In Vitro and In Vivo Study on Humans of Natural Compound Synergy as a Multifunctional Approach to Cellulite-Derived Skin Imperfections
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition of the Nutraceutical Ingredient
2.2. ABTS Assay
2.3. DPPH Assay
2.4. Total Polyphenols Content
2.5. Total Anthocyanins Content
2.6. Proteolytic Activity
2.7. Pancreatic Lipase Activity
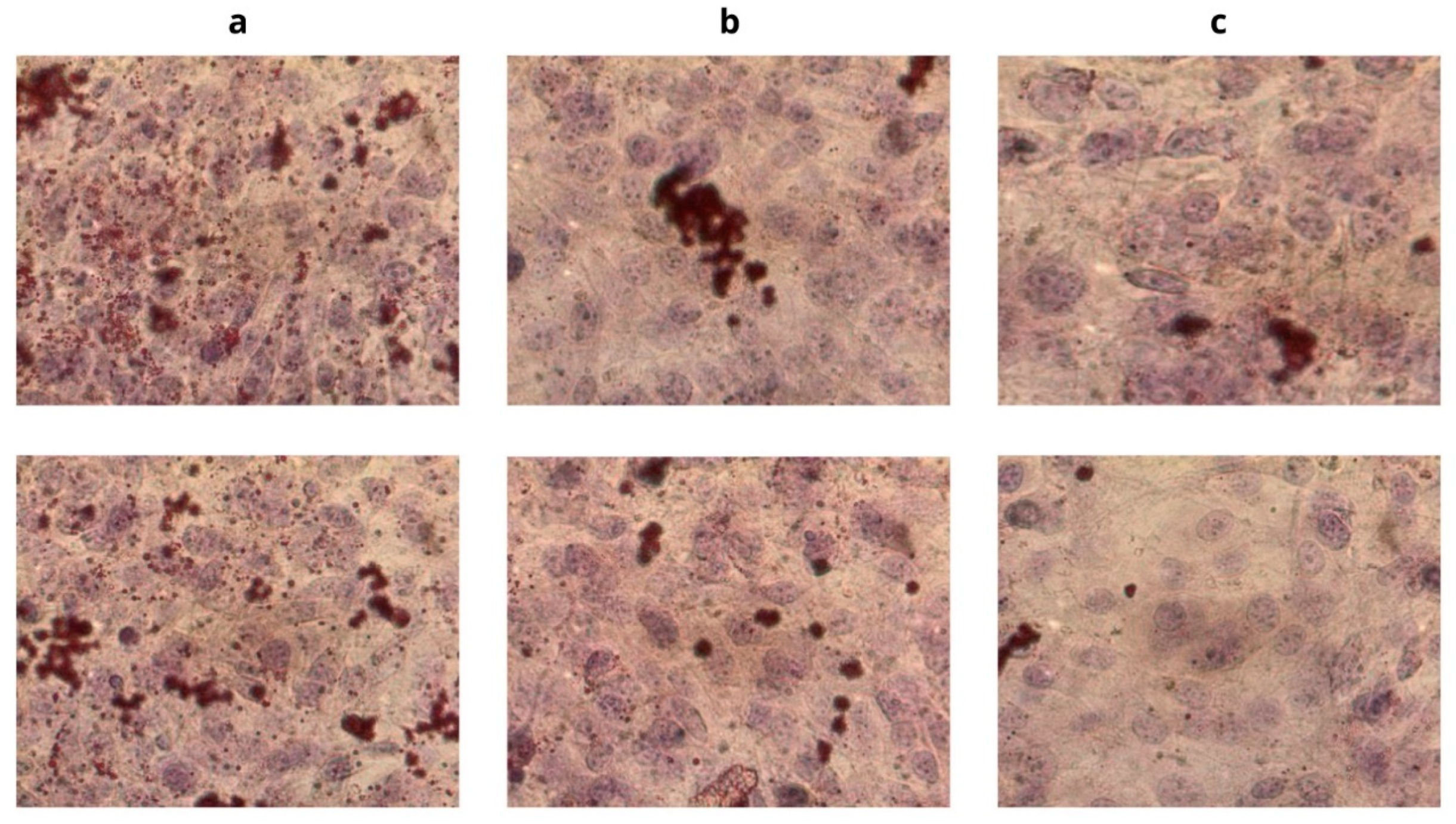
2.8. Oil Red O Staining
2.9. Clinical Study
2.9.1. Study Design and Participants
Inclusion Criteria
Non-Inclusion Criteria
2.9.2. Treatment
2.9.3. Assessments
2.10. Statistical Methods
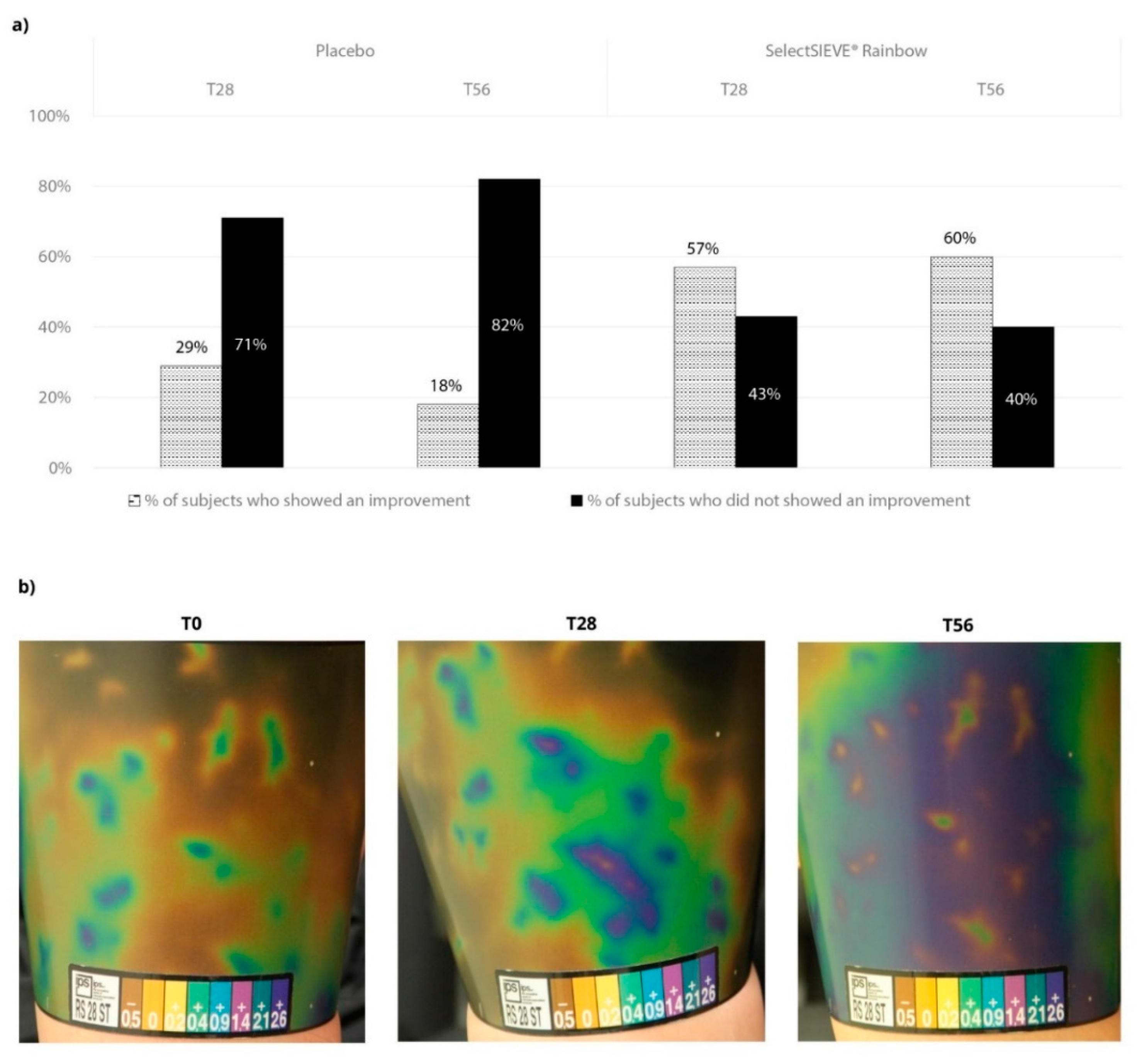
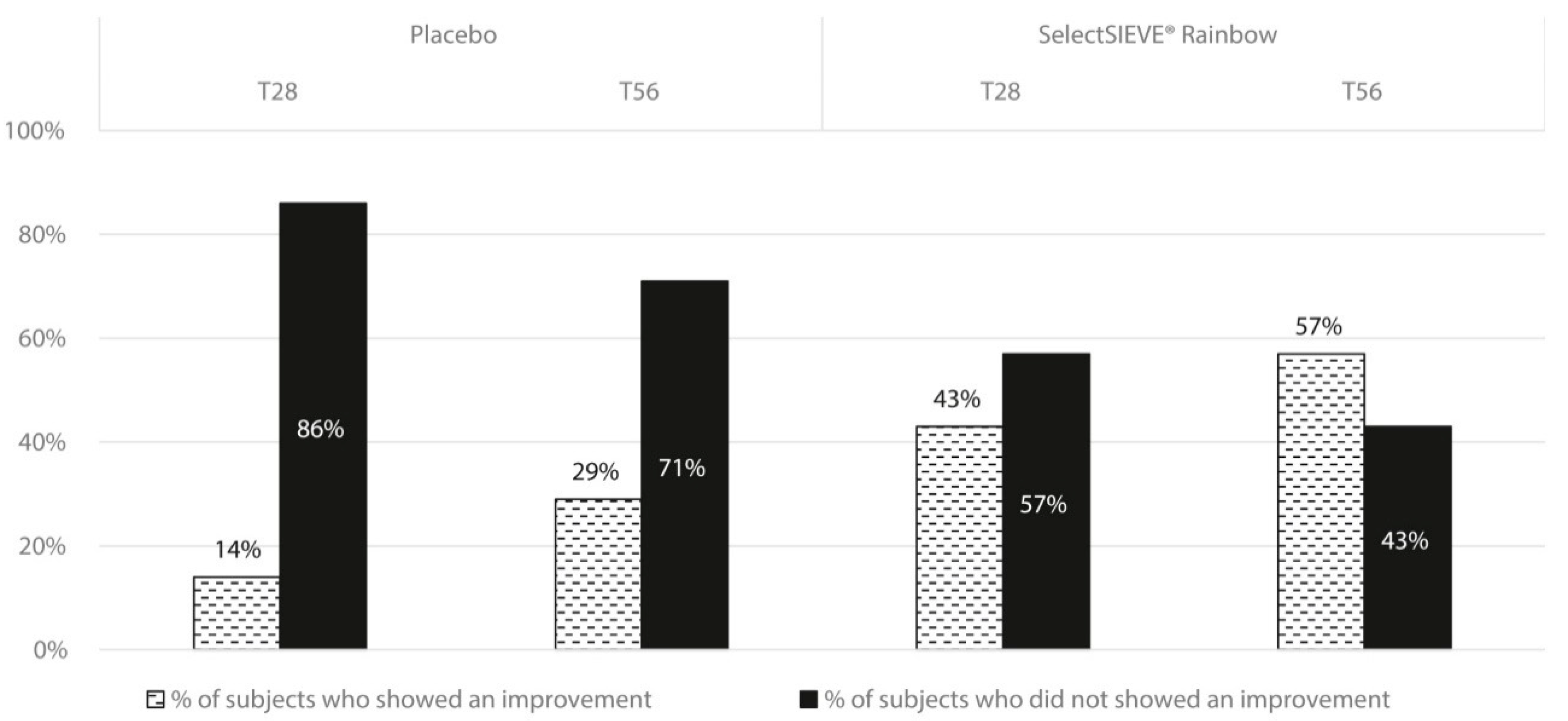
3. Results
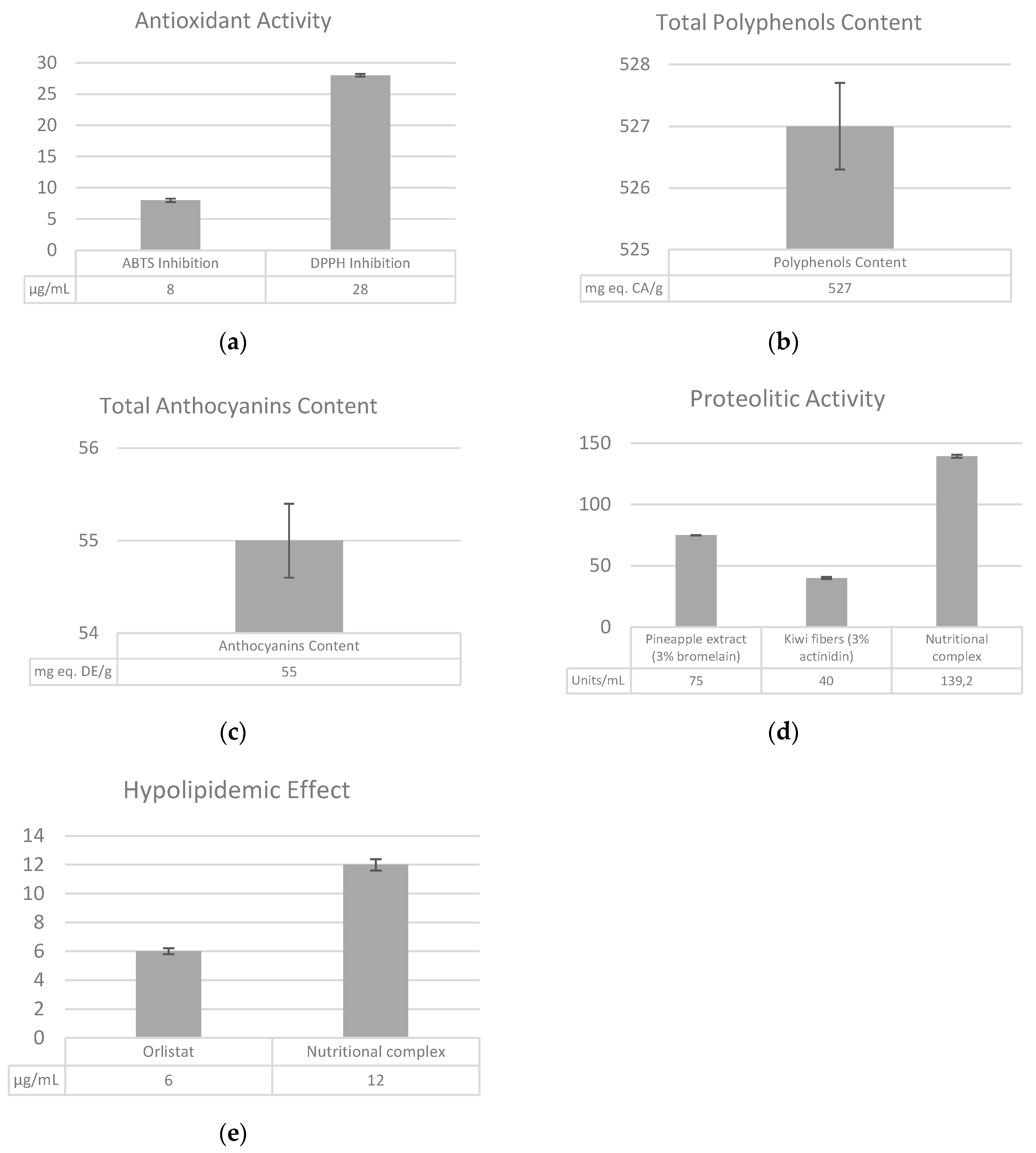
3.1. Evaluation of the Antioxidant Activity and the Total Polyphenols and Anthocyanins Content
3.2. Proteolytic Activity
3.3. Evaluation of the Hypolipidemic Effect
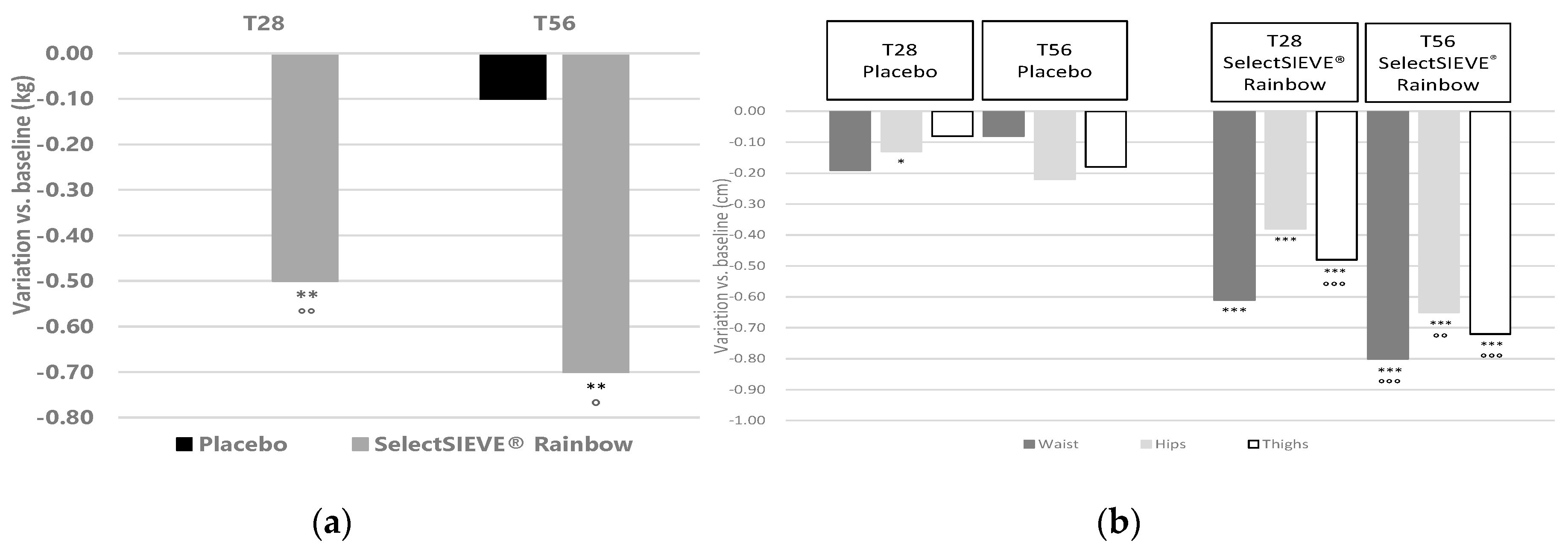
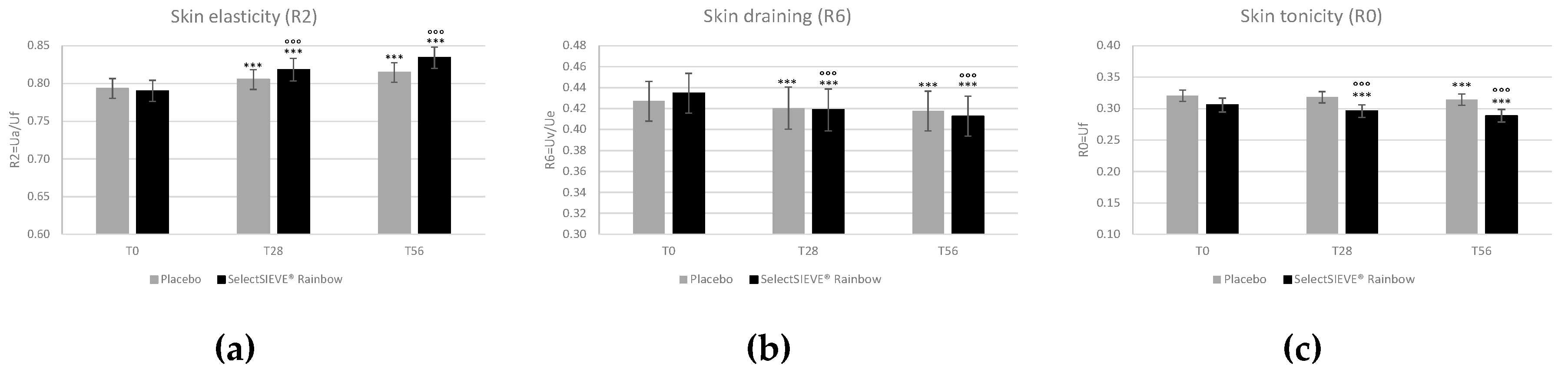
3.4. Clinical Trial
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Emanuele, E.; Minoretti, P.; Altabas, K.; Gaeta, E.; Altabas, V. Adiponectin expression in subcutaneous adipose tissue is reduced in women with cellulite. Int. J. Dermatol. 2011, 50, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Hexsel, D.M.; Siega, C.; Schilling-Souza, J.; Porto, M.D.; Rodrigues, T.C. A bipolar radiofrequency, infrared, vacuum and mechanical massage device for treatment of cellulite: A pilot study. J. Cosmet. Laser Ther. 2011, 13, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.; Victor, F.; Rao, B.; Sadick, N.S. Treatment of cellulite: Part I Pathophysiology. J. Am. Acad. Dermatol. 2010, 62, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Smalls, L.K.; Hicks, M.; Passeretti, D.; Gersin, K.; Kitzmiller, W.J.; Bakhsh, A.; Wickett, R.R.; Whitestone, J.; Visscher, M.O. Effect of weight loss on cellulite: Gynoid lipodystrophy. Plast. Reconstr. Surg. 2006, 118, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.B.R.; Vergnanini, A.L. Cellulite: A review. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 251–262. [Google Scholar] [CrossRef]
- Okay, D.M.; Jackson, P.V.; Marcinkiewicz, M.; Papino, M.N. Exercise and obesity. Prim. Care 2009, 36, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Ab Rahman, W.N.A.W.; Lee, K.S.; Yee, J.C.W.; Gupta, M.; Ming, L.C. Evidence of Garcinia cambogia as a fat burning and appetite suppressing agents. Arch. Pharm. Pract. 2016, 7, 22. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Bosisio, E. Biflavones of Ginkgo biloba stimulate lipolysis in 3T3-L1 adipocytes. Planta Medica 2002, 68, 76–79. [Google Scholar] [CrossRef]
- Zahara, K.; Bibi, Y.; Tabassum, S. Clinical and therapeutic benefits of Centella asiatica. Pure Appl. Biol. 2014, 3, 152. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Radzi, C.W.J.W.M.; Cordell, G.A.; Yaze, I. Potential of traditional medicinal plants for treating obesity: A review. arXiv 2012, arXiv:1208.1923. [Google Scholar]
- Emanuele, E. Cellulite: Advances in treatment: Facts and controversies. Clin. Dermatol. 2013, 31, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.P.; Hexsel, D. Cellulite: Pathophysiology and Treatment; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Sparavigna, A.; Guglielmini, G.; Togni, S.; Cristoni, A.; Maramaldi, G. Evaluation of anti-cellulite efficacy: A topical cosmetic treatment for cellulite blemishes—A multifunctional formulation. Int. J. Cosmet. Sci. 2011, 623, 305. [Google Scholar]
- Hexsel, D.; Orlandi, C.; Zechmeister do Prado, D. Botanical extracts used in the treatment of cellulite. Dermatol. Surg. 2005, 31, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Rona, C.; Berardesca, E. Aging skin and food supplements: The myth and the truth. Clin. Dermatol. 2008, 26, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants–a mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, Ş.; Gülçin, İ. Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Jannesar, M.; Shoushtari, M.S.; Majd, A.; Pourpak, Z. Bee pollen flavonoids as a therapeutic agent in allergic and immunological disorders. Iran. J. Allergy Asthma Immunol. 2017, 16, 171–182. [Google Scholar] [PubMed]
- Marino, A.; Paterniti, I.; Cordaro, M.; Morabito, R.; Campolo, M.; Navarra, M.; Esposito, E.; Cuzzocrea, S. Role of natural antioxidants and potential use of bergamot in treating rheumatoid arthritis. Pharma Nutr. 2015, 3, 53–59. [Google Scholar] [CrossRef]
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 2015, 61, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.W.; Qiu, Z.D.; Wang, W.N.; Sui, X.; Sui, D.J. Flavonoids extraction from propolis attenuates pathological cardiac hypertrophy through PI3K/AKT signaling pathway. J. Evid. Based Complement. Altern. Med. 2016, 2016, 6281376. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Pedro, A.C.; Granato, D.; Rosso, N.D. Extraction of anthocyanins and polyphenols from black rice (Oryza sativa L.) by modeling and assessing their reversibility and stability. Food Chem. 2016, 191, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: A review. Crit. Rev. Food Sci. Nutr. 2016, 5613, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Bratman, S.; Kroll, D.J. Clinical Evaluation of Medicinal Herbs and Other Therapeutic Natural Products; Prima Health: Rocklin, CA, USA, 1999. [Google Scholar]
- Šavikin, K.; Menković, N.; Zdunić, G.; Pljevljakušić, D.; Spasić, S.; Kardum, N.; Konić-Ristić, A. Dietary supplementation with polyphenol-rich chokeberry juice improves skin morphology in cellulite. J. Med. Food 2014, 17, 582–587. [Google Scholar] [CrossRef]
- Wagner, H. New plant phenolics of pharmaceutical interest. Annal. Proc. Phytochem. Soc. Eur. 1985, 25, 409–425. [Google Scholar]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 3161, 149–157. [Google Scholar] [CrossRef]
- Tsuda, T. Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants 2016, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hong, J.; Jeon, R.; Kim, H.S. Adzuki bean ameliorates hepatic lipogenesis and proinflammatory mediator expression in mice fed a high-cholesterol and high-fat diet to induce nonalcoholic fatty liver disease. Nutr. Res. 2016, 36, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, J.H.; Lee, E.B.; Hur, W.; Kwon, O.J.; Park, H.J.; Yoon, S.K. Aronia melanocarpa extract ameliorates hepatic lipid metabolism through PPARγ2 downregulation. PLoS ONE 2017, 12, e0169685. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Horie, K.; Maeda, H.; Tomisawa, T.; Kitajima, M.; Nakamura, T. Blackcurrant Anthocyanins increase the levels of collagen, elastin, and hyaluronic acid in human skin fibroblasts and ovariectomized rats. Nutrients 2018, 10, 495. [Google Scholar] [CrossRef]
- Phetpornpaisan, P.; Tippayawat, P.; Jay, M.; Sutthanut, K. A local Thai cultivar glutinous black rice bran: A source of functional compounds in immunomodulation, cell viability and collagen synthesis, and matrix metalloproteinase-2 and-9 inhibition. J. Funct. Foods 2014, 7, 650–661. [Google Scholar] [CrossRef]
- Etebu, E.; Nwauzoma, A.B. A review on sweet orange (Citrus sinensis L Osbeck): Health, diseases and management. Am. J. Res. Commun. 2014, 2, 33–70. [Google Scholar]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef]
- Ejaz, S.; Ejaz, A.; Matsuda, K.; Chae, W.L. Limonoids as cancer chemopreventive agents. J. Sci. Food Agric. 2006, 86, 339–345. [Google Scholar] [CrossRef]
- Stohs, S.J.; Badmaev, V. A review of natural stimulant and non-stimulant thermogenic agents. Phytother. Res. 2016, 30, 732–740. [Google Scholar] [CrossRef]
- Graziano, A.C.; Cardile, V.; Crascì, L.; Caggia, S.; Dugo, P.; Bonina, F.; Panico, A. Protective effects of an extract from Citrus bergamia against inflammatory injury in interferon-gamma and histamine exposed human keratinocytes. Life Sci. 2012, 90, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.J.; Han, S.C.; Ock, J.W.; Kang, H.K.; Yoo, E.S. Anti-inflammatory effect of quercetagetin, an active component of immature Citrus unshiu, in HaCaT human keratinocytes. Biomol. Ther. 2013, 21, 138. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, S.; Katsumata, S.I.; Suzuki, K.; Ishimi, Y.; Wu, J.; Uehara, M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2009, 46, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Sia, C.L.; Upadhyay, M.; Korzeniewski, K.; Viswanathan, P.; Abuaysheh, S.; Mohanty, P.; Dandona, P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am. J. Clin. Nutr. 2010, 914, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Burke, A.C.; Huff, M.W. Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu. Rev. Nutr. 2016, 36, 275–299. [Google Scholar] [CrossRef]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Kim, M.H.; Ko, H.C.; Kim, S.J. Immature Citrus sunki peel extract exhibits antiobesity effects by β-oxidation and lipolysis in high-fat diet-induced obese mice. Biol. Pharm. Bull. 2012, 35, 223–230. [Google Scholar] [CrossRef]
- Kim, G.S.; Park, H.J.; Woo, J.H.; Kim, M.K.; Koh, P.O.; Min, W.; Ko, Y.G.; Kim, C.H.; Won, C.K.; Cho, J.H. Citrus aurantium flavonoids inhibit adipogenesis through the Akt signaling pathway in 3T3-L1 cells. BMC Complement. Altern. Med. 2012, 12, 31. [Google Scholar] [CrossRef]
- Anilkumar, M. Ethnomedicinal plants as anti-inflammatory and analgesic agents. In Ethnomedicine: A Source of Complementary Therapeutics; Chattopadhyay, D., Ed.; Research Signpost: Kolkata, India, 2010; pp. 267–293. [Google Scholar]
- Hale, L.P.; Greer, P.K.; Trinh, C.T.; Gottfried, M.R. Treatment with oral bromelain decreases colonic inflammation in the IL-10-deficient murine model of inflammatory bowel disease. Clin. Immunol. 2005, 116, 135–142. [Google Scholar] [CrossRef]
- Pandey, P.; Tiwari, S. Therapeutic potential of Indian plants for the treatment of rheumatoid arthritis. J. Pharmacogn. Phytochem. 2018, 7, 37–41. [Google Scholar]
- Secor, E.R.; Carson, W.F.; Cloutier, M.M.; Guernsey, L.A.; Schramm, C.M.; Wu, C.A.; Thrall, R.S. Bromelain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Cell Immunol. 2005, 237, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Graf, J. Herbal anti-inflammatory agents for skin disease. Skin Ther. Lett. 2000, 5, 3–5. [Google Scholar]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Tochi, B.N.; Wang, Z.; Xu, S.Y.; Zhang, W. Therapeutic application of pineapple protease (bromelain): A review. Pak. J. Nutr. 2008, 74, 513–520. [Google Scholar]
- Bhattacharyya, B.K. Bromelain: An overview. Nat. Prod. Rad. 2008, 7, 359–363. [Google Scholar]
- Manzoor, Z.; Nawaz, A.; Mukhtar, H.; Haq, I. Bromelain: Methods of extraction, purification and therapeutic applications. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef]
- Maurer, H.R. Bromelain: Biochemistry, pharmacology and medical use. Cell Mol. Life Sci. 2001, 589, 1234–1245. [Google Scholar] [CrossRef]
- Pavan, R.; Jain, S.; Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef]
- Grover, A.K.; Samson, S.E. Benefits of antioxidant supplements for knee osteoarthritis: Rationale and reality. Nutr. J. 2015, 15, 1. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa ‘Hayward’and Actinidia eriantha ‘Bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef]
- Park, Y.S.; Im, M.H.; Ham, K.S.; Kang, S.G.; Park, Y.K.; Namiesnik, J.; Leontowicz, H.; Leontowicz, M.; Trakhtenberg, S.; Gorinstein, S. Quantitative assessment of the main antioxidant compounds, antioxidant activities and FTIR spectra from commonly consumed fruits, compared to standard kiwi fruit. LWT Food Sci. Technol. 2015, 63, 346–352. [Google Scholar] [CrossRef]
- Cheng, C.H.; Seal, A.G.; Boldingh, H.L.; Marsh, K.B.; MacRae, E.A.; Murphy, S.J.; Ferguson, A.R. Inheritance of taste characters and fruit size and number in a diploid Actinidia chinensis (kiwifruit) population. Euphytica 2004, 138, 185–195. [Google Scholar] [CrossRef]
- D’Evoli, L.; Moscatello, S.; Lucarini, M.; Aguzzi, A.; Gabrielli, P.; Proietti, S.; Battistelli, A.; Famiani, F.; Böhm, V.; Lombardi-Boccia, G. Nutritional traits and antioxidant capacity of kiwifruit (Actinidia deliciosa Planch., cv. Hayward) grown in Italy. J. Food Compost. Anal. 2015, 37, 25–29. [Google Scholar] [CrossRef]
- Deters, A.M.; Schröder, K.R.; Hensel, A. Kiwi fruit (Actinidia chinensis L.) polysaccharides exert stimulating effects on cell proliferation via enhanced growth factor receptors, energy production, and collagen synthesis of human keratinocytes, fibroblasts, and skin equivalents. J. Cell Physiol. Suppl. 2005, 202, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Gammon, C.S.; Beck, K.L.; Conlon, C.A.; Kruger, R. Kiwifruit: Our daily prescription for health. Can. J. Physiol. Pharmacol. 2013, 91, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Wójcik, E.; Szwajgier, D. Characteristics and pro-health properties of mini kiwi (Actinidia argute). Hortic. Environ. Biotechnol. 2019, 60, 217–225. [Google Scholar] [CrossRef]
- Martin, H. Proteinase activities of kiwifruit, pineapple and papaya using ovalbumin, soy protein, casein and bovine serum albumin as substrates. J. Food Nutr. Res. 2017, 5, 214–225. [Google Scholar]
- Ivanova, D.; Gerova, D.; Chervenkov, T.; Yankova, T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol. 2005, 96, 145–150. [Google Scholar] [CrossRef]
- Curcio, M.; Puoci, F.; Iemma, F.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J. Agric. Food Chem. 2009, 57, 5933–5938. [Google Scholar] [CrossRef]
- Pagliarulo, C.; De Vito, V.; Picariello, G.; Colicchio, R.; Pastore, G.; Salvatore, P.; Volpe, M.G. Inhibitory effect of pomegranate (Punica granatum L.) polyphenol extracts on the bacterial growth and survival of clinical isolates of pathogenic Staphylococcus aureus and Escherichia coli. Food Chem. 2016, 190, 824–831. [Google Scholar] [CrossRef]
- Dash, P.; Ghosh, G. Proteolytic and antioxidant activity of protein fractions of seeds of Cucurbita moschata. Food Biosci. 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Kim, S.P.; Nam, S.H.; Friedman, M. Mechanism of the antiadipogenic-antiobesity effects of a rice hull smoke extract in 3t3-l1 preadipocyte cells and in mice on a high-fat diet. Food Funct. 2015, 6, 2939–2948. [Google Scholar] [CrossRef] [PubMed]
- Schunck, M.; Zague, V.; Oesser, S.; Proksch, E. Dietary supplementation with specific collagen peptides has a body mass index-dependent beneficial effect on cellulite morphology. J. Med. Food. 2015, 18, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Wilczyński, S.; Koprowski, R.; Deda, A.; Janiczek, M.; Kuleczka, N.; Błońska-Fajfrowska, B. Thermographic mapping of the skin surface in biometric evaluation of cellulite treatment effectiveness. Skin Res. Technol. 2017, 231, 61–69. [Google Scholar] [CrossRef] [PubMed]
- David, R.B.; Paula, R.F.D.; Schneider, A.P. Lipodistrofia ginoide: Conceito, etiopatogenia e manejo nutricional. Rev. Bras. Nutr. Clin. 2011, 26, 202–206. [Google Scholar]
- Avram, M.M. Cellulite: A review of its physiology and treatment. J. Cosmet. Laser Ther. 2004, 6, 181–185. [Google Scholar] [CrossRef]
- Distante, F.; Bacci, P.A.; Carrera, M. Efficacy of a multifunctional plant complex in the treatment of the so-called ‘cellulite’: Clinical and instrumental evaluation. Int. J. Cosmet. Sci. 2006, 28, 191–206. [Google Scholar] [CrossRef]
- Errasti, M.E.; Prospitti, A.; Viana, C.A.; Gonzalez, M.M.; Ramos, M.V.; Rotelli, A.E.; Caffini, N.O. Effects on fibrinogen, fibrin, and blood coagulation of proteolytic extracts from fruits of Pseudananas macrodontes, Bromelia balansae, and B. hieronymi (Bromeliaceae) in comparison with bromelain. Blood Coagul. Fibrinolysis 2016, 274, 441–449. [Google Scholar] [CrossRef]
- Nakajima, V.M.; Madeira, J.V., Jr.; Macedo, G.A.; Macedo, J.A. Biotransformation effects on anti lipogenic activity of citrus extracts. Food Chem. 2016, 197, 1046–1053. [Google Scholar] [CrossRef]
- van der Heijden, R.A.; Morrison, M.C.; Sheedfar, F.; Mulder, P.; Schreurs, M.; Hommelberg, P.P.; Hofker, M.H.; Schalkwijk, C.; Kleemann, R.; Tietge, U.J.F.; et al. Effects of anthocyanin and flavanol compounds on lipid metabolism and adipose tissue associated systemic inflammation in diet-induced obesity. Mediat. Inflamm. 2016, 2016, 2042107. [Google Scholar] [CrossRef]
- Packianathan, N.; Kandasamy, R. Skin care with herbal exfoliants. Funct. Plant. Sci. Biotechnol. 2011, 5, 94–97. [Google Scholar]
| Description | Botanical Name | Plant Part | Composition |
|---|---|---|---|
| Black rice extract | Oryza sativa L. | Semen (Seed) | 35–45% |
| Kiwi dried powder | Actinidia chinensis Planch. | Fructus (Fruit) | 30–40% |
| Orange extract | Citrus sinensis (L.) Osbeck | Fructus (Fruit) | 20–30% |
| Pineapple extract enriched with Bromelain | Ananas comosus (L.) Merr | Stipites (Stem) | 1–5% |
| (a) Clinical Classification of the Thermographic Stage at T0 | Score | (b) Dermatological Classification of Orange Peel Skin at T0 | Score | |
|---|---|---|---|---|
| IV | Severe | 9 | Slight skin roughness which is not yet clearly visible as “orange peel” skin | 1 |
| Medium | 8 | |||
| Initial | 7 | Appearance of “orange peel” skin in a skin region | 2 | |
| III | Severe | 6 | ||
| Medium | 5 | Appearance of “orange peel” skin in several skin regions | 3 | |
| Initial | 4 | |||
| II | Severe | 3 | Skin appearance like “mattress” | 4 |
| Medium | 2 | |||
| Initial | 1 | (c) Scoring of Thermographic Stage and of Improvement of “Orange Peel” Skin | Score | |
| I | Normality | 0 | ||
| No variation | 1 | |||
| Slight improvement | 2 | |||
| Moderate improvement | 3 | |||
| Remarkable improvement | 4 | |||
| Active | Placebo | |
|---|---|---|
| Sex | ||
| Female | 30 | 30 |
| Age | 48.3 ± 1.3 | 47.0 ± 1.7 |
| Body mass index | 25.3 ± 0.4 | 25.6 ± 0.5 |
| Weight (kg) | 65.5 ± 1.4 | 65.6 ± 2.0 |
| Skin elasticity | ||
| R2 | 0.7902 ± 0.014 | 0.7934 ± 0.013 |
| R6 | 0.4347 ± 0.019 | 0.4270 ± 0.013 |
| R0 | 0.3055 ± 0.011 | 0.3202 ± 0.009 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobile, V.; Cestone, E.; Puoci, F.; Deponti, I.; Pisati, M.; Michelotti, A. In Vitro and In Vivo Study on Humans of Natural Compound Synergy as a Multifunctional Approach to Cellulite-Derived Skin Imperfections. Cosmetics 2020, 7, 48. https://doi.org/10.3390/cosmetics7020048
Nobile V, Cestone E, Puoci F, Deponti I, Pisati M, Michelotti A. In Vitro and In Vivo Study on Humans of Natural Compound Synergy as a Multifunctional Approach to Cellulite-Derived Skin Imperfections. Cosmetics. 2020; 7(2):48. https://doi.org/10.3390/cosmetics7020048
Chicago/Turabian StyleNobile, Vincenzo, Enza Cestone, Francesco Puoci, Ileana Deponti, Marta Pisati, and Angela Michelotti. 2020. "In Vitro and In Vivo Study on Humans of Natural Compound Synergy as a Multifunctional Approach to Cellulite-Derived Skin Imperfections" Cosmetics 7, no. 2: 48. https://doi.org/10.3390/cosmetics7020048
APA StyleNobile, V., Cestone, E., Puoci, F., Deponti, I., Pisati, M., & Michelotti, A. (2020). In Vitro and In Vivo Study on Humans of Natural Compound Synergy as a Multifunctional Approach to Cellulite-Derived Skin Imperfections. Cosmetics, 7(2), 48. https://doi.org/10.3390/cosmetics7020048







