Antioxidant Activities of Jeju Wax Apple (Syzygium samarangense) and Safety of Human Keratinocytes and Primary Skin Irritation Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Extracts
2.2. DPPH Radical Scavenging Activity
2.3. ABTS Radical Scavenging Activity
2.4. DMPD (Dimethyl-4-Phenylenediamine) Radical Scavenging Activity
2.5. Nitrite Scavenging Activity
2.6. Ferrous-Ion Chelating Activity
2.7. Cupric Reducing Antioxidant Capacity (CUPRAC)
2.8. Reducing Power Assay
2.9. Ferric Reducing Antioxidant Power (FRAP)
2.10. Total Phenol Contents
2.11. Total Flavonoid Contents
2.12. Cell Cultures
2.13. Cell Viability Assay
2.14. Skin Primary Irritation Test
2.15. HPLC Fingerprint
2.16. Statistical Analyses
3. Results
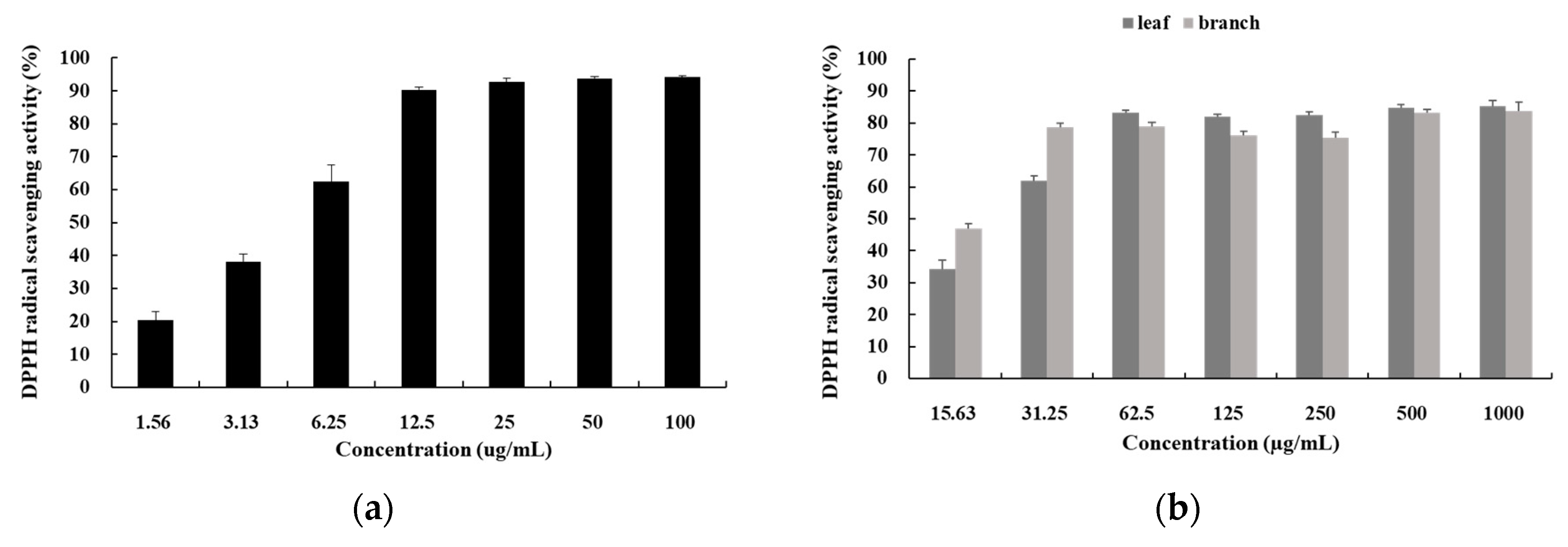
3.1. DPPH Radical Scavenging Activity
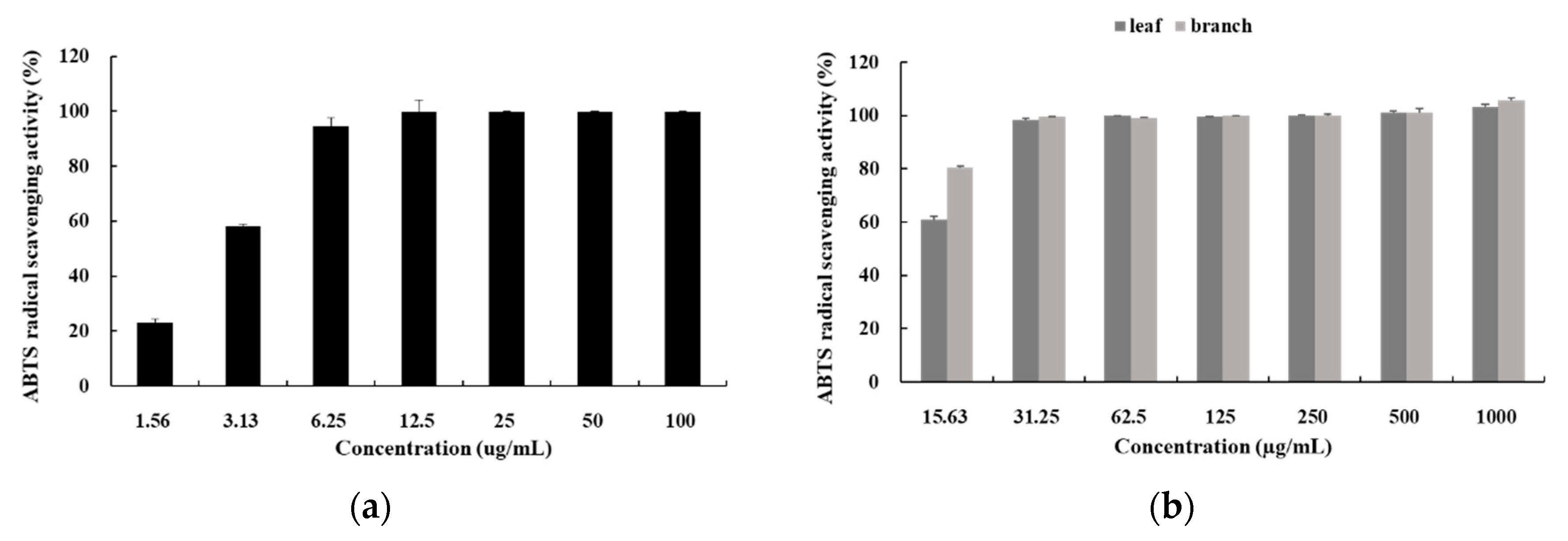
3.2. ABTS Radical Scavenging Activity
3.3. DMPD Radical Scavenging Activity
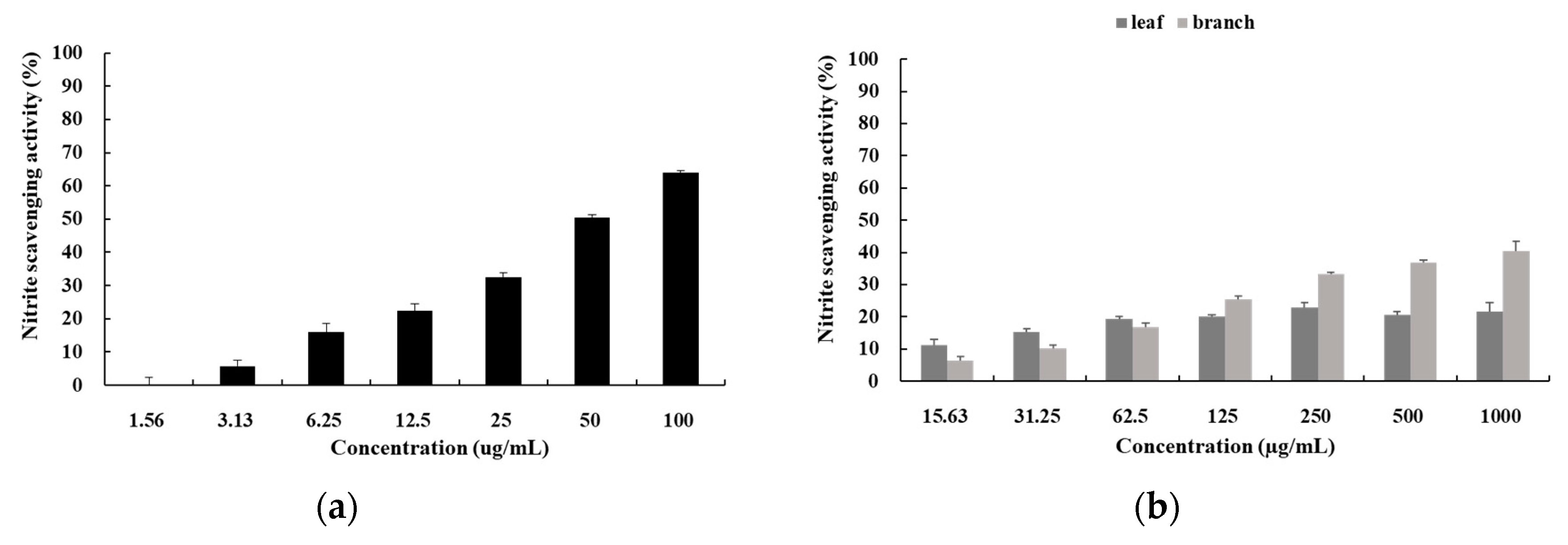
3.4. Nitrite Scavenging Activity
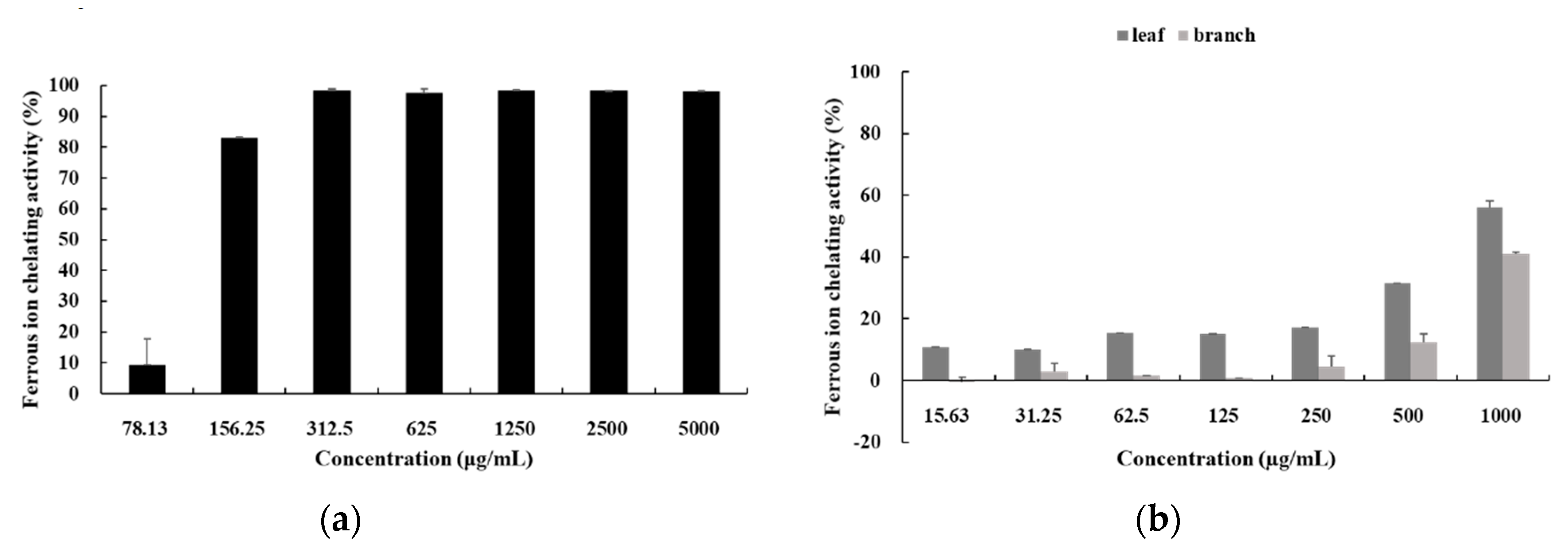
3.5. Ferrous-Ion Chelating Activity
3.6. Cupric Reducing Antioxidant Capacity (CUPRAC)
3.7. Reducing Power Assay
3.8. Ferric Reducing Antioxidant Power (FRAP)
3.9. Total Phenol Content and Total Flavonoid Content
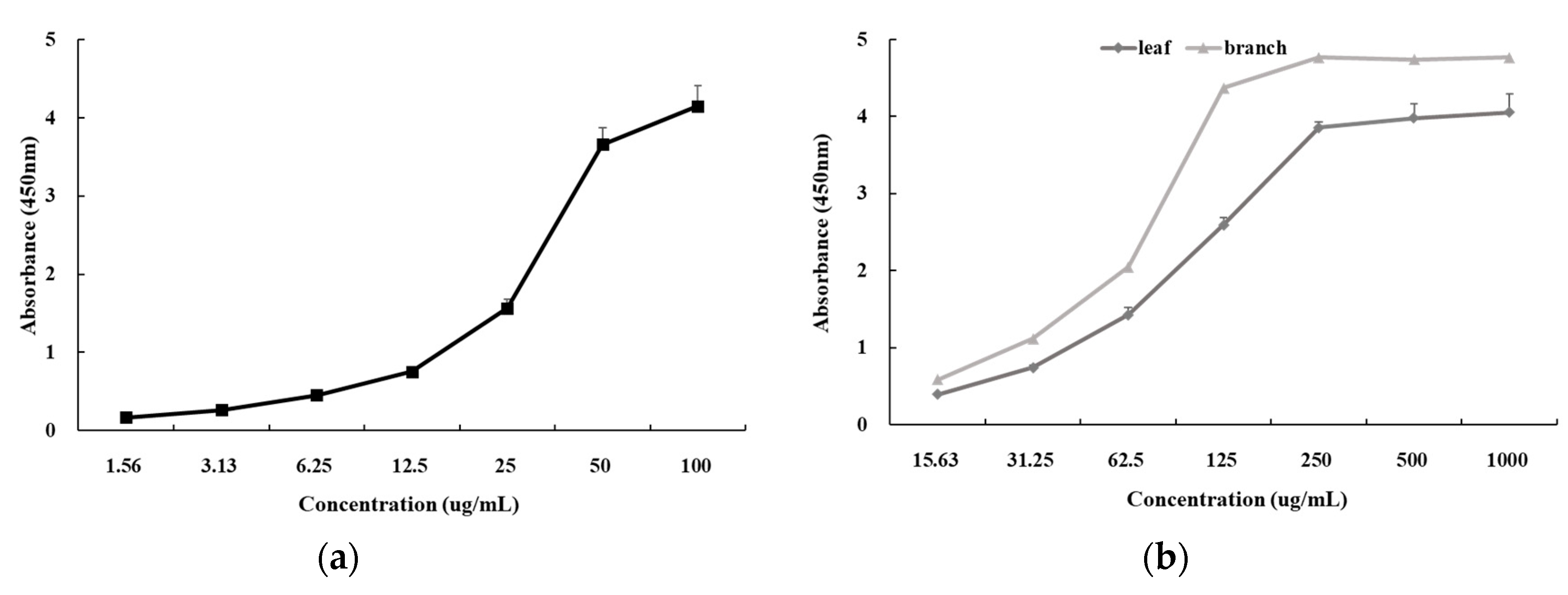
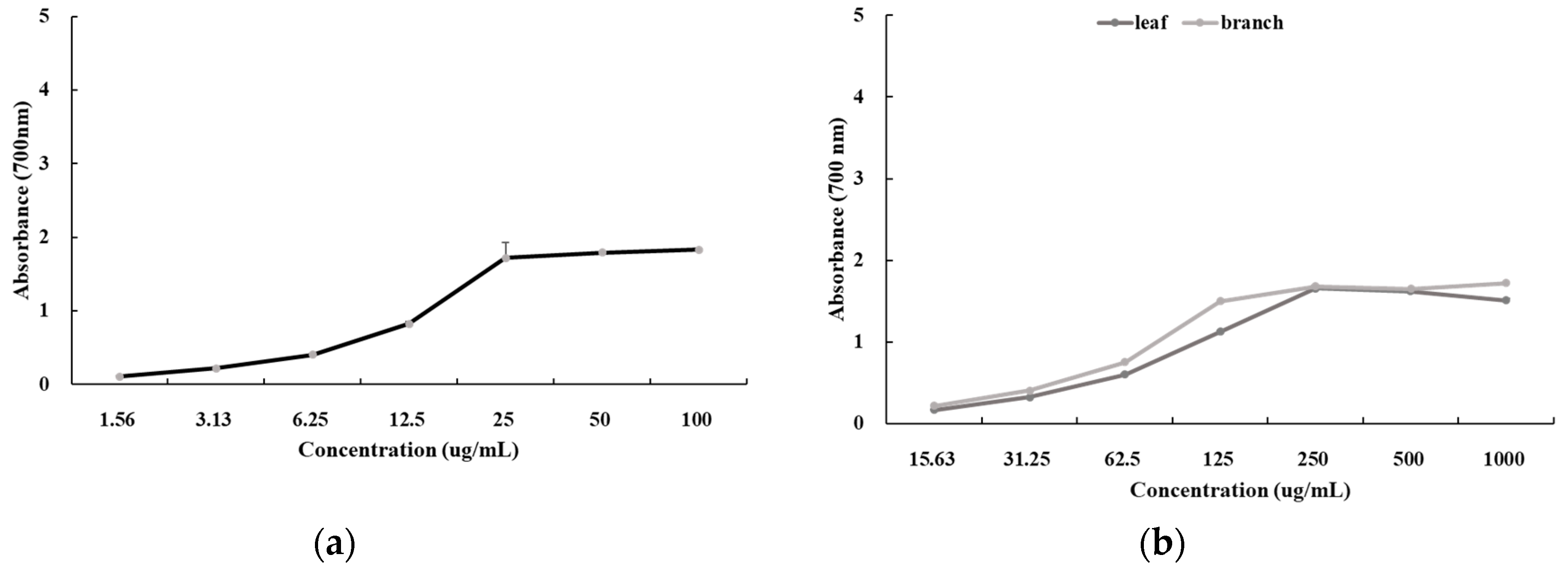
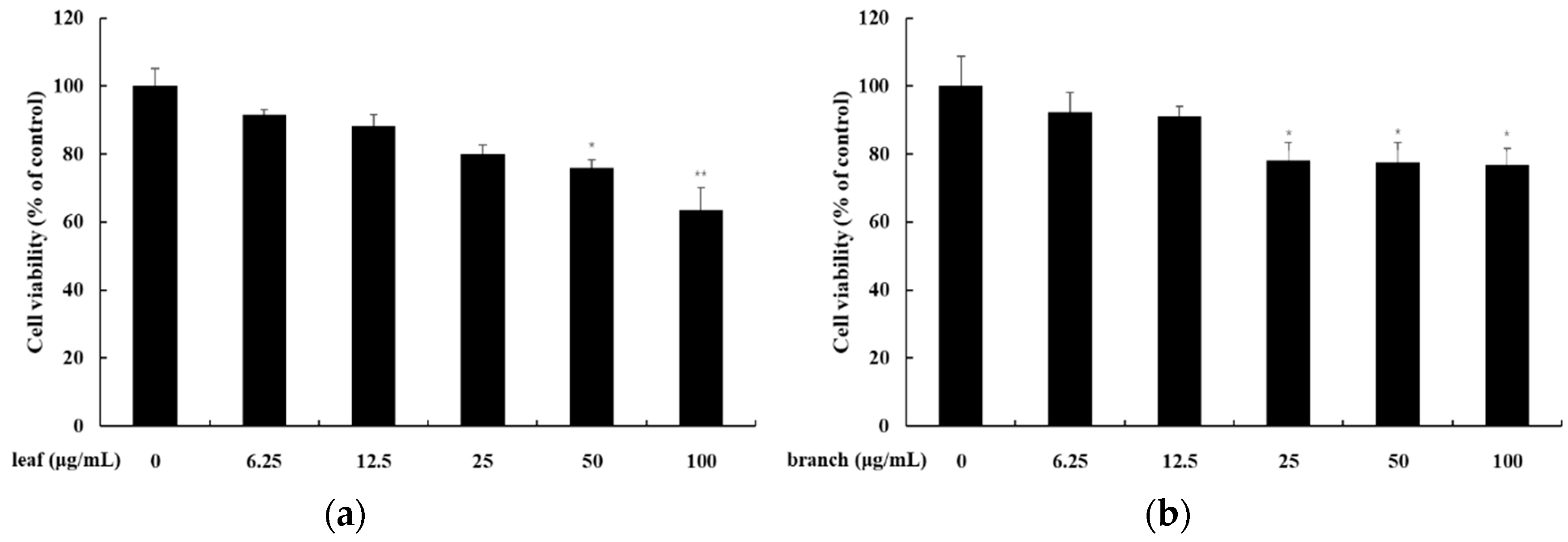
3.10. Cell Viability Assay
3.11. Skin Primary Irritation Test
3.12. HPLC Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kwak, T.S.; Ki, J.H.; Kim, Y.E.; Jeon, H.M.; Kim, S.J. A Study of GIS prediction model of domestic fruit cultivation location changes by the global warming -six tropical and sub-tropical fruits-. J. Korea Spatial Inform. Syst. Soc. 2008, 10, 93–106. [Google Scholar]
- Seong, K.C.; Kim, C.H.; Son, D.; Wi, S.H.; Lim, C.G.; Cheon, S.J.; Kim, S.Y.; Choi, B.Y.; Seo, J.S.; Song, M.G.; et al. The present state and future prospect of tropical/subtropical vegetables in Korea. Korean Soc. Hortic. Sci. 2014, 5, 56. [Google Scholar]
- Kim, C.Y.; Kim, Y.H.; Han, S.H.; Ko, H.C. Current situations and prospects on the cultivation program of tropical and subtropical crops in Korea. Plant Resour. Soc. Korea 2019, 32, 45–52. [Google Scholar]
- Kim, M.J.; Lee, J.Y.; Kim, S.S.; Seong, K.C.; Lim, C.K.; Park, K.J.; An, H.J.; Choi, Y.H.; Kim, S.Y.; Lee, N.H.; et al. Anti-inflammatory activity of wax apple (Syzygium samarangense) extract from Jeju Island. Korean Soc. Biotechnol. Bioeng. 2017, 32, 245–250. [Google Scholar]
- Zhang, Y.J.; Zhou, T.; Wang, F.; Zhou, Y.; Li, Y.; Zhang, J.J.; Zheng, J.; Xu, D.P.; Li, H.B. The effects of Syzygium samarangense, Passiflora edulis and Solanum muricatum on alcohol-induced liver injury. Int. J. Mol. Sci. 2016, 17, 1616. [Google Scholar] [CrossRef] [PubMed]
- Soubir, T. Antioxidant activities of some local bangladeshi fruits (Artocarpus heterophyllus, Annona squamosa, Terminalia bellirica, Syzygium samarangense, Averrhoa carambola and Olea europa). Chin. J. Biotechnol. 2007, 23, 257–261. [Google Scholar]
- Simirgiotis, M.J.; Adachi, S.; To, S.; Yang, H.; Reynertson, K.A.; Basile, M.J.; Gil, R.R.; Weinstein, I.B.; Kennelly, E.J. Cytotoxic chalcones and antioxidants from the fruits of Syzygium samarangense (Wax Jambu). Food Chem. 2008, 107, 813–819. [Google Scholar] [CrossRef]
- Kim, M.J.; Hyun, J.M.; Kim, S.S.; Seong, K.C.; Lim, C.K.; Kang, J.S. In vitro screening of subtropical plants cultivated in Jeju Island for cosmetic ingredients. Orient. J. Chem. 2006, 32, 807–815. [Google Scholar] [CrossRef][Green Version]
- Park, S.H.; Cho, C.H.; Ahn, B.Y. A study on the application of Gastrodiae rhizoma for food stuffs-effects of Gastrodiae rhizoma on the regional cerebral blood flow and blood pressure. J. East Asian Soc. Diet Life 2007, 17, 554–562. [Google Scholar]
- Rhim, T.J. In vitro antioxidant activity of Sanguisorbae Radix ethanol extracts. Korean J. Plant Res. 2013, 26, 149–158. [Google Scholar] [CrossRef][Green Version]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Lee, I.E.; Van Chuyen, N.; Kim, S.B.; Hayase, F. Inhibition of nitrosamine formation by nondialyzable melanoidins. Agr. Biol. Chem. 1987, 51, 1333–1338. [Google Scholar]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, Y.C.; Jung, S.W.; Lee, K.M. Flavor components of citron juice as affected by the extraction method. Korean J. Food Sci. Technol. 1994, 26, 709–712. [Google Scholar]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Kim, I.S.; Yang, M.R.; Lee, O.H.; Kang, S.N. Antioxidant activities of hot water extracts from various spices. Int. J. Mol. Sci. 2011, 12, 4120–4131. [Google Scholar] [CrossRef]
- An, S.M.; Ham, H.; Choi, E.J.; Shin, M.K.; An, S.S.; Kim, H.O.; Koh, J.S. Primary irritation index and safety zone of cosmetics: Retrospective analysis of skin patch tests in 7440 Korean women during 12 years. Int. J. Cosmet. Sci. 2014, 36, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ancerewicz, J.; Migliavacca, E.; Carrupt, P.A.; Testa, B.; Brée, F.; Zini, R.; Tillement, J.P.; Labidalle, S.; Guyot, C.; Chauvet, A.M.; et al. Structure–property relationships of trimetazidine derivatives and model compounds as potential antioxidants. Free Radic. Biol. Med. 1998, 25, 113–120. [Google Scholar] [CrossRef]
- Song, W.Y.; Byeon, S.J.; Choi, J.H. Anti-oxidative and anti-inflammatory activities of Sasa borealis extracts. J. Agric. Life Sci. 2015, 49, 145–154. [Google Scholar] [CrossRef]
- Asghar, M.N.; Khan, I.U.; Arshad, M.N.; Sherin, L. Evaluation of antioxidant activity using an improved DMPD radical cation decolorization assay. Acta. Chim. Slov. 2007, 54, 295–300. [Google Scholar]
- Jin, T.Y.; Park, J.R.; Kim, J.H. Electron donating abilities, nitrite scavenging effects and antimicrobial activities of Smilax china leaf. J. Korean Soc. Food Sci. Nutr. 2004, 33, 621–625. [Google Scholar]
- Kim, D.B.; Shin, G.H.; Lee, J.S.; Lee, O.H.; Park, I.J.; Cho, J.H. Antioxidant and nitrite scavenging activities of Acanthopanax senticosus extract fermented with different mushroom mycelia. Korean J. Food Sci. Technol. 2014, 46, 205–212. [Google Scholar] [CrossRef]
- Kim, E.M.; Won, S.I. Functional composition and antioxidative activity from different organs of native Cirsium and Carduus genera. Korean J. Food Cook Sci. 2009, 25, 406–414. [Google Scholar]
- Rhim, T.J.; Choi, M.Y.; Park, H.J. Antioxidative activity of Rumex crispus L. extract. Korean J. Plant Res. 2012, 25, 568–577. [Google Scholar] [CrossRef][Green Version]
- Kim, K.H.; Kim, H.J.; Byun, M.W.; Yook, H.S. Antioxidant and antimicrobial activities of ethanol extract from six vegetables containing different sulfur compounds. J. Korean Soc. Food Sci. Nutr. 2012, 41, 577–583. [Google Scholar] [CrossRef]
- Hwang, J.S.; Lee, B.H.; An, W.; Jeong, H.R.; Kim, Y.E.; Lee, I.; Lee, H.; Kim, D.O. Total phenolics, total flavonoids, and antioxidant capacity in the leaves, bulbs, and roots of Allium hookeri. Korean J. Food Sci. Technol. 2015, 47, 261–266. [Google Scholar] [CrossRef]
- Jang, S.C.; Park, T.J.; Kim, M.S.; Hong, H.H.; Kim, M.J.; Kim, S.Y. The Anti-inflammatory Activity of Corydalis platycarpa (Maxim.) Makino Crude Extract from Jeju Island. Korean Soc. Biotechnol. Bioeng. J. 2018, 33, 253–260. [Google Scholar]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and skin aging-from the perspective of food nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Akhtar, N.; Menaa, B.; Menaa, A.; Braga, V.A.; Menaa, F. Relative free radicals scavenging and enzymatic activities of Hippophae rhamnoides and Cassia fistula extracts: Importance for cosmetic, food and medicinal applications. Cosmetics 2017, 4, 3. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.J.; Kim, J.A.; Chun, J.Y. Quality characteristics and antioxidative activity of different parts of bitter melon (Momordica charantia L.). J. Korean Soc. Food Sci. Nutr. 2019, 48, 418–423. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.W.; Kim, Y.D. Antimicrobial activity and antioxidant effect of Curcuma longa, Curcuma aromatica and Curcuma zedoaria. Korean J. Food Preserv. 2011, 18, 219–225. [Google Scholar] [CrossRef]
- Ku, T.K.; Yoo, I.S.; Park, A.N. Antioxidant effects of bioactive mango leaves. J. Kor. Soc. Cosm. 2014, 20, 847–8512. [Google Scholar]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Sobeh, M.; Petruk, G.; Osman, S.; El Raey, M.A.; Imbimbo, P.; Monti, D.M.; Wink, M. Isolation of myricitrin and 3,5-di-O-methyl gossypetin from Syzygium samarangense and evaluation of their involvement in protecting keratinocytes against oxidative stress via activation of the Nrf-2 pathway. Molecules 2019, 24, 1839. [Google Scholar] [CrossRef]



| Grade | Description of Clinical Observation |
|---|---|
| +1 | Slight erythema |
| +2 | Moderate erythema, possibly with barely perceptible edema at the margin, papules may be present |
| +3 | Moderate erythema, with generalized edema |
| +4 | Severe erythema with severe edema, with or without vesicles |
| +5 | Severe reaction spread beyond the area of the patch |
| Range of Response | Judgment |
|---|---|
| 0 ≤ R < 0.87 | None to Slight |
| 0.87 ≤ R < 2.42 | Mild |
| 2.42 ≤ R < 3.44 | Moderate |
| 3.44 ≤ R | Severe |
| Grade | Myricitrin | ρ-Coumaric Acid |
|---|---|---|
| Column | YMC-Triact C18 (250 × 4.6mml.D. S-5 μm.12 nm) | YMC-Triact C18 (250 × 4.6 mml.D. S-5 μm.12 nm) |
| Detector | UV 370 nm | UV 310 nm |
| Mobile phase | A: 3% acetic acid, B: MeOH 1 min A 100% 27 min B 63% 32 min A 100% | Water:MeOH:acetic acid |
| Flow rate | 1 mL/min | 1 mL/min |
| Injection volume | 10 µL | 10 µL |
| Column temperature. | 27 °C | 30 °C |
| Sample temperature. | 15 °C | 15 °C |
| Run time | 32 min | 30 min |
| L-Ascorbic Acid | Leaf | Branch | |
|---|---|---|---|
| mmol Fe2+/g | 3.438 ± 0.111 | 0.333 ± 0.006 | 0.371 ± 0.014 |
| Leaf | Branch | |
|---|---|---|
| mg GAE (1)/g | 66.778 ± 1.64 | 76.3820 ± 1.085 |
| mg QE (2)/g | 40.8076 ± 2.226 | 78.057 ± 3.576 |
| No | Test Sample | No. of Responders | 24 h | 48 h | Reaction Grade | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +1 | +2 | +3 | +4 | +1 | +2 | +3 | +4 | 24 h | 48 h | Mean | |||
| 1 | leaf | 6 | 4 | - | - | - | 3 | - | - | - | 2.9 | 2.2 | 2.6 |
| 2 | branch | 2 | 2 | - | - | - | - | - | - | - | 1.5 | 0 | 0.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, S.B.; Bae, S.; Hyun, C.-G. Antioxidant Activities of Jeju Wax Apple (Syzygium samarangense) and Safety of Human Keratinocytes and Primary Skin Irritation Test. Cosmetics 2020, 7, 39. https://doi.org/10.3390/cosmetics7020039
Hyun SB, Bae S, Hyun C-G. Antioxidant Activities of Jeju Wax Apple (Syzygium samarangense) and Safety of Human Keratinocytes and Primary Skin Irritation Test. Cosmetics. 2020; 7(2):39. https://doi.org/10.3390/cosmetics7020039
Chicago/Turabian StyleHyun, Su Bin, Sungmin Bae, and Chang-Gu Hyun. 2020. "Antioxidant Activities of Jeju Wax Apple (Syzygium samarangense) and Safety of Human Keratinocytes and Primary Skin Irritation Test" Cosmetics 7, no. 2: 39. https://doi.org/10.3390/cosmetics7020039
APA StyleHyun, S. B., Bae, S., & Hyun, C.-G. (2020). Antioxidant Activities of Jeju Wax Apple (Syzygium samarangense) and Safety of Human Keratinocytes and Primary Skin Irritation Test. Cosmetics, 7(2), 39. https://doi.org/10.3390/cosmetics7020039





