-10 in the connective tissue, IL-1 in the connective tissue, and IL-10 in the epithelium (Table 3).

Table 3.
Spearman’s rank correlation coefficient revealed correlations between the relative numbers of different factors in rosacea-affected epithelium and subepithelial connective tissue.
4. Discussion
The long-term influence of trigger factors on the skin of the face, leading to dysfunction of the pro- and anti- inflammatory system of the skin, is considered as the main cause of the pathogenesis of rosacea [30]. The expression of IL-1, IL-10, HBD-2, and LL-37 proteins from all researched inflammatory mediators was the highest. In our patients, it can be assumed that the increased release of dominant mediators leads to damage of the facial tissue and the development of rosacea. This is proved by the other scientists who have indicated the role of long-term trigger factors in the development of abnormal immune system responses [31].
It is widely known that rosacea is characterized by inflammatory lesions of epidermal and dermal tissues. IL-1 is a primary cytokine of inflammation which can be suppressed by IL-10. In the present study, we found decreased levels of IL-1α and increased levels of IL-10 in lesion-affected tissues, including normal immune system reaction on inflammation. It is confirmed by the strong positive correlation of IL-1α and IL-10 in the epithelium and in the connective tissue. Other researches confirm ability of IL-10 to inhibit IL-1α and what is more the two ways of inhibition are known: (1) treatment with lipopolysaccharides (LPS), which increase IL-10 production; (2) autocrine IL-10 effects on signal transduction in the production of proIL-1 [32,33]. IL-10 inhibits Th1 and Th2 cytokine production by suppressing the costimulatory molecule CD80 and CD86 expression in dendritic cells and antigen-presenting cells [34]. Furthermore, IL-10 can decrease T-cell, B-cell, and mast cell differentiation [35] and regulate the phase-specific migration of macrophages and secretion of cytokines in damaged tissue. Interestingly, despite our results, a number of studies have demonstrated that IL-1 and other pro-inflammatory cytokine levels and activity were significantly elevated in rosacea-affected skin [36]. As IL-1 is main mediator in cutaneous inflammation, it’s deregulation can cause the development of inflammatory skin disease [7]. Additionally, in our research we can suggest that there are no observable deregulation problems of the IL-1 and IL-10 system.
The HBD-2 level was quite similar in the subepithelium compared to IL-1 and IL-10. Usually, the production of HBD-2 occurs in the uppermost layers of the epidermis. Relevant studies have indicated HBD-2 expression in the epithelium of gastrointestinal and genitor-urinary tracts, and also in the respiratory tract epithelium and in the secretory tubules of the submucosal glands [37]. Interestingly, the expression of HBD-2 also can be induced in immune cells such as monocytes and macrophages by cytokines and LPS [38], which can explain possible rosacea development in the subepithelium.
An interesting finding is the dominant secretion of HBD-2, but not of HBD-4, in the epithelium of rosacea-affected tissue. HBD-2 plays one of the main roles in the development of epithelial inflammation. It is the first human defensin (HBD-2) which is produced after adequate keratinocyte stimulation by UBV; proinflammatory cytokines—for example, IL-1 and TNF-α; and Gram-negative and Gram-positive bacteria and their products, such as bacterial endotoxins [39]. According to other studies, IL-1-mediated stimulation significantly enhances the secretion of HBD-2 in keratinocytes of an infected area [40], which shows HBD-2 as a protective factor during inflammation. Furthermore, HBD-2 promotes the adaptive immune system by inducing the migration of CD45RO memory T cells and shows chemoattractant activity due to binding to the CC chemokine receptor 6 (CCR6) on neutrophils [41]; it also stimulates the migration of monocytes, macrophages, and dendritic cells. As a result, HBD-2 intensifies the inflammatory process to reduce the damage caused by trigger factors.
We detected an elevated level of LL-37 in the rosacea-affected epithelium, which indicates that it is involved in the progression of inflammation. LL-37 normally is produced less in keratinocytes and is converted to smaller peptides with enhanced antimicrobial function and a partial activity which is strictly regulated [42,43]. However, it was found that, in patients with rosacea, the expression of LL-37 is higher and has a different structure [44,45]. UVB irradiation stimulates the increased excretion of extracellular antimicrobial peptides (ATP), which cooperate with LL-37 to fully activate the P2 × 7R on keratinocytes. As a result, LL-37 synergistical working with ATP enhances the secretion of IL-1 by keratinocytes [46] and prolongs inflammation in rosacea-affected tissue.
5. Conclusions
Rosacea-affected facial skin is the main source of inflammatory cytokines. The increased level of anti-inflammatory cytokine IL-10 and decreased level of pro-inflammatory cytokine IL-1 showed the balance between the anti- and pro-inflammatory tissue responses.
The moderate number of HBD-2-positive structures in the connective tissue demonstrates a possible intensification of local immunity increase in the case of rosacea in the subepithelium. The statistically significant difference in the HBD-2 between the rosacea-affected subjects and the control group and also moderate positive structures in the epithelium proves HBD-2′s important role in the common local immune response of rosacea-affected tissue. The last effect seems to be intensified also by the elevated level of LL-37 in the epithelium.
Author Contributions
Conceptualization, M.P.; methodology, M.P. and E.L.; software, E.L.; validation, M.P.; formal analysis, E.L.; investigation, E.L.; resources, M.P., M.R.-K., and J.K.; data curation, M.P.; writing—original draft preparation, E.L.; writing—review and editing, M.P. and E.L.; visualization, E.L.; supervision, M.P.; project administration, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Holmes, A.D.; Spoendlin, J.; Chien, A.L.; Baldwin, H.; Chang, A.L.S. Evidence-based update on rosacea comorbidities and their common physiologic pathways. J. Am. Acad. Dermatol. 2018, 78, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Abram, K.; Silm, H.; Oona, M. Prevalence of Rosacea in an Estonian Working Population Using a Standard Classification. Acta. Derm. Venereol. 2010, 90, 269–273. [Google Scholar] [CrossRef]
- Wilkin, J.; Dahl, M.; Detmar, M.; Drake, L.; Feinstein, A.; Odom, R.; Powell, F. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J. Am. Acad. Dermatol. 2002, 46, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Sulk, M.; Seeliger, S.; Aubert, J.; Schwab, V.D.; Cevikbas, F.; Rivier, M.; Nowak, P.; Voegel, J.J.; Buddenkotte, J.; Steinhoff, M. Distribution and Expression of Non-Neuronal Transient Receptor Potential (TRPV) Ion Channels in Rosacea. J. Investig. Dermatol. 2012, 132, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Buddenkotte, J.; Steinhoff, M. Recent advances in understanding and managing rosacea. F1000Research 2018, 7, 1885. [Google Scholar] [CrossRef] [PubMed]
- Kupper, T.S. Immune and inflammatory processes in cutaneous tissues: Mechanisms and speculations. J. Clin. Investig. 1990, 86, 1783–1789. [Google Scholar] [CrossRef]
- Palmer, G.; Talabot-Ayer, D.; Kaya, G.; Gabay, C. Type I IL-1 Receptor Mediates IL-1 and Intracellular IL-1 Receptor Antagonist Effects in Skin Inflammation. J. Investig. Dermatol. 2007, 127, 1938–1946. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Function and regulation of IL-1α in inflammatory diseases and cancer. Immunol. Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef]
- Newby, C.S.; Barr, R.M.; Greaves, M.W.; Mallet, A.I. Cytokine release and cytotoxicity in human keratinocytes and fibroblasts induced by phenol and sodium dodecyl sulfate. J. Investig. Dermatol. 2000, 115, 292–298. [Google Scholar] [CrossRef]
- Szabowski, A.; Maas-Szabowski, N.; Andrecht, S.; Kolbus, A.; Schorpp-Kistner, M.; Fusenig, N.E.; Angel, P. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell 2000, 103, 745–755. [Google Scholar] [CrossRef]
- Harder, J.; Meyer-Hoffert, U.; Teran, L.M.; Schwichtenberg, L.; Bartels, J.; Maune, S.; Schröder, J.M. Mucoid Pseudomonas aeruginosa, TNF- α, and IL-1 β, but Not IL-6, Induce Human β -Defensin-2 in Respiratory Epithelia. ATS J. 1999, 22, 714–721. [Google Scholar] [CrossRef]
- Kubo, M.; Motomura, Y. Transcriptional regulation of the anti-inflammatory cytokine IL-10 in acquired immune cells. Front. Immunol. 2012, 3, 275. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ohshima, T.; Kondo, T. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem. Biophys. Res. Commun. 1999, 265, 194–199. [Google Scholar] [CrossRef]
- Abdoli, A.; Maspi, N.; Ghaffarifar, F. Wound healing in cutaneous leishmaniasis: A double edged sword of IL-10 and TGF-β. Comp. Immunol. Microbiol. Infect. Dis. 2017, 51, 15–26. [Google Scholar] [CrossRef]
- Taefehshokr, N.; Isazadeh, A.; Oveisi, A.; Key, Y.A.; Taefehshokr, S. Reciprocal role of hBD2 and hBD3 on the adaptive immune response by measuring T lymphocyte proliferation in terms of CD4 and CCR6 expression. Horm. Mol. Biol. Clin. Investig. 2018, 35. [Google Scholar] [CrossRef]
- Johansen, C.; Bertelsen, T.; Ljungberg, C.; Mose, M.; Iversen, L. Characterization of TNF-α– and IL-17A–Mediated Synergistic Induction of DEFB4 Gene Expression in Human Keratinocytes through IκBζ. J. Investig. Dermatol. 2016, 136, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Ganz, T. Defensins of vertebrate animals. Curr. Opin. Immunol. 2002, 14, 96–102. [Google Scholar] [CrossRef]
- Schröder, J.M.; Harder, J. Human beta-defensin-2. Int. J. Biochem. Cell. Biol. 1999, 31, 645–651. [Google Scholar] [CrossRef]
- Baroni, A.; Donnarumma, G.; Paoletti, I.; Longanesi-Cattani, I.; Bifulco, K.; Tufano, M.A.; Carriero, M.V. Antimicrobial human beta-defensin-2 stimulates migration, proliferation and tube formation of human umbilical vein endothelial cells. Peptides 2009, 30, 267–272. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Lu, W. α-Defensins in human innate immunity. Immunol. Rev. 2012, 245, 4–112. [Google Scholar] [CrossRef]
- Schneider, J.J.; Unholzer, A.; Schaller, M.; Schäfer-Korting, M.; Korting, H.C. Human defensins. J. Mol. Med. 2005, 83, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Smiley, A.K.; Gardner, J.; Klingenberg, J.M.; Neely, A.N.; Supp, D.M. Expression of human beta defensin 4 in genetically modified keratinocytes enhances antimicrobial activity. J. Burn. Care. Res. 2007, 28, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Meyer-Hoffert, U.; Wehkamp, K.; Schwichtenberg, L.; Schröder, J.M. Differential Gene Induction of Human β-Defensins (hBD-1, -2, -3, and -4) in Keratinocytes Is Inhibited by Retinoic Acid. J. Investig. Dermatol. 2004, 123, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Fabisiak, A.; Murawska, N.; Fichna, J. LL-37: Cathelicidin-related antimicrobial peptide with pleiotropic activity. Pharmacol. Rep. 2016, 68, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Bandurska, K.; Berdowska, A.; Barczyńska-Felusiak, R.; Krupa, P. Unique features of human cathelicidin LL-37. Biofactors 2015, 41, 289–300. [Google Scholar] [CrossRef]
- da Silva, P.F.; Machado, M.C. The dual role of cathelicidins in systemic inflammation. Immunol. Lett. 2017, 182, 57–60. [Google Scholar] [CrossRef]
- Di Nardo, A.; Vitiello, A.; Gallo, R.L. Cutting edge: Mast Cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 2003, 170, 2274–2278. [Google Scholar] [CrossRef]
- Peric, M.; Lehmann, B.; Vashina, G.; Dombrowski, Y.; Koglin, S.; Meurer, M.; Ruzicka, T.; Schauber, J. UV-B—Triggered induction of vitamin D3 metabolism differentially affects antimicrobial peptide expression in keratinocytes. J. Allergy Clin. Immunol. 2010, 125, 746–749. [Google Scholar] [CrossRef]
- Junga, A.; Pilmane, M.; Ābola, Z.; Volrāts, O. The Distribution of Vascular Endothelial Growth Factor (VEGF), Human Beta-Defensin-2 (HBD-2), and Hepatocyte Growth Factor (HGF) in Intra-Abdominal Adhesions in Children under One Year of Age. Sci. World J. 2018, 2018, 5953095. [Google Scholar] [CrossRef]
- Gerber, P.A.; Buhren, B.A.; Steinhoff, M.; Homey, B. Rosacea: The Cytokine and Chemokine Network. J. Investig. Dermatol. Symp. Proc. 2011, 15, 40–47. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Li, Y.; Deng, Z.; Zhou, L.; Long, J.; Tang, Y.; Zuo, Z.; Zhang, Y.; Xie, H. Artemisinin, a potential option to inhibit inflammation and angiogenesis in rosacea. Biomed. Pharmacother. 2019, 117, 109181. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, J.; Li, D.; Li, P.; Zhou, X.; Li, Y.; He, Z.; Qin, L.; Liang, L.; Luo, X. Interleukin-10 inhibits interleukin-1β production and inflammasome activation of microglia in epileptic seizures. J. Neuroinflamm. 2019, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef]
- Commins, S.; Steinke, J.W.; Borish, L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J. Allergy Clin. Immunol. 2008, 121, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Mamelak, A.J.; La Morgia, S.; Wang, B.; Feliciani, C.; Tulli, A.; Sauder, D.N. The role of interleukin 10 in the pathogenesis and potential treatment of skin diseases. J. Am. Acad. Dermatol. 2004, 50, 657–675. [Google Scholar] [CrossRef]
- Margalit, A.; Kowalczyk, M.J.; Żaba, R.; Kavanagh, K. The role of altered cutaneous immune responses in the induction and persistence of rosacea. J. Dermatol. Sci. 2016, 82, 3–8. [Google Scholar] [CrossRef]
- Marcinkiewicz, M.; Majewski, S. The role of antimicrobial peptides in chronic inflammatory skin diseases. Postepy Dermatol. Alergol. 2016, 33, 6–12. [Google Scholar] [CrossRef]
- Duits, L.A.; Ravensbergen, B.; Rademaker, M.; Hiemstra, P.S.; Nibbering, P.H. Expression of β-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology 2002, 106, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Witthöft, T.; Pilz, C.S.; Fellermann, K.; Nitschke, M.; Stange, E.F.; Ludwig, D. Enhanced human beta-defensin-2 (HBD-2) expression by corticosteroids is independent of NF-kappaB in colonic epithelial cells (CaCo2). Dig. Dis. Sci. 2005, 50, 1252–1259. [Google Scholar] [CrossRef]
- Wang, B.; McHugh, B.J.; Qureshi, A.; Campopiano, D.J.; Clarke, D.J.; Fitzgerald, J.R.; Dorin, J.R.; Weller, R.; Davidson, D.J. IL-1β–Induced Protection of Keratinocytes against Staphylococcus aureus-Secreted Proteases Is Mediated by Human β-Defensin 2. J. Investig. Dermatol. 2017, 137, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chertov, O.; Bykovskaia, S.N.; Chen, Q.; Buffo, M.J.; Shogan, J.; Anderson, M.; Schröder, J.M.; Wang, J.M.; Howard, O.M.Z.; et al. β-Defensins: Linking Innate and Adaptive Immunity Through Dendritic and T Cell CCR6. Science 1999, 286, 525–528. [Google Scholar] [CrossRef]
- Yamasaki, K.; Schauber, J.; Coda, A.; Lin, H.; Dorschner, R.A.; Schechter, N.M.; Bonnart, C.; Descargues, P.; Hovnanian, A.; Gallo, R.L. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006, 20, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M.; Gallo, R.L. Cathelicidins, essential gene-encoded mammalian antibiotics. J. Mol. Med. 2002, 80, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Schauber, J.; Gallo, R.L. The vitamin D pathway: A new target for control of the skin’s immune response. Exp. Dermatol. 2008, 17, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Di Nardo, A.; Bardan, A.; Murakami, M.; Ohtake, T.; Coda, A.; Dorschner, R.A.; Bonnart, C.; Descargues, P.; Hovnanian, A.; et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2000, 13, 975–980. [Google Scholar] [CrossRef]
- Elssner, A.; Duncan, M.; Gavrilin, M.; Wewers, M.D. A Novel P2 × 7 Receptor Activator, the Human Cathelicidin-Derived Peptide LL37, Induces IL-1β Processing and Release. J. Immunol. 2004, 172, 4987–4994. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).