Skin Improvement Effects of Gardeniae fructus Extract in HaCaT Keratinocytes, B16F10 Melanocytes, and CCD-986sk Fibroblast Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Gardeniae Fructus Extract (GF) Preparation
2.2. Cell Culture and UV-B Irradiation
2.3. WST-1 Assay
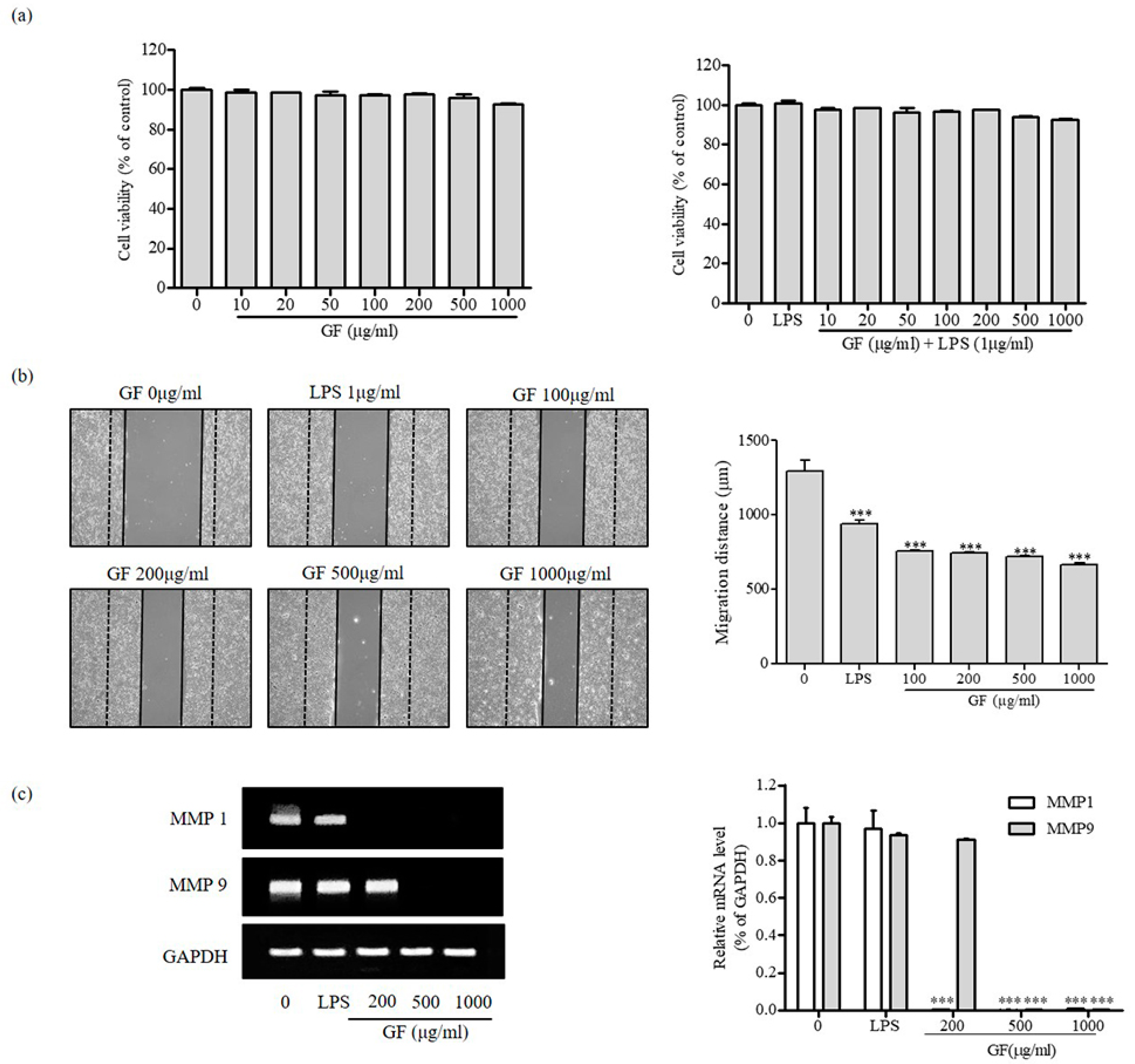
2.4. Cell Migration
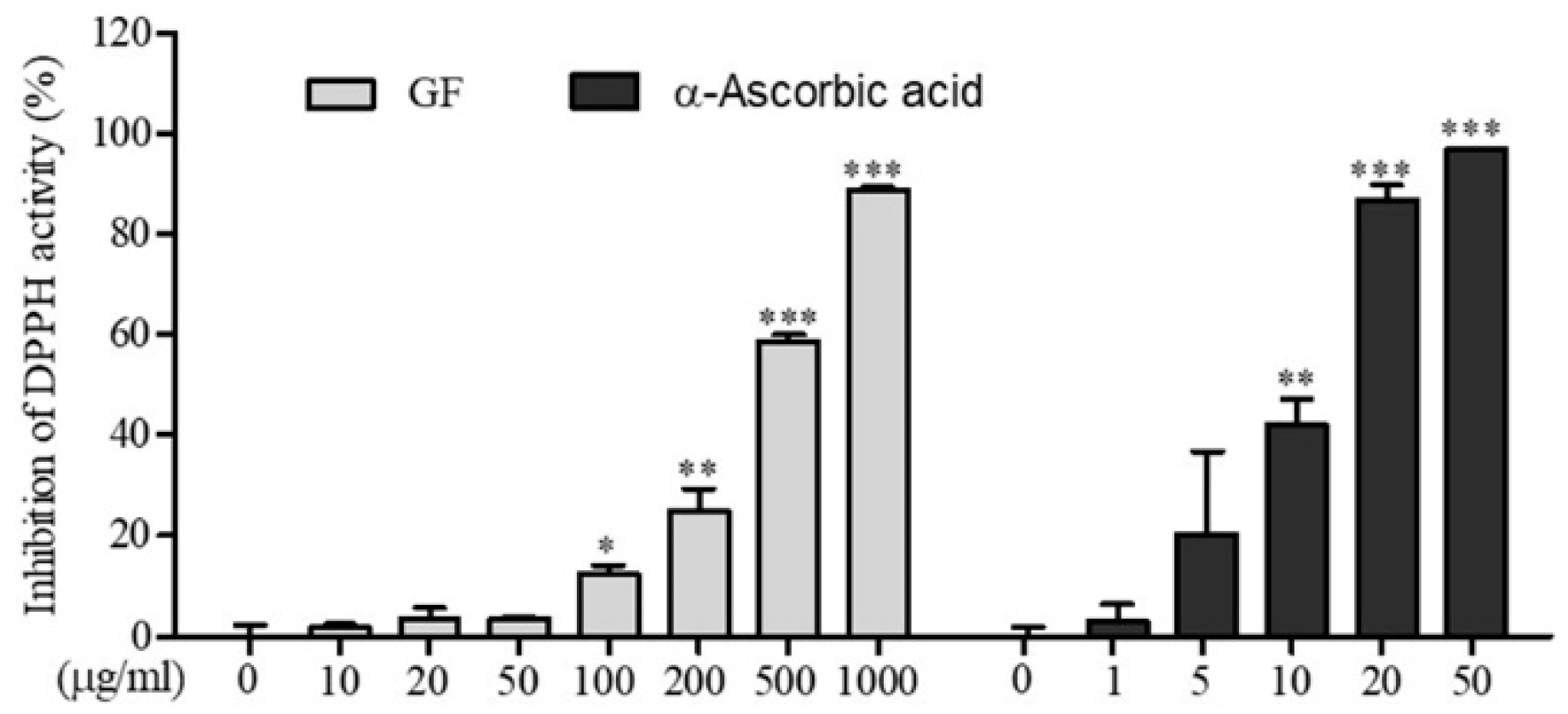
2.5. DPPH Free Radical Scavenging Activity Assay
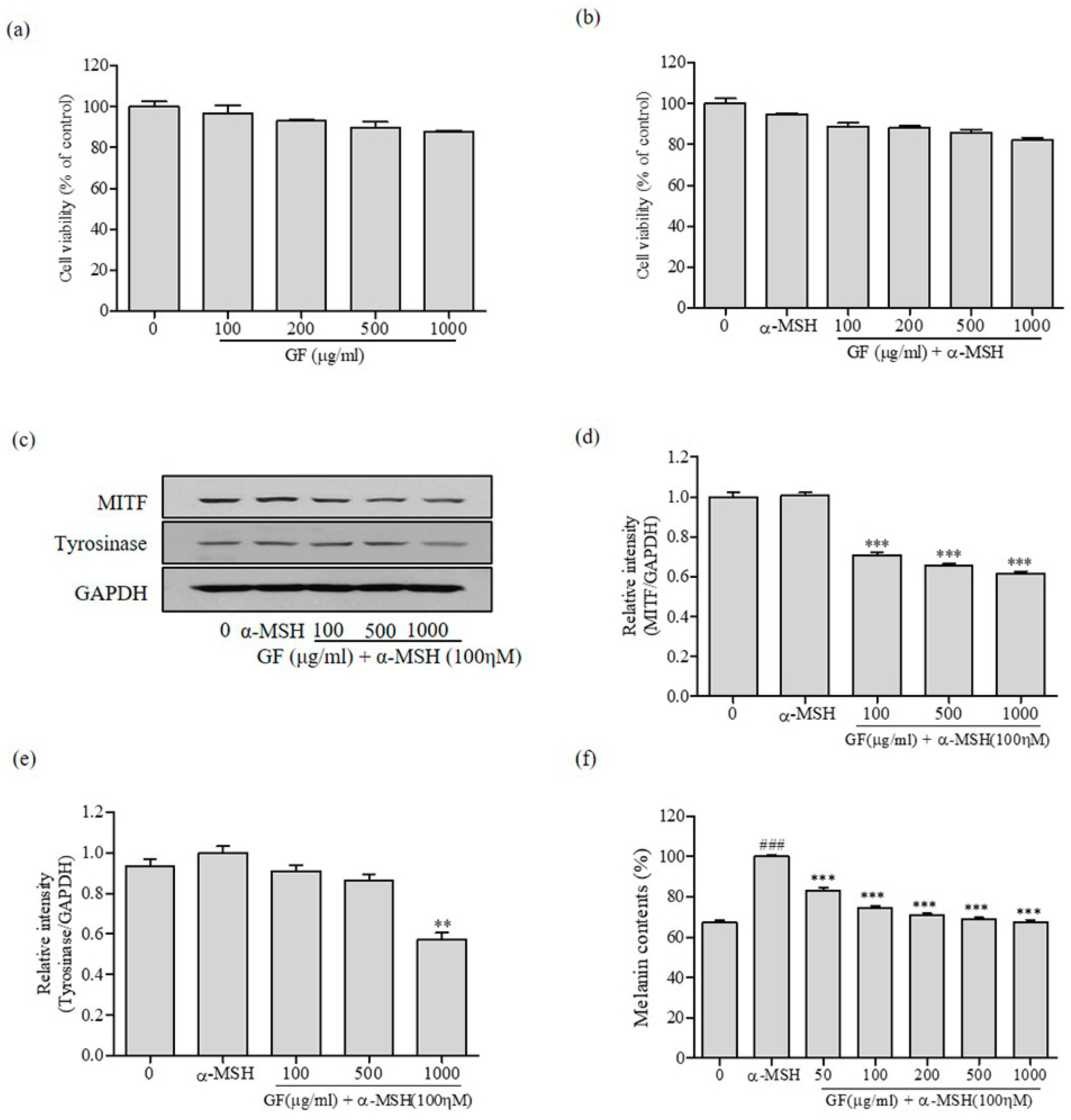
2.6. Melanin Content Assay
2.7. Reverse Transcription-Polymerase Chain Reaction
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. In Vitro Antioxidant Activity of GF
3.2. Inhibition of Melanin Production by GF in B16F10 Melanoma Cells
3.3. Inhibition of MMP1 and MMP9 Expression by GF in HaCaT Keratinocyte Cells
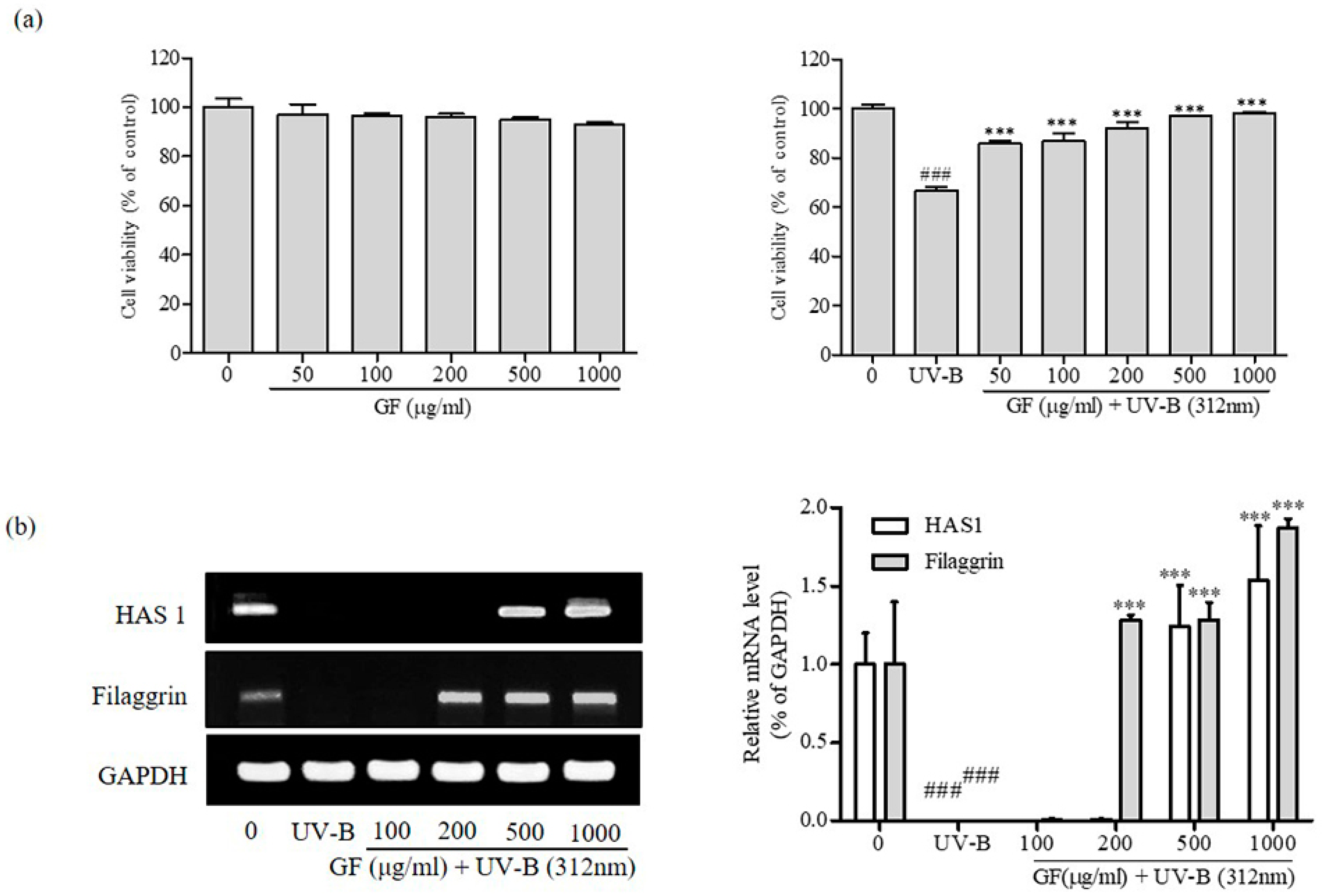
3.4. Activation of HAS1 and Filaggrin Expression by GF in CCD-986sk Fibroblast Cells
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Ethics Approval
Consent for Publication
Availability of Data and Materials
References
- Freeman, B.A.; Crapo, J.D. Biology of disease: Free radicals and tissue injury. Lab. Investig. 1982, 47, 412–426. [Google Scholar] [PubMed]
- Chiang, H.M.; Chen, H.C.; Lin, T.J.; Shih, I.C.; Wen, K.C. Michelia alba extract attenuates UVB-induced expression of matrix metalloproteinases via MAP kinase pathway in human dermal fibroblasts. Food Chem. Toxicol. 2012, 50, 4260–4269. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A. Skin aging and photoaging. Dermatol. Nurs. 1990, 2, 79–82. [Google Scholar] [PubMed]
- Maddodi, N.; Jayanthy, A.; Setaluri, V. Shining light on skin pigmentation: The darker and the brighter side of effects of UV radiation. Photochem. Photobiol. 2012, 88, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Stratigos, A.J.; Rigopoulos, D.; Katsambas, A.D. A review of genetic disorders of hypopigmentation: Lessons learned from the biology of melanocytes. Exp. Dermatol. 2009, 18, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N. Plants and Natural Products for the Treatment of Skin Hyperpigmentation—A Review. Planta Med. 2018, 84, 988–1006. [Google Scholar] [CrossRef] [PubMed]
- Garraway, L.A.; Widlund, H.R.; Rubin, M.A.; Getz, G.; Berger, A.J.; Ramaswamy, S.; Beroukhim, R.; Milner, D.A.; Granter, S.R.; Du, J.; et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 2005, 436, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.J.; Fisher, D.E. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Arch. Biochem. Biophys. 2014, 563, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Ock, S.M.; Kim, H.J.; Lee, Y.B.; Choi, J.M.; Cho, C.S.; Lee, J.Y.; Cho, B.K.; Cho, D.H. Vitamin C attenuates ERK signalling to inhibit the regulation of collagen production by LL-37 in human dermal fibroblasts. Exp. Dermatol. 2010, 19, e258–e264. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Piao, M.J.; Han, X.; Kang, K.A.; Kang, H.K.; Yoon, W.J.; Ko, M.H.; Lee, N.H.; Lee, M.Y.; Chae, S.; et al. Anti-wrinkle effects of Sargassum muticum ethyl acetate fraction on ultraviolet B-irradiated hairless mouse skin and mechanistic evaluation in the human HaCaT keratinocyte cell line. Mol. Med. Rep. 2016, 14, 2937–2944. [Google Scholar] [CrossRef][Green Version]
- Albanesi, C.; De Pita, O.; Girolomoni, G. Resident skin cells in psoriasis: A special look at the pathogenetic functions of keratinocytes. Clin. Dermatol. 2007, 25, 581–588. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. Isolation and culture of skin fibroblasts. Methods Mol. Med. 2005, 117, 83–98. [Google Scholar] [PubMed]
- Choi, J.W.; Kwon, S.H.; Huh, C.H.; Park, K.C.; Youn, S.W. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: A comprehensive and objective approach. Skin Res. Technol. 2013, 19, e349–e355. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Yang, L.; Zhao, S.; Chen, L.; Zhao, F.; Qiu, F. Chemical constituents from the fruit of Gardenia jasminoides and their inhibitory effects on nitric oxide production. Bioorg. Med. Chem. Lett. 2013, 23, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Yang, K.S. Anti-inflammatory activities of crocetin derivatives from processed Gardenia jasminoides. Arch. Pharmacal Res. 2013, 36, 933–940. [Google Scholar] [CrossRef]
- Nagaki, Y.; Hayasaka, S.; Abe, T.; Zhang, X.Y.; Hayasaka, Y.; Terasawa, K. Effects of oral administration of Gardeniae fructus extract and intravenous injection of crocetin on lipopolysaccharide- and prostaglandin E2-induced elevation of aqueous flare in pigmented rabbits. Am. J. Chin. Med. 2003, 31, 729–738. [Google Scholar] [CrossRef]
- Zang, C.X.; Bao, X.Q.; Li, L.; Yang, H.Y.; Wang, L.; Yu, Y.; Wang, X.L.; Yao, X.S.; Zhang, D. The Protective Effects of Gardenia jasminoides (Fructus Gardenia) on Amyloid-beta-Induced Mouse Cognitive Impairment and Neurotoxicity. Am. J. Chin. Med. 2018, 46, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Cai, X.; Zhang, Q.; de Freitas, V.; Mateus, N.; He, J.; Fernandes, I. Gastrointestinal absorption, antiproliferative and anti-inflammatory effect of the major carotenoids of Gardenia jasminoides Ellis on cancer cells. Food Funct. 2017, 8, 1672–1679. [Google Scholar] [CrossRef]
- Lin, W.H.; Kuo, H.H.; Ho, L.H.; Tseng, M.L.; Siao, A.C.; Hung, C.T.; Jeng, K.C.; Hou, C.W. Gardenia jasminoides extracts and gallic acid inhibit lipopolysaccharide-induced inflammation by suppression of JNK2/1 signaling pathways in BV-2 cells. Iran J. Basic Med. Sci. 2015, 18, 555–562. [Google Scholar]
- Jameel, F.; Phang, M.; Wood, L.G.; Garg, M.L. Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation. Lipids Health Dis. 2014, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985, 45, 1474–1478. [Google Scholar] [PubMed]
- Russo, A.; Cardile, V.; Lombardo, L.; Vanella, L.; Vanella, A.; Garbarino, J.A. Antioxidant activity and antiproliferative action of methanolic extract of Geum quellyon Sweet roots in human tumor cell lines. J. Ethnopharmacol. 2005, 100, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Chakraborty, A.K.; Dey, P.; Kar, P.; Guha, P.; Sen, S.; Kumar, A.; Sen, A.; Chaudhuri, T.K. Amelioration of CCl4 induced liver injury in swiss albino mice by antioxidant rich leaf extract of Croton bonplandianus Baill. PLoS ONE 2018, 13, e0196411. [Google Scholar] [CrossRef] [PubMed]
- Kippenberger, S.; Loitsch, S.; Solano, F.; Bernd, A.; Kaufmann, R. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR-regulation by steroid hormones. J. Investig. Dermatol. 1998, 110, 364–367. [Google Scholar] [PubMed]
- Vaalamo, M.; Mattila, L.; Johansson, N.; Kariniemi, A.L.; Karjalainen-Lindsberg, M.L.; Kahari, V.M.; Saarialho-Kere, U. Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J. Investig. Dermatol. 1997, 109, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Thompson, P.; Kelso, I.; Jackson, C. Activated protein C stimulates proliferation, migration and wound closure, inhibits apoptosis and upregulates MMP-2 activity in cultured human keratinocytes. Exp. Cell Res. 2004, 299, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Nakamizo, S.; Egawa, G.; Doi, H.; Natsuaki, Y.; Miyachi, Y.; Kabashima, K. Topical treatment with basic fibroblast growth factor promotes wound healing and barrier recovery induced by skin abrasion. Skin Pharmacol. Physiol. 2013, 26, 22–29. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.H.; Ku, J.M.; Lee, S.H.; Shim, H.J.; Park, D.S.; Sung, J.W.; Shin, Y.C.; Ko, S.-G. Skin Improvement Effects of Gardeniae fructus Extract in HaCaT Keratinocytes, B16F10 Melanocytes, and CCD-986sk Fibroblast Cells. Cosmetics 2019, 6, 48. https://doi.org/10.3390/cosmetics6030048
Hong SH, Ku JM, Lee SH, Shim HJ, Park DS, Sung JW, Shin YC, Ko S-G. Skin Improvement Effects of Gardeniae fructus Extract in HaCaT Keratinocytes, B16F10 Melanocytes, and CCD-986sk Fibroblast Cells. Cosmetics. 2019; 6(3):48. https://doi.org/10.3390/cosmetics6030048
Chicago/Turabian StyleHong, Se Hyang, Jin Mo Ku, Seung Hwan Lee, Ho Jong Shim, Dong Sun Park, Joo Won Sung, Yong Cheol Shin, and Seong-Gyu Ko. 2019. "Skin Improvement Effects of Gardeniae fructus Extract in HaCaT Keratinocytes, B16F10 Melanocytes, and CCD-986sk Fibroblast Cells" Cosmetics 6, no. 3: 48. https://doi.org/10.3390/cosmetics6030048
APA StyleHong, S. H., Ku, J. M., Lee, S. H., Shim, H. J., Park, D. S., Sung, J. W., Shin, Y. C., & Ko, S.-G. (2019). Skin Improvement Effects of Gardeniae fructus Extract in HaCaT Keratinocytes, B16F10 Melanocytes, and CCD-986sk Fibroblast Cells. Cosmetics, 6(3), 48. https://doi.org/10.3390/cosmetics6030048



