Abstract
Intrinsic skin aging and photoaging, from exposure to ultraviolet (UV) radiation, are associated with altered regulation of genes associated with the extracellular matrix (ECM) and inflammation, as well as cellular damage from oxidative stress. The regulatory properties of 1α, 25dihydroxyvitamin D3 (vitamin D) include endocrine, ECM regulation, cell differentiation, photoprotection, and anti-inflammation. The goal of this research was to identify the beneficial effects of vitamin D in preventing intrinsic skin aging and photoaging, through its direct effects as well as its effects on the ECM, associated heat shock proteins (HSP-47, and -70), cellular oxidative stress effects, and inflammatory cytokines [interleukin (IL)-1 and IL-8] in non-irradiated, UVA-radiated, UVB-radiated dermal fibroblasts. With regard to the ECM, vitamin D stimulated type I collagen and inhibited cellular elastase activity in non-irradiated fibroblasts; and stimulated type I collagen and HSP-47, and inhibited elastin expression and elastase activity in UVA-radiated dermal fibroblasts. With regard to cellular protection, vitamin D inhibited oxidative damage to DNA, RNA, and lipids in non-irradiated, UVA-radiated and UVB-radiated fibroblasts, and, in addition, increased cell viability of UVB-radiated cells. With regard to anti-inflammation, vitamin D inhibited expression of Il-1 and IL-8 in UVA-radiated fibroblasts, and stimulated HSP-70 in UVA-radiated and UVB-radiated fibroblasts. Overall, vitamin D is predominantly beneficial in preventing UVA-radiation induced photoaging through the differential regulation of the ECM, HSPs, and inflammatory cytokines, and protective effects on the cellular biomolecules. It is also beneficial in preventing UVB-radiation associated photoaging through the stimulation of cell viability and HSP-70, and the inhibition of cellular oxidative damage, and in preventing intrinsic aging through the stimulation of type I collagen and inhibition of cellular oxidative damage.
1. Introduction
The structural integrity of the extracellular matrix (ECM) is essential to anti-aging [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. The structural ECM is composed largely of collagen and elastin fibers, which are synthesized by the dermal fibroblasts [9,10,11,12,13,14,15,16]. The structural interstitial collagen consists primarily of type I collagen and relies on heat shock protein-47 (HSP-47) for its folding [3,9,10,11,12,17,18]. The elastin fiber, which provides elasticity and resilience to the skin, is predominantly composed of elastin protein and is degraded by elastases [1,11,13,14,15,16,19,20,21,22,23]. Skin aging is associated with general atrophy of the ECM and reduced levels of collagen and elastin proteins and manifests as wrinkles and loss of skin firmness [1,3,9,11,12,13,14,15,16,18,19]. Environmental factors, predominantly ultraviolet radiation (UV) radiation, superimpose on intrinsic skin aging to cause the loss of collagen fibers and the addition of elastotic deposits, which manifests as added wrinkles and coarse skin [1,3,12,14,16,21,22,23]. The expression of collagen is inhibited by UVA-radiation (320–400 nm) and UVB- radiation (290–320 nm) in dermal fibroblasts [3,12]. The expression of elastin is stimulated by UVA radiation and inhibited by UVB radiation in dermal fibroblasts [1,14,16].
The mechanisms of skin aging and photoaging includes oxidative stress from reactive oxygen species (ROS) and inflammation from inflammatory cytokines [4,5,7,8,21,22,23,24,25,26,27,28,29,30]. The ROS are primarily superoxide, hydroxyl radicals, hydrogen peroxide, and singlet oxygen, and the inflammatory cytokines are primarily interleukin (IL)-1, IL-6, IL-8 and tumor necrosis factor-a (TNF-a) [4,5,7,8,28,29,30]. The HSP-70, with its anti-inflammatory activity, mediates photoprotective effect on HSP-70 expressing transgenic mice [21]. The ROS and inflammatory mediators alter cellular signal transduction pathways, such as the mitogen activated protein kinase (MAPK), Janus Kinases (JAK), and nuclear factor-kB (NF-kB) pathways, to alter the expression of the ECM regulatory factors and thereby the ECM proteins [4,5,7,8,17,28]. In addition to the atrophy of the ECM, the oxidative stress and inflammatory mediators damage the biomolecules and the integrity of the cells [4,5,7,8,21,22,23,24,25,26,27]. The ROS directly damage DNA to generate 8-oxo-7, 8-dihydro-2′-deoxyguanosine (8-oxo-dG) and pyrimidine dimmers, proteins to generate carbonyl amino acid derivatives, and lipids to generate lipid peroxides and lipid inflammatory mediators [4,5,24,25,26,27]. Phenolic compounds have anti-oxidative and anti-inflammatory properties, and thereby anti-aging properties, because of their structure and the number and location of their hydroxyl groups [1,2,3,4,5,31,32,33].
The structure of 1α, 25dihydroxyvitamin D3 (vitamin D) and its endocrine role in cellular homeostasis as well as its photoprotective and anti-inflammatory role makes it essential to skin health [34,35,36,37,38,39,40,41,42,43,44,45]. Vitamin D, or its analog, stimulates the expression of collagen and counteracts the glucocorticoid mediated inhibition of collagen synthesis in dermal fibroblasts [35,36]. Vitamin D inhibits the expression of elastin in several cell types [37]. Vitamin D increases the survival of UVB radiated keratinocytes, through the stimulation of p53 and the inhibition of nitric oxide products [38]. It inhibits 8-oxo-dG, thymine dimers, and the cyclobutane pyrimidine dimer in human ex-vivo UVB radiated skin, solar simulated UV radiated mice skin, or keratinocytes [39,40,41,42]. Vitamin D inhibits the expression of IL-6 and IL-8 in IL-1β treated human gingivial or nasal polyposis fibroblasts and stimulates HSP-70 in monocytes [43,44,45].
The hypothesis of this research was that the structure and the endocrine, photoprotective, and anti-inflammatory properties of 1α, 25 dihydroxyvitaminD3 (vitamin D) would lend to anti-aging through the direct inhibition of ROS and elastase activity, and the beneficial regulation of the ECM (collagen, elastin and HSP-47), oxidative stress effects (cell viability, membrane integrity, oxidative DNA/RNA damage, and lipid peroxidation) and inflammatory modulators (IL-1, IL-8, and HSP-70) in non-irradiated, UVA-radiated and UVB-radiated fibroblasts.
2. Results
2.1. Direct Effects of 1α, 25 dihydroxyvitamin D3 (Vitamin D) on ABTS® (2, 2′-azino-di-[3-ethylbenzthiazoline sulphonate]) Oxidation and Elastase Activity
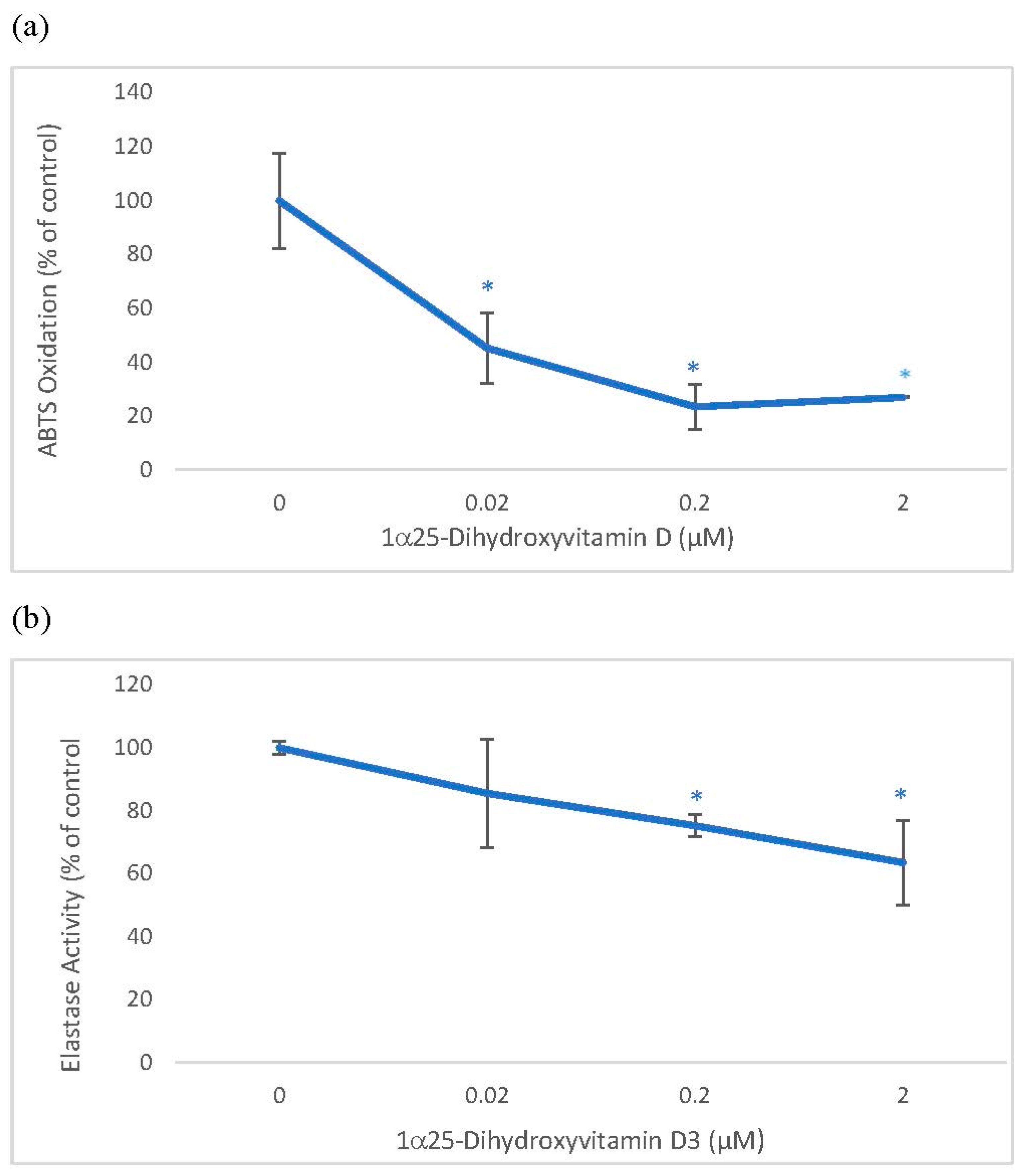
Vitamin D was directly inhibitory to ABTS oxidation and elastase activity. Vitamin D at 0.02, 0.2 and 2 µM significantly inhibited ABTS oxidation to 45%, 24%, and 27% of the control, and at 0.2 and 2 µM, it significantly inhibited elastase activity to 75% and 63% of the control (p < 0.05) (Figure 1).
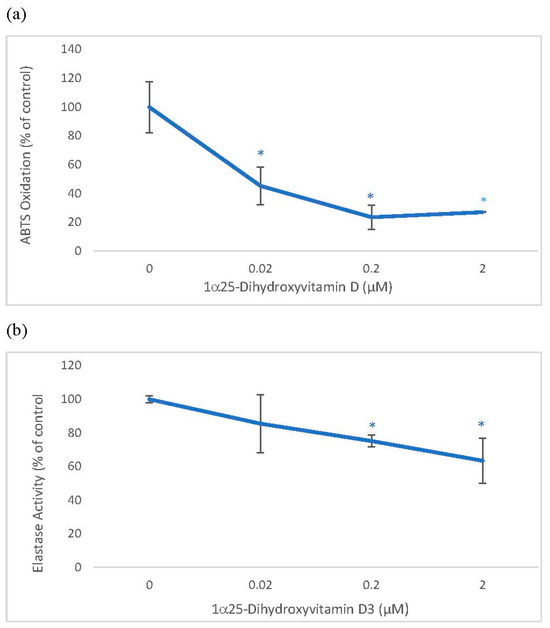
Figure 1.
Direct Effects of 1α, 25 dihydroxyvitaminD3 (Vitamin D) on ABTS® (2, 2′-azino-di-[3-ethylbenzthiazoline sulphonate]) Oxidation and Elastase activity. Vitamin D (0–2 µM) was incubated with respective assay reactants and products measured to determine its effect on ABTS oxidation (a), and elastase activity (b). The effect of vitamin D is represented as % of control (0 µM), represented at 100%; * = p < 0.05, relative to control. Error bars represent standard deviation, n = 4.
2.2. The Effect of 1α, 25 Dihydroxyvitamin D3 (Vitamin D) on Type I Collagen Promoter Activity, Type I Collagen Protein Levels, and Heat Shock Protein-47 (HSP-47) in Non-Irradiated, UVA-Radiated, and UVB-Radiated Fibroblasts
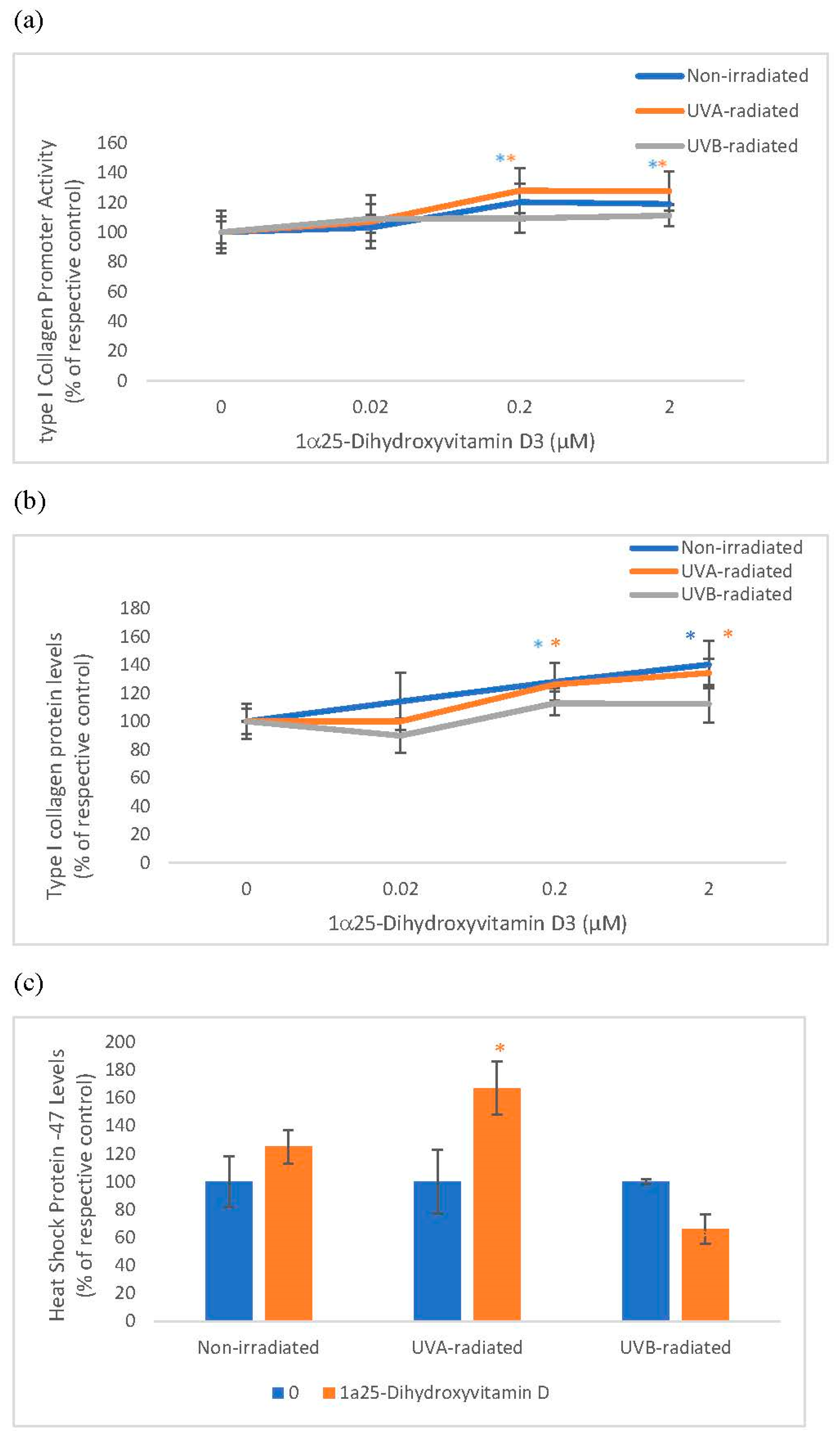
Vitamin D significantly and similarly stimulated collagen expression at promoter and protein levels in non-irradiated and UVA-radiated fibroblasts but not in UVB-radiated fibroblasts (Figure 2). Vitamin D significantly stimulated HSP-47 in UVA-radiated fibroblasts but not in non-radiated and UVB-radiated fibroblasts (Figure 2). UVA and UVB-radiation significantly inhibited collagen expression at promoter and protein levels, and UVA-radiation significantly inhibited HSP-47 in fibroblasts, as previously reported [3,12].
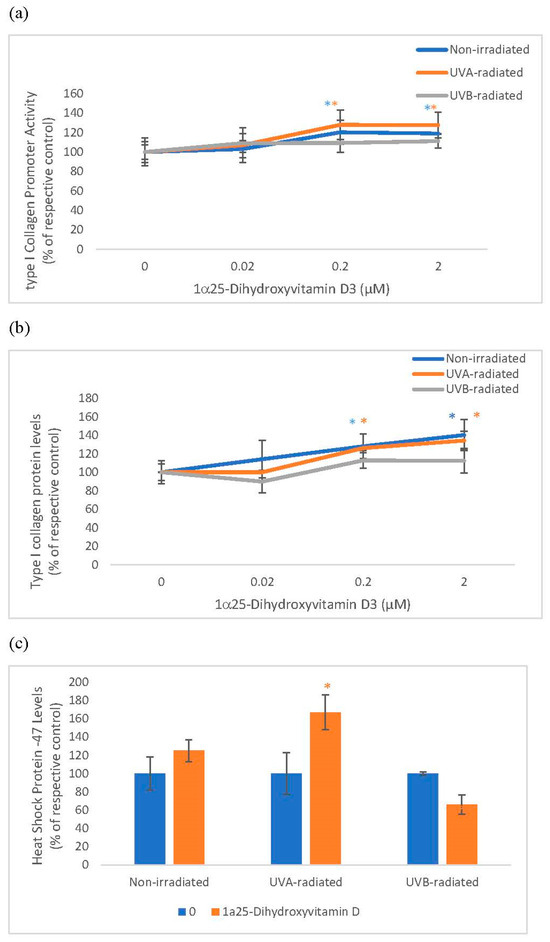
Figure 2.
The effect of 1α, 25 dihydroxyvitaminD3 (vitamin D) on type I Collagen Promoter Activity, type I Collagen Protein Levels, and Heat Shock Protein-47 (HSP-47) in non-irradiated, UVA-radiated, and UVB-radiated fibroblasts. Fibroblasts were co-transfected with COL1α1 promoter-firefly luciferase (pGL4 vector) and TK-hRenilla luciferase plasmids (for normalization) for 24 h, non-irradiated (blue), UVA-radiated (orange), or UVB-radiated (grey), incubated with vitamin D (0–2 µM) for 24 h, and assayed for luciferase activities (Promega, Madison, WI, USA) (a); and for type I collagen (b) and heat shock protein (HSP)-47 (c) protein levels by indirect ELISA (Kirkguaard and Perry Inc, Gaithersburg, MD, USA). * = p < 0.05, relative to respective controls. Error bars represent standard deviation, n = 4.
Relative to the respective controls (100%), vitamin D at 0.2 and 2 µM significantly stimulated the type I collagen promoter activity to 120% and 119% in non-irradiated fibroblasts and to 128% and 128% in UVA-radiated fibroblasts (p < 0.05) (Figure 2a). Relative to the respective controls (100%), vitamin D at 0.2 and 2 µM significantly, though similarly, stimulated type I collagen protein levels to 128% and 126% in non-irradiated fibroblasts and to 140% and 134% in UVA-radiated fibroblasts (p < 0.05) (Figure 2b). In UVA-radiated cells, vitamin D at 0.2 µM stimulated HSP-47 to 167% of the UVA-radiated control (p < 0.05) (Figure 2c).
2.3. The Effect of 1α, 25 Dihydroxyvitamin D3 (Vitamin D) on Elastin Promoter Activity, Elastin Protein Levels, and Elastase Activity in Non-Irradiated, UVA-Radiated, and UVB-Radiated Fibroblasts
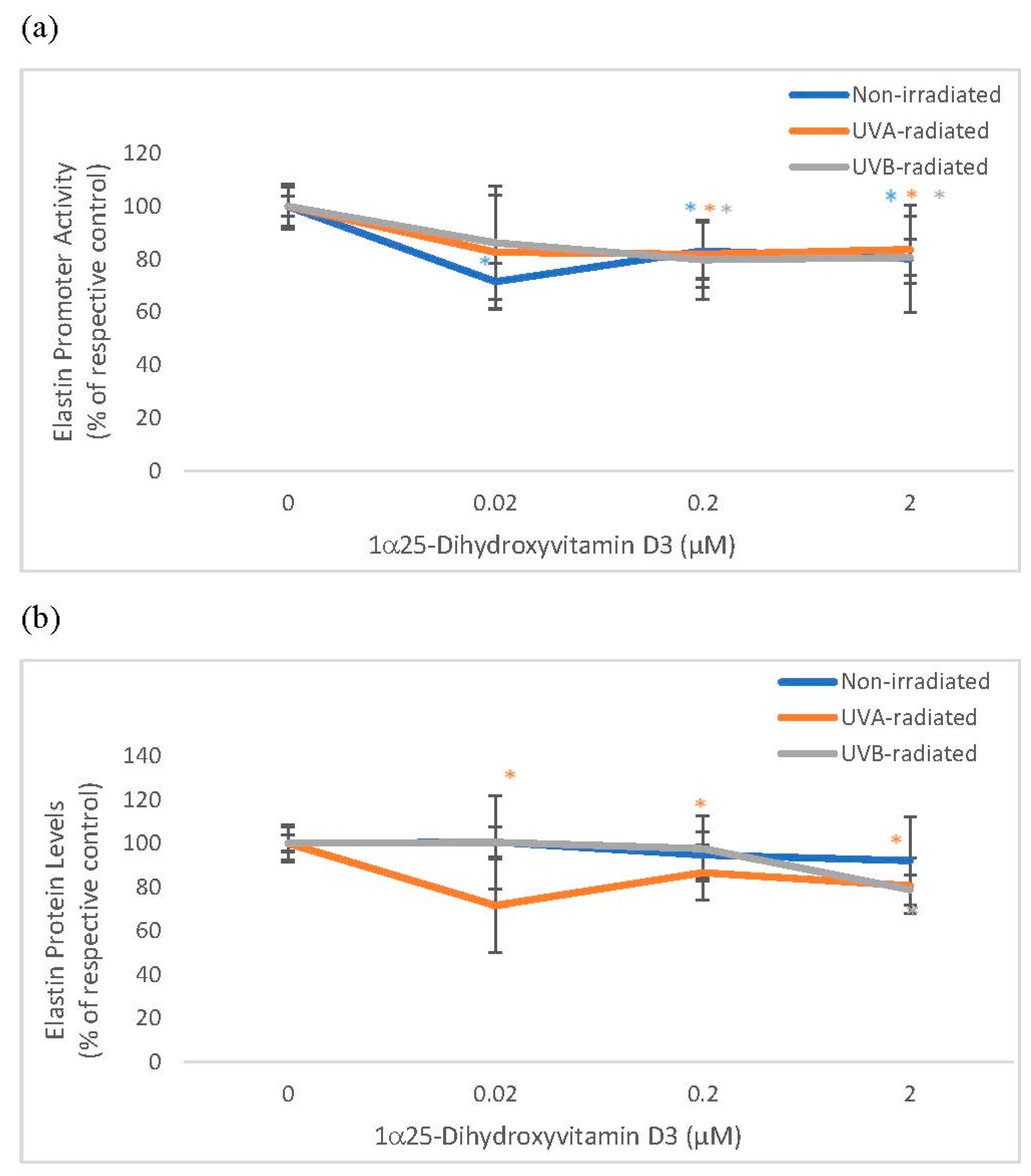
Vitamin D significantly and similarly inhibited elastin promoter activity in non-irradiated and UV-radiated fibroblasts and inhibited elastin protein levels in UVA radiated fibroblasts (Figure 3). Vitamin D significantly inhibited elastase activity in non-radiated and UVA-radiated fibroblasts but not in UVB-radiated fibroblasts (Figure 3). UVA-radiation significantly stimulated elastin expression at promoter and protein levels, and UVB-radiation significantly inhibited elastin expression at promoter and protein levels in fibroblasts, as previously reported [1,14,16]. UV radiation did not significantly alter elastase activity in dermal fibroblasts (data not shown).
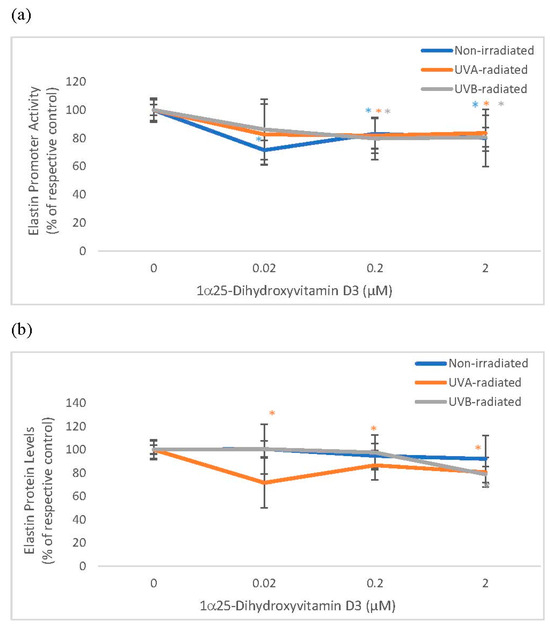
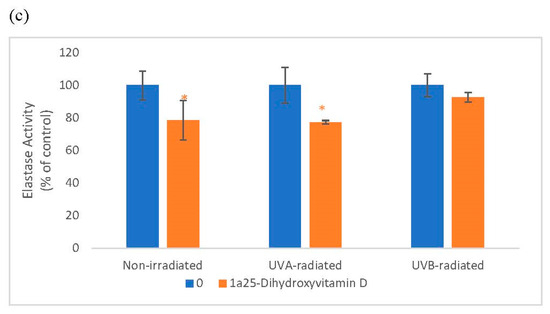
Figure 3.
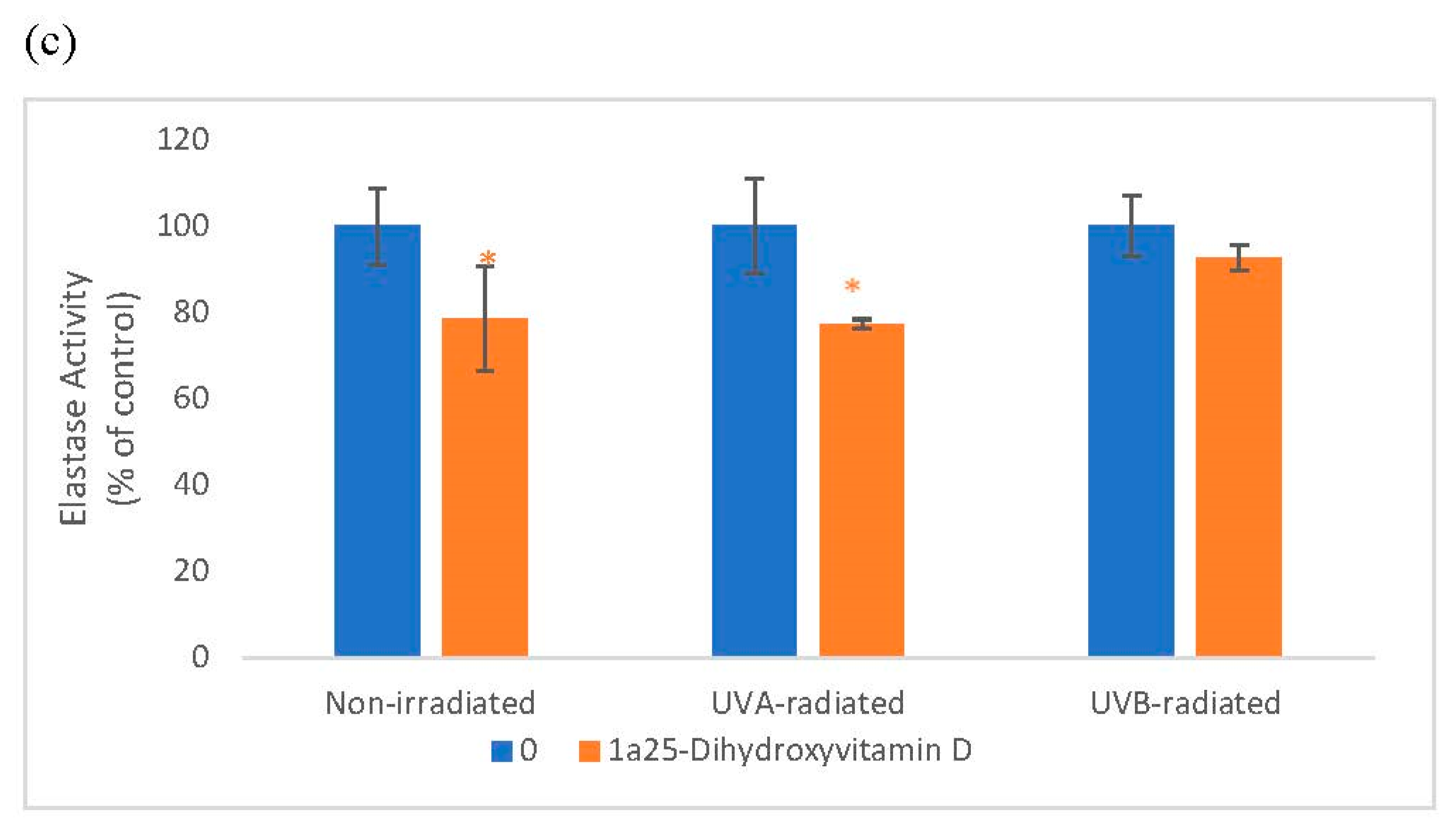
The effect of 1α, 25 dihydroxyvitaminD3 (vitamin D) on Elastin Promoter Activity, Elastin Protein Levels, and Elastase Activity in non-irradiated, UVA-radiated, and UVB-radiated fibroblasts. Fibroblasts were co-transfected with elastin promoter- firefly luciferase (pGL4 vector) and TK-hRenilla luciferase plasmids (for normalization) for 24 h, non-irradiated (blue), UVA-radiated (orange), or UVB-radiated (grey), incubated with vitamin D (0–2 µM) for 24 h, and assayed for luciferase activities (a); and for elastin protein levels by indirect ELISA (Kirkguaard and Perry Inc) (b). Non-irradiated, UVA-radiated, or UVB-radiated fibroblasts were incubated with vitamin D (0 or 0.2 µM) and examined for cellular elastase activity (c). * = p < 0.05, relative to respective controls. Error bars represent standard deviation, n = 4.
Relative to the respective controls (100%), vitamin D at 0.2 and 2 µM significantly inhibited elastin promoter activity to around 80% in non-irradiated, UVA-radiated, and UVB-radiated fibroblasts (p < 0.05) (Figure 3a). Relative to UVA-radiated control (100%), vitamin D at 0.02, 0.2, and 2 µM significantly inhibited elastin protein levels to 72%, 87%, and 81% in UVA-radiated fibroblasts (p < 0.05) (Figure 3b). Relative to the respective controls, vitamin D at 0.2 µM inhibited elastase activity to 79% in non-irradiated fibroblasts, and to 77% in the UVA-radiated control (p < 0.05) (Figure 3c).
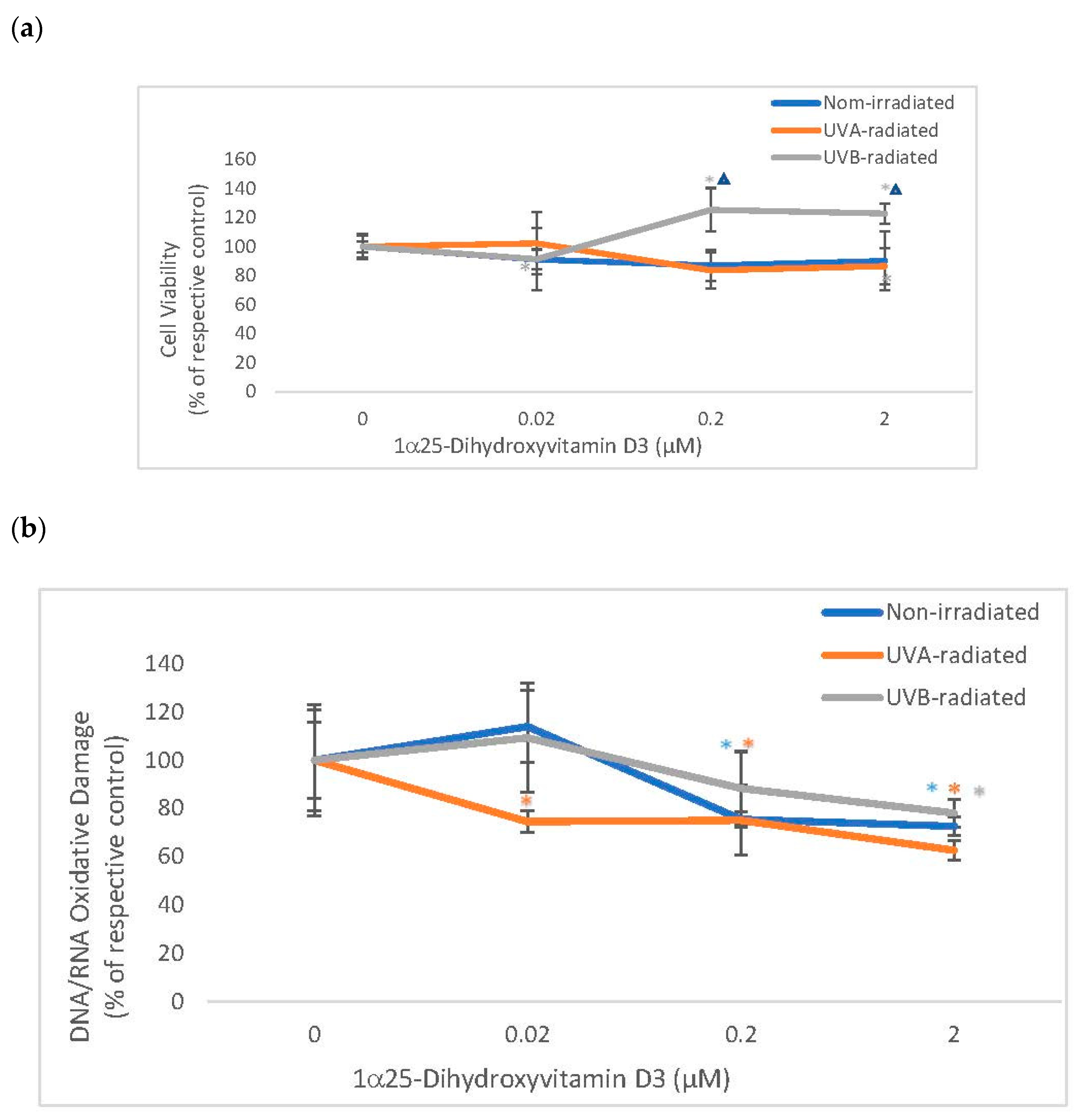
2.4. The Effect of 1α, 25 Dihydroxyvitamin D3 (Vitamin D) on Cell Viability and Oxidative DNA/RNA Damage in Non-Irradiated, UVA-Radiated, and UVB-Radiated Fibroblasts
Vitamin D significantly stimulated the viability of UVB-radiated fibroblasts (which was significantly different from its effect on non-irradiated fibroblasts) and did not alter the viability of non-irradiated or UVA radiated fibroblasts (Figure 4). Vitamin D significantly and similarly inhibited DNA/RNA oxidative damage in non-irradiated and UV-radiated fibroblasts (Figure 4). UVA or UVB radiation did not significantly alter the viability of fibroblasts, as previously reported [1,3,12,14,16]. UV radiation did not significantly cause DNA/RNA oxidative damage in dermal fibroblasts (data not shown).
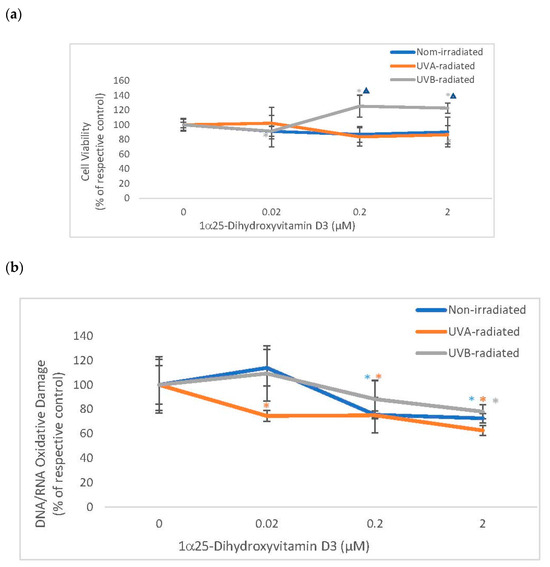
Figure 4.
The effect of 1α, 25dihydroxyvitamin D3 (vitamin D) on Cell Viability and Oxidative DNA/RNA Damage in non-irradiated, UVA-radiated, and UVB-radiated fibroblasts. Non-irradiated (blue), UVA-radiated (orange), or UVB-radiated (grey) fibroblasts were incubated with vitamin D (0–2 µM) and examined for cell viability by MTS assay (Promega) (a), and for DNA/RNA oxidative DNA damage by competitive ELISA (Cayman Chemical) (b). * = p < 0.05, relative to respective controls. ∆ = p < 0.05, between non-irradiated and UVB radiated cells. Error bars represent standard deviation, n = 4.
Relative to UVB-radiated control (100%), vitamin D at 0.2 and 2 µM significantly stimulated viability to 125%, and 123% in UVB-radiated fibroblasts (p < 0.05) (Figure 4a). Relative to the respective controls (100%), vitamin D at 0.2 and 2 µM significantly inhibited DNA/RNA oxidative damage to 76%, and 73% in non-irradiated fibroblasts and to 75%, and 63% in UVA-radiated fibroblasts, and at 2 µM, vitamin D inhibited DNA/RNA oxidative damage to 78% in UVB-radiated fibroblasts (p < 0.05) (Figure 4b).
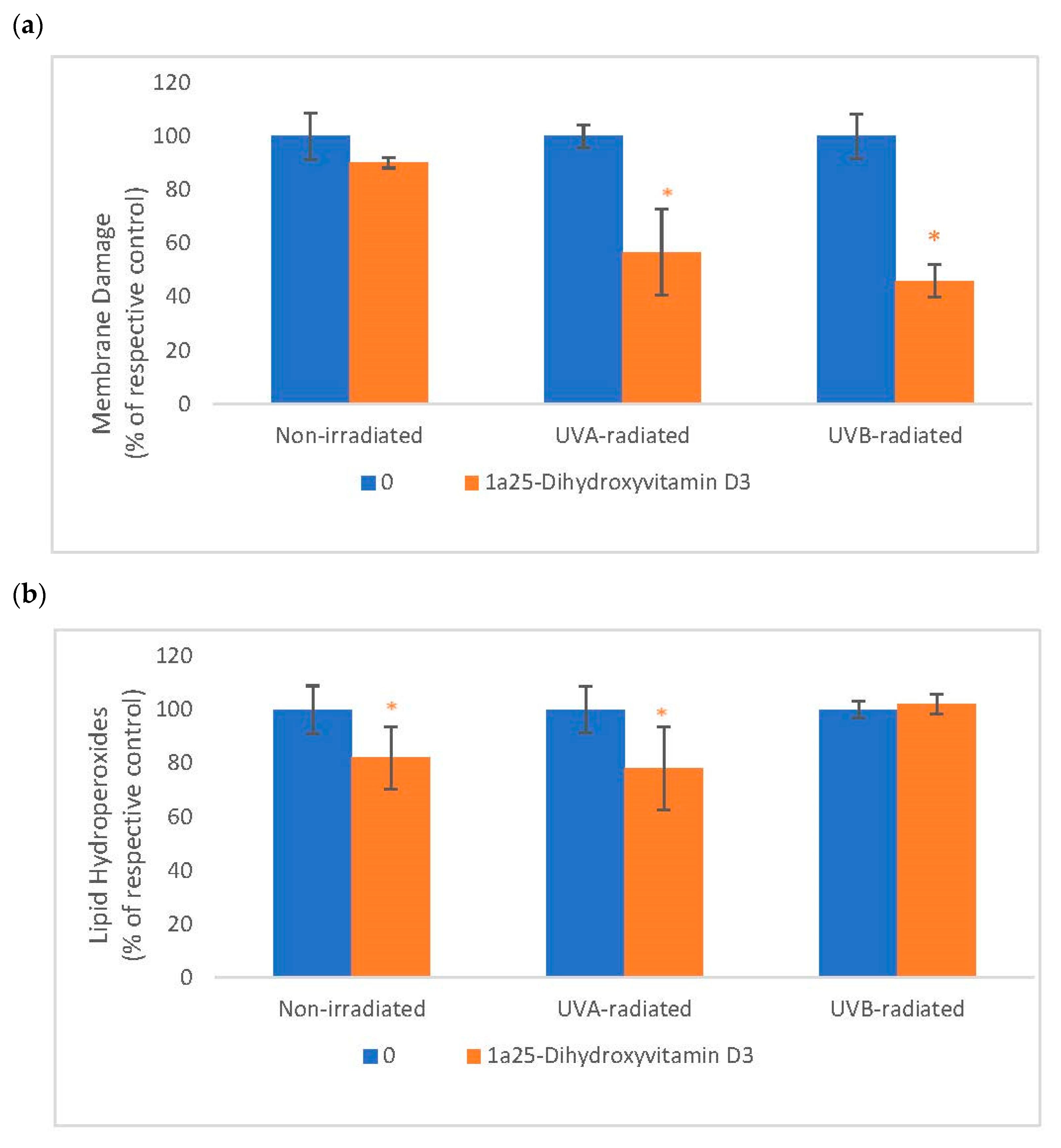
2.5. The Effect of 1α, 25 Dihydroxyvitamin D3 (Vitamin D) on Membrane Damage and Lipid Peroxidation in Non-Irradiated, UVA-Radiated, and UVB-Radiated Fibroblasts
Vitamin D significantly inhibited membrane damage in UVA-radiated and UVB-radiated fibroblasts (Figure 5). Vitamin D significantly inhibited lipid peroxidation in non-irradiated and UVA-radiated fibroblasts (Figure 5). UVA and UVB-radiation did not significantly alter membrane integrity or lipid peroxidation, as previously reported [16].
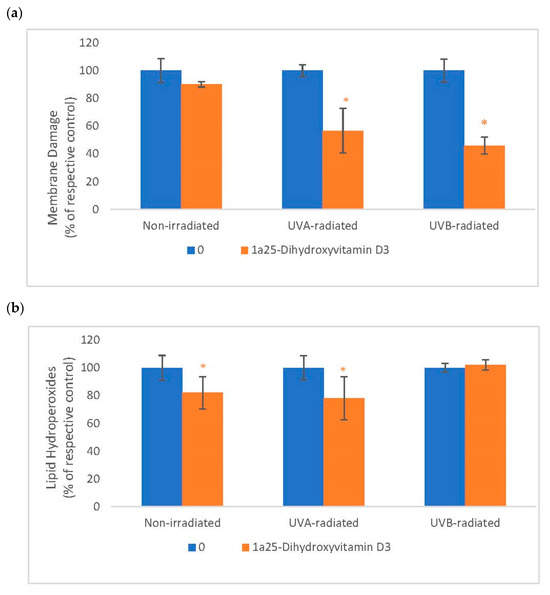
Figure 5.
The effect of 1α, 25dihydroxyvitamin D3 (vitamin D) on Membrane Damage and Lipid Peroxidation in non-irradiated, UVA-radiated, and UVB-radiated fibroblasts. Non-irradiated, UVA-radiated, or UVB-radiated fibroblasts were incubated with vitamin D (0 or 0.2 µM) and examined for membrane damage by LDH assay (Sigma, Ronkonkoma, NY, USA) (a), and for lipid hydroperoxides by Peroxi detect kit (Sigma) (b). * = p < 0.05, relative to respective controls. Error bars represent standard deviation, n = 4.
Relative to respective controls (100%), vitamin D at 0.2 µM significantly inhibited membrane damage to 57% and 46%, respectively, in UVA-radiated and UVB-radiated fibroblasts (p < 0.05) (Figure 5a). Relative to respective controls (100%), vitamin D at 0.2 µM significantly inhibited lipid peroxidation to 82% and 78%, respectively, in non-irradiated and UVA-radiated fibroblasts (p < 0.05) (Figure 5a).
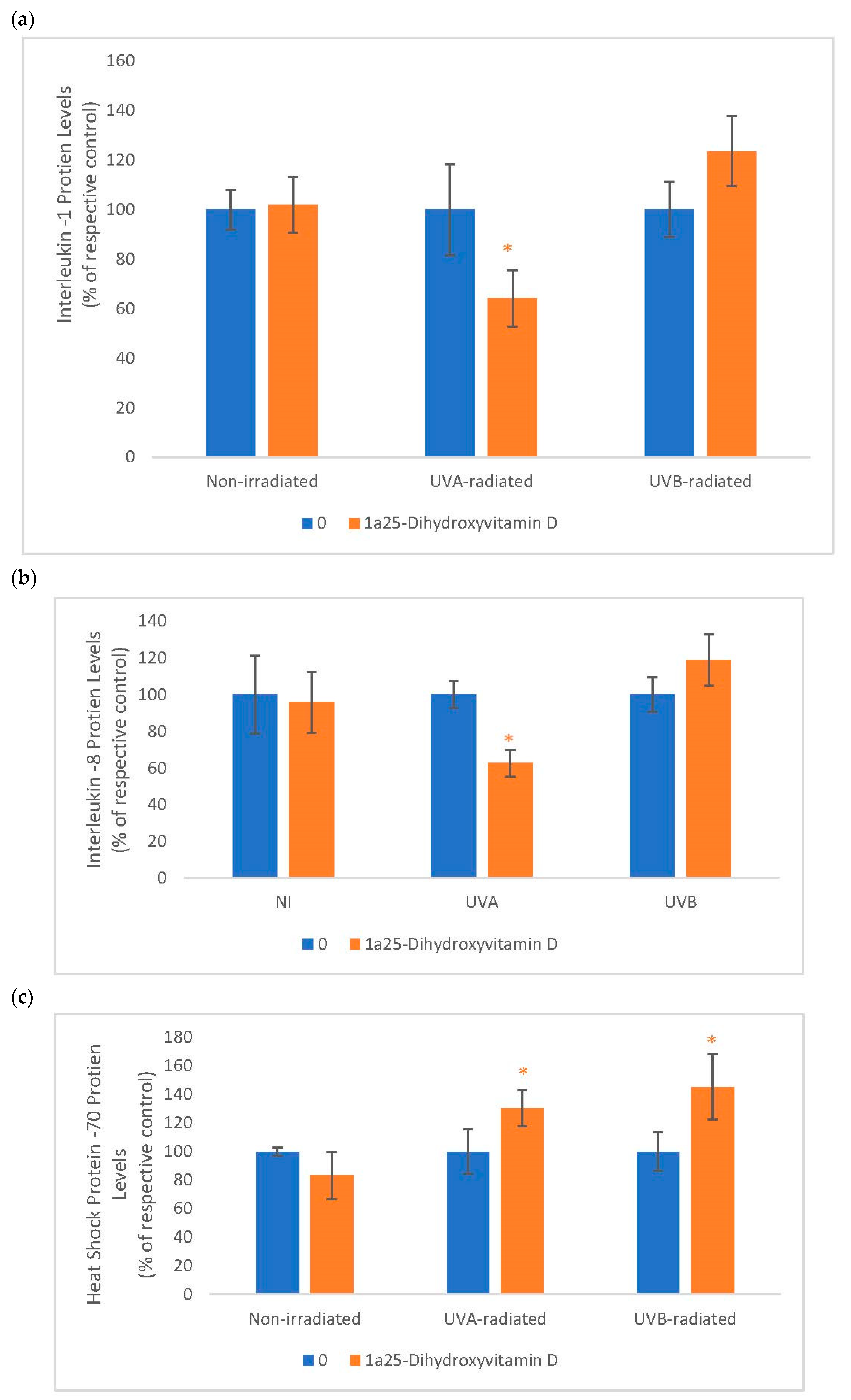
2.6. The Effect of 1α, 25 Dihydroxyvitamin D3 (Vitamin D) on Interleukin-1 (IL-1), Interleukin-8 (IL-8) and Heat Shock Protein-70 (HSP-70) in Non-Irradiated, UVA-Radiated, and UVB-Radiated Fibroblasts
Vitamin D significantly inhibited IL-1 and IL-8 expression in UVA-radiated fibroblasts but not in non-irradiated, and UVB-radiated fibroblasts (Figure 6). It significantly stimulated HSP-70 in UVA-radiated and UVB-radiated fibroblasts but not in non-irradiated fibroblasts (Figure 6). UVA significantly stimulated the expression of IL-1 and IL-8 to 186% and 168% of the control, respectively, and UVB-radiation significantly inhibited the expression HSP-70 to 52% of the control (data not shown).
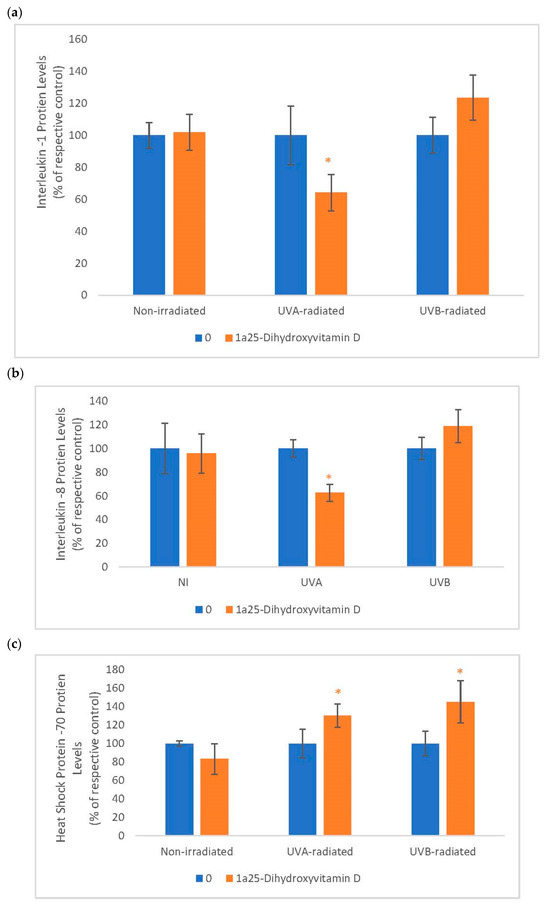
Figure 6.
The effect of 1α, 25dihydroxyvitamin D3 (vitamin D) on interleukin-1 (IL-1), interleukin-8 (IL-8) and heat shock protein-70 (HSP-70) in non-irradiated, UVA-radiated, and UVB-radiated fibroblasts. Non-irradiated, UVA-radiated, or UVB-radiated fibroblasts were incubated with vitamin D (0 or 2 µM) for 24 h, and examined for IL-1 (a), IL-8 (b), and HSP-70 (c) protein levels by indirect ELISA (Kirkguaard and Perry Inc). * = p < 0.05, relative to respective controls. Error bars represent standard deviation, n = 4.
In UVA-radiated cells, vitamin D at 0.2 µM inhibited IL-1 and IL-8 protein levels to 64% and 63% of the UVA-radiated control, respectively (p < 0.05) (Figure 6a). Relative to the respective controls (100%), vitamin D at 0.2 µM significantly stimulated HSP-70 to 130% and 145% in UVA-radiated and UVB-radiated fibroblasts, respectively (p < 0.05) (Figure 6b).
3. Discussion
Skin aging is associated with the altered regulation of genes associated with extracellular matrix and inflammation, as well as cellular oxidative stress effects. The UV radiation is the predominant environmental factor that accelerates skin aging. The regulatory properties of 1α, 25dihydroxyvitamin D3 (vitamin D) include endocrine, ECM regulation, cell differentiation, photoprotection, and anti-inflammation [34,35,36,37,38,39,40,41,42,43,44,45].
Skin aging/photoaging is associated with the loss of structural collagen, co-regulated HSP-47, and solar elastosis from stimulation of elastin and elastases [1,3,12,14,16]. Vitamin D stimulates collagen expression and inhibits elastin expression in dermal fibroblasts [35,36,37]. We herein report that vitamin D stimulates the expression of type I collagen, transcriptionally, in non-irradiated and UVA-radiated fibroblasts, though not in UVB-radiated fibroblasts. Further, it stimulates the expression of HSP-47 in the UVA-radiated fibroblasts. Vitamin D inhibits the elastin protein levels in UVA-radiated fibroblasts, transcriptionally, and elastase activity directly, as well as in non-irradiated and UVA-radiated fibroblasts. It is inferred that vitamin D is beneficial in preventing intrinsic skin aging through the stimulation of type I collagen and the inhibition of cellular elastase activity, as well as in preventing UVA-radiation associated photoaging through the stimulation of type I collagen and HSP-47, and the inhibition of elastin and elastase activity in dermal fibroblasts.
A primary mechanism of skin aging/photoaging is cellular oxidative stress, which directly damages DNA, lipids, proteins, and cell survival, and alters signal transduction pathways and gene expression [4,5,7,8,17]. Vitamin D improves the survival of UVB radiated keratinocytes or skin through the inhibition of oxidative damage [38,39,40,41,42]. We herein report that vitamin D has direct anti-oxidative activity, improves the viability of UVB-radiated dermal fibroblasts, and inhibits oxidative DNA/RNA damage in non-irradiated as well as UVA or UVB radiated fibroblasts. Further, vitamin D inhibits membrane damage in UVA-radiated and UVB-radiated fibroblasts and lipid peroxidation in non-irradiated and UVA-radiated fibroblasts. It is inferred that vitamin D is beneficial in preventing intrinsic skin aging and UVA or UVB-radiation associated photoaging through the inhibition of oxidative damage to DNA, RNA, and lipids in non-irradiated, UVA-radiated, and UVB-radiated cells. In addition, vitamin D is shown to stimulate the cell viability of UVB-radiated cells
In addition to oxidative stress, another predominant mechanism of skin aging/photoaging is inflammation, which alters signal transduction pathways and gene expression, including HSP-70, through inflammatory mediators, such as IL-1 and IL-8 [4,5,7,8,28,29,30]. Vitamin D inhibits the expression IL-8 and stimulates HSP-70 [43,44,45]. We herein report that vitamin D inhibits the expression of IL-1 and IL-8 in UVA or UVB radiated fibroblasts and stimulates HSP-70 in UVA-radiated and UVB-radiated fibroblasts. It is inferred that vitamin D is predominantly beneficial in preventing UVA-radiation associated photoaging through the inhibition the inflammatory cytokines, as well as the stimulation of HSP-70.
4. Conclusions
Skin aging/photoaging is associated with damage to the ECM and cellular structure and an increase in inflammatory cytokines. Vitamin D is predominantly beneficial in preventing UVA-radiation induced photoaging through the differential regulation of the ECM, HSPs, and inflammatory cytokines, and protective effects on cellular biomolecules. Its beneficial effects in preventing UVB-radiation associated photoaging are accomplished through stimulation of cell viability and HSP-70 and the inhibition of cellular oxidative damage. Further, preventing intrinsic skin aging is accomplished through the stimulation of type I collagen and the inhibition of cellular oxidative damage.
5. Methods
5.1. Antioxidant Activity
The direct antioxidant activity of vitamin D was determined by an ABTS® (2, 2′-azino-di-[3-ethylbenzthiazoline sulphonate]) assay, which is based on the inhibition of the oxidation of ABTS® to an ABTS® radical by metmyoglobin (Cayman Chemical Antioxidant Assay kit) [2,3]. Vitamin D at 0, 0.02, 0.2, or 2 µM was incubated with ABTS and metmyoglobin, and the inhibition of ABTS oxidation was measured spectrophotometrically at 405 nm.
5.2. Elastase Activity
The direct inhibitory effect of vitamin D on elastase activity was determined by an elastase activity inhibition assay, which is based on the inhibition of the conversion of an elastase substrate to a colored product (Elastin products Co, Owensville, Missouri) [1,6,11]. Vitamin D at 0, 0.02, 0.2, or 2 µM was incubated with elastase for 10 min followed by the addition of its substrate, and the product was measured spectrophotometrically at 410 nm. In addition, cells that had been dosed with 0 or 0.2 µM vitamin D were assayed for elastase activity.
5.3. Cell Culture
Human neonatal dermal fibroblasts (American Type Culture Collection, ATCC) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum, 1% penicillin/streptomycin, and 1% L-glutamine (Sigma, Ronkonkoma, NY, USA). For the experiments, cells were dosed for 24 h with 0, 0.02, 0.2, or 2 µM of vitamin D to determine the collagen and elastin promoter activities/protein levels, cell viability and oxidative DNA damage; and with 0 or 2 µM to determine cellular elastase activity, membrane damage, lipid peroxidation, and the expression of HSP-47, IL-1β, IL-8, and HSP-70 [9,10,11,12,13,14,15,16]. In parallel, cells were exposed to 2.5J UVA-radiation or 2.5 mJ UVB-radiation prior to dosing with vitamin D, as previously described (UV lamps: gifts from Dr. Salvador Gonzalez, Massachusetts General Hospital, Boston, MA, USA) [1,3,12,14,16].
5.4. Promoter Activities
Fibroblasts were co-transfected with COL1α1 promoter-firefly luciferase or elastin promoter-firefly luciferase (pGL4 vector) (gifts from Dr. Joel Rosenbloom, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA, USA) and thymidine kinase (TK) promoter-hRenilla luciferase plasmids (Promega, Madison, WI, USA) (for normalization of transfection efficiency) using an escort for 24 h, prior to the dosing with UV-radiation and/or vitamin D (Sigma, Ronkonkoma, NY, USA) [1,3,9,11,12]. The cells were measured for firefly and renilla luciferase activities sequentially using a dual luciferase reporter assay (Promega, Madison, WI, USA) [1,3,9,11,12].
5.5. Protein Levels
The total protein content in the media and cells, following experiments, was measured using a Pierce BCA (bicinchonini acid) protein assay, by incubating aliquots of the samples with a mix of BCA and cupric ion and measuring the formation of BCA-cuprous ion, which is proportional to the total protein content (Thermo Scientific, Waltham, MA, USA). The protein content in the samples was similar (data not shown).
The expression of the type I collagen, elastin, HSP-47, IL-1β, IL-8, and HSP-70 proteins was measured by indirect ELISA (Kirkguaard and Perry Laboratories, Inc, Gaithersburg, MD, USA) [1,2,3,9,10,11,12,13,14,15,16]. Aliquots of 9100 ul of the samples were placed in a 96 well plate for 24 h at 4 °C, blocked with bovine serum albumin for 30 min at room temperature and incubated with respective primary antibodies (Millipore, Burlington, MA, USA; Elastin products Co, Owensville, Missouri; StressGen, Farmingdale, NY; Sigma, Ronkonkoma, NY, USA) for 1 h at room temperature. The plates were washed with a wash buffer, incubated with a secondary antibody linked to peroxidase for 1 h at room temperature, washed, and incubated with a peroxidase substrate until color development, which was measured spectrophotometrically at 405 nm.
5.6. Cell Viability
The cells were examined for cell viability using a CellTiter 96® Aqueous One Solution reagent (MTS: tetrazolium compound (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS) + electron coupling reagent (phenazine ethosulfate; PES)) (Promega, Madison, WI, USA) [1,2,3,9,10,11,12,13,14,15,16]. The cells were incubated with aliquots of an MTS solution (yellow) for 30 min at 37 °C, and the conversion of the tertrazolium reagent to formazan (brown) by viable cells was measured spectrophotometrically at 490 nm.
5.7. Oxidative DNA/RNA Damage
The oxidative DNA damage was measured using a DNA/RNA Oxidative Damage ELISA Kit, a competitive ELISA in which the cellular oxidized guanosine or guanine residues compete with acetylcholinesterase linked to 8-OH-dG (tracer) for an antibody to oxidatively damaged guanine, and the tracer is quantitated by incubation with its substrate converted to a yellow product (Cayman Chemical, Ann Arbor, MI, USA). Aliquots of cells or a buffer (to quantitate the maximum tracer binding/activity), tracer, and antibody were added to the ELISA plate, incubated for 24 h at 4 °C, washed, and incubated with a substrate, and the product was measured spectrophotometrically at 412 nm. The readings were subtracted from the maximum tracer binding to determine the cellular DNA/RNA oxidative damage.
5.8. Membrane Damage
The media were examined for lactate dehydrogenase (LDH), which is indicative of membrane damage. Aliquots of the media were incubated with a mix of LDH substrate, a cofactor, and tertrazolium dye, and the reduction of the tertrazolium dye was measured spectrophotometrically at 490 nm (Sigma, Tox-7, Ronkonkoma, NY, USA) [16].
5.9. Lipid Peroxidation
The lipid hydroperoxides were measured using the PeroxiDetect kit, which is based on the hydroperoxides oxidizing ferrous to ferric ion, which reacts with xylenol orange (“3,3′-bis[N,N-bis(carboxymethyl)aminomethyl]o-cresolsulfonephthalein, sodium salt”) to a form a blue product (Sigma, Ronkonkoma, NY, USA). Aliquots of the media were incubated with a mix of ferrous ion/xylene orange for 30 min at room temperature, and the product was measured spectrophotmetrically at 560 nm (Sigma, Ronkonkoma, NY, USA) [2].
Data Analysis
The data were analyzed for significant differences by ANOVA and student t-tests at a 95% confidence interval. The direct antioxidant and elastase inhibitory effects of vitamin D were analyzed relative to the control (0 dose, 100%). The effects of UV radiation on the cellular targets were analyzed relative to the non-irradiated control cells. The effects of vitamin D on non-irradiated cells were analyzed relative to the non-irradiated control cells (0 dose, 100%). The effects of vitamin D on UV (UVA or UVB)-radiated fibroblasts were analyzed relative to the UV radiation effect alone (UVA or UVB-radiated respective control).
Author Contributions
N.P. is the principal investigator and the co-authors are her research students who provided technical assitance.
Funding
This research received no external funding.
Acknowledgments
Michael Pirro, Mounika Parvataneni, Tishawn Reid for their technical contribution.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Philips, N.; Chalensouk-Khaosaat, J.; González, S. Stimulation of the elastin and fibrillin in non-irradiated or UVA radiated fibroblasts, and direct inhibition of elastase or matrix metalloptoteinases activity by nicotinamide or its derivatives. J. Cosmet. Sci. 2017, 68, 1–10. [Google Scholar]
- Philips, N.; Samuel, P.; Lozano, T.; Gvaladze, A.; Guzman, B.; Siomyk, H.; Haas, G. Effects of Humulus lupulus extract or its Components on Viability, Lipid Peroxidation, and expression of Vascular Endothelial Growth Factor in Melanoma Cells and Fibroblasts. Madr. J. Clin. Res. 2017, 1, 15–19. [Google Scholar] [CrossRef]
- Philips, N.; Chalensouk-Khaosaat, J.; Gonzalez, S. Stimulation of the Fibrillar Collagen and Heat Shock Proteins by Nicotinamide or Its Derivatives in Non-Irradiated or UVA Radiated Fibroblasts, and Direct Anti-Oxidant Activity of Nicotinamide Derivatives. Cosmetics 2015, 2, 146–161. [Google Scholar] [CrossRef]
- Philips, N.; Chalensouk-Khaosaat, J.; Devmurari, A.; Patel, H. Polyphenolic nanobiomaterials as emerging therapies for combating physiology and clinical aspects of photoaging and photocarcinogenesis. In Skin Aging and Photoaging: Physiology, Clinical Aspects and Emerging Therapies 2015; Nova Publishers Inc.: Hauppauge, NY, USA, 2015; Chapter 6; ISBN 978-1-63482-907-6. [Google Scholar]
- Philips, N.; Siomyk, H.; Bynum, D.; Gonzalez, S. Skin Cancer, Polyphenols, and Oxidative Stress. Cancer 2014, 1, 265–270. [Google Scholar]
- Philips, N.; Gonzalez, S. Beneficial Regulation of Elastase Activity and Expression of Tissue Inhibitors of Matrixmetalloproteinases, Fibrillin, Transforming Growth Factor-β, and Heat Shock Proteins by P. leucotomos in Nonirradiated or Ultraviolet-Radiated Epidermal Keratinocytes. ISRN Oxidative Med. 2013, 2013, 257463. [Google Scholar] [CrossRef]
- Philips, N.; Samuel, M.; Parakandi, H.; Siomyk, H.; Gopal, S.; Jia, H.; Ret, M.; Shahin, H. Vitamins in the Therapy of Inflammatory and Oxidative Diseases. In Frontiers in Clinical Drug Research—Anti Allergy Agents; Bentham Science: Potomac, MD, USA, 2013; pp. 240–264. [Google Scholar]
- Philips, N.; Samuel, P.; Siomyk, H.; Parakandi, H.; Gopal, S.; Shahin, H. Improved cell metabolism and strengthening of the extracellular matrix by nicotinamide, and copper for anti-skin aging. In Skin Aging: New Research; Nova Science: Hauppauge, NY, USA, 2012; pp. 43–58. [Google Scholar]
- Philips, N.; Samuel, P.; Parakandi, H.; Gopal, S.; Siomyk, H.; Ministro, A.; Thompson, T.; Borkow, G. Beneficial Regulation of Fibrillar Collagens, Heat Shock Protein-47, Elastin Fiber Components, Transforming Growth Factor-β1, Vascular Endothelial Growth Factor and Oxidative Stress Effects by Copper in Dermal Fibroblasts. Connect. Tissue Res. 2012, 53, 373–378. [Google Scholar] [CrossRef]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of cell proliferation, and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef]
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.; Conte, J.; Natrajan, P.; Haas, G.; Gonzalez, S. Direct inhibition of e.lastase and matrixmetalloproteinases, and stimulation of biosynthesis of fibrillar collagens, elastin and fibrillins by xanthohumol. J. Cosmet. Sci. 2010, 61, 125–132. [Google Scholar]
- Philips, N.; Conte, J.; Chen, Y.-J.; Natrajan, P.; Taw, M.; Keller, T.; Givant, J.; Tuason, M.; Dulaj, L.; Leonardi, D.; et al. Beneficial regulation of matrixmetalloproteinases and their inhibitors, fibrillar collagens and transforming growth factor-β by Polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Arch. Dermatol. Res. 2009, 301, 487–495. [Google Scholar] [CrossRef]
- Philips, N.; Tuason, M.; Chang, T.; Lin, Y.; Tahir, M.; Rodriguez, S. Differential Effects of Ceramide on Cell Viability and Extracellular Matrix Remodeling in Keratinocytes and Fibroblasts. Skin Pharmacol. Physiol. 2009, 22, 151–157. [Google Scholar] [CrossRef]
- Philips, N.; Keller, T.; Hendrix, C.; Hamilton, S.; Arena, R.; Tuason, M.; Gonzalez, S. Regulation of the extracellular matrix remodeling by lutein in dermal fibroblasts, melanoma cells, and ultraviolet radiation exposed fibroblasts. Arch. Dermatol. Res. 2007, 8, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Burchill, D.; O’Donoghue, D.; Keller, T.; Gonzalez, S. Identification of Benzene Metabolites in Dermal Fibroblasts as Nonphenolic: Regulation of Cell Viability, Apoptosis, Lipid Peroxidation and Expression of Matrix Metalloproteinase 1 and Elastin by Benzene Metabolites. Skin Pharmacol. Physiol. 2004, 17, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Smith, J.; Keller, T.; Gonzalez, S. Predominant effects of Polypodium leucotomos on membrane integrity, lipid peroxidation, and expression of elastin and matrixmetalloproteinase-1 in ultraviolet radiation exposed fibroblasts, and keratinocytes. J. Dermatol. Sci. 2003, 32, 1–9. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Kaiser, C.A.; Krieger, M.; Scott, M.P.; Bretscher, A.; Ploegh, H.; Matsudaira, P. Molecular Cell Biology; W.H. Freeman and Company: Stuttgart, Germany, 2016. [Google Scholar]
- Callaghan, T.M.; Wilhelm, K.-P. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part 2: Clinical perspectives and clinical methods in the evaluation of ageing skin. Int. J. Cosmet. Sci. 2008, 30, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Mecham, R.P.; Davis, E.C. Elastic Fiber Structure and Assembly. In Extracellular Matrix Assembly and Structure; Academic Press: Cambridge, MA, USA, 1994; pp. 281–314. [Google Scholar]
- Tsuji, N.; Moriwaki, S.; Suzuki, Y.; Takema, Y.; Imokawa, G. The role of elastases secreted by fibroblasts in wrinkle formation: Implication through selective inhibition of elastase activity. Photochem. Photobiol. 2001, 71, 283–290. [Google Scholar] [CrossRef]
- Matsuda, M.; Hoshino, T.; Yamashita, Y.; Tanaka, K.-I.; Maji, D.; Sato, K.; Adachi, H.; Sobue, G.; Ihn, H.; Funasaka, Y.; et al. Prevention of UVB Radiation-induced Epidermal Damage by Expression of Heat Shock Protein 70. J. Biol. Chem. 2009, 285, 5848–5858. [Google Scholar] [CrossRef] [PubMed]
- Labat-Robert, J.; Fourtanier, A.; Boyer-Lafargue, B.; Robert, L. Age dependent increase of elastase type protease activity in mouse skin. J. Photochem. Photobiol. B Biol. 2000, 57, 113–118. [Google Scholar] [CrossRef]
- Sellheyer, K. Pathogenesis of solar elastosis: Synthesis or degradation? J. Cutan. Pathol. 2003, 30, 123–127. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2009, 302, 71–83. [Google Scholar] [CrossRef]
- Walschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B 2001, 63, 41–45. [Google Scholar] [CrossRef]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new? J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Derm. 2007, 157, 874. [Google Scholar] [CrossRef] [PubMed]
- Kindt, T.J.; Goldsby, R.A.; Osborne, B.A. Kuby Immunology; W.H. Freeman and Company: Stuttgart, Germany, 2007. [Google Scholar]
- Philips, N.; Samuel, P.; Samuel, M.; Perez, G.; Khundoker, R.; Alahmade, G. Interleukin-4 Signaling Pathway and Effects in Allergic Diseases. Curr. Signal Transduct. Ther. 2018, 13, 1–5. [Google Scholar] [CrossRef]
- Philips, N.; Samuel, M. Inhibition of interleukin-4 signalling in the treatment of atopic dermatitis and allergic asthma. Glob. J. Allergy 2017, 3, 019–021. [Google Scholar] [CrossRef]
- Cheng, L.X.; Tang, J.J.; Luo, H.; Jin, X.L.; Dai, F.; Yang, J.; Qian, Y.P.; Li, X.Z.; Zhou, B. Antioxidant and antiproliferative activities of hydroxyl-substituted Schiff bases. Bioorg. Med. Chem. Lett. 2010, 20, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Chan, P.T.; Ho, K.Y.; Fung, K.P.; Wang, J. Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem. Phys. Lipids 1996, 79, 157–163. [Google Scholar] [CrossRef]
- Gazák, R.; Sedmera, P.; Vrbacký, M.; Vostálová, J.; Drahota, Z.; Marhol, P.; Walterová, D.; Kren, V. Molecular mechanisms of silybin and 2,3-dehydrosilybin antiradical activity—role of individual hydroxyl groups. Free Radic. Biol. Med. 2009, 46, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, B.; Wood, D. The Endocrine System at a Glance; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dobak, J.; Grzybowski, J.; Liu, F.T.; Landon, B.; Dobke, M. 1, 25-Dihydroxyvitamin D3 increases collagen production in dermal fibroblasts. J. Dermatol. Sci. 1994, 8, 18–24. [Google Scholar] [CrossRef]
- Norsgaard, H.; Kurdykowski, S.; Descargues, P.; Gonzalez, T.; Marstrand, T.; Dünstl, G.; Røpke, M. Calcipotriol counteracts betamethasone-induced decrease in extracellular matrix components related to skin atrophy. Arch. Dermatol. Res. 2014, 306, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Botney, M.D.; Mecham, R.R.; Parks, W.C. Inhibition of Tropoelastin Expression by 1, 25-Dihydroxyvitamin D3. Connect. Tissue Res. 1991, 26, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dixon, K.M.; Deo, S.S.; Holliday, C.J.; Slater, M.; Halliday, G.M.; Reeve, V.E.; Mason, R.S. Photoprotection by 1,25 Dihydroxyvitamin D3 Is Associated with an Increase in p53 and a Decrease in Nitric Oxide Products. J. Investig. Derm. 2007, 127, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Song, E.J.; Gordon-Thomson, C.; Cole, L.; Stern, H.; Halliday, G.M.; Damian, D.L.; Reeve, V.E.; Mason, R.S. 1α, 25-Dihydroxyvitamin D3 reduces several types of UV-induced DNA damage and contributes to photoprotection. J. Steroid Biochem. Mol. Biol. 2013, 136, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.; Deo, S.; Wong, G.; Slater, M.; Norman, A.; Bishop, J.; Posner, G.; Ishizuka, S.; Halliday, G.; Reeve, V.; et al. Skin cancer prevention: A possible role of 1,25dihydroxyvitamin D3 and its analogs. J. Steroid Biochem. Mol. Biol. 2005, 97, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.M.; Norman, A.W.; Sequeira, V.B.; Mohan, R.; Rybchyn, M.S.; Reeve, V.E.; Halliday, G.M.; Mason, R.S. 1α,25(OH)2-Vitamin D and a Nongenomic Vitamin D Analogue Inhibit Ultraviolet Radiation-Induced Skin Carcinogenesis. Cancer Prev. Res. 2011, 4, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Thomson, C.; Gupta, R.; Tongkao-On, W.; Ryan, A.; Halliday, G.M.; Mason, R.S. 1α,25 Dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin. Photochem. Photobiol. Sci. 2012, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Nakashyan, V.; Tipton, D.A.; Karydis, A.; Livada, R.; Stein, S.H. Effect of 1,25(OH)2D3 and 20(OH)D3 on interleukin-1β-stimulated interleukin-6 and -8 production by human gingival fibroblasts. J. Periodontal Res. 2017, 52, 832–841. [Google Scholar] [CrossRef]
- Rostkowska-Nadolska, B.; Sliupkas-Dyrda, E.; Potyka, J.; Kusmierz, D.; Fraczek, M.; Krecicki, T.; Kubik, P.; Zatonski, M.; Latocha, M. Vitamin D derivatives: Calcitriol and tacalcitol inhibits interleukin-6 and interleukin-8 expression in human nasal polyp fibroblast cultures. Adv. Med. Sci. 2010, 55, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Polla, B.S.; Healy, A.M.; Wojno, W.C.; Krane, S.M. Hormone 1 alpha,25-dihydroxyvitamin D3 modulates heat shock response in monocytes. Am. J. Physiol. Cell Physiol. 1987, 252, C640–C649. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).