Abstract
The exudate of Commiphora myrrha (myrrh) has been known for centuries as one of the most popular natural skin remedies. The characterization and safety assessment of myrrh ingredients are challenging due to the chemical variability of commercially available sources, as well as potential adulteration. Human and animal data have reported potential concerns about myrrh as a skin sensitizer, although no specific chemical entity has been identified as a potential culprit yet. In the present work, the in chemico high-throughput method using dansylated cysteamine (HTS-DCYA) was applied to extract and fractions of myrrh samples in an attempt to identify potential skin sensitizers. Nine oxo-furanogermacranes and the sesquiterpenoid alismol were isolated as major constituents. Five of the compounds were identified as weakly to moderately reactive in HTS-DCYA and could therefore trigger the molecular initiating event leading to skin sensitization. The reactive compounds were identified as 6-oxofuranodienones (2 and 5) and methoxyfuranogermacrenones (7 and 9). The reaction adducts of 2 with DCYA was confirmed by HPLC-DAD-MS and by HPLC-MS/MS experiments. A comparison of the chemical profile of myrrh samples available in-house confirmed a certain degree of chemical variability, with compounds 1, 7, and 9 occurring in four of the six samples.
1. Introduction
The genus Commiphora (Burseraceae) includes approximately 200 species, typically characterized as small trees or shrubs producing reddish-brown resinous exudates with an acrid taste and a balsamic odor. According to the United States Pharmacopoeia [1], myrrh is defined as the oleo-gum-resin obtained from the stems and branches of C. molmol (syn. C. myrrha, C. myrrha var. molmol, or Balsamodendrum myrrha) and/or other related species of Commiphora other than C. mukul. Native to east/northeast Africa (especially Ethiopia, Somalia, and Kenya), southwest Asia, and Arabia, C. myrrha is commonly imported as gum [2]. Myrrh, probably one of the most renowned natural remedies, has a long tradition of use throughout history, especially in Traditional Chinese Medicine as well as in perfumery. Myrrh has been widely used as a skin remedy for the treatment of wounds, infections, acne and boils, and as an oral preparation for sore throats, canker sores, and gingivitis [3]. Myrrh can be very challenging to authenticate and characterize due to the variability and chemical complexity of the exudates. Along with the intrinsic variability of myrrh sources and confusion in taxonomical classification, adulteration is often reported, due to either economic motives, unintentional contamination, or misidentification [2,4].
Allergic contact dermatitis (ACD) has been reported as a side effect of topical exposure to myrrh and its preparations in human patch tests [5,6,7,8]. ACD is a type IV form of delayed allergic reaction; the first exposure to the allergen (sensitization phase) is typically asymptomatic. Further exposures to the same allergen in now-sensitized subjects (elicitation phase) will provoke an exacerbated immunoreaction resulting in typical inflammation symptoms (redness, itchiness, and swelling). ACD is a chronic pathology with occasionally severe outcomes, and only complete avoidance of contact with the chemical sensitizers can prevent its reoccurrence [9].
In vivo animal models such as the local lymph node assay (LLNA) have been considered for decades to be the gold standard for the characterization of the sensitization stage. Myrrh preparations were classified as weakly sensitizing in LLNA studies [10]. Nonetheless, the chemical constituent(s) responsible for such adverse effects has not yet been identified. A systematic investigation of the skin sensitization adverse effects of plants like myrrh can be extremely costly in terms of resources and animal welfare. Furthermore, regulatory agencies worldwide have been intensifying efforts aimed to reduce the use of animal methods for the safety evaluation of ingredients in cosmetic and personal care preparations [11].
The safety assessment of botanical cosmetic ingredients is an essential piece of information due to their widespread use and consequent consumer exposure. According to the adverse outcome pathway (AOP) paradigm for skin sensitization, four main key events have to occur for a chemical to act as a sensitizer. The haptenation step, i.e., the covalent binding of the chemical to skin protein, is considered the molecular initiating event (MIE). In chemico methods can represent a powerful tool at pre-screening stages by providing information about the likelihood of a molecule to covalently bind to skin proteins [12]. An in chemico approach for the rapid investigation of the presence of potential skin sensitizers in complex botanical mixtures was recently described [13]. The high-throughput screening method using dansylated cysteamine (HTS-DCYA™) enables the stable derivatization of thio-reactive chemical sensitizers as fluorescent dansylated adducts, which can be quantified through a fluorescence assay in a 96-well format.
In the present work, the oleo-gum-resinous extract and fractions from myrrh were evaluated for their skin sensitization potential using the in chemico HTS-DCYA™ assay followed by isolation of candidate skin sensitizers. Ten major constituents were isolated and tested for their thio-reactivity which could be a potential source of ACD.
2. Materials and Methods
2.1. Plant Material
The commercial myrrh sample (NCNPR accession code # 3350) was purchased from Frontier Natural Products. Specimens of the material are deposited at the botanical repository at the National Center for Natural Products Research (NCNPR, The University of Mississippi, Oxford, MS, USA). Additional myrrh oleo-gum-resins were obtained from the Williams Ware House (NCNPR accession code #2092) or from the Missouri Botanical Garden (NCNPR accession codes #6452, 8549, and 8986).
2.2. Chemicals and Reagents
The fluorescent compounds DCYA and DCYA disulfide were synthesized as previously described [14]. Trans-cinnamaldehyde (CINN, CAS 14371-10-9) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile HPLC grade (ACN) and standardized pH 10 ± 0.02 buffer (cat. N. SB116-500) were purchased from Fisher Scientific (Suwanee, GA, USA). Silica gel 60 (175–225 µm) thin-layer chromatography (TLC) plates (cat. N. 105553) were purchased from Merck (Darmstadt, Germany). Polymer-supported maleimide (SiliaBond® Maleimide, ≥0.64 mmol/g) was purchased from SiliCycle (Quebec City, Quebec, Canada). Flash column chromatography purifications were performed on a Biotage Isolera Four, using SNAP cartridges or Flash+ cartridges. Silica gel cartridges were KP-Sil (40−63 μm, 60 Å) or KP-C18-HS (35−70 μm, 90 Å). Gravity column chromatography (CC) purifications were performed using silica gel (40−63 μm, 60 Å, Sorbtech) or reversed-phase RP-C18 silica (Polarbond, J.T. Baker - Avantor, Radnor, PA, USA).
2.3. Solvent Extraction
The oleo-gum-resin (201.0 g) was extracted with 1 L of methanol (1:5 w/v) by sonication (30 min) at room temperature. The extract was filtered over Celite, and the soluble fraction was dried under vacuum; the procedure was repeated two more times to obtain a total of 111.9 g (55.7% yield) of dry myrrh methanol extract (MME).
2.4. Fractionation
Fifty-six grams of the obtained MME were further fractionated by silica gel dry column vacuum chromatography (DCVC) using a gradient of hexane/EtOAc from 100% to 20% hexane, followed by washing with EtOAc, acetone, and methanol. Forty-nine fractions were obtained and combined based on their TLC profiles into nine main fractions: F1 (0.835g), F2 (0.868 g), F3 (3.615 g), F4 (3.778 g), F5 (3.744 g), F6 (4.072 g), F7 (4.449 g), F8 (9.508 g), and F9 (11.806 g).
The main reactive fractions F2–F4 were further purified by fractionation on Sephadex followed by column chromatography. The identity of the isolated compounds was confirmed by 1 and 2D NMR data, GC-MS, and comparison with literature data [15]. Compounds 1, 4, and 6 were obtained from F2; compounds 2, 5, and 9 were obtained from F3; and compounds 3, 7, 8, and 10 were isolated from fraction F4.
Curzerenone (1, 585.3 mg, pale yellow oil) and furandienone (2, 150.5 mg, pale yellow oil) were isolated on C-18 reverse phase (gradient elution 60% to 70% methanol in H2O). Compound 4 (83.6 mg, pale yellow oil) was obtained by gradient elution on silica gel (40% to 50% CH2Cl2 in hexane). Compound 5 (178.1 mg, pale yellow oil) was eluted on C-18 reverse phase (60% methanol in H2O). Compound 6 (138.1 mg, off-white solid) was purified using silica gel in 100% hexane. Compound 7 (239.5 mg, yellow solid) was obtained from purification on silica gel (4% EtOAc in hexane). Compound 9 (1.152 g, yellow solid) was separated using silica gel (4% EtOAc in hexane). Alismol (3, 24 mg, colorless oil), the epoxygermacrane 8 (68.0 mg, off-white solid), and the acetoxygermacrane 10 (QZ-1-85-G, 515.3 mg, yellow solid) were isolated on silica gel (1%–5% EtOAc in hexane).
2.5. GC-MS Analysis
Gas chromatographic analyses were conducted using an Agilent 7890A GC system equipped with a 5975C quadrupole mass spectrometer and a 7693 autosampler (Agilent Technologies, Santa Clara, CA, USA). The separation was performed on a DB-5MS capillary column (Agilent J&W Scientific, Folsom, CA, USA) with dimensions of 30 m × 0.25 mm i.d. × 0.25 µm film thickness. The MME was diluted to 5 mg/mL in HPLC-plus-grade acetone (>99.9%, Sigma-Aldrich), and 0.5 µL of the sample solution was injected. Helium was used as the carrier gas at a flow rate of 1 mL/min. The inlet temperature was set to 260 °C with a split injection at the split ratio of 30:1. The oven temperature program was as follows: the temperature was initially 60 °C (held for 2 min), increased to 280 °C at a rate of 5.0 °C /min, and isothermal for 10 min at 280 °C. Mass spectra were recorded from m/z 40 to 500 amu in scan mode with the electron ionization (EI) source at 70 eV. The ion source and quadrupole temperatures were 230 °C and 150 °C, respectively. Data acquisition was performed using an Agilent MSD Chemstation (F.01.03.2357). Agilent MassHunter Qualitative Analysis and Quantitative Analysis (version B.07.00) were used for data analysis. Peak identity was confirmed with available standards, and the NIST database (version 2.2) was used for tentative compound identification.
2.6. Sample Preparation Procedure for HTS-DCYA
Twenty milligrams of plant extract (or fraction) were accurately weighed and dissolved to a final concentration of 20 mg/mL in ACN. The samples were sonicated then centrifuged at 900 g for 15 min, and the precipitate was discarded. The soluble fraction was further diluted to obtain two additional concentrations (5 and 10 mg/mL) in ACN.
2.7. HTS-DCYA
The myrrh extract and fractions were tested at three concentrations (5, 10, and 20 mg/mL) as previously described [13]. Negative and positive controls (NC and PC) were included to estimate the minimal and maximal fluorescence response. Blank controls (Bl) containing both the test chemical and DCYA were used to estimate the potential interference of the test chemical on DCYA fluorescence emission. Pure compounds were tested at a final concentration of 5 mM in ACN [16]. Briefly, a solution of DCYA in ACN (2.5 mM) was mixed with an equal volume of test chemical. The DCYA–test article solution was then split into two polypropylene vials (150 µL each). Thirty microliters of aqueous pH 10 buffer were added to the reaction vials (R) and to the negative control (NC), while ACN was added in place of buffer to Bl and PC. Solvent (ACN) was added in place of the test article in both PC and NC. After 24 min incubation, the unreacted DCYA was removed by addition of SiliaBond® Maleimide resin (30 mg/vial), and the obtained suspension was incubated for 60 min at room temperature. Sixty microliters of supernatant were diluted to a final volume of 1 mL with ACN. The samples were then diluted 2× into solvent-resistant polypropylene 96-well plates, and each plate was read in triplicate using fluorescence end-point readings. The fluorescence readings were performed on a SpectraMax M5 multi-mode microplate reader, and data were acquired and processed using SoftMax Pro 5 (Molecular Devices, Sunnyvale, CA, USA). The readings were recorded at λ520 nm (excitation λ350 nm, cutoff 420 nm) at 23 °C. The reactivity index (RI) (referred to as the total reactivity index (TRI) for extracts and fractions) was calculated based on the average of nine readings per sample as follows:
2.8. UPLC-DAD-MS and MS-MS Analysis
Characterization of DCYA adducts was performed using an Agilent 1260 HPLC instrument equipped with a diode array (DAD) detector and a quadrupole MS system (Agilent 6130). The column was an Agilent SB-C18 RRHD column (2.1 × 100 mm, 1.8 µm). The mobile phase was water (A) and acetonitrile (B), both containing 0.2% acetic acid. The gradient (flowrate 0.2 ml/min) was developed as follows: 45% to 60% B in 3 minutes then to 70% B in 17 minutes and to 90% in 3 minutes, followed by column wash at 90% B for 4 minutes. The reaction adducts were monitored at 330 nm. Positive and negative ESI spectra were obtained from m/z 100 to 1000 amu.
3. Results
3.1. Reactivity of Myrrh Fractions
The commercial oleo-gum of myrrh was extracted in methanol followed by solvent evaporation to obtain MME. The TLC fingerprint of MME was characterized by the presence of multiple non-polar compounds. Therefore, the total extract was fractionated by DCVC using a low-polarity gradient of hexane/ethyl acetate. Nine major fractions (F1–F9) were obtained and tested in the HTS-DCYA assay along with the crude extract. The reactivity of the whole extract was below a TRI of 50 at any tested concentration (Figure 1), whereas all fractions scored a TRI between 34 and 82 at 20 mg/mL. A concentration-dependent increase in fluorescence response (TRI) was obtained for fractions F1–F5 and, to a minor extent, for the polar fraction F9. Qualitative evaluation of the crude extract profile by TLC showed the presence of multiple fluorescent spots after reaction with DCYA, mainly in fractions F2–F4. The remaining fractions were also positive in the fluorescence assay, but no clear, evident fluorescent adduct(s) was identified by qualitative TLC experiments.
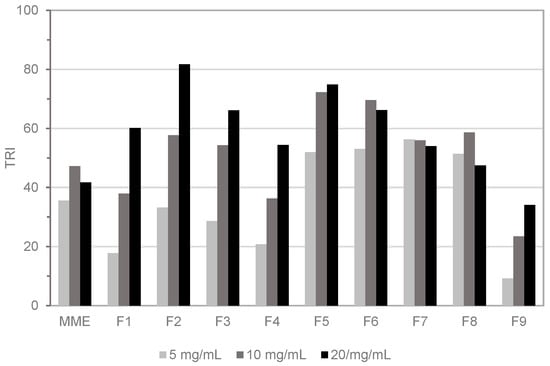
Figure 1.
High-throughput screening using dansylated cysteamine (HTS-DCYA) results of myrrh methanol extract (MME) and fractions TRI = total reactive index.
3.2. GC-MS Profile of Reactive Fractions
Skin sensitizers are typically small, often volatile, lipophilic molecules. Both the MME and fractions F1–F9 were thus examined by GC-MS analyses. The profile of the crude extract (Figure 2) showed three main peaks and seven less abundant peaks along with multiple minor peaks. The main peaks were present in higher amounts in the nonpolar fractions (F2–F4). Based on the chromatographic data and reactivity with DCYA, fractions F2, F3, and F4 were subjected to further isolation. Repeated purification steps enabled the isolation of ten main compounds (1–10) (Figure 3) from these fractions.
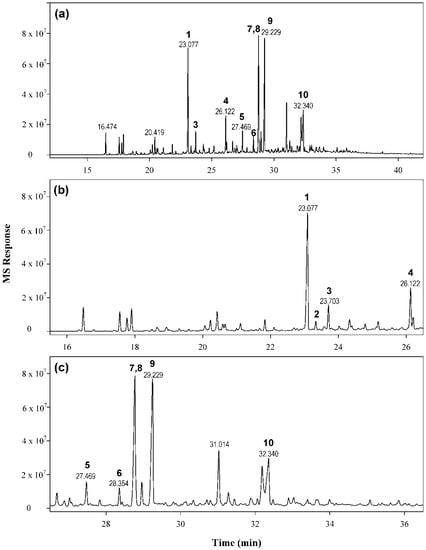
Figure 2.
Characterization of the volatile fraction of the crude methanol extract of myrrh. (a) Total Ion Chromatogram (TIC) MS chromatogram of MME; (b) expansion between 16 and 26 minutes, (c) expansion between 26 and 37 minutes.
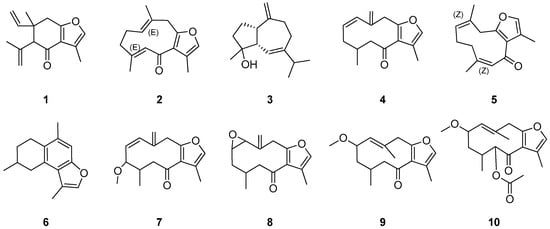
Figure 3.
Structures of main constituents isolated from myrrh.
The most reactive fraction (F2) contained predominantly compound 1 (m/z 230, rt 23.08 min) along with five minor compounds, including unidentified mono-oxygenated compounds having m/z 204 and compounds 4 and 6. The major peaks in fraction F3 corresponded to a compound with m/z 262 (9, rt 29.23 min), while minor amounts of compounds 1, 2 (rt 23.33 min, m/z 230), 7 (rt 28.76 min, m/z 260), and 5 (m/z 230, rt 27.47 min) were found. The epoxy derivative 8 (rt 28.80 min, m/z 246) and the acetoxy derivative 10 (rt 32.19, m/z 320) were the major constituents of fraction F4, along with 9, 3 (rt 23.70, m/z 220), 7, and traces of other compounds.
3.3. Thio-Reactivity of Myrrh Sesquiterpenoids
Structural elucidation of the isolated compounds by NMR resulted in the identification of nine known furanosesquiterpenoids (Figure 3) and one sesquiterpene (alismol). The spectroscopic data were compatible with those in the previously reported literature [15,17,18,19,20]. The reactivity of the individual pure compounds isolated was also tested using the HTS-DCYA method. Although the furanosesquiterpenoids shared close structural similarity, chemical reactivity varied significantly among the compounds (Figure 4). Five compounds were found to be reactive toward DCYA, whereas two were very weakly reactive. Based on thio-reactivity, both 6-oxofuranodienones (2 and 5) and the epoxyderivative (8) were classified as moderately reactive, while the methoxy furano-germacrenones 7 and 9 were weakly reactive but still comparable to the positive control. The five remaining compounds 1, 3, 4, 6, and 10 were found to be minimally or not reactive.
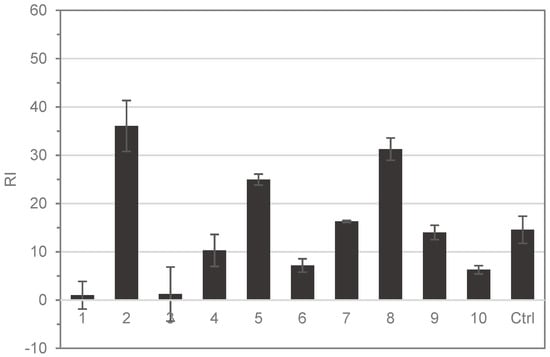
Figure 4.
HTS-DCYA results of isolated compounds (RI = reactive index). Cinnamaldehyde was used as the positive control (Ctrl).
In order to confirm whether the increased fluorescence response was related to the formation of covalent fluorescent adduct(s), the reaction of 2 with DCYA was analyzed by HPLC-DAD-MS as an example. The comparison of control chromatograms with the reaction enabled the identification of a newly formed peak (rt 10.5 min) having m/z 573 (ESI+). The calculated mass was compatible with a dioxygenated analog of a DCYA adduct with furanodienone 2. Since both the DCYA and the furanone moiety could be potentially subjected to oxidization, HPLC-TOF-MS/MS experiments were further performed (Figure 5). The accurate mass values of the corresponding m/z 311, 263, and 245 fragmentation peaks substantiated the hypothesis of two extra oxygen atoms on the furandienone moiety (Figure 6). The furanodienones being highly susceptible to oxidization, it was not clear whether the corresponding adduct was formed due to the presence of minor reactive oxidized impurities or to in situ activation of 2 in the experimental conditions.
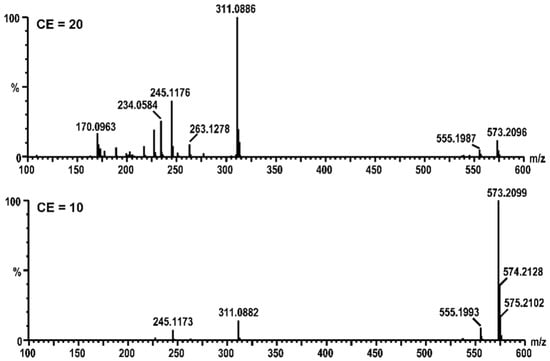
Figure 5.
HPLC-MS/MS fragmentation profiles of the DCYA adduct having m/z 573 at different collision energies (CE).
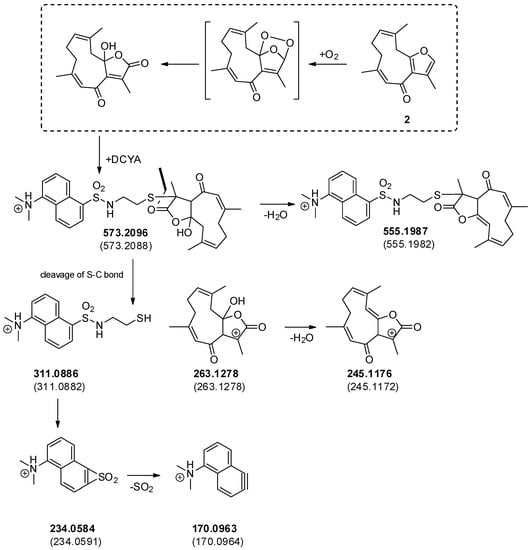
Figure 6.
Possible pathways leading to the formation of oxidized DCYA adduct with 2 and the corresponding fragmentation mechanism. Experimental (bold) and calculated mass values (in parentheses) are reported.
3.4. Chemical Profile of Various Myrrh Specimens
An analytical comparison of methanol extracts from the six different samples from an in-house repository highlighted the known issue of the chemical variability of myrrh exudates. The six extracts possessed qualitative and quantitative differences in terms of chemical markers. Compounds 1, 7, and 9 were found to be major constituents of four of the six samples. On the other hand, the chromatographic profiles of myrrh samples 2 and 3 (Figure 7) were significantly distinct from those of the other samples. The attempt to collect an authenticated oleo-resin sample of C. myrrha was unsuccessful; thus, it is not possible to compare the chemical fingerprint with a reliable reference sample.
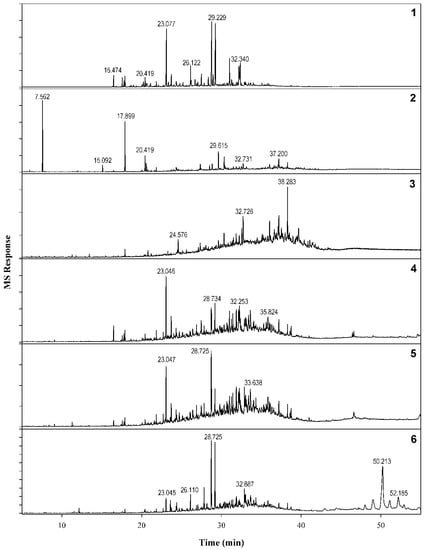
Figure 7.
Comparative GC-MS profiles of various samples of C. myrrha. Specimens: (1) #3550, (2) #2092, (3) #5369, (4) #6452, (5) #8549, and (6) #8986.
4. Discussion
The high-throughput chemical method HTS-DCYA™ for the identification of potential skin sensitizers in botanical ingredients can provide rapid reactivity screening of complex mixtures. The reactivity data obtained can provide insight into the presence of potential sensitizers while minimizing the need for animal testing.
The method was tested here on a myrrh extract to identify potential sources of concern due to reported skin sensitization literature on myrrh. Reactivity-guided fractionation led to the isolation of ten known compounds commonly occurring in various Commiphora species. Three of the minor compounds isolated were found to be more reactive than the positive control (cinnamaldehyde) and thus potentially able to trigger the MIE for skin sensitization. Two of the major constituents of the analyzed extract were also reactive, although to a minor extent.
Oxygenated furanoterpenoids are reported constituents of C. myrrha but also of other related Commiphora species, including C. sphaeorocarpa [17], C. erythreaea [21,22], C. opobalsamum [23,24], and C. holtziana [25]. The presence of the compounds here isolated in at least four of the analyzed extracts shows nonetheless that, in spite of the authenticity of the botanical source, 6-oxofuranoterpenoids are commonly found in commercial C. myrrha sources.
Further experiments on the ability of myrrh compounds to elicit inflammatory responses in keratinocytes and dendritic cells (key events 2 and 3) [26,27,28] are required to understand whether these compounds may be responsible for the reported ACD adverse effects of myrrh. It thus seems that the rare occurrence of skin sensitization related to myrrh may be related to the presence of multiple minor reactive compounds in combination with weakly reactive methoxygermacranes. The common occurrence of myrrh sesquiterpenoids in other related Commiphora species [15] suggests that these other sources should also be monitored for evidence of potential skin sensitization adverse effects.
Author Contributions
Conceptualization, C.A. and A.G.C.; methodology, Q.Z. and Y.L.; formal analysis, M.W.; investigation, C.A., Q.Z., Y.L., Y.S. and Y.T.; resources, C.A. and A.G.C.; writing—original draft preparation, C.A.; writing—review and editing, A.G.C.; supervision, C.A.; project administration, A.G.C.
Funding
This research was funded by USDA “Discovery & Development of Natural Products for Pharmaceutical & Agrichemical Applications” grant number 58-606-6-015.
Acknowledgments
The authors are sincerely thankful to Ikhlas Khan and Wei Wang for their contribution in providing the resources necessary to the success of the project. We thank the KUMS Deputy of Research and Technology for providing financial support to Y.S. for a visiting scholarship at NCNPR. We are thankful for the support from the Pharmaceutical First-Class Discipline of Hunan Province, P.R. China. We also thank Yan-Hong Wang for their assistance with HPLC-MS-MS experiments.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ACN, acetonitrile; ACD, allergic contact dermatitis; AOP, adverse outcome pathway; CINN, cinnamaldehyde; DCVC, dry column vacuum chromatography; DCYA, dansyl cysteamine; GC-MS, gas chromatography coupled to mass spectrometry; HTS, high-throughput screening; LLNA, local lymph node assay; MIE, molecular initiating event; MME, myrrh methanol extract; NMR, nuclear magnetic resonance; RI, reactive index; TRI, total reactive index; TLC, thin-layer chromatography.
References
- United States Pharmacopeial Convention. Myrrh Topical Solution. In United States Pharmacopeia, 7th ed.; National Formulary 29th Edition (USP 34-NF 29); United States Pharmacopeial Convention: Rockville, MD, USA, 2011; pp. 3582–3583. [Google Scholar]
- Hanuš, L.O.; Řezanka, T.; Dembitsky, V.M.; Moussaieff, A. Myrrh-commiphora chemistry. Biomed. Pap. 2005, 149, 3–28. [Google Scholar] [CrossRef]
- El Ashry, E.; Rashed, N.; Salama, O.; Saleh, A. Components, therapeutic value and uses of myrrh. Pharmazie 2003, 58, 163–168. [Google Scholar] [PubMed]
- Tucker, A.O. Frankincense and myrrh. Econ. Bot. 1986, 40, 425–433. [Google Scholar] [CrossRef]
- Lee, T.; Lam, T. Allergic contact dermatitis due to a Chinese orthopaedic solution Tieh Ta Yao Gin. Contact Dermat. 1993, 28, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lam, T. Myrrh is the putative allergen in bonesetter’s herbs dermatitis. Contact Dermat. 1993, 29, 279. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.; Rivara, G.; Cattarini, G.; Cozzani, E.; Guarrera, M. Allergic contact dermatitis from myrrh. Contact Dermat. 1999, 41, 230–231. [Google Scholar] [CrossRef]
- Al-Suwaidan, S.N.; Rab, M.O.G.; Al-Fakhiry, S.; Hoqail, I.A.; Al-Maziad, A.; Sherif, A.B. Allergic contact dermatitis from myrrh, a topical herbal medicine used to promote healing. Contact Dermat. 1998, 39, 137. [Google Scholar] [CrossRef] [PubMed]
- Kimber, I.; Basketter, D.; Gerberick, G.; Ryan, C.; Dearman, R. Chemical allergy: Translating biology into hazard characterization. Toxicol. Sci. 2011, 120 (Suppl. 1), S238–S268. [Google Scholar] [CrossRef]
- Lv, L.; Yan, G.-Y.; Zhao, Y.-L.; He, X.-J.; Jiang, X.; Zhuo, Y.-Q.; Wang, Y.-L.; Wang, L.; Cen, X.-B. Investigation of the dermal sensitizing potential of traditional medical extracts in local lymph node assays. Exp. Biol. Med. 2009, 234, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.B.; Strickland, J.; Allen, D.; Casati, S.; Zuang, V.; Barroso, J.; Whelan, M.; Régimbald-Krnel, M.; Kojima, H.; Nishikawa, A. International regulatory requirements for skin sensitization testing. Regul. Toxicol. Pharmacol. 2018, 95, 52–65. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 442C: In Chemico Skin Sensitisation: Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef]
- Avonto, C.; Chittiboyina, A.G.; Sadrieh, N.; Vukmanovic, S.; Khan, I.A. In chemico skin sensitization risk assessment of botanical ingredients. J. Appl. Toxicol. 2018, 38, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Chittiboyina, A.G.; Avonto, C.; Rua, D.; Khan, I.A. Alternative testing methods for skin Sensitization: NMR spectroscopy for probing the reactivity and classification of potential skin sensitizers. Chem. Res. Toxicol. 2015, 28, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Dekebo, A.; Dagne, E.; Sterner, O. Furanosesquiterpenes from Commiphora sphaerocarpa and related adulterants of true myrrh. Fitoterapia 2002, 73, 48–55. [Google Scholar] [CrossRef]
- Avonto, C.; Chittiboyina, A.G.; Rua, D.; Khan, I.A. A fluorescence high throughput screening method for the detection of reactive electrophiles as potential skin sensitizers. Toxicol. Appl. Pharmacol. 2015, 289, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Dekebo, A.; Dagne, E.; Hansen, L.K.; Gautun, O.R.; Aasen, A.J. Crystal structures of two furanosesquiterpenes from Commiphora sphaerocarpa. Tetrahedron Lett. 2000, 41, 9875–9878. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hatakeyama, S.; Tanaka, N.; Fukuda, Y.; Murakami, N.; Yamahara, J. Orientalols A, B, and C, sesquiterpene constituents from Chinese Alismatis rhizoma, and revised structures of alismol and alismoxide. Chem. Pharm. Bull. 1992, 40, 2582–2584. [Google Scholar] [CrossRef]
- Zhu, N.; Kikuzaki, H.; Sheng, S.; Sang, S.; Rafi, M.M.; Wang, M.; Nakatani, N.; DiPaola, R.S.; Rosen, R.T.; Ho, C.-T. Furanosesquiterpenoids of Commiphora myrrha. J. Nat. Prod. 2001, 64, 1460–1462. [Google Scholar] [CrossRef]
- Brieskorn, C.H.; Noble, P. Three new furanogermacrenes from myrrh. Tetrahedron Lett. 1980, 21, 1511–1514. [Google Scholar] [CrossRef]
- Santoro, E.; Messina, F.; Marcotullio, M.C.; Superchi, S. Absolute configuration of bioactive furanogermacrenones from Commiphora erythraea (Ehrenb) Engl. by computational analysis of their chiroptical properties. Tetrahedron 2014, 70, 8033–8039. [Google Scholar] [CrossRef]
- Cenci, E.; Messina, F.; Rossi, E.; Epifano, F.; Marcotullio, M.C. Antiviral furanosesquiterpenes from Commiphora erythraea. Nat. Prod. Commun. 2012, 7, 143–144. [Google Scholar] [CrossRef]
- Shen, T.; Wan, W.; Yuan, H.; Kong, F.; Guo, H.; Fan, P.; Lou, H. Secondary metabolites from Commiphora opobalsamum and their antiproliferative effect on human prostate cancer cells. Phytochemistry 2007, 68, 1331–1337. [Google Scholar] [CrossRef]
- Yang, J.-L.; Shi, Y.-P. Cycloartane-type triterpenoids and sesquiterpenoids from the resinous exudates of Commiphora opobalsamum. Phytochemistry 2012, 76, 124–132. [Google Scholar] [CrossRef]
- Provan, G.J.; Gray, A.I.; Waterman, P.G. Sesquiterpenes from the myrrh-type resins of some Kenyan Commiphora species. Flavour Fragr. J. 1987, 2, 109–113. [Google Scholar] [CrossRef]
- OECD. Test No. 442D: In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2018. [Google Scholar] [CrossRef]
- OECD. Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2018. [Google Scholar] [CrossRef]
- Chittiboyina, A.G.; Khan, I.A.; Avonto, C. Methods for Detecting and Categorizing Skin Sensitizers. U.S. Patent 10261017, 16 April 2019. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).