Inhibitory Effect of Manassantin B Isolated from Saururus chinensis on Skin Heat Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
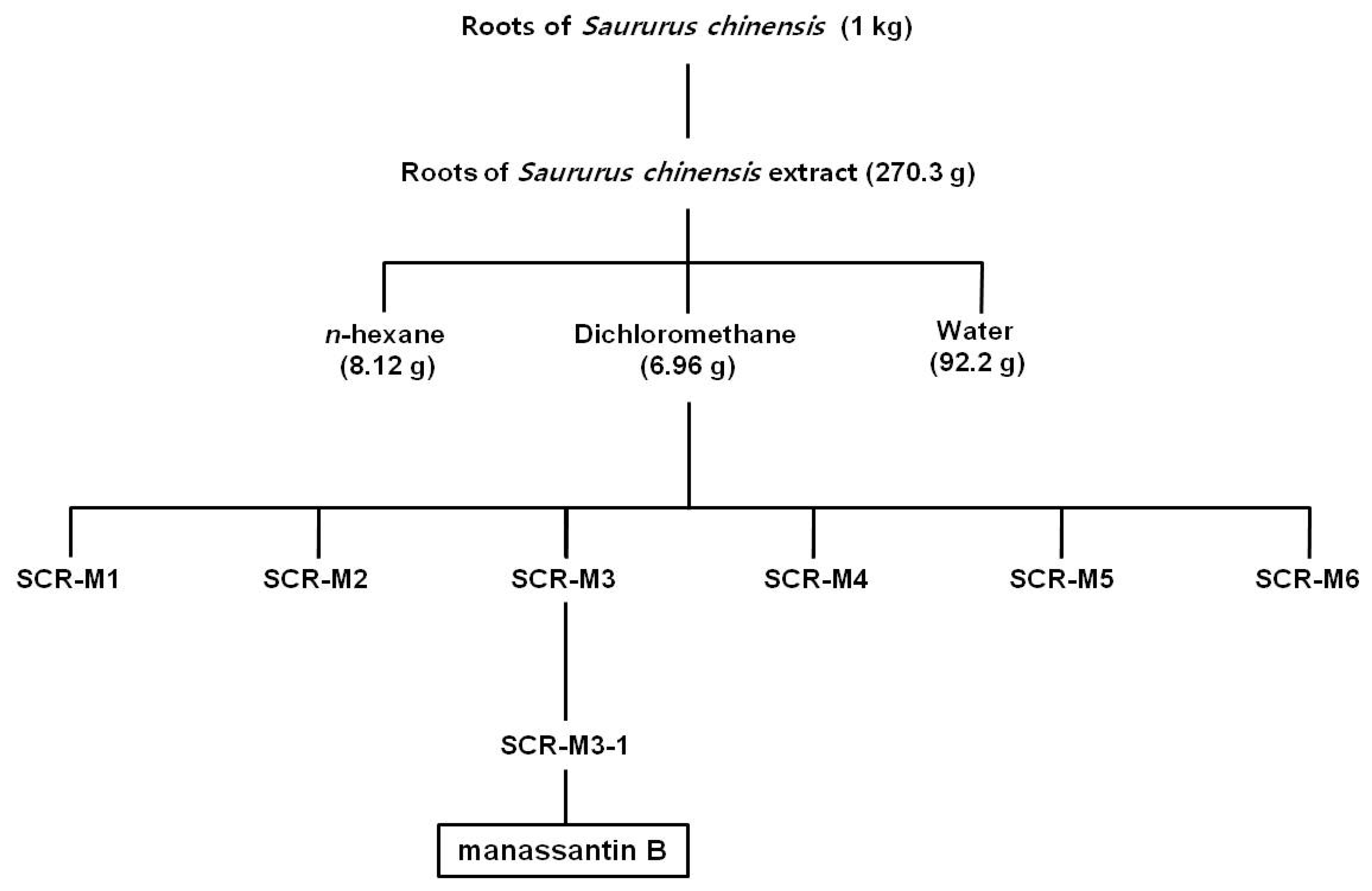
2.2. Isolation of Manassantin B
2.3. Cell Subculture
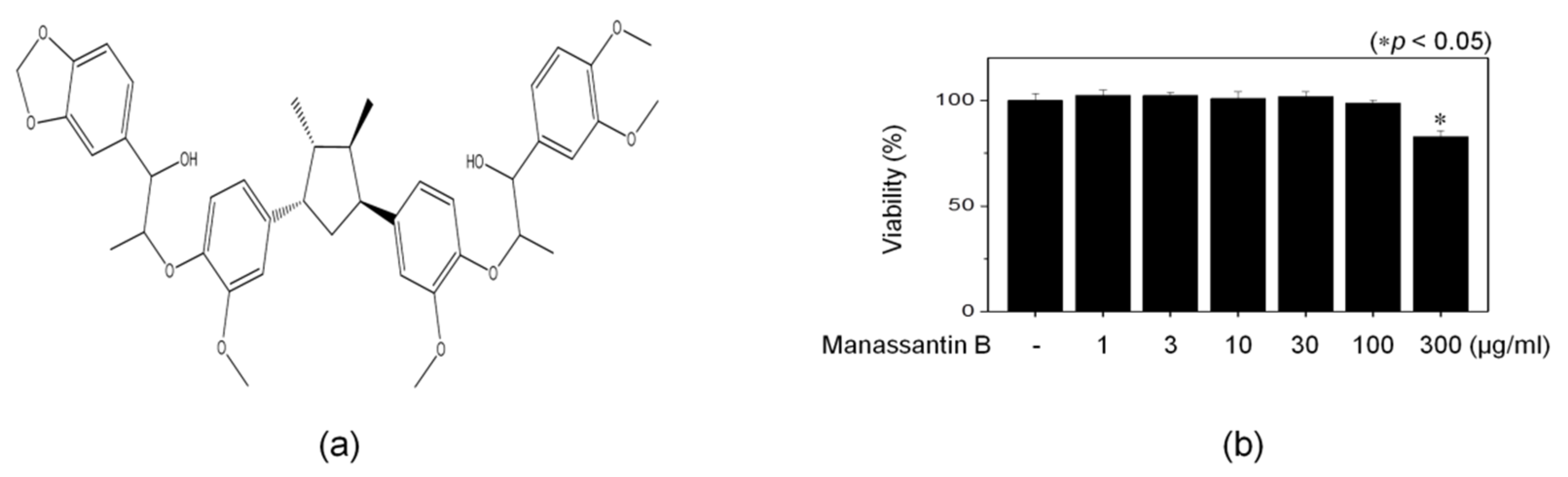
2.4. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.5. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.6. Western Blotting
2.7. Procollagen Synthesis Assay (ELISA)
2.8. Statistics
3. Results and Discussion
3.1. Isolation of MB
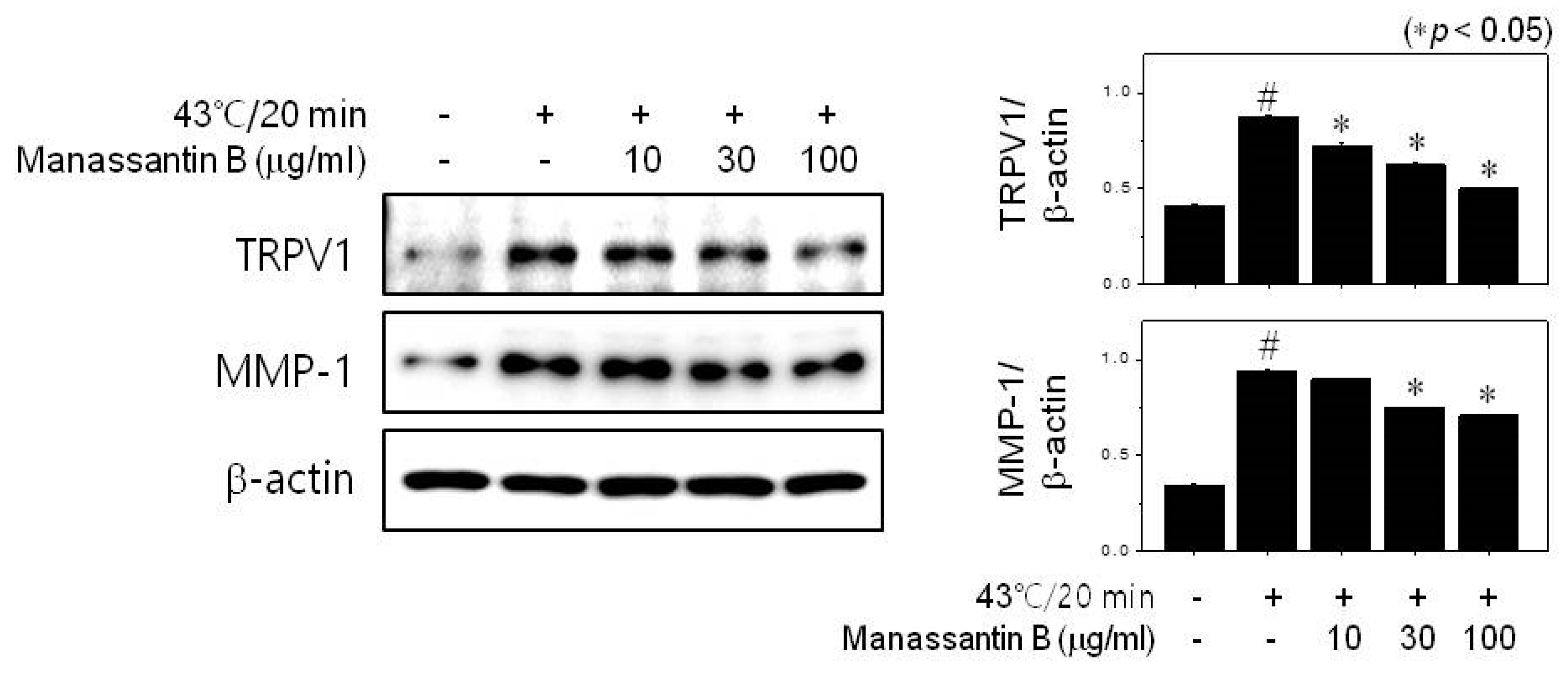
3.2. MB Inhibits MMP-1 Expression through TRPV1 Activation
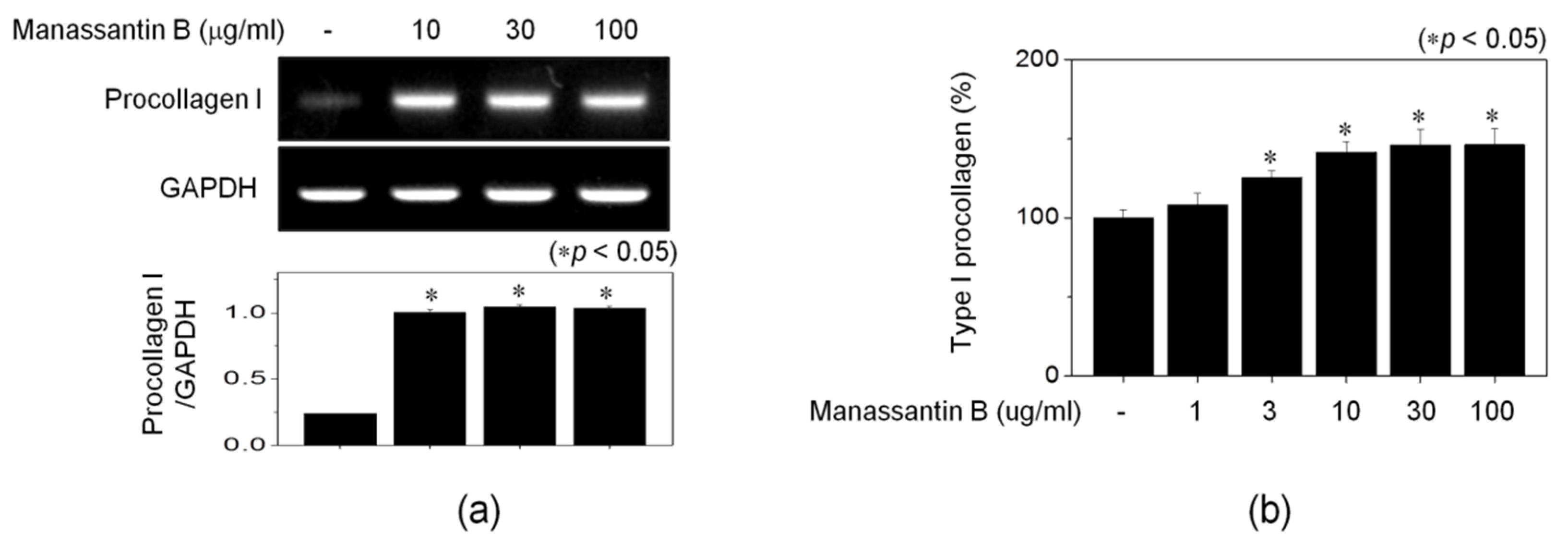
3.3. MB Increases Procollagen Expression
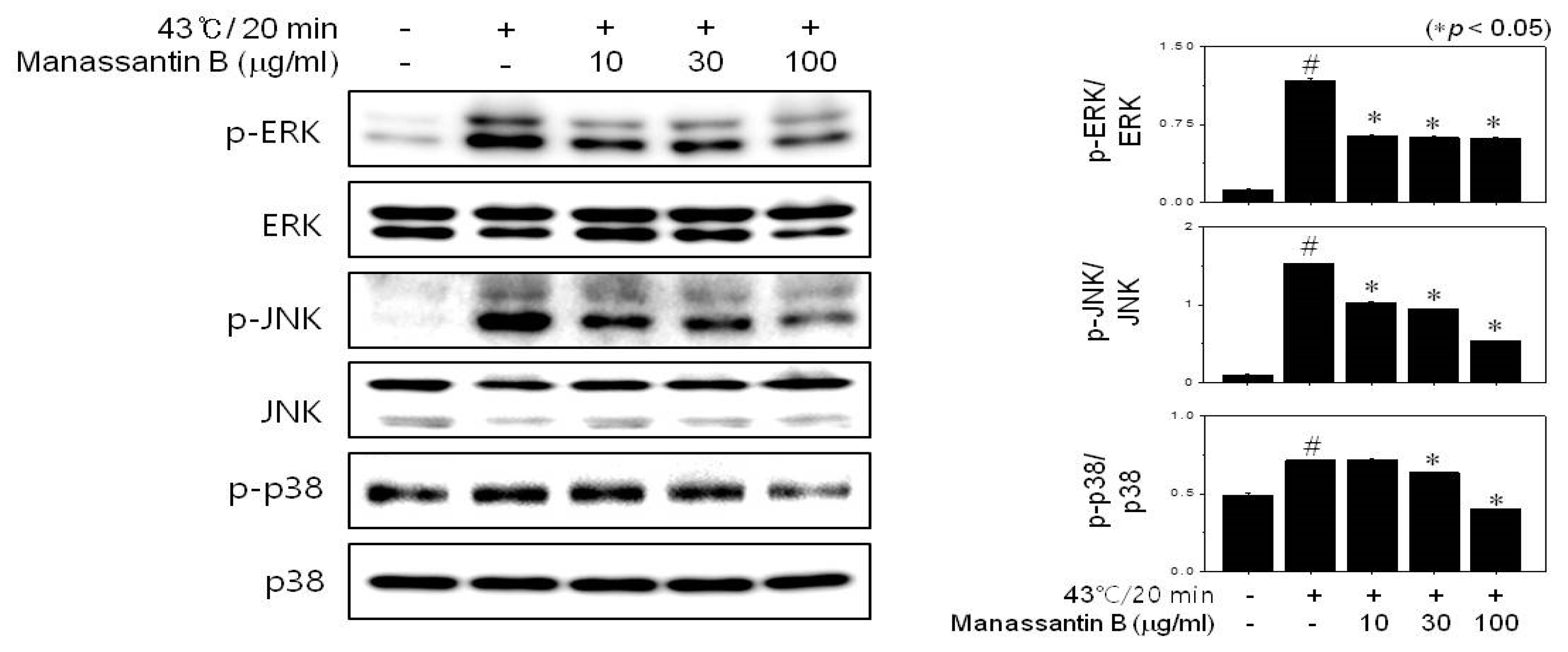
3.4. MB Inhibits Heat-Induced Mitogen-Activated Protein Kinase (MAPK) Activation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cho, S.; Shin, M.H.; Kim, Y.K.; Seo, J.E.; Lee, Y.M.; Park, C.H.; Chung, J.H. Effects of infrared radiation and heat on human skin aging in vivo. J. Investig. Derm. Symp Proc. 2009, 14, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, D.H.; Cho, S.; Chung, J.H. Minimal heating dose A novel biological unit to measure infrared irradiation. Photodermatol. Photoimmunol. Photomed. 2006, 22, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Chung, J.H. Thermal aging: A new concept of skin aging. J. Dermatol. Sci. Suppl. 2006, 2, S13–S22. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Derm. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaW, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodeling. Nat. Rev. Mol. Cell Biol 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, M.J.; Ahn, J.; Kim, S.; Kim, H.H.; Kim, K.H.; Eun, H.C.; Chung, J.H. Heat shock-induced matrix metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and JNK activation and via an autocrine interleukin-6 loop. J. Invest. Derm. 2004, 123, 1012–1019. [Google Scholar] [CrossRef]
- Lee, Y.M.; Li, W.H.; Kim, Y.K.; Kim, K.H.; Chung, J.H. Heat-induced MMP-1 expression is mediated by TRPV1 through PKCalpha signaling in HaCaT cells. Exp. Derm. 2008, 17, 864–870. [Google Scholar] [CrossRef]
- Zheng, J. Molecular mechanism of TRP channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar] [CrossRef]
- Klionsky, L.; Tamir, R.; Holzinger, B.; Bi, X.; Talvenheimo, J.; Kim, H.; Martin, F.; Louis, J.C.; Treanor, J.J.; Gavva, N.R. A polyclonal antibody to the prepore loop of transient receptor potential vanilloid type 1 blocks channel activation. J. Pharm. Exp. 2006, 319, 192–198. [Google Scholar] [CrossRef]
- Morales-Lazaro, S.L.; Liorente, I.; Sierra-Ramirez, F.; Lopez-Romero, A.E.; Oritiz-Renteria, M.; Serrano-Flores, B.; Simon, S.A.; Islas, L.D.; Rosenbaum, T. Inhibition of TRPV1 channels by a naturally occurring omega-9 fatty acid reduces pain and itch. Nat. Commun. 2016, 127, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Lee, Y.M.; Kim, J.Y.; Kang, S.; Kim, S.; Kim, K.H.; Park, C.H.; Chung, J.H. Transient receptor potential vanilloid-1 mediates heat-shock-induced matrix metalloproteinase-1 expression in human epidermal keratinocytes. J. Investig. Derm. 2007, 127, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Son, K.N.; Song, I.S.; Shin, Y.H.; Pai, T.K.; Chung, D.K.; Baek, N.I.; Lee, J.J.; Kim, J. Inhibition of NF-IL6 activity by manassantin B, a dilignan isolated from Saururus chinensis, in phorbol myristate acetate-stimulated U937 promonocytic cells. Mol. Cells 2005, 20, 105–111. [Google Scholar] [PubMed]
- Rho, M.C.; Kwon, O.E.; Kim, K.; Lee, S.W.; Chung, M.Y.; Kim, Y.H.; Hayashi, M.; Lee, H.S.; Kim, Y.K. Inhibitory effects of manassantin A and B isolated from the roots of Saururus chinensis on PMA-induced ICAM-1 expression. Planta Med. 2003, 69, 1147–1149. [Google Scholar] [CrossRef]
- Hodges, T.W.; Hossain, C.F.; Kim, Y.P.; Zhou, Y.D.; Nagle, D.G. Molecular-targeted antitumor agents the Saururus cernuus dineolignans manassantin B and 4-O-demethylmanassantin B are potent inhibitors of hypoxia-activated HIF-1. J. Nat. Prod. 2004, 67, 767–771. [Google Scholar] [CrossRef]
- Park, H.C.; Bae, H.B.; Jeong, C.W.; Lee, S.H.; Jeung, H.J.; Kwak, S.H. Effect of manassantin B, a lignan isolated from Saururus chinensis, on lipopolysaccharide-induced interleukin-1beta in RAW 264.7 cells. Korean J. Anesth. 2012, 62, 161–165. [Google Scholar] [CrossRef][Green Version]
- Lu, Y.; Hwang, S.L.; Son, J.K.; Chang, H.W. Manassantin B isolated from Saururus chinensis inhibits cyclooxygenase-2-dependent prostaglandin D2 generation by blocking Fyn-mediated nuclear factor-kappaB and mitogen activated protein kinase pathways in bone marrow derived-mast cells. Biol. Pharm. Bull. 2013, 36, 1370–1374. [Google Scholar] [CrossRef][Green Version]
- Seo, C.S.; Lee, W.H.; Chung, H.W.; Chang, E.J.; Lee, S.H.; Jahng, Y.; Hwang, B.Y.; Son, J.K.; Han, S.B.; Kim, Y. Manassantin A and B from Saururus chinensis inhibiting cellular melanin production. Phytother. Res. 2009, 23, 1531–1536. [Google Scholar] [CrossRef]
- Chang, H.; Choi, H.; Joo, K.M.; Kim, D.; Lee, T.R. Manassantin B inhibits melanosome transport in melanocytes by disrupting the melanophilin-myosin Va interaction. Pigment. Cell Melanoma Res. 2012, 25, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Huh, M.S.; Kim, Y.C.; Hattori, M.; Otake, T. Lignan, sesquilignans and dilignans, novel HIV-1 protease and cytopathic effect inhibitors purified from the rhizomes of Saururus chinensis. Antivir. Res. 2010, 85, 425–428. [Google Scholar] [CrossRef]
- Lee, W.S.; Lee, D.W.; Baek, Y.I.; An, S.; Cho, K.H.; Choi, Y.K.; Kim, H.C.; Park, H.Y.; Bae, K.H.; Jeong, T.S. Human ACAT-1 and -2 inhibitory activities of saucerneol B, manassantin A and B isolated from Saururus chinensis. Bioorg. Med. Chem. Lett. 2004, 14, 3109–3112. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lafond, J.; Pelletier, R.M. A novel technical approach for the measurement of individual ACAT-1 and ACAT-2 enzymatic activity in the testis. Methods Mol. Biol. 2009, 550, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Xu, B.; Wu, T.; Xu, J.; Yuan, Y.; Gu, Q. Potential antiviral lignans from the roots of Saururus chinensis with activity against Epstein-Barr virus lytic replication. J. Nat. Prod. 2014, 77, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, X.; Xu, T.; Yang, X.; Liu, Y. Lignans from Saururus chinensis. Chem. Nat. Compd. 2010, 46, 450–451. [Google Scholar] [CrossRef]
- He, X.; Dai, J.; Fan, Y.; Zhang, C.; Zhao, X. Regulation function of MMP-1 downregulated by siRNA on migration of heat-denatured dermal fibroblasts. Bioengineered 2017, 8, 686–692. [Google Scholar] [CrossRef]
- Li, D.Q.; Meller, D.; Liu, Y.; Tseng, S.C. Overexpression of MMP-1 and MMP-3 by cultured conjunctivochalasis fibroblasts. Invest. Ophthalmol Vis. Sci. 2000, 41, 404–410. [Google Scholar]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis A dynamic view. Dev. Biol 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Reunanen, N.; Li, S.P.; Ahonen, M.; Foschi, M.; Han, J.; Kahari, V.M. Activation of p38 alpha MAPK enhances collagenase-1 (matrix metalloproteinase (MMP)-1) and stromelysin-1 (MMP-3) expression by mRNA stabilization. J. Biol. Chem. 2002, 277, 32360–32368. [Google Scholar] [CrossRef]
- Westermarck, J.; Kahari, V.M. Regulation of matrix metalloproteinase expression in tumor invasion. Faseb J. 1999, 13, 781–792. [Google Scholar] [CrossRef]
- Lewis, T.S.; Shapiro, P.S.; Ahn, N.G. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 1998, 74, 49–139. [Google Scholar] [CrossRef] [PubMed]
- Garrington, T.P.; Johnson, G.L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 1999, 11, 211–218. [Google Scholar] [CrossRef]
- Lam, E.; Kilani, R.T.; Li, Y.; Tredget, E.E.; Ghahary, A. Stratifin-induced matrix metalloproteinase-1 in fibroblast is mediated by c-fos and p38 mitogen-activated protein kinase activation. J. Invest. Derm. 2005, 125, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, H.S.; Choi, J.-Y.; Lee, K.-S.; Lee, J.-N.; Lee, C.M.; Park, S.-M. Inhibitory Effect of Manassantin B Isolated from Saururus chinensis on Skin Heat Aging. Cosmetics 2020, 7, 47. https://doi.org/10.3390/cosmetics7020047
Ryu HS, Choi J-Y, Lee K-S, Lee J-N, Lee CM, Park S-M. Inhibitory Effect of Manassantin B Isolated from Saururus chinensis on Skin Heat Aging. Cosmetics. 2020; 7(2):47. https://doi.org/10.3390/cosmetics7020047
Chicago/Turabian StyleRyu, Hwa Sun, Jeong-Yeon Choi, Kyeong-Sun Lee, Jung-No Lee, Chun Mong Lee, and Sung-Min Park. 2020. "Inhibitory Effect of Manassantin B Isolated from Saururus chinensis on Skin Heat Aging" Cosmetics 7, no. 2: 47. https://doi.org/10.3390/cosmetics7020047
APA StyleRyu, H. S., Choi, J.-Y., Lee, K.-S., Lee, J.-N., Lee, C. M., & Park, S.-M. (2020). Inhibitory Effect of Manassantin B Isolated from Saururus chinensis on Skin Heat Aging. Cosmetics, 7(2), 47. https://doi.org/10.3390/cosmetics7020047




