The Beneficial Regulation of Extracellular Matrix and Heat Shock Proteins, and the Inhibition of Cellular Oxidative Stress Effects and Inflammatory Cytokines by 1α, 25 dihydroxyvitaminD3 in Non-Irradiated and Ultraviolet Radiated Dermal Fibroblasts
Abstract
1. Introduction
2. Results
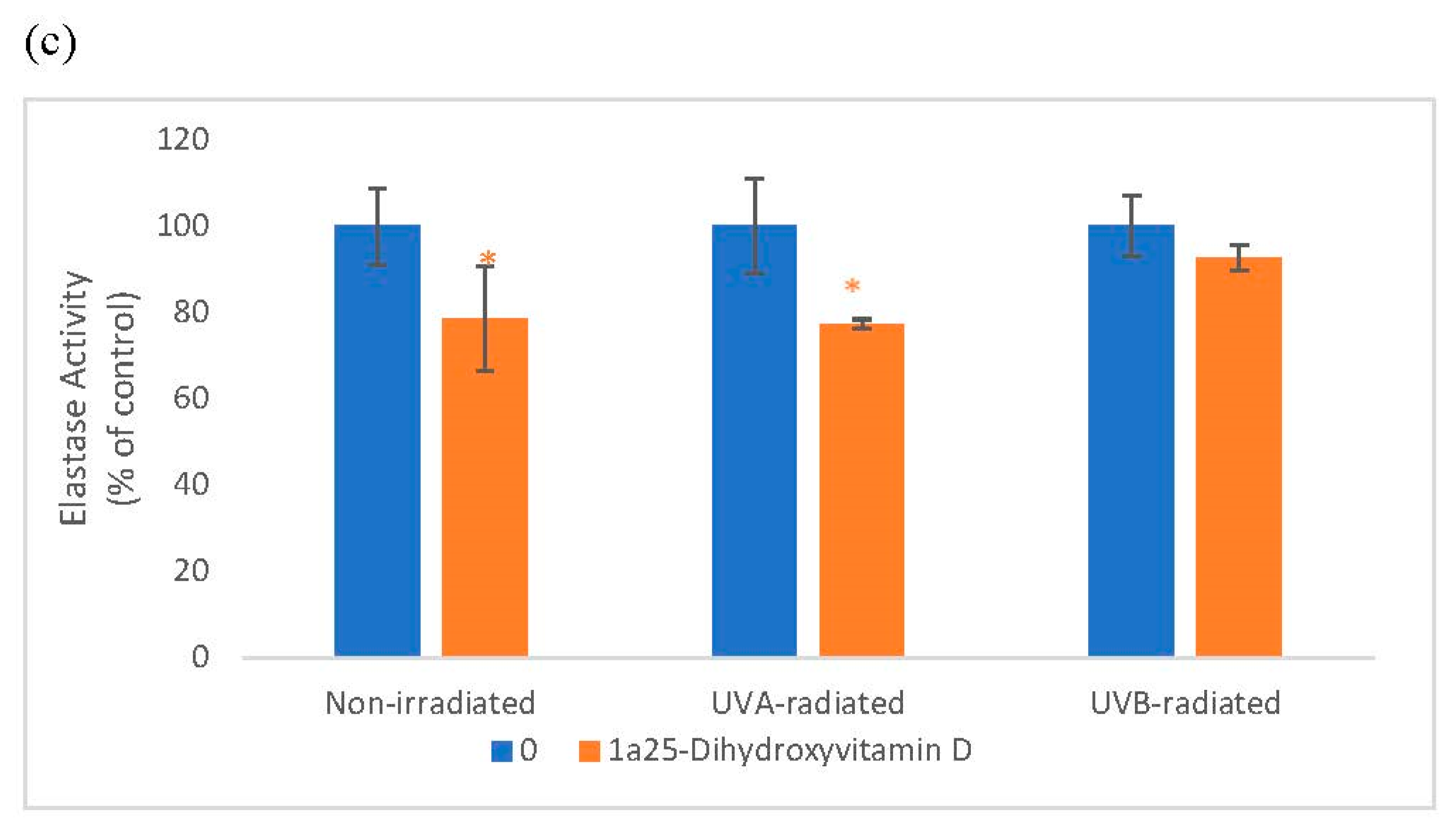
2.1. Direct Effects of 1α, 25 dihydroxyvitamin D3 (Vitamin D) on ABTS® (2, 2′-azino-di-[3-ethylbenzthiazoline sulphonate]) Oxidation and Elastase Activity
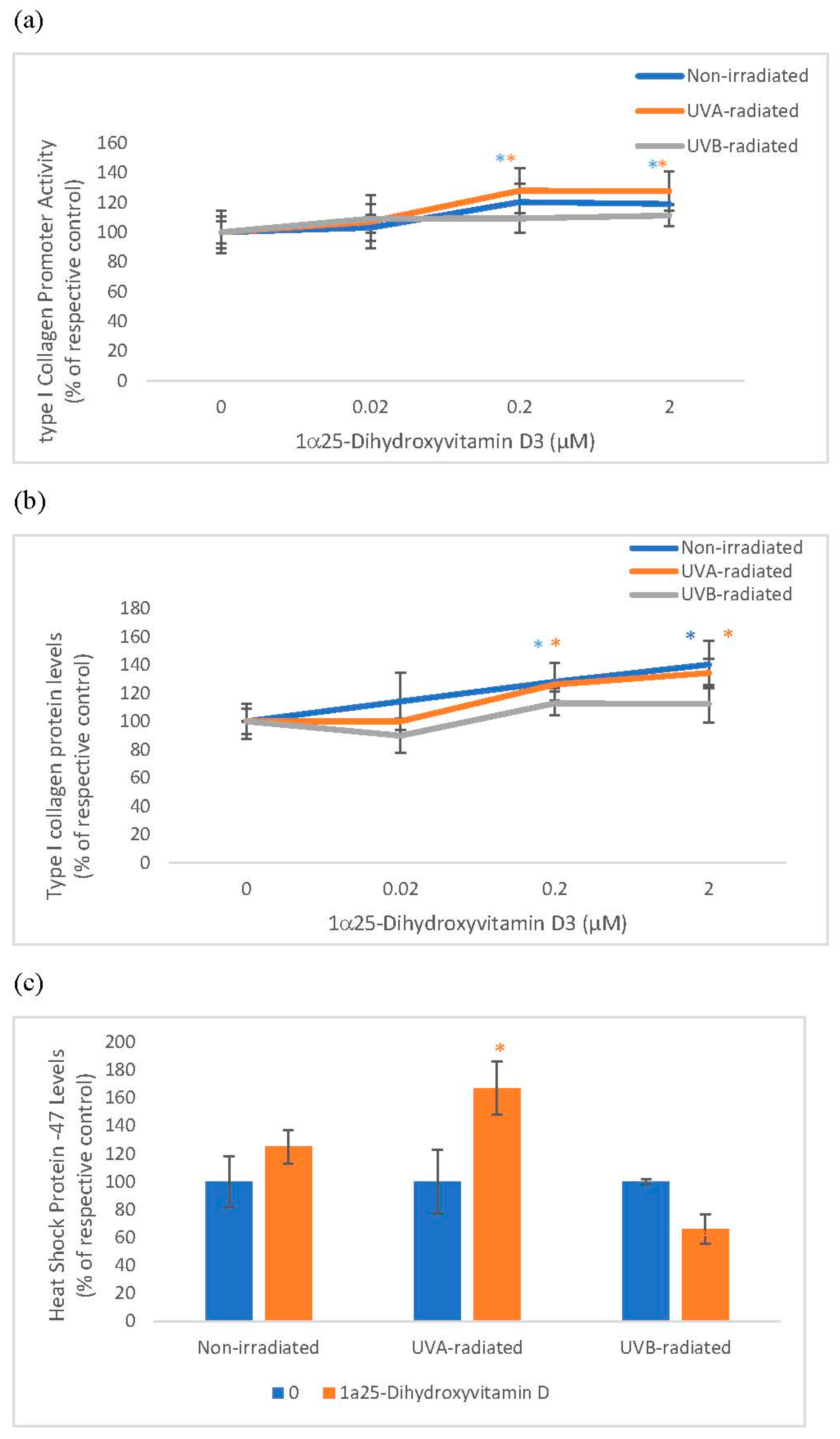
2.2. The Effect of 1α, 25 Dihydroxyvitamin D3 (Vitamin D) on Type I Collagen Promoter Activity, Type I Collagen Protein Levels, and Heat Shock Protein-47 (HSP-47) in Non-Irradiated, UVA-Radiated, and UVB-Radiated Fibroblasts
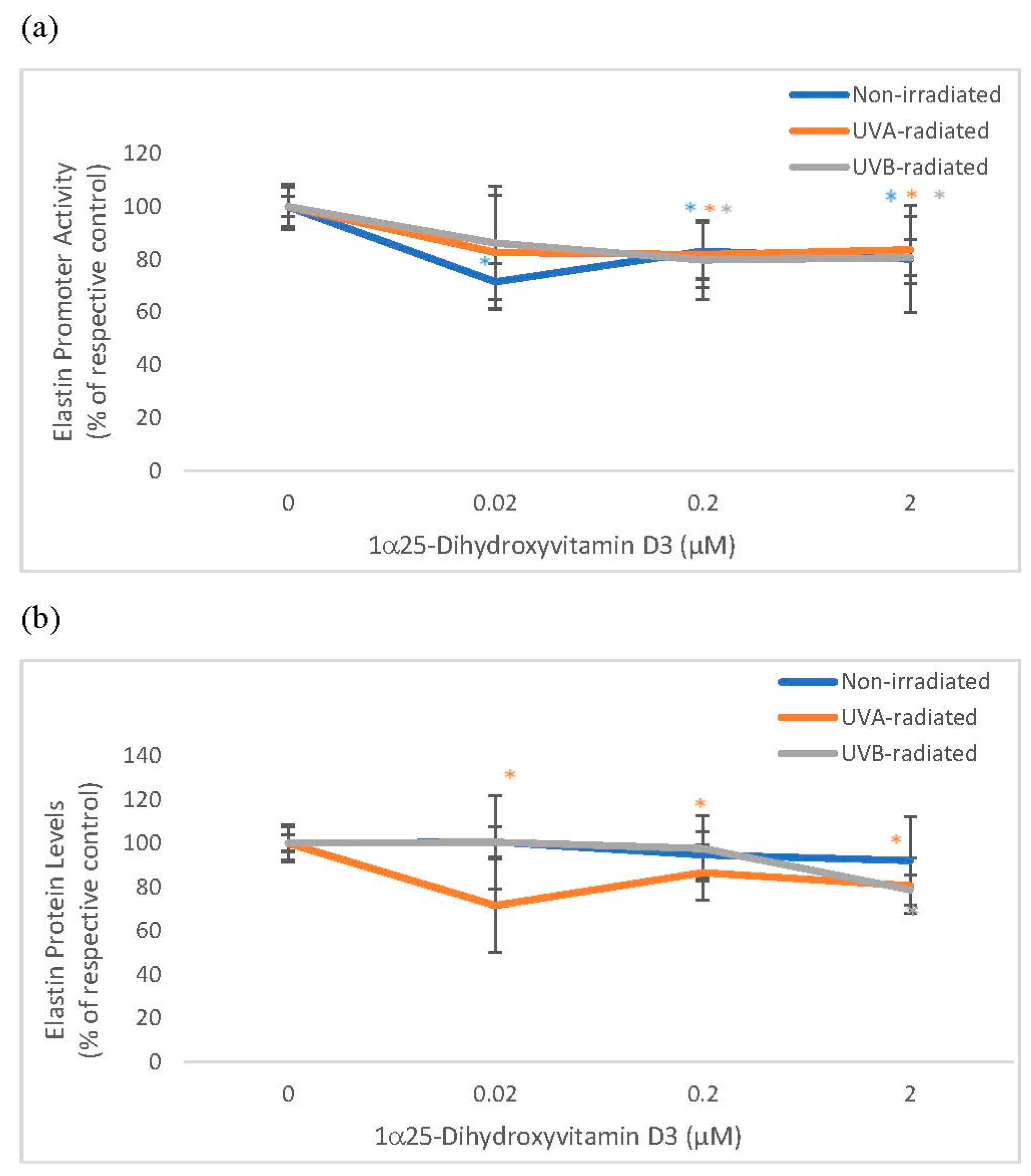
2.3. The Effect of 1α, 25 Dihydroxyvitamin D3 (Vitamin D) on Elastin Promoter Activity, Elastin Protein Levels, and Elastase Activity in Non-Irradiated, UVA-Radiated, and UVB-Radiated Fibroblasts
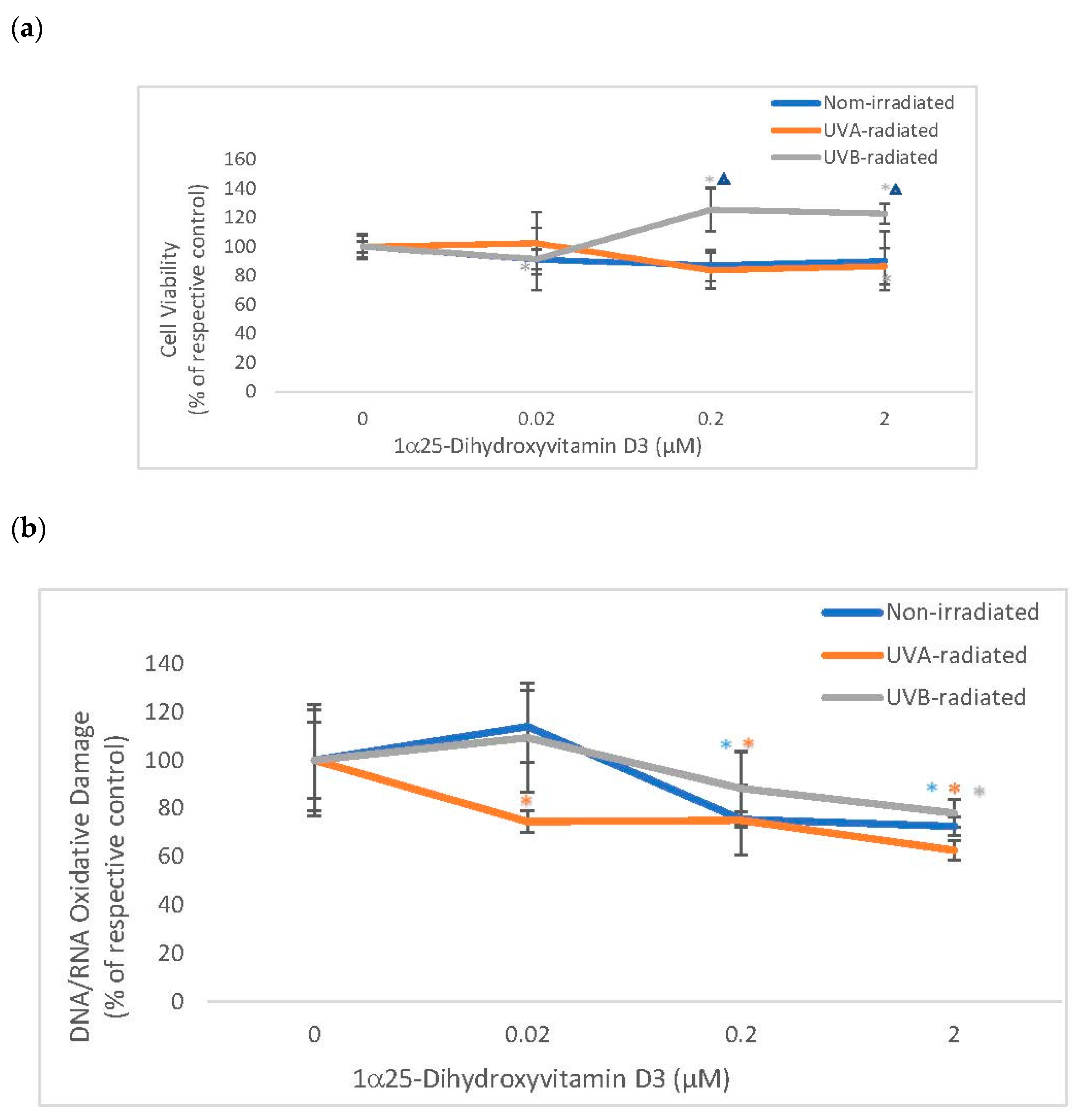
2.4. The Effect of 1α, 25 Dihydroxyvitamin D3 (Vitamin D) on Cell Viability and Oxidative DNA/RNA Damage in Non-Irradiated, UVA-Radiated, and UVB-Radiated Fibroblasts
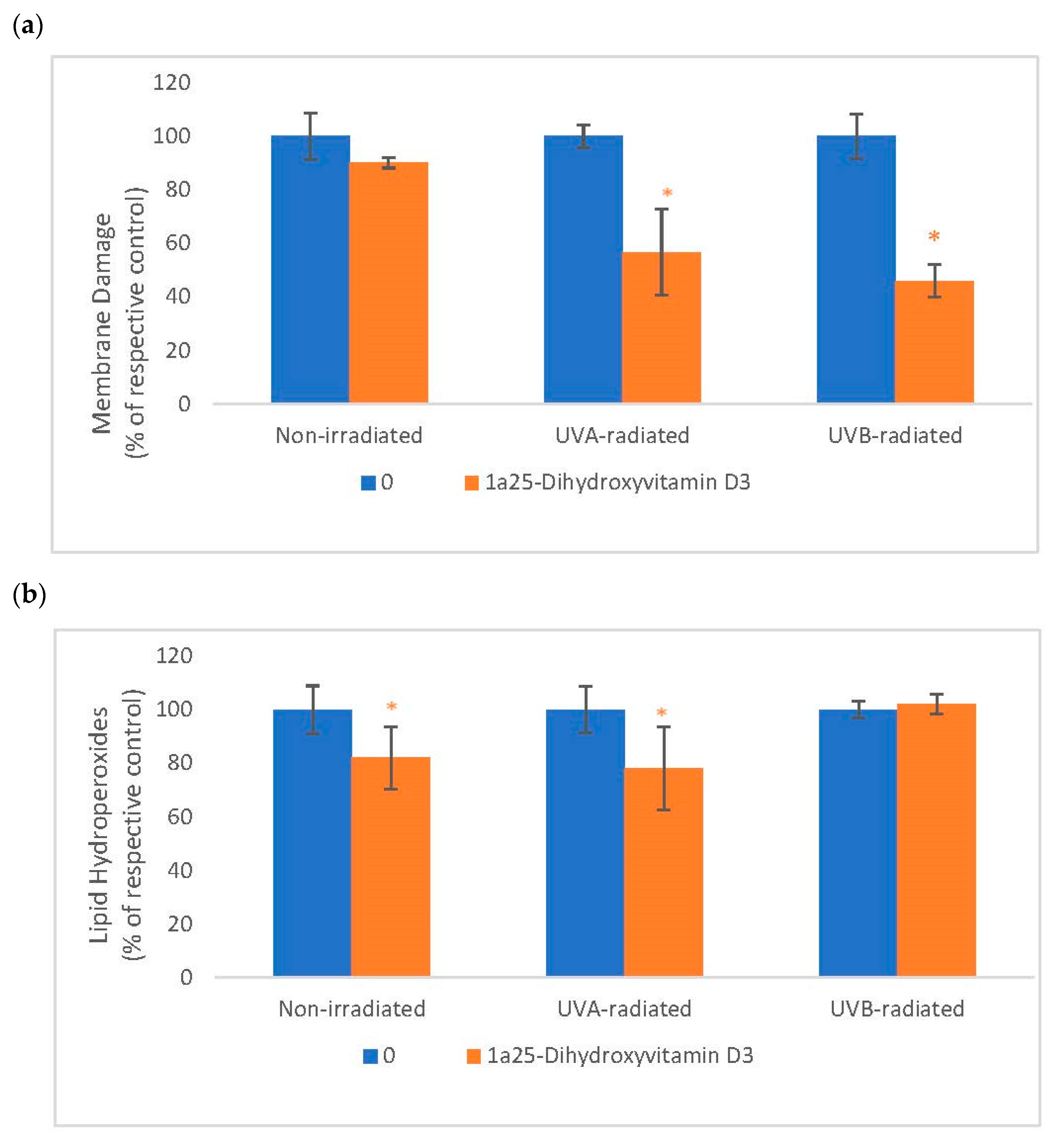
2.5. The Effect of 1α, 25 Dihydroxyvitamin D3 (Vitamin D) on Membrane Damage and Lipid Peroxidation in Non-Irradiated, UVA-Radiated, and UVB-Radiated Fibroblasts
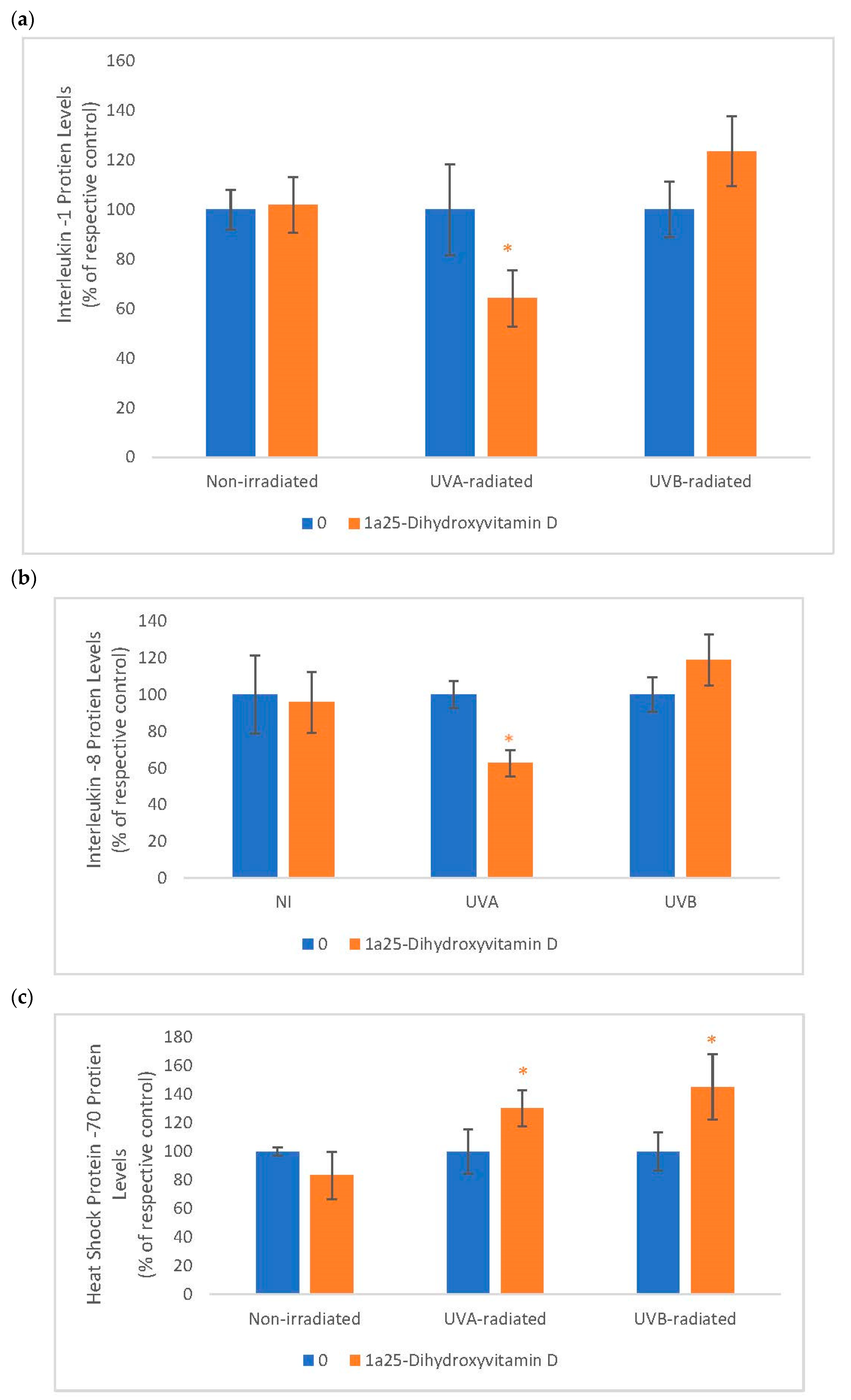
2.6. The Effect of 1α, 25 Dihydroxyvitamin D3 (Vitamin D) on Interleukin-1 (IL-1), Interleukin-8 (IL-8) and Heat Shock Protein-70 (HSP-70) in Non-Irradiated, UVA-Radiated, and UVB-Radiated Fibroblasts
3. Discussion
4. Conclusions
5. Methods
5.1. Antioxidant Activity
5.2. Elastase Activity
5.3. Cell Culture
5.4. Promoter Activities
5.5. Protein Levels
5.6. Cell Viability
5.7. Oxidative DNA/RNA Damage
5.8. Membrane Damage
5.9. Lipid Peroxidation
Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Philips, N.; Chalensouk-Khaosaat, J.; González, S. Stimulation of the elastin and fibrillin in non-irradiated or UVA radiated fibroblasts, and direct inhibition of elastase or matrix metalloptoteinases activity by nicotinamide or its derivatives. J. Cosmet. Sci. 2017, 68, 1–10. [Google Scholar]
- Philips, N.; Samuel, P.; Lozano, T.; Gvaladze, A.; Guzman, B.; Siomyk, H.; Haas, G. Effects of Humulus lupulus extract or its Components on Viability, Lipid Peroxidation, and expression of Vascular Endothelial Growth Factor in Melanoma Cells and Fibroblasts. Madr. J. Clin. Res. 2017, 1, 15–19. [Google Scholar] [CrossRef]
- Philips, N.; Chalensouk-Khaosaat, J.; Gonzalez, S. Stimulation of the Fibrillar Collagen and Heat Shock Proteins by Nicotinamide or Its Derivatives in Non-Irradiated or UVA Radiated Fibroblasts, and Direct Anti-Oxidant Activity of Nicotinamide Derivatives. Cosmetics 2015, 2, 146–161. [Google Scholar] [CrossRef]
- Philips, N.; Chalensouk-Khaosaat, J.; Devmurari, A.; Patel, H. Polyphenolic nanobiomaterials as emerging therapies for combating physiology and clinical aspects of photoaging and photocarcinogenesis. In Skin Aging and Photoaging: Physiology, Clinical Aspects and Emerging Therapies 2015; Nova Publishers Inc.: Hauppauge, NY, USA, 2015; Chapter 6; ISBN 978-1-63482-907-6. [Google Scholar]
- Philips, N.; Siomyk, H.; Bynum, D.; Gonzalez, S. Skin Cancer, Polyphenols, and Oxidative Stress. Cancer 2014, 1, 265–270. [Google Scholar]
- Philips, N.; Gonzalez, S. Beneficial Regulation of Elastase Activity and Expression of Tissue Inhibitors of Matrixmetalloproteinases, Fibrillin, Transforming Growth Factor-β, and Heat Shock Proteins by P. leucotomos in Nonirradiated or Ultraviolet-Radiated Epidermal Keratinocytes. ISRN Oxidative Med. 2013, 2013, 257463. [Google Scholar] [CrossRef]
- Philips, N.; Samuel, M.; Parakandi, H.; Siomyk, H.; Gopal, S.; Jia, H.; Ret, M.; Shahin, H. Vitamins in the Therapy of Inflammatory and Oxidative Diseases. In Frontiers in Clinical Drug Research—Anti Allergy Agents; Bentham Science: Potomac, MD, USA, 2013; pp. 240–264. [Google Scholar]
- Philips, N.; Samuel, P.; Siomyk, H.; Parakandi, H.; Gopal, S.; Shahin, H. Improved cell metabolism and strengthening of the extracellular matrix by nicotinamide, and copper for anti-skin aging. In Skin Aging: New Research; Nova Science: Hauppauge, NY, USA, 2012; pp. 43–58. [Google Scholar]
- Philips, N.; Samuel, P.; Parakandi, H.; Gopal, S.; Siomyk, H.; Ministro, A.; Thompson, T.; Borkow, G. Beneficial Regulation of Fibrillar Collagens, Heat Shock Protein-47, Elastin Fiber Components, Transforming Growth Factor-β1, Vascular Endothelial Growth Factor and Oxidative Stress Effects by Copper in Dermal Fibroblasts. Connect. Tissue Res. 2012, 53, 373–378. [Google Scholar] [CrossRef]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of cell proliferation, and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef]
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.; Conte, J.; Natrajan, P.; Haas, G.; Gonzalez, S. Direct inhibition of e.lastase and matrixmetalloproteinases, and stimulation of biosynthesis of fibrillar collagens, elastin and fibrillins by xanthohumol. J. Cosmet. Sci. 2010, 61, 125–132. [Google Scholar]
- Philips, N.; Conte, J.; Chen, Y.-J.; Natrajan, P.; Taw, M.; Keller, T.; Givant, J.; Tuason, M.; Dulaj, L.; Leonardi, D.; et al. Beneficial regulation of matrixmetalloproteinases and their inhibitors, fibrillar collagens and transforming growth factor-β by Polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Arch. Dermatol. Res. 2009, 301, 487–495. [Google Scholar] [CrossRef]
- Philips, N.; Tuason, M.; Chang, T.; Lin, Y.; Tahir, M.; Rodriguez, S. Differential Effects of Ceramide on Cell Viability and Extracellular Matrix Remodeling in Keratinocytes and Fibroblasts. Skin Pharmacol. Physiol. 2009, 22, 151–157. [Google Scholar] [CrossRef]
- Philips, N.; Keller, T.; Hendrix, C.; Hamilton, S.; Arena, R.; Tuason, M.; Gonzalez, S. Regulation of the extracellular matrix remodeling by lutein in dermal fibroblasts, melanoma cells, and ultraviolet radiation exposed fibroblasts. Arch. Dermatol. Res. 2007, 8, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Burchill, D.; O’Donoghue, D.; Keller, T.; Gonzalez, S. Identification of Benzene Metabolites in Dermal Fibroblasts as Nonphenolic: Regulation of Cell Viability, Apoptosis, Lipid Peroxidation and Expression of Matrix Metalloproteinase 1 and Elastin by Benzene Metabolites. Skin Pharmacol. Physiol. 2004, 17, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Smith, J.; Keller, T.; Gonzalez, S. Predominant effects of Polypodium leucotomos on membrane integrity, lipid peroxidation, and expression of elastin and matrixmetalloproteinase-1 in ultraviolet radiation exposed fibroblasts, and keratinocytes. J. Dermatol. Sci. 2003, 32, 1–9. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Kaiser, C.A.; Krieger, M.; Scott, M.P.; Bretscher, A.; Ploegh, H.; Matsudaira, P. Molecular Cell Biology; W.H. Freeman and Company: Stuttgart, Germany, 2016. [Google Scholar]
- Callaghan, T.M.; Wilhelm, K.-P. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part 2: Clinical perspectives and clinical methods in the evaluation of ageing skin. Int. J. Cosmet. Sci. 2008, 30, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Mecham, R.P.; Davis, E.C. Elastic Fiber Structure and Assembly. In Extracellular Matrix Assembly and Structure; Academic Press: Cambridge, MA, USA, 1994; pp. 281–314. [Google Scholar]
- Tsuji, N.; Moriwaki, S.; Suzuki, Y.; Takema, Y.; Imokawa, G. The role of elastases secreted by fibroblasts in wrinkle formation: Implication through selective inhibition of elastase activity. Photochem. Photobiol. 2001, 71, 283–290. [Google Scholar] [CrossRef]
- Matsuda, M.; Hoshino, T.; Yamashita, Y.; Tanaka, K.-I.; Maji, D.; Sato, K.; Adachi, H.; Sobue, G.; Ihn, H.; Funasaka, Y.; et al. Prevention of UVB Radiation-induced Epidermal Damage by Expression of Heat Shock Protein 70. J. Biol. Chem. 2009, 285, 5848–5858. [Google Scholar] [CrossRef] [PubMed]
- Labat-Robert, J.; Fourtanier, A.; Boyer-Lafargue, B.; Robert, L. Age dependent increase of elastase type protease activity in mouse skin. J. Photochem. Photobiol. B Biol. 2000, 57, 113–118. [Google Scholar] [CrossRef]
- Sellheyer, K. Pathogenesis of solar elastosis: Synthesis or degradation? J. Cutan. Pathol. 2003, 30, 123–127. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2009, 302, 71–83. [Google Scholar] [CrossRef]
- Walschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B 2001, 63, 41–45. [Google Scholar] [CrossRef]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new? J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Derm. 2007, 157, 874. [Google Scholar] [CrossRef] [PubMed]
- Kindt, T.J.; Goldsby, R.A.; Osborne, B.A. Kuby Immunology; W.H. Freeman and Company: Stuttgart, Germany, 2007. [Google Scholar]
- Philips, N.; Samuel, P.; Samuel, M.; Perez, G.; Khundoker, R.; Alahmade, G. Interleukin-4 Signaling Pathway and Effects in Allergic Diseases. Curr. Signal Transduct. Ther. 2018, 13, 1–5. [Google Scholar] [CrossRef]
- Philips, N.; Samuel, M. Inhibition of interleukin-4 signalling in the treatment of atopic dermatitis and allergic asthma. Glob. J. Allergy 2017, 3, 019–021. [Google Scholar] [CrossRef]
- Cheng, L.X.; Tang, J.J.; Luo, H.; Jin, X.L.; Dai, F.; Yang, J.; Qian, Y.P.; Li, X.Z.; Zhou, B. Antioxidant and antiproliferative activities of hydroxyl-substituted Schiff bases. Bioorg. Med. Chem. Lett. 2010, 20, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Chan, P.T.; Ho, K.Y.; Fung, K.P.; Wang, J. Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem. Phys. Lipids 1996, 79, 157–163. [Google Scholar] [CrossRef]
- Gazák, R.; Sedmera, P.; Vrbacký, M.; Vostálová, J.; Drahota, Z.; Marhol, P.; Walterová, D.; Kren, V. Molecular mechanisms of silybin and 2,3-dehydrosilybin antiradical activity—role of individual hydroxyl groups. Free Radic. Biol. Med. 2009, 46, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, B.; Wood, D. The Endocrine System at a Glance; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dobak, J.; Grzybowski, J.; Liu, F.T.; Landon, B.; Dobke, M. 1, 25-Dihydroxyvitamin D3 increases collagen production in dermal fibroblasts. J. Dermatol. Sci. 1994, 8, 18–24. [Google Scholar] [CrossRef]
- Norsgaard, H.; Kurdykowski, S.; Descargues, P.; Gonzalez, T.; Marstrand, T.; Dünstl, G.; Røpke, M. Calcipotriol counteracts betamethasone-induced decrease in extracellular matrix components related to skin atrophy. Arch. Dermatol. Res. 2014, 306, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Botney, M.D.; Mecham, R.R.; Parks, W.C. Inhibition of Tropoelastin Expression by 1, 25-Dihydroxyvitamin D3. Connect. Tissue Res. 1991, 26, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dixon, K.M.; Deo, S.S.; Holliday, C.J.; Slater, M.; Halliday, G.M.; Reeve, V.E.; Mason, R.S. Photoprotection by 1,25 Dihydroxyvitamin D3 Is Associated with an Increase in p53 and a Decrease in Nitric Oxide Products. J. Investig. Derm. 2007, 127, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Song, E.J.; Gordon-Thomson, C.; Cole, L.; Stern, H.; Halliday, G.M.; Damian, D.L.; Reeve, V.E.; Mason, R.S. 1α, 25-Dihydroxyvitamin D3 reduces several types of UV-induced DNA damage and contributes to photoprotection. J. Steroid Biochem. Mol. Biol. 2013, 136, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.; Deo, S.; Wong, G.; Slater, M.; Norman, A.; Bishop, J.; Posner, G.; Ishizuka, S.; Halliday, G.; Reeve, V.; et al. Skin cancer prevention: A possible role of 1,25dihydroxyvitamin D3 and its analogs. J. Steroid Biochem. Mol. Biol. 2005, 97, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.M.; Norman, A.W.; Sequeira, V.B.; Mohan, R.; Rybchyn, M.S.; Reeve, V.E.; Halliday, G.M.; Mason, R.S. 1α,25(OH)2-Vitamin D and a Nongenomic Vitamin D Analogue Inhibit Ultraviolet Radiation-Induced Skin Carcinogenesis. Cancer Prev. Res. 2011, 4, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Thomson, C.; Gupta, R.; Tongkao-On, W.; Ryan, A.; Halliday, G.M.; Mason, R.S. 1α,25 Dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin. Photochem. Photobiol. Sci. 2012, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Nakashyan, V.; Tipton, D.A.; Karydis, A.; Livada, R.; Stein, S.H. Effect of 1,25(OH)2D3 and 20(OH)D3 on interleukin-1β-stimulated interleukin-6 and -8 production by human gingival fibroblasts. J. Periodontal Res. 2017, 52, 832–841. [Google Scholar] [CrossRef]
- Rostkowska-Nadolska, B.; Sliupkas-Dyrda, E.; Potyka, J.; Kusmierz, D.; Fraczek, M.; Krecicki, T.; Kubik, P.; Zatonski, M.; Latocha, M. Vitamin D derivatives: Calcitriol and tacalcitol inhibits interleukin-6 and interleukin-8 expression in human nasal polyp fibroblast cultures. Adv. Med. Sci. 2010, 55, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Polla, B.S.; Healy, A.M.; Wojno, W.C.; Krane, S.M. Hormone 1 alpha,25-dihydroxyvitamin D3 modulates heat shock response in monocytes. Am. J. Physiol. Cell Physiol. 1987, 252, C640–C649. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philips, N.; Ding, X.; Kandalai, P.; Marte, I.; Krawczyk, H.; Richardson, R. The Beneficial Regulation of Extracellular Matrix and Heat Shock Proteins, and the Inhibition of Cellular Oxidative Stress Effects and Inflammatory Cytokines by 1α, 25 dihydroxyvitaminD3 in Non-Irradiated and Ultraviolet Radiated Dermal Fibroblasts. Cosmetics 2019, 6, 46. https://doi.org/10.3390/cosmetics6030046
Philips N, Ding X, Kandalai P, Marte I, Krawczyk H, Richardson R. The Beneficial Regulation of Extracellular Matrix and Heat Shock Proteins, and the Inhibition of Cellular Oxidative Stress Effects and Inflammatory Cytokines by 1α, 25 dihydroxyvitaminD3 in Non-Irradiated and Ultraviolet Radiated Dermal Fibroblasts. Cosmetics. 2019; 6(3):46. https://doi.org/10.3390/cosmetics6030046
Chicago/Turabian StylePhilips, Neena, Xinxing Ding, Pranathi Kandalai, Ilonka Marte, Hunter Krawczyk, and Richard Richardson. 2019. "The Beneficial Regulation of Extracellular Matrix and Heat Shock Proteins, and the Inhibition of Cellular Oxidative Stress Effects and Inflammatory Cytokines by 1α, 25 dihydroxyvitaminD3 in Non-Irradiated and Ultraviolet Radiated Dermal Fibroblasts" Cosmetics 6, no. 3: 46. https://doi.org/10.3390/cosmetics6030046
APA StylePhilips, N., Ding, X., Kandalai, P., Marte, I., Krawczyk, H., & Richardson, R. (2019). The Beneficial Regulation of Extracellular Matrix and Heat Shock Proteins, and the Inhibition of Cellular Oxidative Stress Effects and Inflammatory Cytokines by 1α, 25 dihydroxyvitaminD3 in Non-Irradiated and Ultraviolet Radiated Dermal Fibroblasts. Cosmetics, 6(3), 46. https://doi.org/10.3390/cosmetics6030046



