Abstract
The current study aims to utilize the concept of the hydrophilic–lipophilic balance (HLB) value of ingredients for the development of a stable emulsion-based moisturizing cream and lotion for cosmetic application. The combination of a hydrophilic and lipophilic emulsifier such as glyceryl stearate (HLB value 3.8) and PEG-100 stearate (HLB value 18.8) were found to be effective to emulsify the chosen oil phase system at a specific concentration to achieve the required HLB for the development of the stable emulsion-based system. The developed formulation was characterized for pH, viscosity, spreadability, rheology, and droplet morphology. The influence of carbopol® ETD 2020 and the concentration of the oil phase on the rheology of the product was investigated and found to be significant to achieve the required thickening to convert the lotion into a cream. The formulation system developed through utilizing the concept of HLB was compared to a product developed through the conventional approach. It was observed that the utilization of the HLB method for the development of an emulsion-based product is a promising strategy compared to the conventional method. The physical stability and thermodynamic stability tests were carried out under different storage conditions. It was observed that the developed formulation was able to retain its integrity without showing any signs of instability during storage.
1. Introduction
The appearance of the skin and its function are well maintained by a significant balance between the water content and lipid content of the stratum corneum (SC) [1,2]. The balance can be disturbed by exposure to external stimuli such as humidity; ultraviolet radiation (UV); temperature; and endogenic factors, i.e., hormones [2,3]. Besides this, the regular application of soap; face wash; detergent; and topical irritants, including alcohol and hot water, can also strip lipids from the skin surface [2,4]. The disruption of this balance results in a dermatological condition known as dry skin, a condition observed mainly in patients with atopic dermatitis [2,5]. In these circumstances, suitable cosmetic products are used to improve skin hydration, preserve the skin’s natural condition, and avoid dryness of the skin. Moisturizers can improve skin dryness by maintaining water in the SC and creating an exogenous barrier to avoid transepidermal water loss (TEWL), which results in soft and moisturized skin [6,7]. Additionally, moisturizers help in treating skin dryness, ruggedness, and desquamation in non-treated psoriasis patients and in patients with acne who receive oral isotretinoin or topical tretinoin [6,8]. Moisturizers are also used to restore the skin barrier function in rosacea patients when used as an adjunctive therapy [9].
The hydration of the skin is restored by moisturizers through different mechanisms. For example, water from the aqueous phase may infiltrate the skin and contribute to an instant hydration effect. Additionally, humectants retain and attract large quantities of water to SC or the emollients from the lipid phase minimize the TEWL by filling the gaps between the desquamating corneocytes which helps to create smooth skin texture [6]. Additionally, emollients may interact with the intercellular skin lipids and restore the role of the skin as a barrier function and reduce the further loss of water. Emollient also shows occlusive properties to prevent water evaporation by creating a hydrophobic film over the skin [6]. Despite the important role of skin moisturizers to maintain the dignity and vitality of the skin to provide a healthy appearance, most of the available moisturizers are based on synthetic ingredients with known toxicity. Therefore, there is a considerable need to search for semi-synthetic/natural alternatives. In the vast majority, cosmetic preparations (lotion, cream, emulsion) are a biphasic semisolid formulation in which hydrophilic–lipophilic balance (HLB) plays a crucial role to balance the interfacial tension between the two immiscible liquids. A deep understanding of the HLB value helps to determine the required HLB (rHLB) which eventually helps to establish a stable biphasic emulsion formulation [10]. The HLB value permits the ability of the surfactant/emulsifier to stabilize the water-in-oil or oil-in-water emulsions. Surfactants with HLB values in the range of 4 to 8 usually stabilize water-in-oil emulsions (HLB number 4–6 results in the formation of poor emulsion; HLB number 6–8 results in the formation of a milky emulsion after vigorous agitation), while HLB values in the range of 8 to 18 stabilize oil-in-water emulsions (HLB number 8–10 results in the formation of a stable milky emulsion; HLB number 10–13 results in the formation of a translucent to clear emulsion; HLB number >13 results in the formation of a clear emulsion) [11]. The present investigation focuses on the formulation design of stable moisturizers, utilizing HLB concepts, their characterization, and the comparative evaluation of moisturizing cream with lotion.
2. Materials and Method
2.1. Materials
Cetyl alcohol, disodium EDTA, glycerin, propylene glycol, mineral oil, and sodium hydroxide were obtained from Sigma-Aldrich (Taufkirchen, Germany). Alpha-Tocopherol was obtained from BASF Lampertheim (Lampertheim, Germany). Almond oil was obtained from Gustav Heess (Leonberg, Germany). The acrylic acid copolymer was obtained from Ashland Specialty Ingredients (Wilmington, DE, USA). Carbopol ETD 2020 was obtained from Lubrizol India Private Limited (Mumbai, India). Glyceryl stearate and PEG-100 stearate were obtained from Croda India (Mumbai, India). Dimethicone and dimethiconol were obtained from Dow Corning (Midland, MI, USA). Phenoxyethanol and ethylhexylglycerin were obtained from Salicylate and Chemical Private Limited (Mumbai, India). Aloe vera extract was obtained from Acetar Bio-Tech Inc (Xian, China). All the ingredients were of cosmetic/pharmaceutical grade.
2.2. Preparation of Moisturizing Cream and Lotion
The emulsions were prepared following the design shown in Table 1 by adopting the reported method with a slight modification [12,13]. The aqueous phase and the oil phase was prepared separately, as per the scheme shown in Table 1. In a clean glass beaker, the required quantity of purified water was taken and heated up to 70 °C. Disodium EDTA was added into it under stirring until a clear solution was obtained. Carbopol ETD 2020 was added into a purified water solution under stirring until a lump-free dispersion was obtained. Glycerin, acrylic acid copolymer, propylene glycol, and Aloe vera extract were added into the aqueous dispersion system of carbopol under continuous stirring by maintaining the temperature of the aqueous phase to 70 °C. Similarly, for the preparation of the oil phase, mineral oil, glyceryl stearate, PEG-100 stearate, almond oil, and cetyl alcohol were taken into a clean glass beaker and heated all together up to 70 °C under continuous stirring until the clear phase was obtained. The aqueous phase and the oil phase were maintained at 70 °C with continuous stirring. The aqueous phase was poured into the oil phase under high-shear homogenization (using T 25 digital Ultra-Turrax, IKA-Werke, Staufen, Germany). The two phases were homogenized at 6500 rpm for 15 min at 70 °C and then cooled to room temperature. When the temperature of the developed emulsion system reached 50–55 °C, a post-emulsification material such as dimethicone, dimethiconol, alpha-tocopherol, phenoxyethanol and ethylhexylglycerin was added under continuous stirring and the mixture was cooled to room temperature. The initial pH of the developed moisturizing formulation was recorded and adjusted to a final pH of 5–6 with NaOH solution (1N).

Table 1.
Percentage composition of different formulations of moisturizing cream lotion.
2.3. Characterization of Moisturizing Cream and Lotion
2.3.1. Organoleptic Characteristics
The optimized formulations of the moisturizing cream and lotion were characterized for various organoleptic characteristics, such as physical appearance, color, texture, phase separation, and homogeneity, by visual observation. The samples were placed between the thumb and index finger and then the homogeneity and texture characteristics of the developed moisturizing formulationwere assessed.
2.3.2. Spreadability
The spreadability of the optimized moisturizer formulation was determined by keeping a small amount of the sample between two glass plates [14]. Further, weight was added gradually to the upper glass plate with time intervals of 1 min. The spreading diameter of the tested sample was measured each time after the addition of weight. The results are expressed in terms of the spreading area as a function of the applied weight [14,15,16].
2.3.3. pH
Accurately weighed quantities of the moisturizing cream and lotion were dissolved with a known quantity of deionized water to make a 10% w/v dispersion system. The pH of this 10% w/v dispersion of moisturizing cream and lotion was determined using a digital pH meter (Mettler-Toledo Ingold Inc., Billerica, MA, USA). A pH measurement of the sample was performed in triplicate, and the average value was reported [14].
2.3.4. Rheology
A Brookfield RS/-CPS rheometer (cone plate model, instrument type RS3CPS115LS) was used with a spindle C50-1 to determine the viscosity of each formulation (cream and lotion), exploiting the software Rheo 3000 V1.2 (2008) for the analysis of the result. The tests were carried out at room temperature (25 °C). All the measurements were made in triplicate and the average value was reported.
2.3.5. Droplet Morphology
The optimized formulation of the moisturizing cream and lotion was assessed for the droplet morphology using a polarized microscope (Nikon Instruments Inc., Melville, NY, USA) after appropriate dilution with distilled water.
2.4. Stability Study
The optimized formulations of the moisturizing cream and lotion were evaluated for their thermodynamic [17,18] and physical stability.
2.4.1. Thermodynamic Stability
The optimized formulations of the moisturizing cream and lotion were characterized for the heating-cooling cycle (3 alternate cycles at 45 °C and 4 °C for 48 h) and observed for any sign of instability (such as change in color, precipitation, and phase separation) [17,18]. Furthermore, samples of moisturizing cream and lotion were also subjected to a centrifugation cycle (3500 rpm for 30 min) and freeze-thaw cycle (3 alternate cycles at −21 °C and 25 °C for 24 h) to observe the chances of phase separation and change in appearance under stress conditions [19].
2.4.2. Physical Stability
Assessments of the physical stability of the optimized moisturizing cream and lotion were performed at different storage conditions (40 ± 2 °C/75% ± 5% RH and 25 ± 2 °C/60% ± 5% RH for 30 days) to observe the overall stability of the developed formulation system. The parameters observed to assess the overall system stability were change in pH, color, odor, and consistency [20,21].
3. Results and Discussion
3.1. Preparation of Moisturizing Cream and Lotion
All the ingredients chosen for the formulation development were of cosmeceutical/pharmaceutical acceptable grade. Disodium EDTA was used as a chelating agent. Glycerine and propylene glycol were used as humectants. Acrylic acid copolymer, carbopol ETD 2020, and cetyl alcohol were added as thickening agents. Aloe vera extract, alpha-tocopherol, and almond oil were used as skin conditioning agents. Additionally, dimethicone and dimethiconol were used as emollient/skin conditioning agents. Mineral oil was used as an occlusive, while glyceryl stearate and PEG-100 stearate were used as emulsifiers. Phenoxyethanol and ethylhexylglycerin were added as preservatives, and sodium hydroxide solution (1N) was used as a pH adjuster. It was found that the formulations F1, F2, and F3, containing the ingredients at different proportions (shown in Table 1), were not able to form a stable emulsion system after the homogenization of the oil and aqueous phase, as shown in Figure 1. This is because the HLB contribution from the emulsifier in formulations F1, F2, and F3 was less than the rHLB of the oil phase (Table 2). To design an emulsion-based stable formulation, we utilized the concept of rHLB for the complete and uniform homogenization of the oil and aqueous phase of the formulation components.
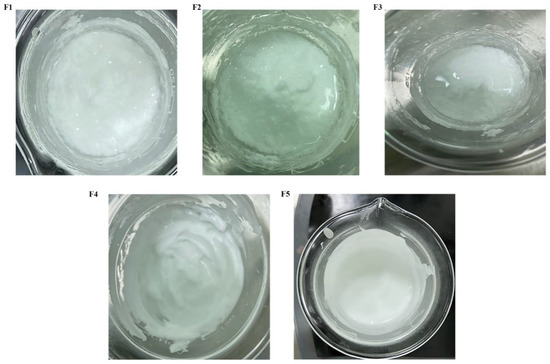
Figure 1.
Appearance and various types of instability observed in different formulations. (F1) congealing and creaming were observed, (F2) formation of lumps and phase separation were observed, (F3) oil separation was observed, (F4) stable moisturizing cream, and (F5) stable moisturizing lotion.

Table 2.
HLB contribution by the oil phase of different formulations to determine the rHLB of the emulsifier.
Utilization of rHLB Concept for Development of Emulsion-Based Stable Formulation
For the formulation design of an emulsion-based stable cream or lotion, a formulation system with different proportions of oil and water phases needed a suitable emulsifier with an rHLB value for the complete emulsification of two immiscible phases [22,23]. The HLB value is not just an essential parameter for the selection of an emulsifier but also an important step in the formulation of the stable emulsion-based product. It can be seen as a significant factor in determining the quality control of the product [24,25,26,27,28]. Therefore, the HLB of each ingredient needs to be known before proceeding with formulation development. Additionally, topical cosmetic products must take into account the HLB value of the finished product for the moisture and oil equilibrium of the skin. Therefore, the formulation’s system must impart the appropriate HLB value for skin conditions. Additionally, emulsion-based topical formulations, specifically where synthetic surfactants are used, with a combination of emulsifiers with rHLB values equal to the oil phase give a stable system [29].
The HLB value of the oil and emulsifier was determined according to the reported method with slight modifications [12,13,30]. The HLB three-step system was followed for the preparation of a stable formulation vis-a-vis(a) the determination of the HLB requirement of oil, (b) the determination of the most effective emulsifier (HLB value), and (c) the determination of the emulsifier concentration required to achieve the desired stability/rheology. The rHLB for the oil phase is the sum of the HLB in combination with an emulsifier, as shown in Table 2.
To determine the rHLB of two emulsifier combinations in the development of the moisturizing cream and lotion, they were calculated by the reported method [12,13,30]. The quantity of emulsifier (A) required to blend with any other emulsifier (B) to reach the rHLB was calculated as follows [23,30]:
Finally, the quantity of two emulsifiers (glyceryl stearate and PEG-100 stearate) needed to achieve the rHLB for the oil phase was calculated (as shown in Table 3) to obtain a stable system of moisturizing cream and lotion.

Table 3.
The hydrophilic–lipophilic balance (HLB) contribution by individual emulsifiers of different formulations to achieve the required hydrophilic–lipophilic balance (rHLB).
It was found that the overall HLB value contributed by the oil phase of formulations F1, F2, and F3 was 12.0, 11.11, and 10.58, respectively (Table 2). However, the overall HLB value contributed by the emulsifier combinations of formulation F1, F2, and F3 was 10.78, 9.8, and 9.24, respectively (Table 3). This difference in the contributed HLB of the oil phase and rHLB achieved by the emulsifier combination is the reason for the instability observed in the case formulations F1, F2, and F3. However, the overall HLB contributed by the oil phase of formulations F4 and F5 was 11.32 (Table 2), and the rHLB achieved by the emulsifier combination in the case of F4 and F5 was 11.32 (Table 3). The stability of the formulations F4 and F5 compared to F1, F2, and F3 is due tothe fact that the HLB contribution by the oil phase is equal to the rHLB achieved by the emulsifier combination.
3.2. Characterization of Optimized Moisturizing Cream and Lotion
3.2.1. Organoleptic Characteristics
Organoleptic properties play a crucial role in cosmetics due to their ability to improve consumer compliance by elevating the elegance and aesthetics of a formulation. The results of the various organoleptic properties of the optimized moisturizing cream and lotion are shown in Table 4. It was found that both of the formulations (F4 and F5) had a cosmetically appealing appearance, smooth texture, and homogenous dispersion, with no signs of phase separation.

Table 4.
Organoleptic characteristics of the optimized moisturizing cream (F4) and lotion (F5).
3.2.2. Spreadability
The spreadability of semi-solid cosmetics—i.e., the ability of a cream or gel to spread uniformly on the skin—plays a significant role in the effectiveness of the topical application. The efficacy of moisturizing cream and lotion depends on their ability to spread over the skin easily. The spreading behavior of the semi-solid preparation helps to apply the product to the skin evenly. Therefore, the developed formulation must have good spreadability and meet the ideal reliability in topical applications [31]. Additionally, this is considered a significant element of the marketing.
The cosmetic formulations in the form of cream and lotion with plastic or pseudo-plastic property are thick under static conditions and get thinner after the application of shearstress. The resulting behavior is helpful in the easy spreadability of the formulation over the skin. The spreadability investigation of the moisturizing cream and lotion revealed that an increase in applied force in the form of weight enhances the spreading area of the developed moisturizing cream and lotion (illustrated in Figure 2). The observation of the results confirmed that the developed moisturizing cream and lotion have optimum spreadability behavior and are found to be suitable for topical application.
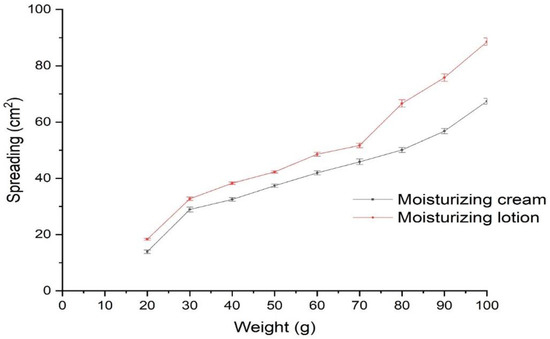
Figure 2.
Spreadability behavior of the moisturizing cream (F4) and lotion (F5).
3.2.3. pH
The initial pH of the cream and lotion has been recorded. The pH of normal skin lies in the range of 5.0–6.0 [14]. The pH of the developed formulation just after the preparation was found to be more acidic than that of the pH of normal skin. The pH of the developed moisturizing cream and lotion has been adjusted by adding NaOH solution (1N) drop by drop under continuous mixing. The final pHs of the moisturizing cream (F4) and lotion (F5) were found to be 5.20 ± 0.025 and 5.25 ± 0.03, respectively. It seems that the developed moisturizer does not cause skin irritation.
3.2.4. Viscosity
The consistency of the semisolid preparation is one of the key attributes of topical formulations, as it is applied to the skin as a thin layer. Usually, the consistency of any semisolid formulation is indicated by its viscosity [32]. It was observed that the viscosity of the cream and lotion was inversely related to the shear stress. The viscosity decreases with an increase in shear stress, indicating the non-Newtonian behavior of flow; this behavior is favored because of its low resistance to flow when applied under high shear conditions [30,33,34]. The viscosity of the developed cream and lotion formulation was determined by a Brookfield rotational rheometer (Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA) of the cone and plate type using spindle C50-1. The viscosity of the moisturizing cream and lotion formulations was found to be 8427.52 ± 7.36 Pa·s and 312.26 ± 1.68 Pa·s at a shear stress of 50.56 Pa and 5.62 Pa and a shear rate of 0.01 s−1 and 0.02 s−1, respectively. The results of the rheological behavior of the moisturizing cream and lotion are shown in Figure 3. Figure 3 illustrates that the rheological behavior of the moisturizing cream exhibits a pseudo-plastic flow, while the moisturizing lotion exhibits a Newtonian flow.
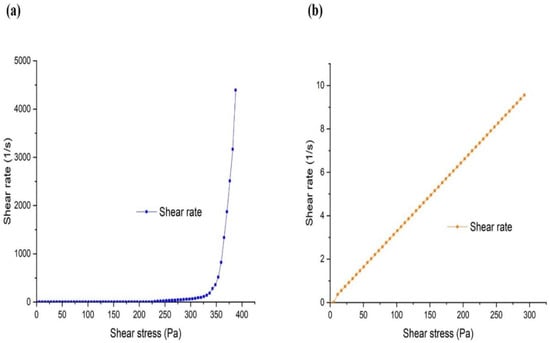
Figure 3.
Rheological behavior of (a) the moisturizing cream (F4) and (b) the lotion (F5).
3.2.5. Droplet Morphology
The droplet morphology of the optimized formulation of the moisturizing cream and lotion was found to be spherical under polarized microscopy (Figure 4).
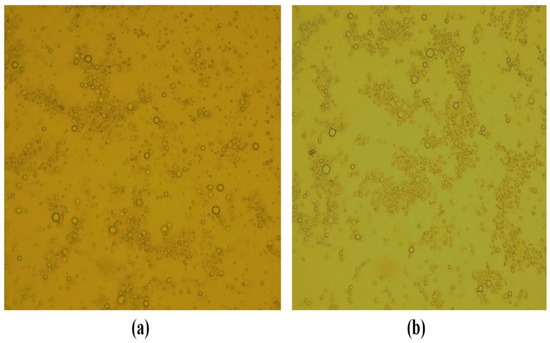
Figure 4.
Morphological analysis of (a) the moisturizing cream (F4) and (b) the lotion (F5) under a polarized microscope.
3.3. Stability Study
The majority of cosmetic and pharmaceutical creams comprises of water-in-oil (w/o) or oil-in-water (o/w) type emulsions. They are thermodynamically unstable and typically break into two separate phases [35]. This instability could be reflected at different times and through a variety of destabilizing physicochemical processes—for example, phase inversion, creaming, coalescence, and flocculation [36].
3.3.1. Thermodynamic Stability
The thermodynamically stable formulation system is in its state of lowest energy or in chemical equilibrium with its environment, which ultimately helps to detect the presence of metastable formulations. The optimized formulation of the cream and lotion based on the HLB system study were subjected to stress tests, including heating-cooling and freeze-thaw cycles, as well as a centrifugation cycle. The optimized moisturizing cream and lotion did not exhibit any sign of instability (such as precipitation, phase separation, and creaming) under the given stress conditions. Furthermore, no cracking and coalescence were found during the thermodynamic stability tests.
3.3.2. Physical Stability
To make the stability test expressive, it is necessary to determine the variables that will be evaluated in order to identify the acceptance requirements and/or methods that can quantify the variations in attributes over time. Physical stability tests were carried out under different storage conditions (40 ± 2 °C/75% ± 5% RH and 25 ± 2 °C/60% ± 5% RH for 30 days). All the observations were recorded at different timeintervals (0, 1, 2, 3, and 4 weeks). The parameters observed for physical stability were the visual appearance (color, odor, and consistency), homogeneity, and phase behavior. These parameters must also take into account the key product characteristics, as these features remain unchanged or alteredwithout compromising the product efficiency and presentation [37,38]. The stability study demonstrated that both the moisturizing cream and lotion were able to retain their integrity without showing any signs of instability (Table 5).

Table 5.
Physical stability of the optimized moisturizing cream and lotion.
4. Conclusions
In this investigation, the rHLB for the oil phase used was observed to be between 10.5 and 12. However, the emulsifier blends used in the formulations F1-F3 were incapable of achieving the rHLB and ultimately resulted in the instability of the formulation system. The rHLB concept was applied to calculate the required quantity of the emulsifier blend for the successful development of stable cream and lotion. Thus, our study demonstrated the successful development of an emulsion-based moisturizing cream and lotion (F4 and F5) using biocompatible ingredients such as Aloe vera extract, glycerin, carbopol ETD 2020, dimethicone, almond oil, and other excipients as emollients, humectants and skin conditioning agents. The developed moisturizing cream has favorable physicochemical properties for dermal use, exhibiting pseudo-plastic flow behavior due to a balanced o/w cream base. The vehicle used in the optimized formulation may, therefore, be a promising tool for developing an efficient topical delivery system, in particular for treating skin dryness and/or skin disorders such as atopic dermatitis.
Author Contributions
Conceptualization, M.S.A. and J.A.; Methodology, M.S.A. and J.A.; Software, M.Z.A. and J.A.; Validation, M.S.A. and J.A.; Formal Analysis, S.A., M.S.A., M.Z.A. and J.A.; Investigation, S.A., M.S.A., M.Z.A. and J.A.; Resources, M.S.A. and J.A.; Data Curation, S.A., M.S.A., M.Z.A. and J.A.; Writing—Original Draft Preparation, M.S.A., M.Z.A. and J.A.; Writing—Review and Editing, M.S.A., M.Z.A. and J.A.; Visualization, M.S.A., M.Z.A. and J.A.; Supervision, J.A.; Project Administration, M.S.A. and J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rawlings, A.V.; Harding, C.R. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17, 43–48. [Google Scholar] [CrossRef]
- Dal’Belo, S.E.; Gaspar, L.R. Moisturizing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Ski. Res. Technol. 2006, 12, 241–246. [Google Scholar] [CrossRef]
- Flynn, T.C.; Petros, J.; E Clark, R.; E Viehman, G. Dry skin and moisturizers. Clin. Dermatol. 2001, 19, 387–392. [Google Scholar] [CrossRef]
- Yosipovitch, G. Dry skin and impairment of barrier function associated with itch—New insights. Int. J. Cosmet. Sci. 2004, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sator, P.-G.; Schmidt, J.B.; Hönigsmann, H. Comparison of epidermal hydration and skin surface lipids in healthy individuals and in patients with atopic dermatitis. J. Am. Acad. Dermatol. 2003, 48, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.; Sidou, F.; Brocard, S. Effect of a new moisturizing lotion on immediate and cumulative skin hydration: Two randomized, intra-individual, vehicle- and comparator-controlled studies. J. Dermatol. Treat. 2010, 22, 221–225. [Google Scholar] [CrossRef]
- Lodén, M. The clinical benefit of moisturizers. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 672–688. [Google Scholar] [CrossRef] [PubMed]
- Laquieze, S.; Czernielewski, J.; Rueda, M.-J. Beneficial effect of a moisturizing cream as adjunctive treatment to oral isotretinoin or topical tretinoin in the management of acne. J. Drugs Dermatol. 2007, 5, 985–990. [Google Scholar]
- Laquieze, S.; Czernielewski, J.; Baltás, E. Beneficial use of Cetaphil® Moisturizing Cream as part of a daily skin care regimen for individuals with rosacea. J. Dermatol. Treat. 2007, 18, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Meher, J.G.; Yadav, N.P.; Sahu, J.J.; Sinha, P. Determination of required hydrophilic–lipophilic balance of citronella oil and development of stable cream formulation. Drug Dev. Ind. Pharm. 2012, 39, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Somasundaran, P. Handbook for Cleaning/Decontamination of Surfaces; Elsevier Science BV: Amsterdam, The Netherlands, 2007; pp. 103–123. [Google Scholar]
- Ferreira, M.R.A.; Santiago, R.R.; De Souza, T.P.; Egito, E.S.T.; Oliveira, E.E.; Soares, L.A.L. Development and Evaluation of Emulsions from Carapaguianensis (Andiroba) Oil. AAPS PharmSciTech 2010, 11, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Saengsorn, K.; Jimtaisong, A. Determination of hydrophilic–lipophilic balance value and emulsion properties of sachainchi oil. Asian Pac. J. Trop. Biomed. 2017, 7, 1092–1096. [Google Scholar] [CrossRef]
- Chen, M.X.; Alexander, K.S.; Baki, G. Formulation and Evaluation of Antibacterial Creams and Gels Containing Metal Ions for Topical Application. J. Pharm. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shankar, N.B.; Kumar, R.P.; Kumar, N.U.; Brata, B.B. Development and Characterization of Bioadhesive Gel of Microencapsulated Metronidazole for Vaginal Use. Iran. J. Pharm. Res. 2010, 9, 209–219. [Google Scholar]
- Mutimer, M.N.; Riffkin, C.; Hill, J.A.; Glickman, M.E.; Cyr, G.N. Modern ointment base technology. II. Comparative evaluation of bases. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1956, 45, 212–218. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Mohammad, Z.A.; Javed, A. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterial 2020, 10, 848. [Google Scholar]
- Akhter, S.; Anwar, M.; Siddiqui, M.A.; Ahmad, I.; Ahmad, J.; Ahmad, M.Z.; Bhatnagar, A.; Ahmad, F.J. Improving the topical ocular pharmacokinetics of an immunosuppressant agent with mucoadhesivenanoemulsions: Formulation development, in-vitro and in-vivo studies. Colloids Surf. Biointerfaces 2016, 148, 19–29. [Google Scholar] [CrossRef]
- Hoopfer, D.; Holloway, C.; Gabos, Z.; Alidrisi, M.; Chafe, S.; Krause, B.; Lees, A.; Mehta, N.; Tankel, K.; Strickland, F.; et al. Three-Arm Randomized Phase III Trial: Quality Aloe and Placebo Cream Versus Powder as Skin Treatment During Breast Cancer Radiation Therapy. Clin. Breast Cancer 2015, 15, 181–190.e4. [Google Scholar] [CrossRef]
- ICH. Available online: https://www.ich.org/page/quality-guidelines (accessed on 27 April 2020).
- James, K.C.; Leach, R.H. A stability study of chloramphenicol in topical formulations*. J. Pharm. Pharmacol. 1970, 22, 607–611. [Google Scholar] [CrossRef]
- Pasquali, R.C.; Taurozzi, M.P.; Bregni, C. Some considerations about the hydrophilic–lipophilic balance system. Int. J. Pharm. 2008, 356, 44–51. [Google Scholar] [CrossRef]
- The HLB System—A Time Saving Guide to Emulsifier Selection. Available online: https://www.formulation.org.uk/images/stories/Archive/ICI/1980ICI_Surfactants_-_The_HLB_system_-_A_time_saving_guide_to_emulsfier_selection.pdf (accessed on 20 April 2020).
- Fernandes, C.P.; Mascarenhas, M.P.; Zibetti, F.M.; Lima, B.G.; Oliveira, R.P.; Rocha, L.; Falcão, D.Q. HLB value, an important parameter for the development of essential oil phytopharmaceuticals. Rev. Bras. Farm. 2013, 23, 108–114. [Google Scholar] [CrossRef]
- Yadav, N.P.; Meher, J.G.; Pandey, N.; Luqman, S.; Yadav, K.S.; Chanda, D. Enrichment, Development, and Assessment of Indian Basil Oil Based Antiseptic Cream Formulation Utilizing Hydrophilic-Lipophilic Balance Approach. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, T.; Dobler, D.; Schlupp, P.; Nissing, C.; Garn, H.; Runkel, F. Development of multiple W/O/W emulsions as dermal carrier system for oligonucleotides: Effect of additives on emulsion stability. Int. J. Pharm. 2010, 398, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.C. Classification of surface-active agents by HLB. J. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Griffin, W.C. Calculation of HLB values of non-ionic surfactants. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- Orafidiya, L.O.; Oladimeji, F. Determination of the required HLB values of some essential oils. Int. J. Pharm. 2002, 237, 241–249. [Google Scholar] [CrossRef]
- Sinko, P.J.; Singh, Y. Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences; Walter Kluer: Alphen aan den Rijn, The Netherlands, 2011; pp. 472–477. [Google Scholar]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Júnior, L.J.; Almeida, J.R.G.D.S. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Shinde, U.; Pokharkar, S.; Modani, S. Design and Evaluation of Microemulsion Gel System of Nadifloxacin. Indian J. Pharm. Sci. 2012, 74, 237–247. [Google Scholar] [CrossRef]
- Bousmina, M. Rheology of polymer blends: Linear model for viscoelastic emulsions. Rheol. Acta 1999, 38, 73–83. [Google Scholar] [CrossRef]
- Bousmina, M.; Aouina, M.; Chaudhry, B.; Guénette, R.; Bretas, R.E.S. Rheology of polymer blends: Non-linear model for viscoelastic emulsions undergoing high deformation flows. Rheol. Acta 2001, 40, 538–551. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, B.; Khan, M.; Mahmood, T.; Khan, H.; Iqbal, M.; Bashir, S. Formulation development and moiturising effects of a topical cream of Aloe vera extract. World Acad. Sci. Eng. Technol. 2011, 51, 172–179. [Google Scholar]
- Masmoudi, H.; Le Dréau, Y.; Piccerelle, P.; Kister, J. The evaluation of cosmetic and pharmaceutical emulsions aging process using classical techniques and a new method: FTIR. Int. J. Pharm. 2005, 289, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Ueoka, A.R.; Moraes, C.P. Development and Stability Evaluation of Liquid Crystal-Based Formulations Containing Glycolic Plant Extracts and Nano-Actives. Cosmetics 2018, 5, 25. [Google Scholar] [CrossRef]
- Vieira, R.; Fernandes, A.R.; Kaneko, T.M.; Consiglieri, V.; Pinto, C.A.S.D.O.; Pereira, C.S.C.; Baby, A.R.; Velasco, M.V.R. Physical and physicochemical stability evaluation of cosmetic formulations containing soybean extract fermented by Bifidobacteriumanimalis. Braz. J. Pharm. Sci. 2009, 45, 515–525. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).