Efficacy and Safety of an Oral Nutritional (Dietary) Supplement Containing Pinus pinaster Bark Extract and Grape Seed Extract in Combination with a High SPF Sunscreen in the Treatment of Mild-to-Moderate Melasma: A Prospective Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Investigational Products
2.4. MASI
2.5. Physician’s Global Assessment
2.6. Patient’s Global Assessment
2.7. Instrumental Evaluation of Efficacy
2.7.1. Measurements of Skin Depigmenting Properties by Mexameter®
2.7.2. Melasma Assessment by VISIA®-CA and Image J
2.8. Subject Self-Assessment of the Properties of the Product
2.9. Evaluation of Tolerability
2.9.1. Self-Evaluation
2.9.2. Visual Examination by Dermatologist
2.10. Statistical Analyses
3. Results
3.1. Subjects
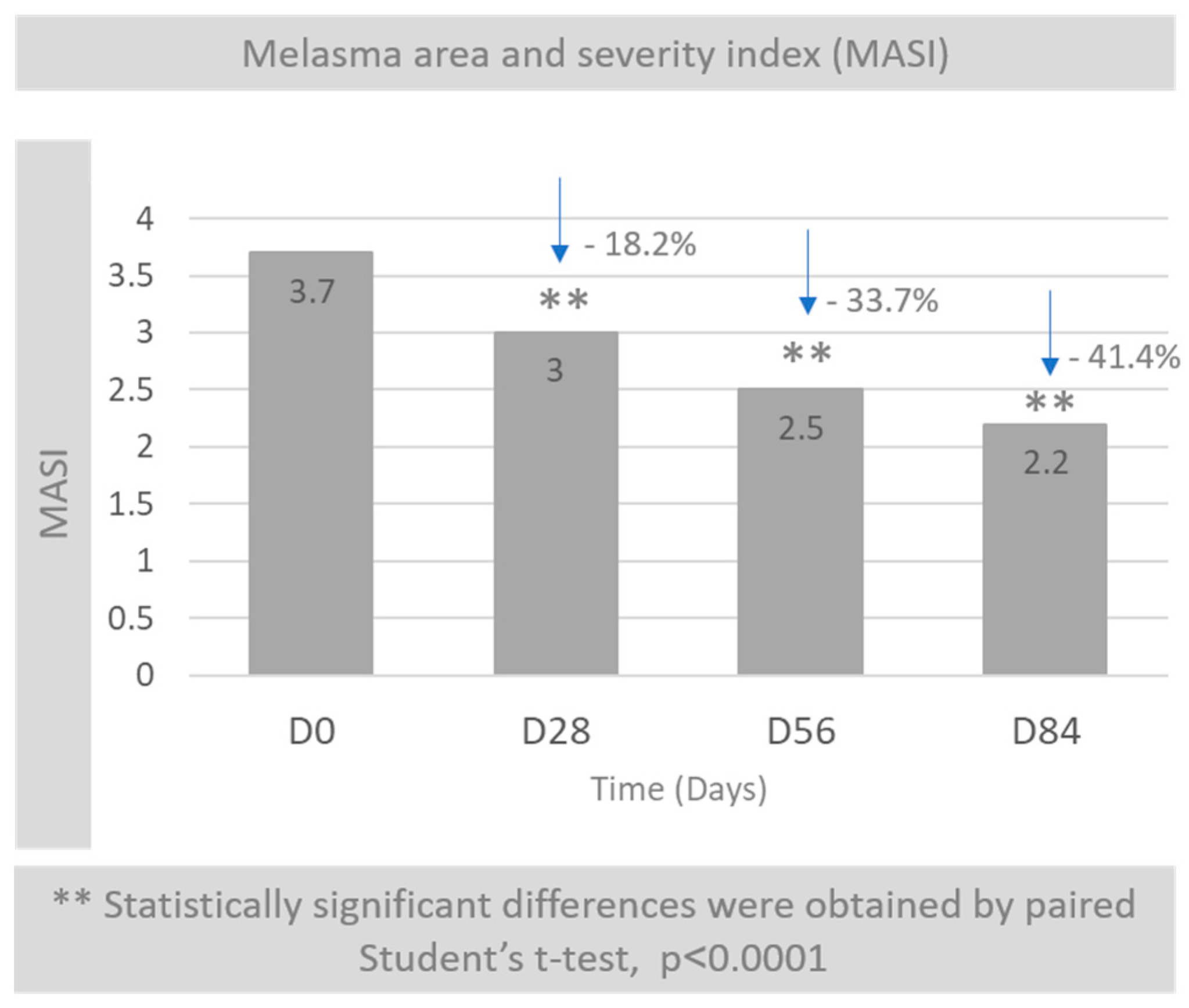
3.2. Melasma Area and Severity Index (MASI)
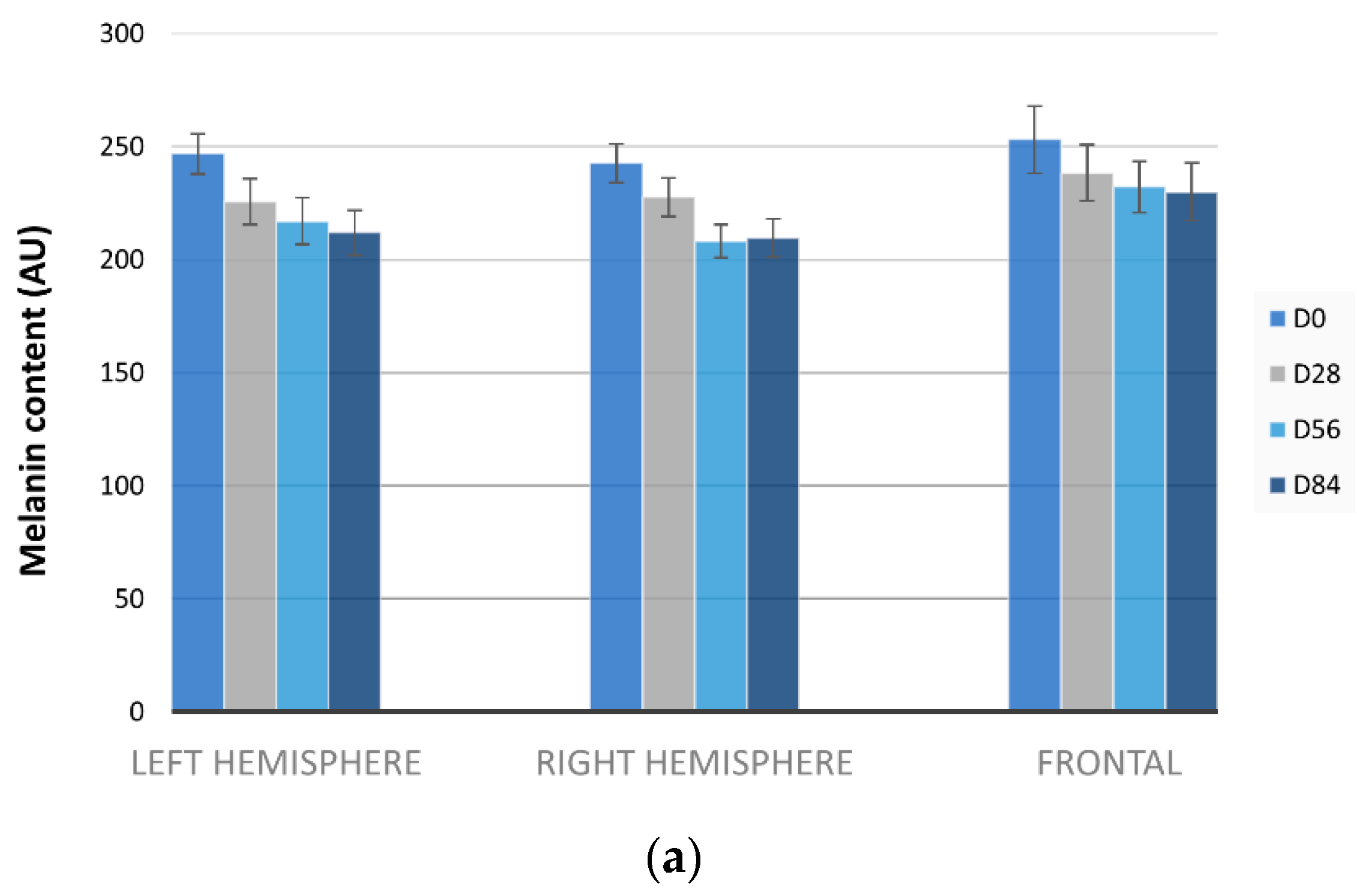
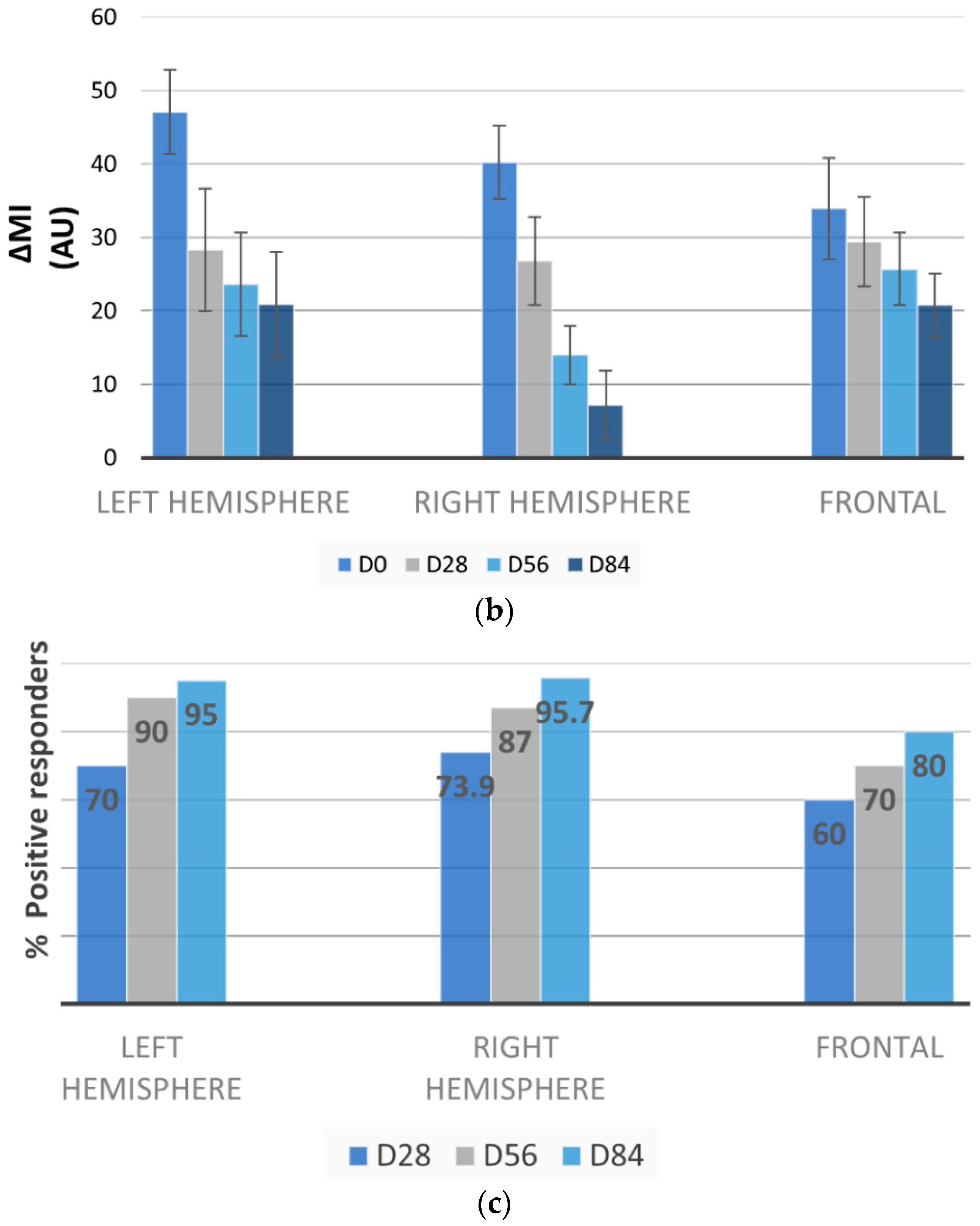
3.3. Melasma Decrease by Mexameter®
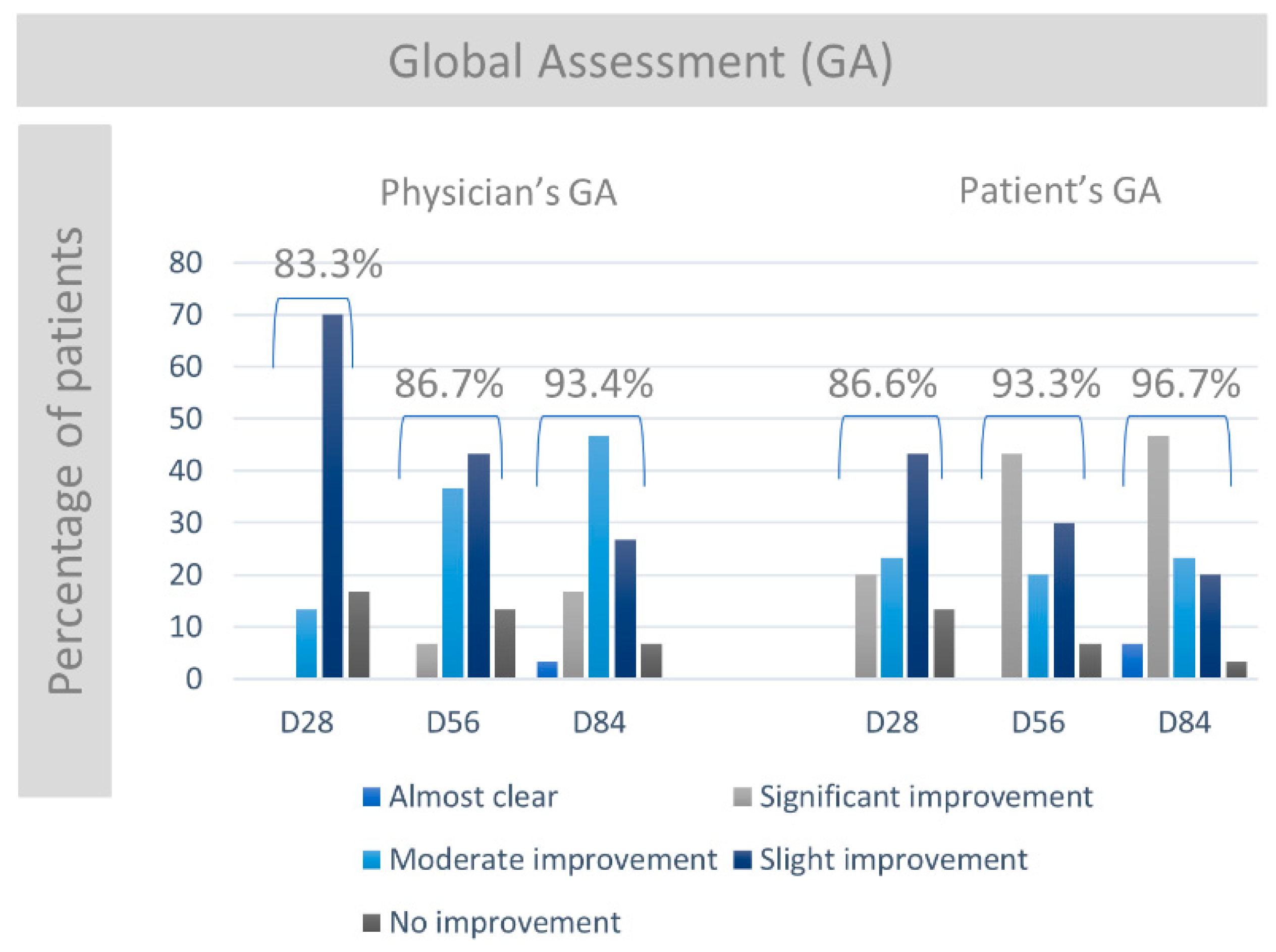
3.4. Physician’s and Patient’s Global Assessment
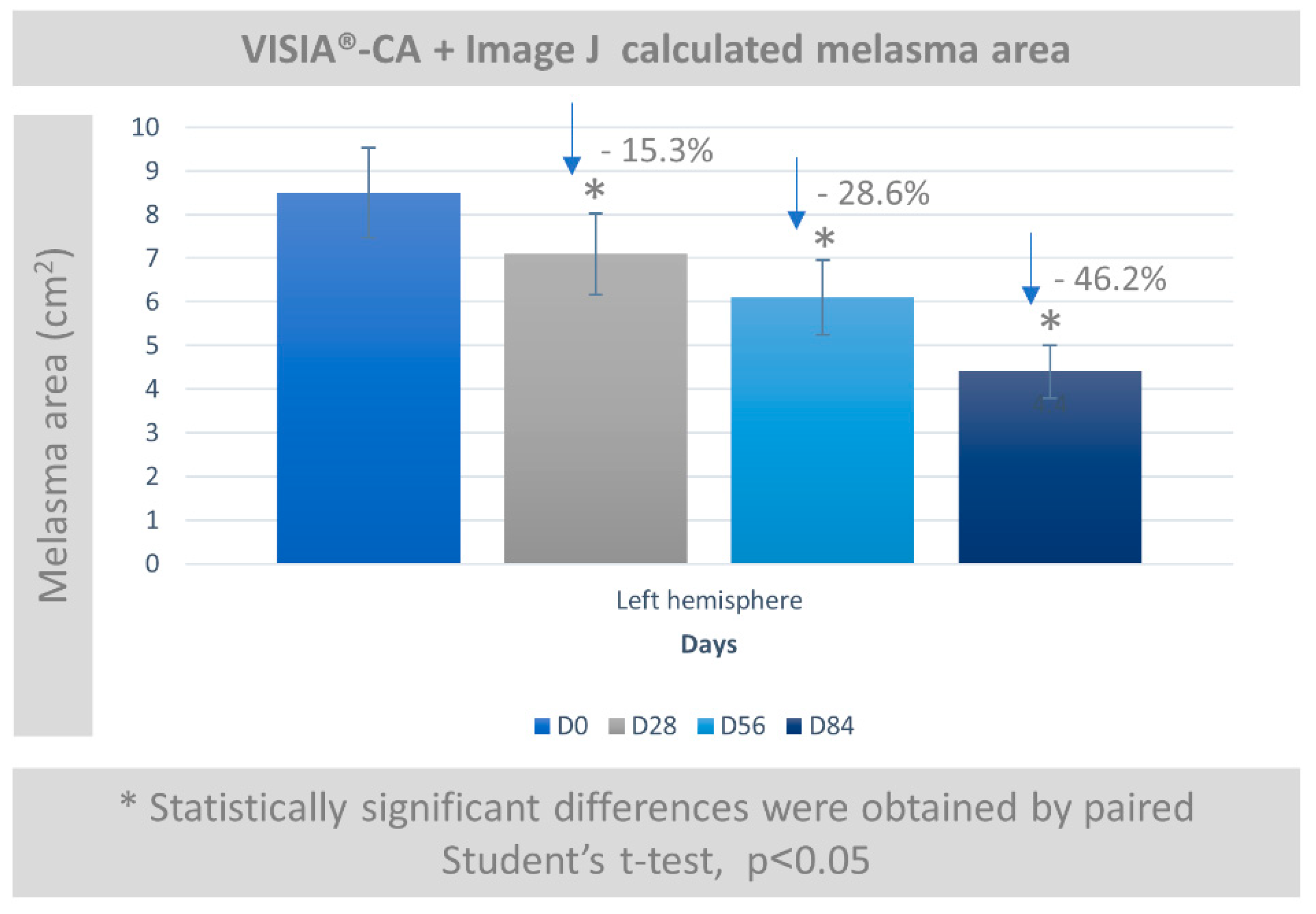
3.5. Melasma Assessment by VISIA-CA® and Image J
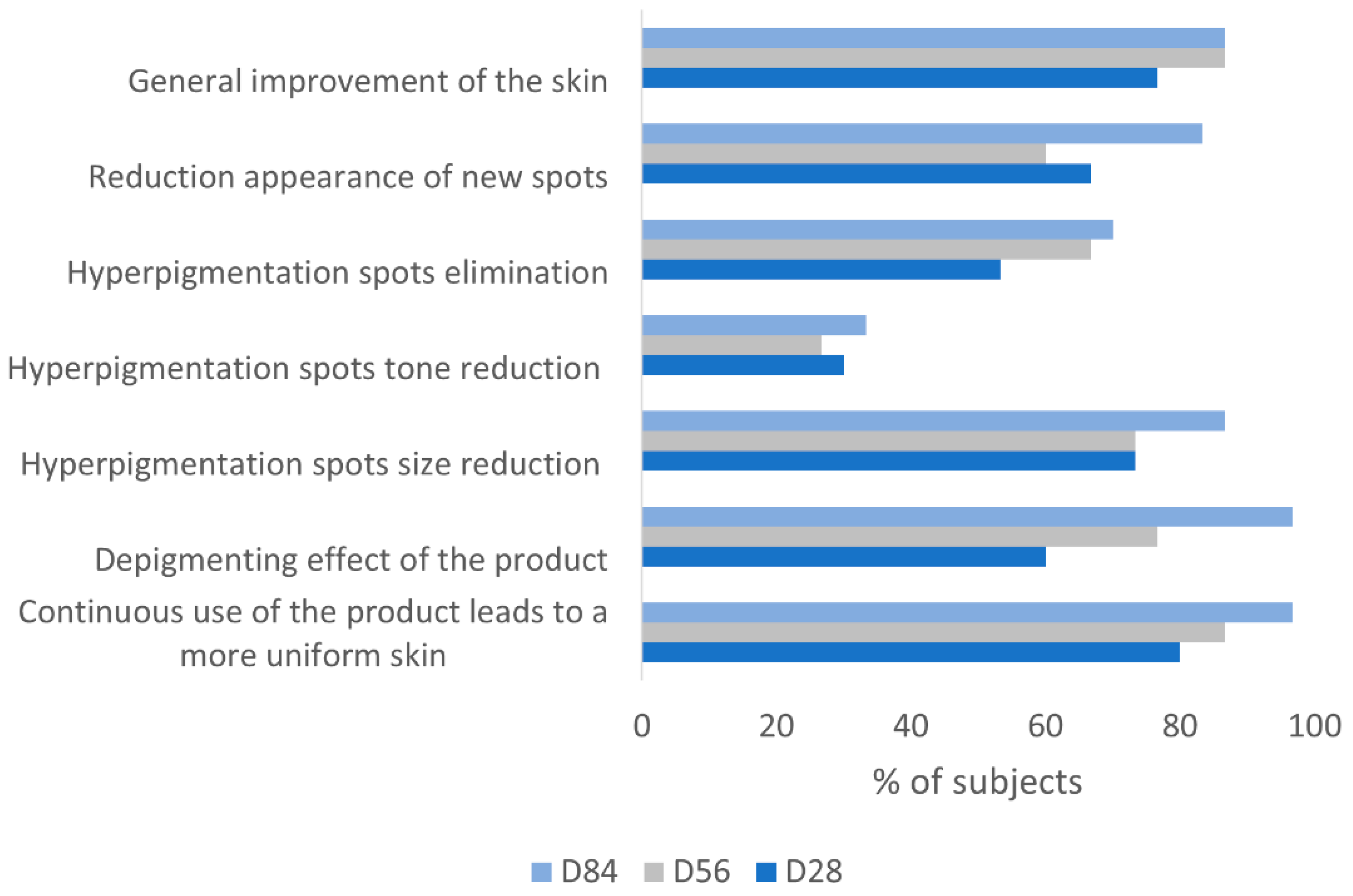
3.6. Subjects Self-Assessment of the Depigmenting Properties of the Product
3.7. Exploratory Analysis: Anti-ageing Parameters
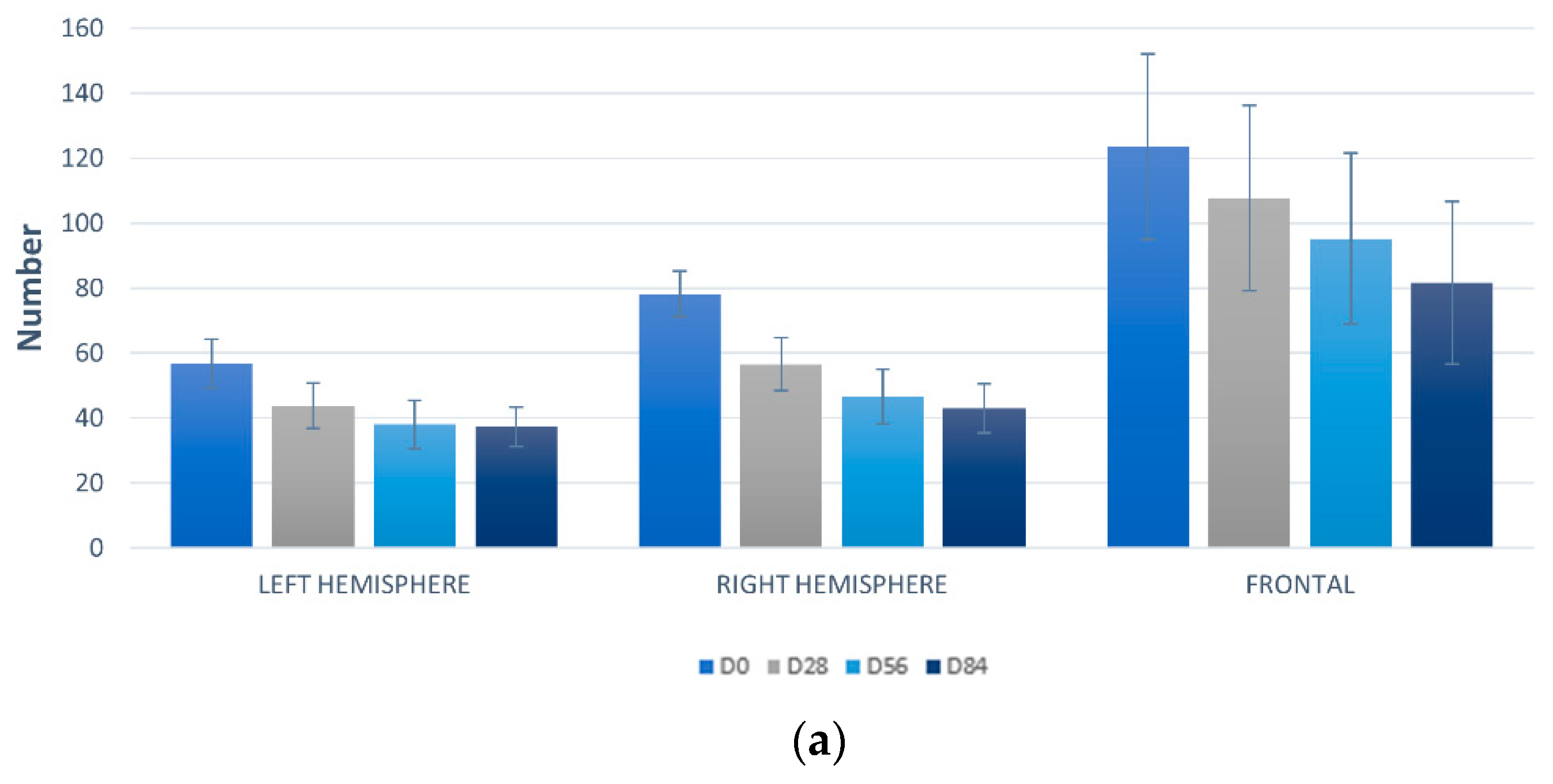
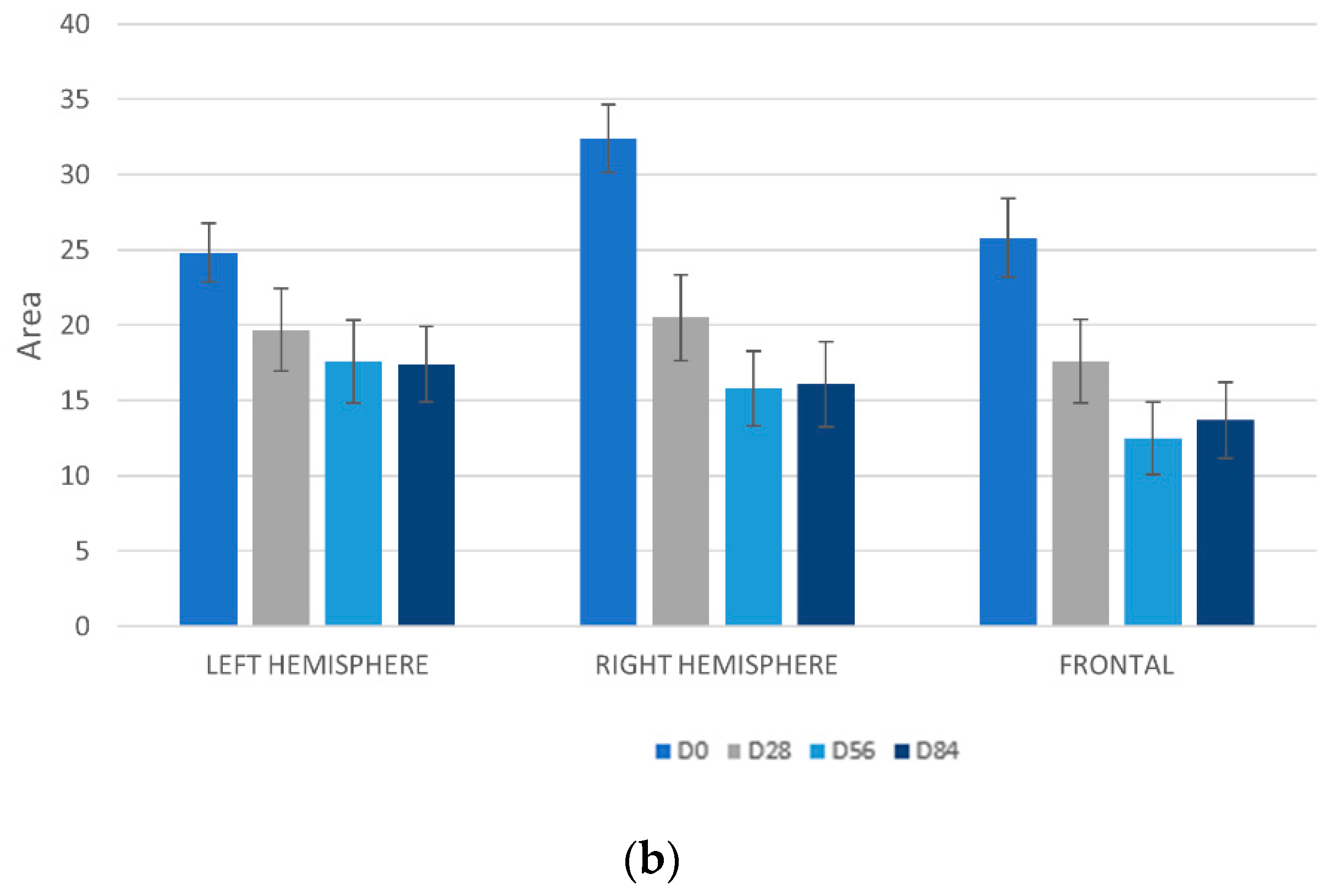
3.7.1. Detection UV Spots by VISIA-CA®
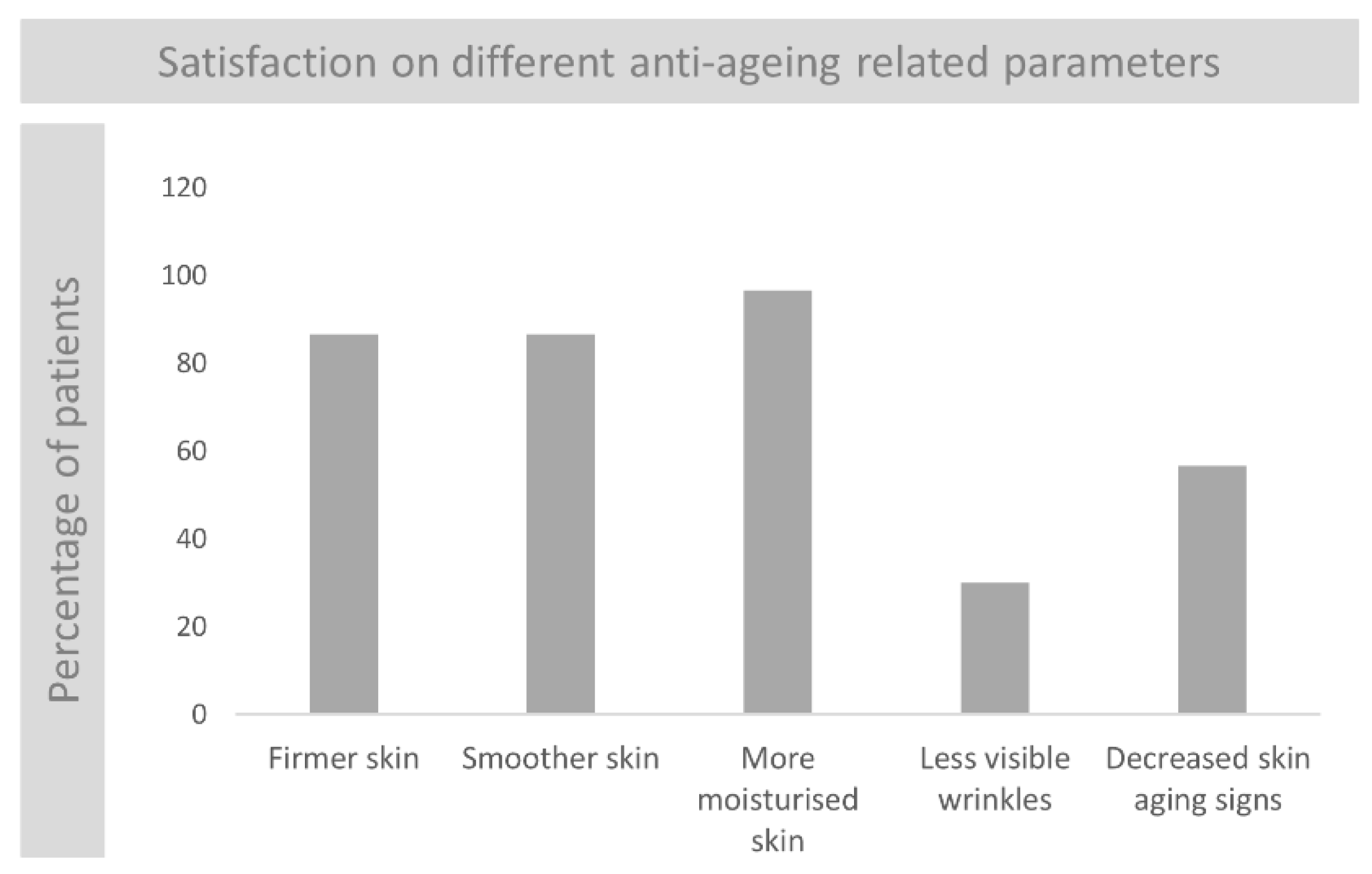
3.7.2. Subjects Self-Assessment of the Anti-Ageing Properties of the Product
3.7.3. Evaluation of Tolerability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Passeron, T.; Picardo, M. Melasma, a photoaging disorder. Pigment Cell Melanoma Res. 2018, 31, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.C.; Miot, L.D.; Miot, H.A. Melasma: A clinical and epidemiological review. An. Bras. Dermatol. 2014, 89, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.H.; Yoon, K.H.; Lee, E.S.; Kim, J.; Lee, K.B.; Yim, H.; Sohn, S.; Im, S. Melasma: Histopathological characteristics in 56 Korean subjects. Br. J. Dermatol. 2002, 146, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.E.; Yamada, N.; Bhawan, J. Light microscopic, immunohistochemical, and ultrastructural alterations in subjects with melasma. Am. J. Dermatopathol. 2005, 27, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Suzuki, I.; Lee, D.J.; Ha, J.; Reiniche, P.; Aubert, J.; Deret, S.; Zugaj, D.; Voegel, J.J.; Ortonne, J.P. Transcriptional profiling shows altered expression of wnt pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J. Investig. Dermatol. 2011, 131, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015, 28, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Duteil, L.; Cardot-Leccia, N.; Queille-Roussel, C.; Maubert, Y.; Harmelin, Y.; Boukari, F.; Ambrosetti, D.; Lacour, J.P.; Passeron, T. Differences in visible light-induced pigmentation according to wavelengths: A clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014, 27, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, M.L.W.; Levin, M.K. The role of systemic treatments for skin lightening. J. Cosmet. Dermatol. 2018, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.L.; Baibergenova, A. Melasma: Systematic review of the systemic treatments. Int. J. Dermatol. 2017, 56, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Mu, Y.; Gulati, O. Treatment of melasma with Pycnogenol. Phytother. Res. 2002, 16, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y.; Kawachi, Y.; Otsuka, F. Oral intake of proanthocyanidin-rich extract from grape seeds improves chloasma. Phytother. Res. 2004, 18, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Saliou, C. Role of vitamins in skin care. Nutrition 2001, 17, 839–844. [Google Scholar] [CrossRef]
- Eberlein-Konig, B.; Placzek, M.; Przybilla, B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and D-alpha-tocopherol (vitamin E). J. Am. Acad. Dermatol. 1998, 38, 45–48. [Google Scholar] [CrossRef]
- Placzek, M.; Gaube, S.; Kerkmann, U.; Gilbertz, K.P.; Herzinger, T.; Haen, E.; Przybilla, B. Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-alpha-tocopherol. J. Investig. Dermatol. 2005, 124, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; De Rosa, V.; Rachidi, W.; Diamond, A.M. Does a role for selenium in DNA damage repair explain apparent controversies in its use in chemoprevention? Mutagenesis 2013, 28, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.C.; Surjana, D.; Halliday, G.M.; Damian, D.L. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in primary melanocytes. Exp. Dermatol. 2014, 23, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough-Green, C.K.; Griffiths, C.E.; Finkel, L.J.; Hamilton, T.A.; Bulengo-Ransby, S.M.; Ellis, C.N.; Voorhees, J.J. Topical retinoic acid (tretinoin) for melasma in black patients. A vehicle-controlled clinical trial. Arch. Dermatol. 1994, 130, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Kern, H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: A clinical study using solar simulated radiation. Free Radic. Biol. Med. 1998, 25, 1006–1012. [Google Scholar] [CrossRef]
- Favrot, C.; Beal, D.; Blouin, E.; Leccia, M.T.; Roussel, A.M.; Rachidi, W. Age-Dependent Protective Effect of Selenium against UVA Irradiation in Primary Human Keratinocytes and the Associated DNA Repair Signature. Oxid. Med. Cell. Longev. 2018, 5895439. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, S.; Gündüz, Ö.; Sayan, C.D. Retrospective Analysis of Endemic Melasma subjects. Dermatol. Rep. 2017, 9, 7027. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.M.; Robillard, N.F. The controlled use test in a cosmetic product safety substantation program. J. Toxicol. Cutan. Ocul. Toxicol. 1982, 20, 117–132. [Google Scholar]
- Keswick, B.H.; Ertel, K.D.; Visscher, M.O. Comparison of exaggerated and normal use techniques for assessing the mildness of personal cleansers. J. Soc. Cosm. Chem. 1992, 43, 187–193. [Google Scholar]
- Jackwerth, B.; Krächter, H.U.; Matthies, W. Dermatological test methods for optimising mild tenside preparations. Parfüm. Kosmet. 1993, 74, 134–141. [Google Scholar]
- Pandya, A.; Berneburg, M.; Ortonne, J.P.; Picardo, M. Guidelines for clinical trials in melasma. Pigmentation Disorders Academy. Br. J. Dermatol. 2006, 156 (Suppl. 1), 21–28. [Google Scholar] [PubMed]
- Pandya, A.G. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J. Am. Acad. Dermatol. 2011, 64, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Alewaeters, K.; Barel, A.O. Comparative Study of Skin Color Using Different Bioengineering Methods. Skin Res. Technol. 1999, 5, 129. [Google Scholar]
- Costa, A.; Moisés, T.A.; Cordero, T.; Alves, C.R.; Marmirori, J. Association of emblica, licorice and belides as an alternative to hydroquinone in the clinical treatment of melasma. An. Bras. Dermatol. 2010, 85, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Dell’Acqua, G.; Wagner, C. Lightening and Illuminating Skin with Acetylated Hydroxystilbenes from Rheum rhaponticum. Cosmet. Toilet. 2011, 126, 634–642. [Google Scholar]
| Subject Baseline Characteristics | |
| Total | 30 women |
| Age - Mean (Range) | 39 (23–63) |
| Skin Phototype (Fitzpatrick *) | |
| II | 3 (10%) |
| III | 23 (76.7%) |
| IV | 4 (13.3%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aladrén, S.; Garre, A.; Valderas-Martínez, P.; Piquero-Casals, J.; Granger, C. Efficacy and Safety of an Oral Nutritional (Dietary) Supplement Containing Pinus pinaster Bark Extract and Grape Seed Extract in Combination with a High SPF Sunscreen in the Treatment of Mild-to-Moderate Melasma: A Prospective Clinical Study. Cosmetics 2019, 6, 15. https://doi.org/10.3390/cosmetics6010015
Aladrén S, Garre A, Valderas-Martínez P, Piquero-Casals J, Granger C. Efficacy and Safety of an Oral Nutritional (Dietary) Supplement Containing Pinus pinaster Bark Extract and Grape Seed Extract in Combination with a High SPF Sunscreen in the Treatment of Mild-to-Moderate Melasma: A Prospective Clinical Study. Cosmetics. 2019; 6(1):15. https://doi.org/10.3390/cosmetics6010015
Chicago/Turabian StyleAladrén, Sonia, Aurora Garre, Palmira Valderas-Martínez, Jaime Piquero-Casals, and Corinne Granger. 2019. "Efficacy and Safety of an Oral Nutritional (Dietary) Supplement Containing Pinus pinaster Bark Extract and Grape Seed Extract in Combination with a High SPF Sunscreen in the Treatment of Mild-to-Moderate Melasma: A Prospective Clinical Study" Cosmetics 6, no. 1: 15. https://doi.org/10.3390/cosmetics6010015
APA StyleAladrén, S., Garre, A., Valderas-Martínez, P., Piquero-Casals, J., & Granger, C. (2019). Efficacy and Safety of an Oral Nutritional (Dietary) Supplement Containing Pinus pinaster Bark Extract and Grape Seed Extract in Combination with a High SPF Sunscreen in the Treatment of Mild-to-Moderate Melasma: A Prospective Clinical Study. Cosmetics, 6(1), 15. https://doi.org/10.3390/cosmetics6010015





