Protective Effects of Salicornia europaea on UVB-Induced Misoriented Cell Divisions in Skin Epithelium
Abstract
1. Introduction
2. Materials and Methods
2.1. S. europaea Extract
2.2. Subjects
2.3. S. europaea Treatment
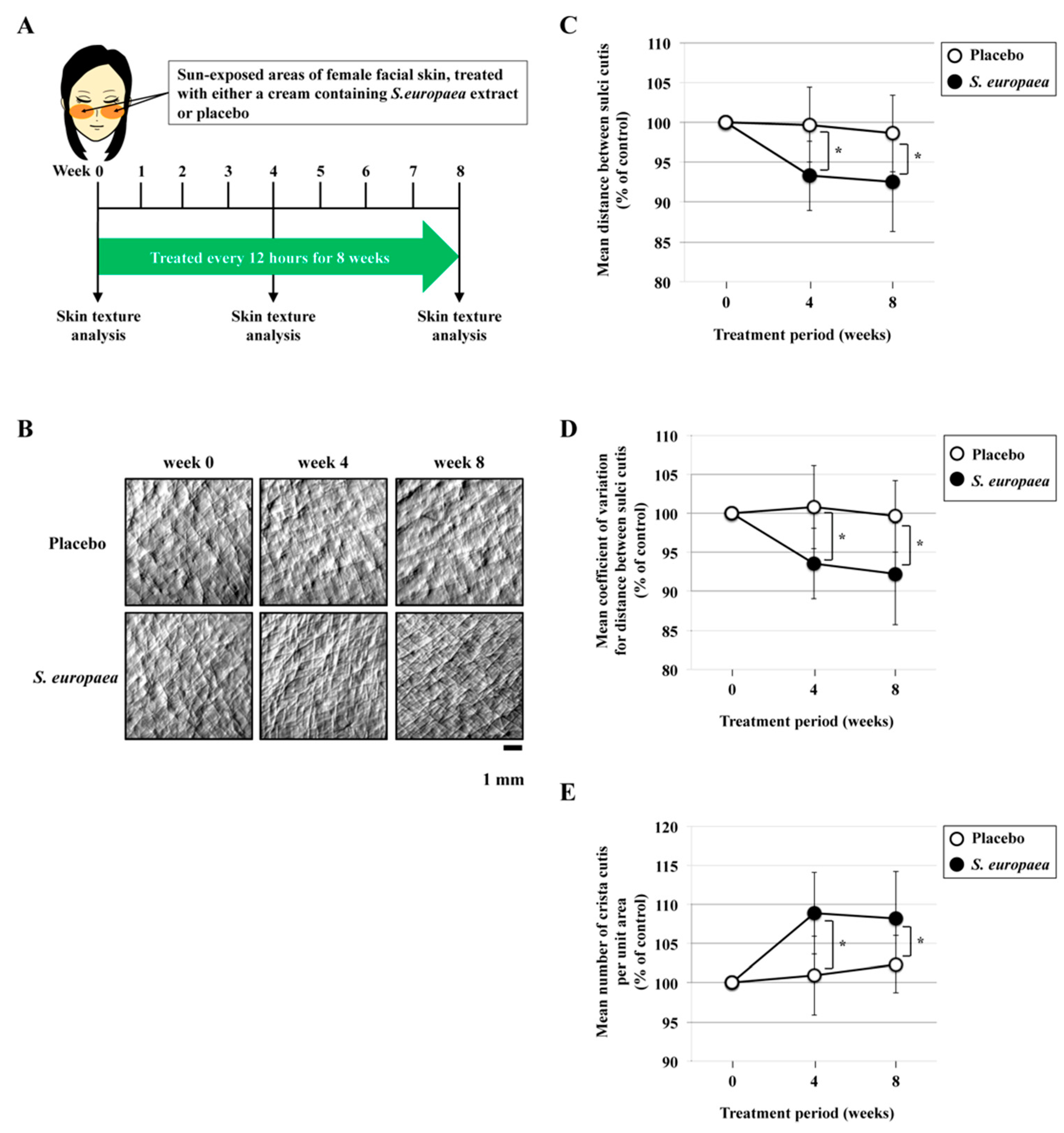
2.4. Skin Texture
2.5. Analyses with a Multilayered, Highly Differentiated In Vitro Skin Model
2.6. Statistics
3. Results
3.1. Effects of S. europaea on Skin Texture in Sun-Exposed Areas
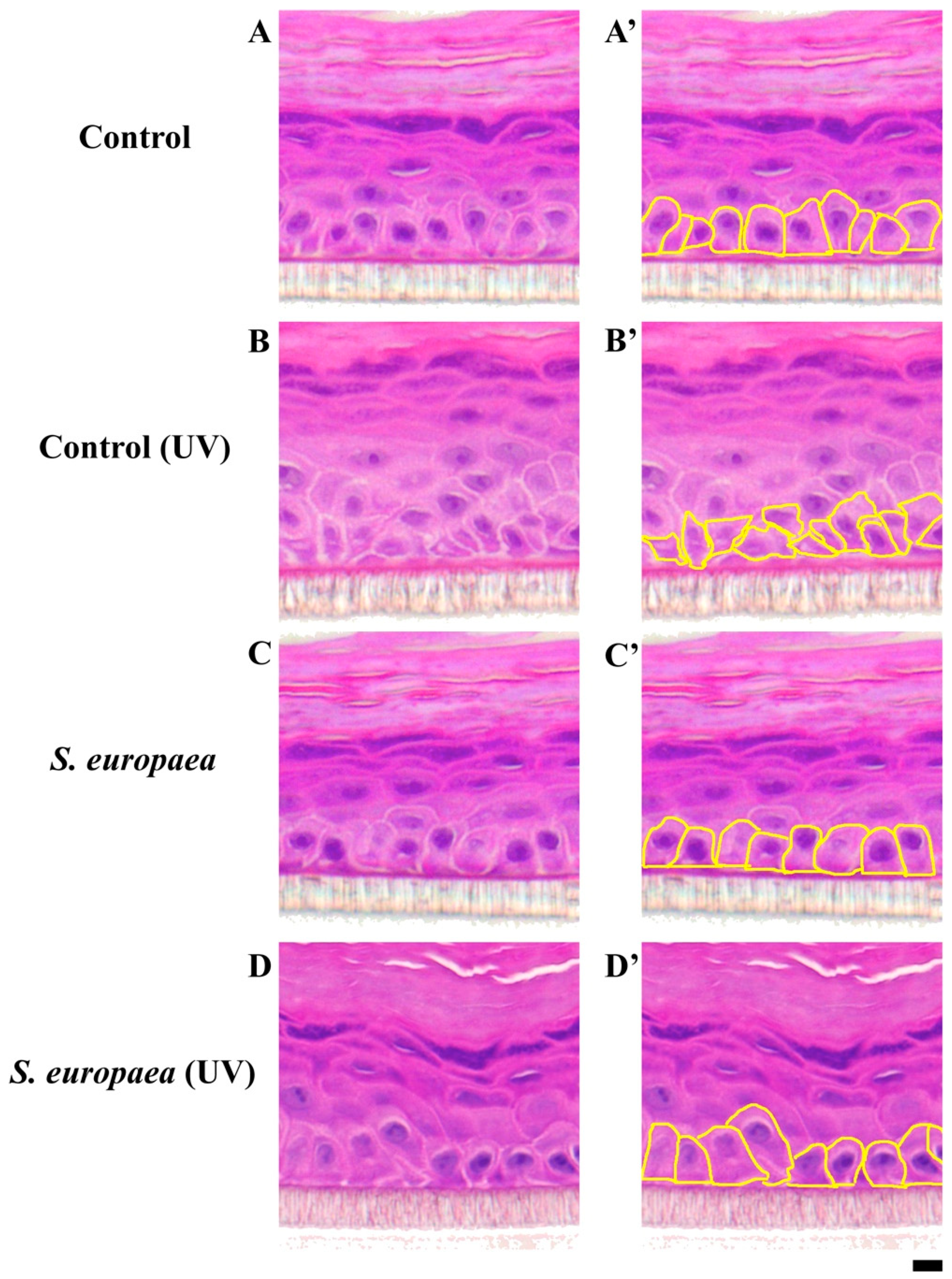
3.2. Effects of S. europaea on the Morphology and Arrangement of Epidermal Cells Located in the Basal Layer Exposed to UVB
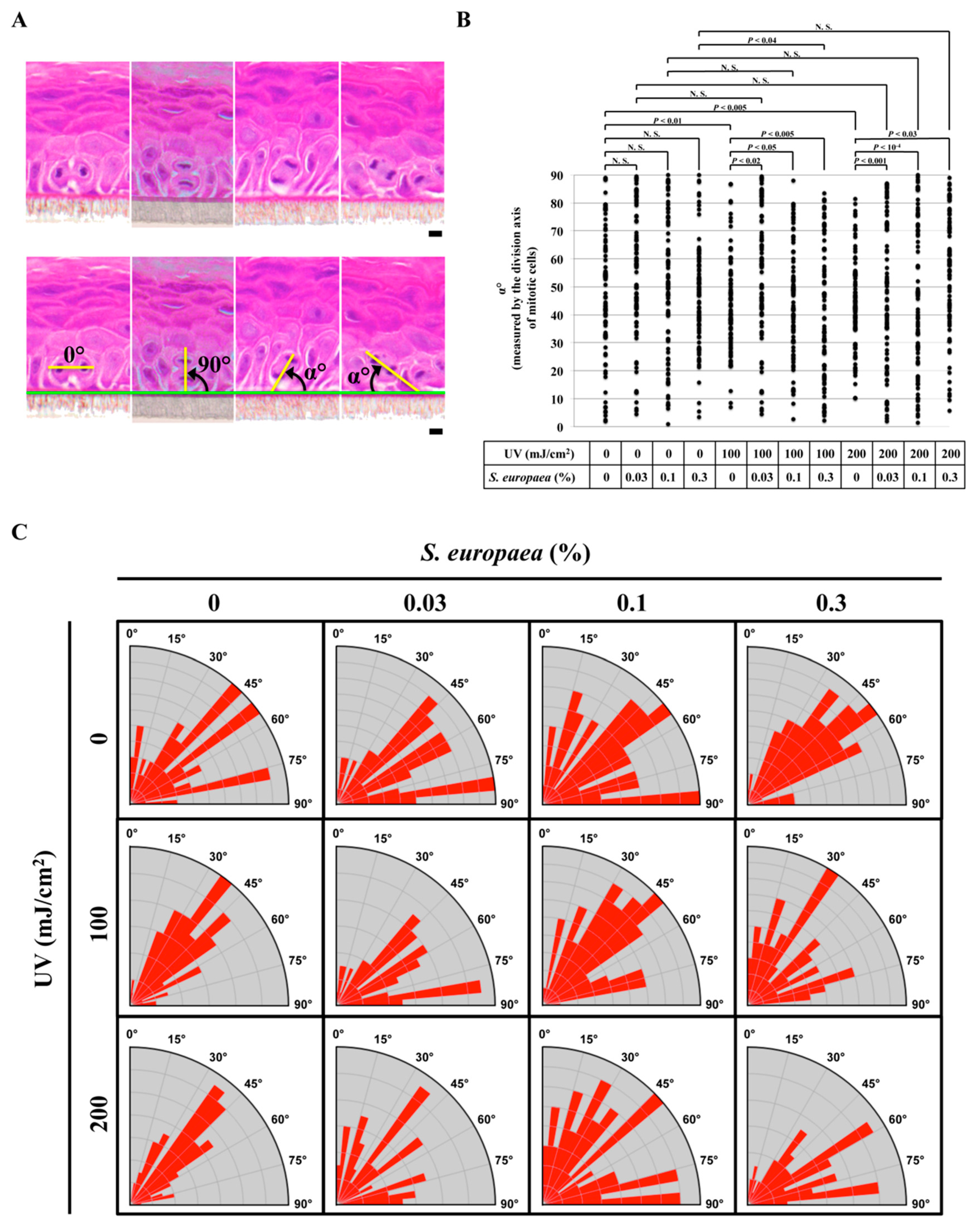
3.3. Effects of S. europaea on the Oriented Cell Division of Basal Keratinocytes Post UVB Exposure
3.4. Effects of S. europaea on Stratification during Skin Differentiation after UVB Eexposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Lippens, S.; Denecker, G.; Ovaere, P.; Vandenabeele, P.; Declercq, W. Death penalty for keratinocytes: Apoptosis versus cornification. Cell Death Differ. 2005, 12 (Suppl. 2), 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Lippens, S.; Hoste, E.; Vandenabeele, P.; Agostinis, P.; Declercq, W. Cell death in the skin. Apoptosis Int. J. Program. Cell Death 2009, 14, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef]
- Lechler, T.; Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 2005, 437, 275–280. [Google Scholar] [CrossRef]
- Yang, S.; Ma, K.; Geng, Z.; Sun, X.; Fu, X. Oriented cell division: New roles in guiding skin wound repair and regeneration. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef]
- Matsumura, S.; Hamasaki, M.; Yamamoto, T.; Ebisuya, M.; Sato, M.; Nishida, E.; Toyoshima, F. ABL1 regulates spindle orientation in adherent cells and mammalian skin. Nat. Commun. 2012, 3, 626. [Google Scholar] [CrossRef]
- Seldin, L.; Muroyama, A.; Lechler, T. NuMA-microtubule interactions are critical for spindle orientation and the morphogenesis of diverse epidermal structures. Elife 2016, 5. [Google Scholar] [CrossRef]
- Morrow, A.; Underwood, J.; Seldin, L.; Hinnant, T.; Lechler, T. Regulated spindle orientation buffers tissue growth in the epidermis. Elife 2019, 8. [Google Scholar] [CrossRef]
- Xie, W.; Zhou, J. Regulation of mitotic spindle orientation during epidermal stratification. J. Cell. Physiol. 2017, 232, 1634–1639. [Google Scholar] [CrossRef]
- Clydesdale, G.J.; Dandie, G.W.; Muller, H.K. Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol. Cell Biol. 2001, 79, 547–568. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Miyamura, Y.; Coelho, S.G.; Schlenz, K.; Batzer, J.; Smuda, C.; Choi, W.; Brenner, M.; Passeron, T.; Zhang, G.; Kolbe, L.; et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment. Cell Melanoma Res. 2011, 24, 136–147. [Google Scholar] [CrossRef]
- Tran, T.T.; Schulman, J.; Fisher, D.E. UV and pigmentation: Molecular mechanisms and social controversies. Pigment. Cell Melanoma Res. 2008, 21, 509–516. [Google Scholar] [CrossRef]
- Chen, H.; Weng, Q.Y.; Fisher, D.E. UV signaling pathways within the skin. J. Investig. Dermatol. 2014, 134, 2080–2085. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Dupont, E.; Gomez, J.; Bilodeau, D. Beyond UV radiation: A skin under challenge. Int. J. Cosmet. Sci. 2013, 35, 224–232. [Google Scholar] [CrossRef]
- Natarajan, V.T.; Ganju, P.; Ramkumar, A.; Grover, R.; Gokhale, R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Biol. 2014, 10, 542–551. [Google Scholar] [CrossRef]
- Abreu Velez, A.M.; Howard, M.S. Tumor-suppressor Genes, Cell Cycle Regulatory Checkpoints, and the Skin. N. Am. J. Med. Sci. 2015, 7, 176–188. [Google Scholar] [CrossRef]
- Gandarillas, A. The mysterious human epidermal cell cycle, or an oncogene-induced differentiation checkpoint. Cell Cycle 2012, 11, 4507–4516. [Google Scholar] [CrossRef] [PubMed]
- Doi, N.; Kunimatsu, Y.; Fujiura, K.; Togari, H.; Minagi, K.; Nakaoji, K.; Hamada, K.; Temme, A.; Tatsuka, M. RhoGDIbeta affects HeLa cell spindle orientation following UVC irradiation. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Lee, S.K.; Jo, J.R.; Kim, M.E.; So, H.A.; Cho, C.W.; Seo, Y.W.; Kim, J.I. Hypolipidemic effect of Salicornia herbacea in animal model of type 2 diabetes mellitus. Nutr. Res. Pract. 2007, 1, 371–375. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kong, C.S.; Um, Y.R.; Lim, S.Y.; Yea, S.S.; Seo, Y. Evaluation of Salicornia herbacea as a potential antioxidant and anti-inflammatory agent. J. Med. Food 2009, 12, 661–668. [Google Scholar] [CrossRef]
- Sung, J.H.; Park, S.H.; Seo, D.H.; Lee, J.H.; Hong, S.W.; Hong, S.S. Antioxidative and skin-whitening effect of an aqueous extract of Salicornia herbacea. Biosci. Biotechnol. Biochem. 2009, 73, 552–556. [Google Scholar] [CrossRef]
- Oe, M.; Sakai, S.; Yoshida, H.; Okado, N.; Kaneda, H.; Masuda, Y.; Urushibata, O. Oral hyaluronan relieves wrinkles: A double-blinded, placebo-controlled study over a 12-week period. Clin. Cosmet. Investig. Dermatol. 2017, 10, 267–273. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef]
- Clark, A.; Hessler, J.L. Skin Care. Facial Plast. Surg. Clin. N. Am. 2015, 23, 285–295. [Google Scholar] [CrossRef]
- Kaur, A.; Thatai, P.; Sapra, B. Need of UV protection and evaluation of efficacy of sunscreens. J. Cosmet. Sci. 2014, 65, 315–345. [Google Scholar] [PubMed]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.D.; Yoon, D.Y.; Lee, S.; Han, S.B.; Kim, Y. Antimelanogenic chemicals with in vivo efficacy against skin pigmentation in guinea pigs. Arch. Pharm. Res. 2014, 37, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Morpurgo, G.; Catacuzzeno, L.; Peruzzi, S.; Blasi, P.; Fioretti, B. Are tyrosinase inhibitors in sunscreens and cosmetics enhancing UV carcinogenicity? Exp. Dermatol. 2015, 24, 546–547. [Google Scholar] [CrossRef]
- Desmedt, B.; Courselle, P.; De Beer, J.O.; Rogiers, V.; Grosber, M.; Deconinck, E.; De Paepe, K. Overview of skin whitening agents with an insight into the illegal cosmetic market in Europe. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 943–950. [Google Scholar] [CrossRef]
- Lee, C.M. Fifty years of research and development of cosmeceuticals: A contemporary review. J. Cosmet. Dermatol. 2016. [Google Scholar] [CrossRef]
- Fuchs, E. Skin stem cells: Rising to the surface. J. Cell Biol. 2008, 180, 273–284. [Google Scholar] [CrossRef]
- Williams, S.E.; Beronja, S.; Pasolli, H.A.; Fuchs, E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature 2011, 470, 353–358. [Google Scholar] [CrossRef]
- Hanafusa, H.; Kedashiro, S.; Tezuka, M.; Funatsu, M.; Usami, S.; Toyoshima, F.; Matsumoto, K. PLK1-dependent activation of LRRK1 regulates spindle orientation by phosphorylating CDK5RAP2. Nat. Cell Biol. 2015, 17, 1024–1035. [Google Scholar] [CrossRef]
- Kulukian, A.; Holland, A.J.; Vitre, B.; Naik, S.; Cleveland, D.W.; Fuchs, E. Epidermal development, growth control, and homeostasis in the face of centrosome amplification. Proc. Natl. Acad. Sci. USA 2015, 112, E6311–E6320. [Google Scholar] [CrossRef]
- Morin, X.; Bellaiche, Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell 2011, 21, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Boulan, E.; Macara, I.G. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 2014, 15, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, B.; Wang, T.; Chen, S.; Li, H.; Zhang, Y.; Dai, S. Mechanisms of plant salt response: Insights from proteomics. J. Proteome Res. 2012, 11, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Kim, K.R.; Choi, S.W.; Woo, M.H.; Choi, J.H. Antioxidant and antithrombus activities of enzyme-treated Salicornia herbacea extracts. Ann. Nutr. Metab. 2007, 51, 119–125. [Google Scholar] [CrossRef]
- Im, S.A.; Lee, Y.R.; Lee, Y.H.; Oh, S.T.; Gerelchuluun, T.; Kim, B.H.; Kim, Y.; Yun, Y.P.; Song, S.; Lee, C.K. Synergistic activation of monocytes by polysaccharides isolated from Salicornia herbacea and interferon-gamma. J. Ethnopharmacol. 2007, 111, 365–370. [Google Scholar] [CrossRef]
- Park, S.H.; Ko, S.K.; Choi, J.G.; Chung, S.H. Salicornia herbacea prevents high fat diet-induced hyperglycemia and hyperlipidemia in ICR mice. Arch. Pharm. Res. 2006, 29, 256–264. [Google Scholar] [CrossRef]
- Panth, N.; Park, S.H.; Kim, H.J.; Kim, D.H.; Oak, M.H. Protective Effect of Salicornia europaea Extracts on High Salt Intake-Induced Vascular Dysfunction and Hypertension. Int. J. Mol. Sci. 2016, 17, 1176. [Google Scholar] [CrossRef]
- Karadeniz, F.; Kim, J.A.; Ahn, B.N.; Kwon, M.S.; Kong, C.S. Effect of Salicornia herbacea on osteoblastogenesis and adipogenesis in vitro. Mar. Drugs 2014, 12, 5132–5147. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Yun, H.J.; Choi, J.H.; Chun, H.K.; Chung, Y.C.; Kim, S.K.; Kim, B.H.; Kwon, K.I.; Jeong, T.C.; Lee, K.Y.; et al. 3-Caffeoyl, 4-dihydrocaffeoylquinic acid from Salicornia herbacea inhibits tumor cell invasion by regulating protein kinase C-delta-dependent matrix metalloproteinase-9 expression. Toxicol. Lett. 2010, 198, 200–209. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, Y.A.; Kim, M.M.; Park, J.S.; Kim, J.A.; Kim, S.K.; Lee, B.J.; Nam, T.J.; Seo, Y. Flavonoid glycosides isolated from Salicornia herbacea inhibit matrix metalloproteinase in HT1080 cells. Toxicol. In Vitro 2008, 22, 1742–1748. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, H.S.; Shin, K.H.; Kim, B.K.; Lee, S. Constituents of the halophyte Salicornia herbacea. Arch. Pharm. Res. 2004, 27, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Isca, V.M.S.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. An overview of Salicornia genus: The phytochemical and pharmacological profile. In Natural Products: Research Review; Gupta, V.K., Ed.; Daya Publishing House: New Delhi, India, 2014; Volume 2, pp. 145–164. [Google Scholar]
- Patel, S. Salicornia: Evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech. 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.T.; Jang, Y.; Myung, S.W.; Lee, J.M.; Kim, H.S.; Ko, S.C.; Lee, S.H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doi, N.; Togari, H.; Minagi, K.; Nakaoji, K.; Hamada, K.; Tatsuka, M. Protective Effects of Salicornia europaea on UVB-Induced Misoriented Cell Divisions in Skin Epithelium. Cosmetics 2020, 7, 44. https://doi.org/10.3390/cosmetics7020044
Doi N, Togari H, Minagi K, Nakaoji K, Hamada K, Tatsuka M. Protective Effects of Salicornia europaea on UVB-Induced Misoriented Cell Divisions in Skin Epithelium. Cosmetics. 2020; 7(2):44. https://doi.org/10.3390/cosmetics7020044
Chicago/Turabian StyleDoi, Natsumi, Hiro Togari, Kenji Minagi, Koichi Nakaoji, Kazuhiko Hamada, and Masaaki Tatsuka. 2020. "Protective Effects of Salicornia europaea on UVB-Induced Misoriented Cell Divisions in Skin Epithelium" Cosmetics 7, no. 2: 44. https://doi.org/10.3390/cosmetics7020044
APA StyleDoi, N., Togari, H., Minagi, K., Nakaoji, K., Hamada, K., & Tatsuka, M. (2020). Protective Effects of Salicornia europaea on UVB-Induced Misoriented Cell Divisions in Skin Epithelium. Cosmetics, 7(2), 44. https://doi.org/10.3390/cosmetics7020044




