Valorisation of Ribes nigrum L. Pomace, an Agri-Food By-Product to Design a New Cosmetic Active
Abstract
1. Introduction
2. Materials and Methods
2.1. Blackcurrant Pomace
2.2. Blackcurrant Pomace Extraction
2.2.1. Solid–Liquid Extraction
2.2.2. Ultrasonic-Assisted Maceration
2.3. Fractionation of R. Nigrum Pomace Extract
2.4. Bioassays
2.4.1. DPPH Radical Scavenging Assay
2.4.2. Tyrosinase Assays
2.4.3. Lipoxygenase Assay
2.4.4. Elastase Assay
2.4.5. Hyaluronidase Assay
2.4.6. Collagenase Assay
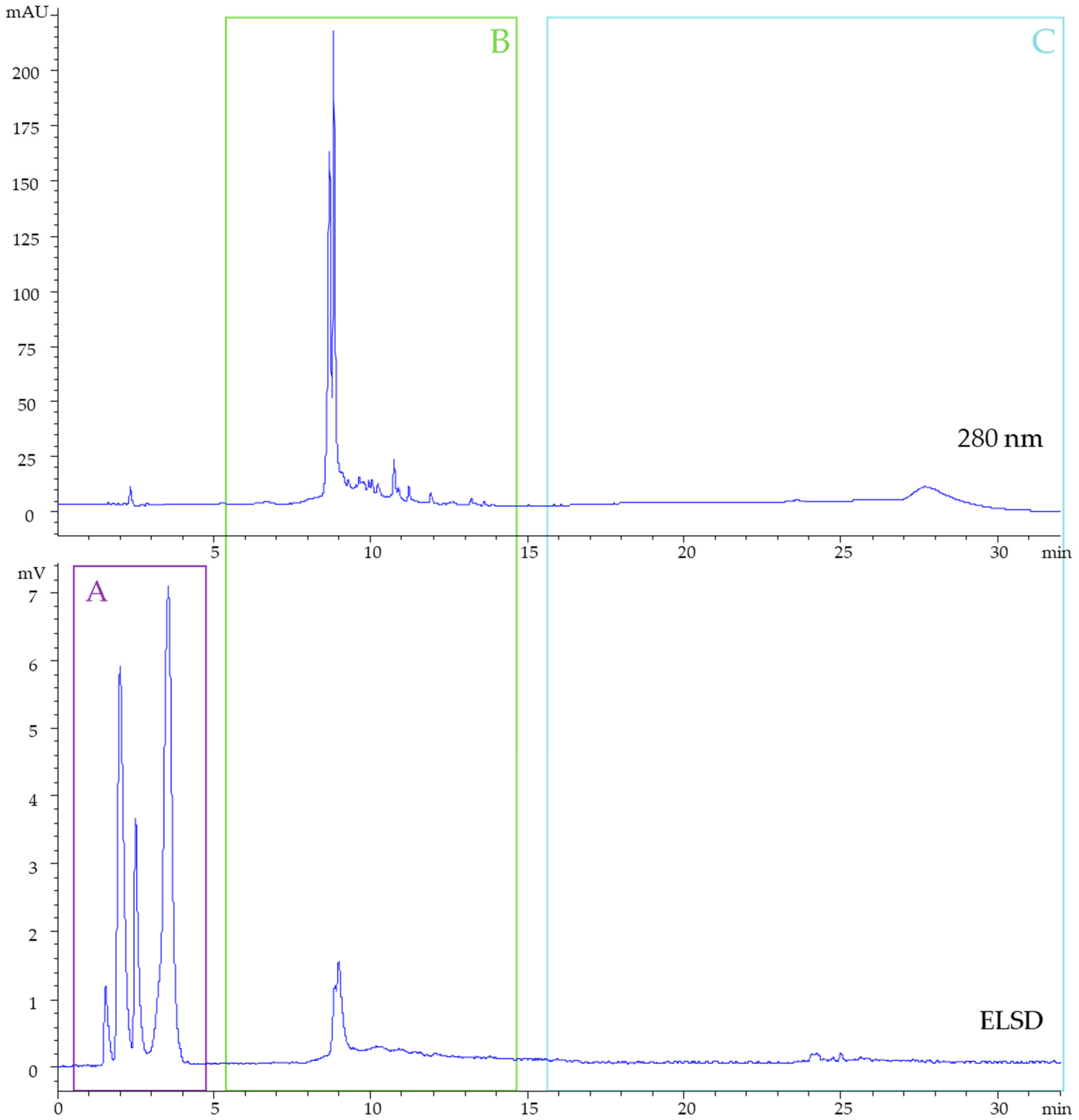
2.5. High Performance Liquid Chromatography
2.6. High Performance Thin-Layer Chromatography
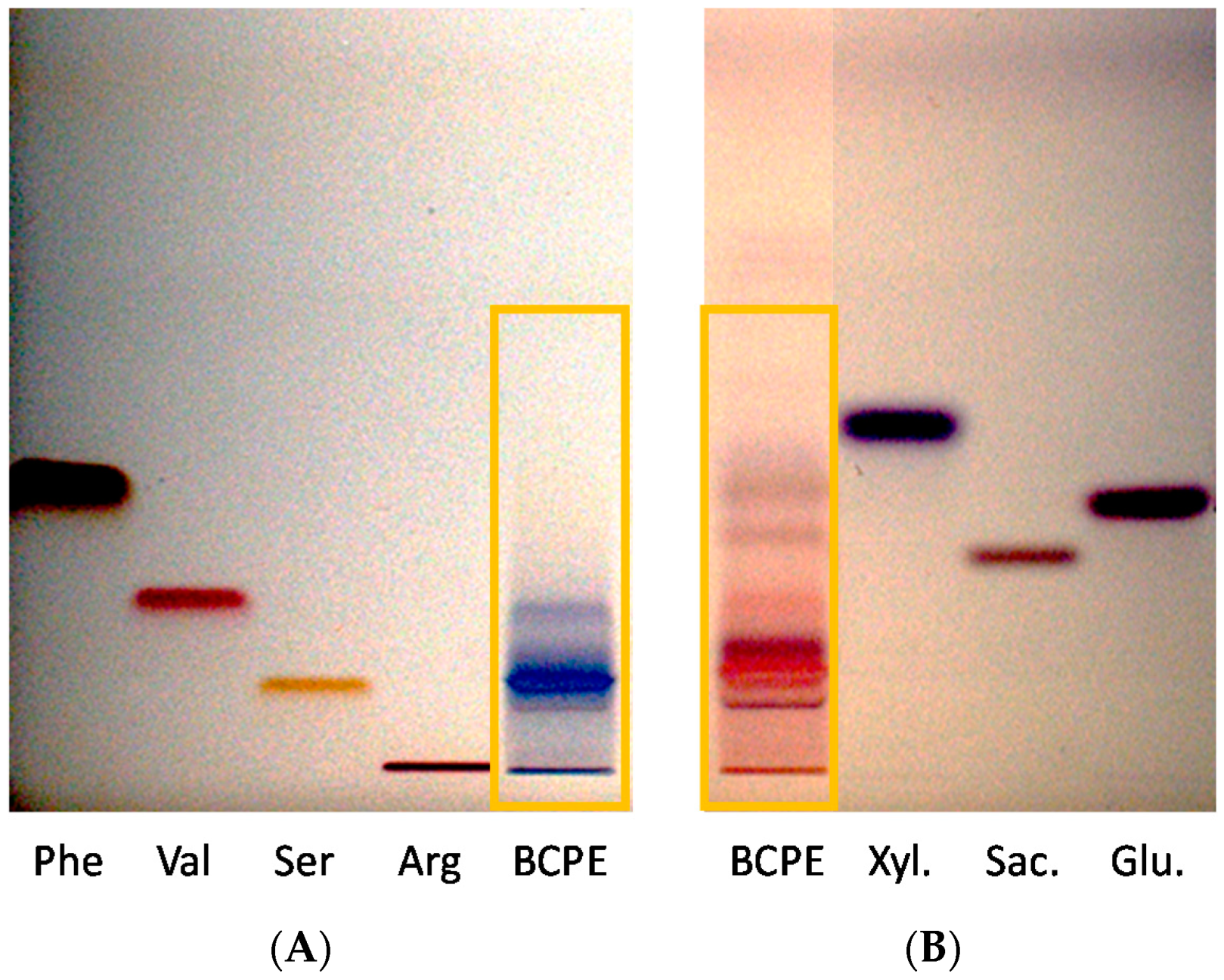
2.6.1. Amino Acids
2.6.2. Sugars
3. Results and Discussion
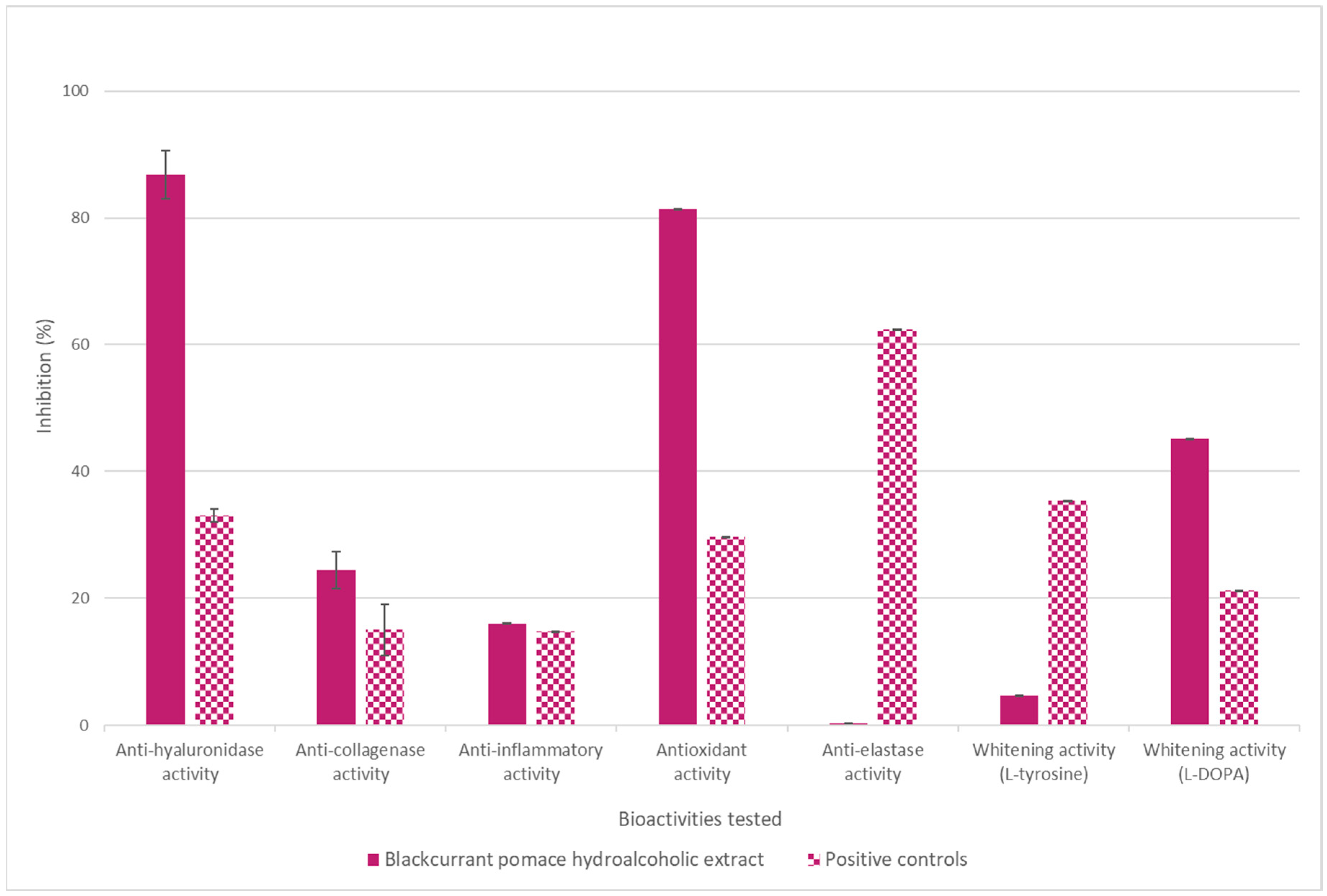
3.1. Hydroalcoholic Blackcurrant Pomace Extract
3.2. Liquid Cosmetic Ingredient
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Gremmen, B. Chapter 2—Economics of food waste co-product exploitation. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Waldron, K., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2009; pp. 23–32. ISBN 978-1-84569-391-6. [Google Scholar]
- May, N.; Guenther, E. Shared benefit by material flow cost accounting in the food supply chain—The case of berry pomace as upcycled by-product of a black currant juice production. J. Clean. Prod. 2020, 245, 118946. [Google Scholar] [CrossRef]
- Dwyer, K.; Hosseinian, F.; Rod, M. The market potential of grape waste alternatives. J. Food. Res. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste. Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Galanakis, C.M. Handbook of Grape Processing by-Products: Sustainable Solutions, 1st ed.; Academic Press: Cambridge, MA, USA, 2017; ISBN 978-0-12-809870-7. [Google Scholar]
- Plainfossé, H.; Burger, P.; Michel, T.; Landreau, A.; Fernandez, X. Actifs cosmétiques pour la réparation cutanée. Tech. De. L’Ingenieur 2017, 1, J3002. [Google Scholar]
- Zhang, S.; Duan, E. Fighting against skin aging. Cell Transpl. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Rodrigues, F.; de la Luz Cádiz-Gurrea, M.; Nunes, M.A.; Pinto, D.; Vinha, A.F.; Linares, I.B.; Oliveira, M.B.P.P.; Carretero, A.S. 12—Cosmetics. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 393–427. ISBN 978-0-12-813572-3. [Google Scholar]
- WHO Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 11 March 2020).
- Plainfossé, H.; Burger, P.; Verger-Dubois, G.; Azoulay, S.; Fernandez, X. Design methodology for the development of a new cosmetic active based on Prunus domestica L. leaves extract. Cosmetics 2019, 6, 8. [Google Scholar] [CrossRef]
- U.S. National Plant Germplasm System Ribes. Nigrum. L. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxonomydetail.aspx?id=31845 (accessed on 6 March 2020).
- Jain, P.; Pandey, R.; Shukla, S.S. Chapter 4—Natural sources of anti-inflammation. In Inflammation: Natural Resources and Its Applications; Springer Briefs in Immunology; Springer: New Delhi, India, 2015; pp. 25–133. [Google Scholar]
- Lim, T.K. Ribes nigrum. In Edible Medicinal and Non-Medicinal Plants: Volume 4, Fruits; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 27–42. ISBN 978-94-007-4053-2. [Google Scholar]
- Vagiri, M.R. Black Currant (Ribes. Nigrum. L.)–An Insight into the Crop; Faculty of Landscape Planning, Horticulture and Agricultural Science, Swedish University of Agricultural Sciences: Alnarp, Sweden, 2012; pp. 1–58. [Google Scholar]
- Jensen, S. Judgement on the harvest of the year 2009. In Proceedings of the 15th European Blackcurrant Conference, Nyborg, Denmark, 7–10 June 2009. [Google Scholar]
- Sójka, M.; Król, B. Composition of industrial seedless black currant pomace. Eur. Food Res. Technol. 2009, 228, 597–605. [Google Scholar] [CrossRef]
- Struck, S.; Plaza, M.; Turner, C.; Rohm, H. Berry pomace–a review of processing and chemical analysis of its polyphenols. Int. J. Food Sci. Technol. 2016, 51, 1305–1318. [Google Scholar] [CrossRef]
- Nawirska, A.; Kwaśniewska, M. Dietary fibre fractions from fruit and vegetable processing waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Technische Universität Dresden, Berrypom—SUSFOOD ERA-Net Research Project. Available online: https://berrypom.mw.tu-dresden.de/ (accessed on 6 March 2020).
- Reißner, A.-M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and physicochemical properties of dried berry pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef]
- Plainfossé, H.; Burger, P.; Azoulay, S.; Landreau, A.; Verger-Dubois, G.; Fernandez, X. Development of a natural anti-age ingredient based on Quercus pubescens Willd leaves axtract–A case study. Cosmetics 2018, 5, 15. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics 2016, 3, 36. [Google Scholar] [CrossRef]
- Lu, W.; Zhao, X.; Xu, Z.; Dong, N.; Zou, S.; Shen, X.; Huang, J. Development of a new colorimetric assay for lipoxygenase activity. Anal. Biochem. 2013, 441, 162–168. [Google Scholar] [CrossRef]
- Debelle, L.; Tamburro, A.M. Elastin: Molecular description and function. Int. J. Biochem. Cell Biol. 1999, 31, 261–272. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Roy, A.; Sahu, R.K.; Matlam, M.; Deshmukh, V.K.; Dwivedi, J.; Jha, A.K. In vitro techniques to assess the proficiency of skin care cosmetic formulations. Pharm. Rev. 2013, 7, 97–106. [Google Scholar] [CrossRef]
- Camag Laboratory Guidelines GDL 50.000.02 Preparation and use of derivatization reagents for TLC/HPTLC 2017.
- Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Waksmundzka-Hajnos, M.; Sherma, J.; Kowalska, T. Thin Layer Chromatography in Phytochemistry; CRC Press: Boca Raton, CA, USA, 2008; ISBN 978-1-4200-4678-6. [Google Scholar]
- White, B.L.; Howard, L.R.; Prior, R.L. Polyphenolic composition and antioxidant capacity of extruded cranberry pomace. J. Agric. Food Chem. 2010, 58, 4037–4042. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Cyboran, S.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Biological activity of blackcurrant extracts (Ribes nigrum L.) in relation to erythrocyte membranes. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Galli, R.L.; Meterko, V.; Carey, A.; Bielinski, D.F.; McGhie, T.; Joseph, J.A. Dietary supplementation with fruit polyphenolics ameliorates age-related deficits in behavior and neuronal markers of inflammation and oxidative stress. AGE 2005, 27, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Tabart, J.; Franck, T.; Kevers, C.; Pincemail, J.; Serteyn, D.; Defraigne, J.-O.; Dommes, J. Antioxidant and anti-inflammatory activities of Ribes nigrum extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
- Slimestad, R.; Solheim, H. Anthocyanins from black currants (Ribes nigrum L.). J. Agric. Food Chem. 2002, 50, 3228–3231. [Google Scholar] [CrossRef] [PubMed]
- Kapasakalidis, P.G.; Rastall, R.A.; Gordon, M.H. Extraction of polyphenols from processed black currant (Ribes nigrum L.) residues. J. Agric. Food Chem. 2006, 54, 4016–4021. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Pecher, V.; Renimel, I.; Pasquier, L.; André, P.; Elfakir, C. Two-step Centrifugal Partition Chromatography (CPC) fractionation of Butea monosperma (Lam.) biomarkers. Sep. Purif. Technol. 2011, 80, 32–37. [Google Scholar] [CrossRef]
- Plundrich, N.; Grace, M.H.; Raskin, I.; Lila, M.A. Bioactive polyphenols from muscadine grape and blackcurrant stably concentrated onto protein-rich matrices for topical applications. Int. J. Cosmet. Sci. 2013, 35, 394–401. [Google Scholar] [CrossRef]
- Burger, P.; Plainfossé, H.; Brochet, X.; Chemat, F.; Fernandez, X. Extraction of natural fragrance ingredients: History overview and future trends. Chem. Biodivers. 2019, 16, e1900424. [Google Scholar] [CrossRef]
- Rutkowska, M.; Namieśnik, J.; Konieczka, P. Chapter 10—Ultrasound-Assisted Extraction. In The Application of Green Solvents in Separation Processes; Pena-Pereira, F., Tobiszewski, M., Eds.; Elsevier Sciences: Amsterdam, The Netherlands, 2017; pp. 301–324. ISBN 978-0-12-805297-6. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Cermak, S.C.; Isbell, T.A. Pilot-plant distillation of meadowfoam fatty acids. Ind. Crop. Prod. 2002, 15, 145–154. [Google Scholar] [CrossRef]
- Kleiman, R.; Dwyer, K. A natural alternative. Inf.-Int. News Fats Oils Relat. Mater. 2006, 17, 346–347. [Google Scholar]
- Padmawar, A.; Herbals, A.; Bhadoriya, U.; Mechanics, M. Glycol and glycerin: Pivotal role in herbal industry as solvent/co-solvent. World J. Pharm. Med. Res. 2018, 4, 153–155. [Google Scholar]
- Joshi, L.S.; Pawar, H.A. Herbal cosmetics and cosmeceuticals: An overview. Nat. Prod. Chem. Res. 2015, 3, 170. [Google Scholar] [CrossRef]



| Assay | Positive Control |
|---|---|
| DPPH Assay | Rosmarinus officinalis L. commercial extract (INCI: ROSMARINUS OFFICINALIS EXTRACT; 3.433 mg/mL in DMSO) |
| Tyrosinase Assay | Phenylethyl resorcinol (4-(1-phenylethyl)benzene-1,3-diol; INCI: PHENYLETHYL RESORCINOL; 3.433 mg/mL in DMSO) |
| Lipoxygenase Assay | Arnica montana L. commercial extract (INCI: ARNICA MONTANA EXTRACT; 3.433 mg/mL in DMSO) |
| Elastase Assay | Rubus idaeus L. commercial extract (INCI: RUBUS IDAEUS EXTRACT; 3.433 mg/mL in DMSO) |
| Hyaluronidase Assay | Rubus idaeus L. commercial extract (INCI: RUBUS IDAEUS EXTRACT; 3.433 mg/mL in DMSO) |
| Collagenase Assay | Betulinic acid (3.433 mg/mL in DMSO) |
| R. Nigrum Pomace Extract | F1 | F2 | F3 | F4 | F5 | F6 | F7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Mass (mg) | 647.0 | 416.0 | 62.9 | 96.4 | 21.4 | 10.2 | 18.3 | 1.1 | |
| Yield (%) | 100.0 | 64.3 | 9.7 | 14.9 | 3.3 | 1.6 | 2.8 | 0.2 | |
| Bioactivities | Anti-hyaluronidase | ++++ | - | ++++ | ++++ | ++++ | ++++ | ++++ | - |
| Anti-collagenase | +++ | + | ++++ | +++ | ++ | + | - | - | |
| Anti-inflammatory | - | - | +++ | +++ | ++++ | ++ | - | - | |
| Antioxidant | +++ | - | ++++ | ++++ | ++++ | +++ | - | - | |
| Anti-elastase | - | - | - | - | - | - | - | - | |
| Whitening (L-tyrosine) | ++ | - | ++ | ++ | +++ | ++ | - | - | |
| Whitening (L-DOPA) | + | - | + | + | + | ++ | + | - |
| Conventional Maceration | Ultrasound-Assisted Extraction | |||||||
|---|---|---|---|---|---|---|---|---|
| Solvent | EtOH | H2O/EtOH 20/80 | H2O/EtOH 50/50 | H2O/EtOH 80/20 | H2O/EtOH 50/50 | H2O/EtOH 50/50 | H2O/EtOH 50/50 | |
| Maceration parameters | RT, 2 h | RT, 2 h | RT, 2 h | RT, 2 h | RT, 6 h | RT, 20 min | RT, 60 min | |
| Extraction yield (%) | 3.6 ± 0.3% | 2.2 ± 0.1% | 3.2 ± 0.2% | 3.2 ± 0.2% | 2.8 ± 0.6% | 3.6% | 3.9% | |
| Bioactivities | Anti-hyaluronidase | + | - | ++++ | + | +++ | ++++ | ++++ |
| Anti-collagenase | - | - | +++ | - | +++ | ++++ | ++++ | |
| Anti-inflammatory | - | - | - | - | - | ++ | + | |
| Antioxidant | ++ | ++ | +++ | +++ | +++ | ++++ | ++++ | |
| Anti-elastase | - | - | - | - | - | - | - | |
| Whitening (L-tyrosine) | + | - | ++ | ++ | + | ++ | ++ | |
| Whitening (L-DOPA) | + | - | + | + | + | + | ++ | |
| Solvent | Sunflower Seed Oil | Propylene Glycol | Glycerine | Propylene Glycol | Glycerine | Propylene Glycol | Glycerine | |
|---|---|---|---|---|---|---|---|---|
| Maceration parameters | RT, 7 h | RT, 7 h | RT, 7 h | RT, 12 h | RT, 12 h | RT, 24 h | RT, 24 h | |
| Bioactivities | Anti-hyaluronidase | - | + | - | + | - | +++ | - |
| Anti-collagenase | - | +++ | +++ | ++ | ++ | ++++ | +++ | |
| Anti-inflammatory | - | - | - | - | - | - | - | |
| Antioxidant | - | ++ | ++ | ++ | ++ | +++ | ++ | |
| Anti-elastase | + | - | - | - | - | - | - | |
| Whitening (L- tyrosine) | - | - | - | - | - | - | - | |
| Whitening (L- DOPA) | - | - | - | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plainfossé, H.; Trinel, M.; Verger-Dubois, G.; Azoulay, S.; Burger, P.; Fernandez, X. Valorisation of Ribes nigrum L. Pomace, an Agri-Food By-Product to Design a New Cosmetic Active. Cosmetics 2020, 7, 56. https://doi.org/10.3390/cosmetics7030056
Plainfossé H, Trinel M, Verger-Dubois G, Azoulay S, Burger P, Fernandez X. Valorisation of Ribes nigrum L. Pomace, an Agri-Food By-Product to Design a New Cosmetic Active. Cosmetics. 2020; 7(3):56. https://doi.org/10.3390/cosmetics7030056
Chicago/Turabian StylePlainfossé, Hortense, Manon Trinel, Grégory Verger-Dubois, Stéphane Azoulay, Pauline Burger, and Xavier Fernandez. 2020. "Valorisation of Ribes nigrum L. Pomace, an Agri-Food By-Product to Design a New Cosmetic Active" Cosmetics 7, no. 3: 56. https://doi.org/10.3390/cosmetics7030056
APA StylePlainfossé, H., Trinel, M., Verger-Dubois, G., Azoulay, S., Burger, P., & Fernandez, X. (2020). Valorisation of Ribes nigrum L. Pomace, an Agri-Food By-Product to Design a New Cosmetic Active. Cosmetics, 7(3), 56. https://doi.org/10.3390/cosmetics7030056





