Abstract
Overexposure to sunlight is widely accepted as the underlying cause of cutaneous melanoma. UV radiation induces the formation of DNA photoproducts that, if unrepaired, can induce carcinogenic mutations. Recent data indicate that sorbates can be useful to widen the protection against UV radiation by acting as a triplet-state quencher in the melanocyte. The aim of the present work was to prepare an after sun formulation containing ethylsorbate or sorbic acid in order to take advantage of the triplet-state quenching activity of these molecules and protect the skin from UV-induced damages. Ethylsorbate and sorbic acid were characterized in terms of solubility and partition coefficient, and their transdermal permeation and skin accumulation were studied in vitro from simple solutions and in the presence of cyclodextrins (alpha and hydroxypropylbeta) as a complexing agent. The goal was to reduce as much as possible sorbates permeation while sustaining their skin levels. The obtained results indicated that the addition of alphacyclodextrins determined a 6-folds (ethylsorbate ) or 4-folds (sorbic acid) reduction of the transdermal permeation. Sorbic acid and alphacyclodextrin (1:1 molar ratio) were then formulated in an after sun vehicle using 1.5% hyaluronic acid (sodium salt) as a thickener and hydrating agent. The addition of hyaluronic acid gave rise to a formulation with good cosmetic properties and good sorbate (0.2–0.3 µmol/cm2) skin levels (stratum corneum + viable epidermis) and thus a potential protection against post-exposure UV damage.
1. Introduction
Cutaneous melanoma is a very aggressive form of skin cancer originating from melanocytes that become cancerous cells due to aberrant changes at the biochemical level. Overexposure to sunlight is widely accepted as the underlying cause of cutaneous melanoma and it has been estimated that four out of five cases of skin cancer could be prevented, through the limitation of exposure to the mid-day sun and the use of sunscreens [1,2]. Mutations in sunlight-induced melanoma are due to UV photon absorption by DNA. The result is the formation of cyclobutane pyrimidine dimers (CPD), DNA photoproducts that, if unrepaired, can induce carcinogenic mutations. These CPDs form picoseconds after an ultraviolet (UV) photon is absorbed, so they originate only during UV exposure and can be prevented by UV filters. However, recently, some researchers demonstrated that in melanocytes, CPDs are continuously generated for hours (at least 3) after exposure to UVA. These CPDs are called “dark CPD” and are due to the chemiexcitation of melanin derivatives and the formation of a melanin quantum triplet state that acts as a CPD maker [3]. The same researchers demonstrated that this process could be prevented by melanin triple state quenchers such as vitamin E and sorbates [3,4]. These substances could in principle be added to after sun formulations to strengthen the prevention against carcinogenic events.
The aim of the present work was to produce an after sun formulation containing sorbates to take advantage of the triplet quenching activity of these molecules and protect the skin against UV damage. In particular, ethylsorbate and sorbic acid were used. The specific goals were (1) to characterize ethylsorbate and sorbic acid; (2) to validate a procedure for the extraction from stratum corneum and viable skin; (3) to evaluate ethylsorbate and sorbic acid permeation and skin retention from solutions; (4) to formulate sorbic acid in an after sun formulation using hyaluronic acid (sodium salt) as a thickener and hydrating agent. In order to reduce sorbates penetration and promote their skin retention, sorbate-cyclodextrins mixtures were evaluated. Cyclodextrins, cyclic oligosaccharides with a hydrophilic surface and a lipophilic cavity, were selected thanks to their property of including molecules and in particular conditions that reduce their skin permeation. A number of studies have indeed demonstrated that the use of excess cyclodextrins (i.e., more than the amount needed to solubilize the compound) results in the reduction of transmembrane penetration [5].
Ethylsorbate is a liquid compound and is FDA-approved as a synthetic flavoring substance permitted for direct addition to food for human consumption [6]. Sorbic acid (Hexa-2,4-dienoic acid) is a medium-chain fatty acid frequently used as an anti-microbial food preservative agent and also used in cosmetic formulation as a preservative.
2. Materials and Methods
2.1. Materials
Sorbic acid (SA, MW = 112.13; pKa = 4.76) and ethylsorbate (ES, MW = 140.18, density: 0.933 g/mL) (Figure 1) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Alpha cyclodextrin (alphaCD, MW 972.86 Da) was from Wacker Chemie (Lyon, France) while hydroxypropyl betacyclodextrin (HPbetaCD, MW ≈ 1396 Da) from Sigma-Aldrich (St. Louis, MO, USA). Transcutol was from Gattefossé (Saint Priest, France) and sodium hyaluronate (HA, MW 1000 kDa) from IBSA Farmaceutici Italia Srl (Lodi, Italy). All other chemicals were of analytical grade.
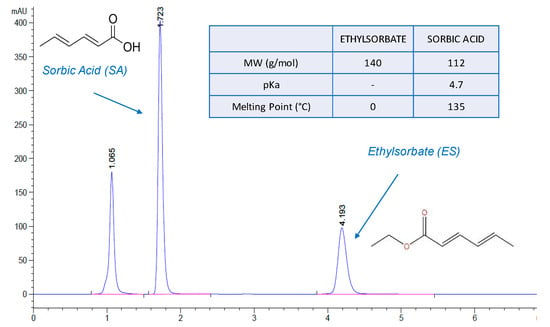
Figure 1.
Panel a: Chromatogram related to the injection of a standard solution containing sorbic acid and ethylsorbate. In the figure, the chemical structure of the two compounds is also reported, together with the relevant physicochemical properties.
2.2. HPLC analysis
Sorbic acid and ethylsorbate were quantified by HPLC-UV (Infinity 1260, Agilent Technologies, Santa Clara, CA, USA), with a reverse-phase Nova-Pack C18 cartridge (150 × 3.9 mm, 4 µm) (Waters, Milford, MA, USA) and a C18 guard column (Security Guard™ Cartridge, Phenomenex, Torrance, CA, USA) both thermostatted at 40 °C. The mobile phase, pumped at 1.0 ml/min, was a 60:40 (v/v) mixture methanol: water containing 1% acetic acid. The injection volume was 20 µL, and absorbance was monitored at 258 nm. In these conditions, sorbic acid and ethylsorbate retention times were about 1.7 and 4.2 min respectively (Figure 1). The HPLC methods were validated for sensitivity, precision and accuracy. In the case of sorbic acid and ethysorbate, calibration curves were prepared in the concentration intervals 1 × 10−4(LOQ)–5 × 10−2 µmol/mL and 7 × 10−3(LOQ)–3.6 × 10−1 µmol/mL respectively. RE% and RSD% were lower than 5 and 2.5% in all cases.
2.3. Determination of the Partition/Distribution Coefficient of Sorbic Acid and Ethylsorbate
Partition coefficients of sorbic acid and ethylsorbate between isopropyl miristate (IPM) and saline solution or between octanol (OCT) and saline solution were determined using the shake flask method, following the guidelines of the European Chemical Bureau (European-Chemical-Bureau, Dir 92/69/EEC; O.J. L383 A). Briefly, before the partition coefficient determination, the two phases of the solvent system were mutually saturated by shaking for 3 h at the same temperature (20 ± 2 °C) and in the same ratio of the partitioning experiments. The organic phase:water phase ratio was 1:1 in case of sorbic acid and 1:10 in case of ethylsorbate. Each compound was then individually dissolved in the saturated water phase at a concentration of 100 µg/mL. 1.0 mL of this solution was transferred to 2.0 mL vials and added to either 1.0 mL (sorbic acid) or 0.1 mL of saline-saturated organic phase. Each sample was submitted to 100 rotations and then the two phases were separated. SA and ES were quantified in both phases. The aqueous phase was directly injected in HPLC, while the organic phase was diluted 1:2 (SA) or 1:10 (ES) with methanol. The partition/distribution coefficients KIPM/Saline and KOCT/Saline of SA and ES were calculated as:
where [IPM] and [OCT] were the concentrations in the organic phase and [Saline] was the concentration in the aqueous phase; VIPM and VOCT were the volumes of the organic phase; VSaline was the volume of the aqueous phase.
2.4. Skin Retention and Permeation Experiments
For permeation experiments, porcine skin was used. The tissue was isolated from Landrace and Large White animals supplied from a local slaughterhouse (Annoni S.p.A., Parma, Italy). The skin, excised from the outer part of pig ears, was separated from the underlying cartilage with a scalpel and frozen at −20 °C until the day of the experiment. The skin was then thawed and mounted on vertical diffusion cells (DISA, Milano, Italy; 0.6 cm2 surface area) with the stratum corneum facing the donor compartment. The receptor compartment was filled with about 4 mL of either 0.9% NaCl or PBS pH 7.4; preliminary experiments demonstrated that the different receptor solution did not have any influence on permeation and retention. The formulations evaluated are reported in Table 1. About 200 µL or 200 mg of the formulations were applied for 5 h in the donor compartment and 300 µL of the receptor fluid were sampled every hour. Each condition was replicated 3 to 6 times. At the end of the experiment, the receptor solution was sampled, the donor formulation was carefully removed with absorbent paper, the tissue was rinsed with 10 mL of distilled water and blotted dry with filter paper. Then, the skin (0.6 cm2, area in contact with the donor formulation) was stripped (20 strips, Scotch Book Tape, 3M, USA) for SC sampling, and the remaining skin sample (0.6 cm2), was minced. Both tissues were extracted using a validated method (see item 2.5). Extraction and permeation samples were analyzed by HPLC.

Table 1.
Composition, concentration and pH of the different vehicles tested on porcine skin for the determination of sorbic acid/ethysorbate permeation and retention.
2.5. Validation of the Extraction Procedure
In order to set up and validate the procedure for sorbic acid and ethylsorbate skin extraction, skin samples (area 0.6 cm2) were tape-stripped 20 times, to separate the SC. Tissues (i.e., SC and the remaining stripped skin) were separately spiked with a known amount of SA or ES and then extracted in different conditions. After centrifugation and/or filtration, samples were analyzed by HPLC for the determination of the recovery %.
2.6. Solubility and Phase Solubility Diagram
For the determination of ES solubility, an excess amount was added to water or to saline solution (NaCl 0.9%). After magnetic stirring for 24 h, the dispersion was centrifuged (10 min, 12,000 rpm), and the water phase was sampled for the HPLC quantification of ES dissolved. If the water phase was not clear, a second centrifugation was performed.
In order to build the SA phase solubility diagram in the presence of cyclodextrins, an excess amount of SA was added to 1 mL of distilled water or aqueous solutions of alpha or HP beta cyclodextrin (from 10 to 90 mM) and magnetically stirred for 24 h at 25 °C. Thereafter, the suspensions were filtered (0.45 µm, PTFE) and the filtrate was diluted 1:100 with saline solution. SA concentration in each solution was determined by HPLC. Each experiment was replicated three times.
2.7. Statistical Analysis
The significance of the differences between conditions was assessed using a Student’s t-test. Differences were considered statistically significant when p < 0.05. In the Figures, for the sake of clarity, the experimental points are represented as the mean value ± standard error of the mean (sem), as indicated in the legend. The number of replicates is always included between three and six.
3. Results and Discussion
3.1. Basic Studies
3.1.1. Partition Coefficient
The partition coefficient of ethylsorbate and sorbic acid was evaluated using 0.9% NaCl as the water phase and both octanol and isopropylmiristate (IPM) as the lipophilic phase. While octanol represented the reference solvent, IPM was selected since it better mimicked the lipophilicity of the stratum corneum [7]. The results are presented in Table 2. The pH of the aqueous phase after sorbic acid addition (100 µg/mL) was 4.8, thus the value reported for sorbic acid represents the distribution coefficient at pH 4.8 (pKa = 4.7; unionized form: 52%). This value was—as expected—lower than the one reported in the literature for the unionized form (1.3) [8].

Table 2.
Physico-chemical characteristics of ethylsorbate (ES) and sorbic acid (SA). Partition (ethylsorbate) and distribution (sorbic acid, pH 4.8) coefficients were obtained using 0.9% NaCl as the water phase and either octanol (LogK OCT/Saline) or IPM (LogK IPM/Saline) as the lipophilic phase.
As expected, a much higher partition was found for ethylsorbate, with no difference between octanol and IPM. In the case of sorbic acid, even if the difference was not statistically significant (p = 0.052), the value found in octanol was higher than in IPM, in agreement with the solubility parameters of SA (11.97) and closer to octanol (10.3 ) than IPM (8.02) [9,10].
3.1.2. Solubility
In the case of ethylsorbate, the solubility was evaluated in water and in saline solution. The measure of solubility in water was challeging, due to (1) the difficulty in ethyilsorbate—water separation (the solution was often opalescent after centrifugation) and (2) the volatility of ES, whose concentration in water tended to decrease by increasing the centrifugation time. The presence of NaCl favoured the phase separation and the obtained solubility was 1.40 ± 0.30 mg/mL.
Sorbic acid solubility in water was 2.00 ± 0.10 mg/mL (Table 2) in agreement with literature data (1.9 mg/mL) [11]. Figure 2 reports the solubility diagram in the presence of either alpha or HPbeta CD. The shape of the curve related to alpha CD suggesting a type B diagram [12], forming a 1:1 inclusion compound with a defined solubility of about 60 mM. The profile obtained with HPbetaCD could be attributed to a type AL diagram [12], at least until a 90 mM CD concentration. Up to 60 mM CD concentration, the affinity of sorbic acid is higher for alphaCD with respect to HPbetaCD. The higher affinity of SA for the smaller cavity of the alphaCD with respect to the bigger betaCD ring was also demonstrated by cyclodextrin-modified capillary electrophoresis [13].
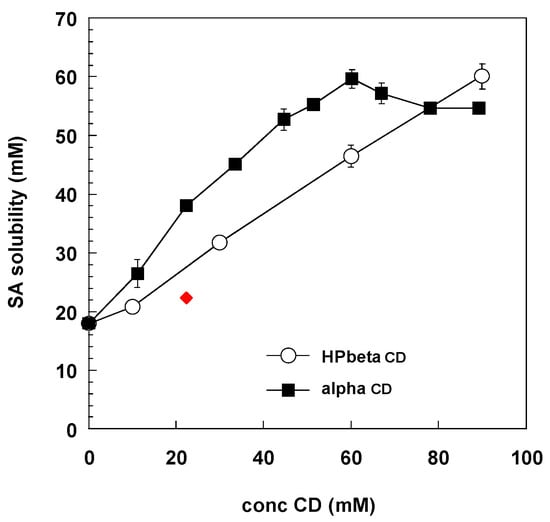
Figure 2.
Solubility diagram of SA in the presence of alpha (full square) and HPbeta (void circle) cyclodextrins. The red diamonds represent the concentrations of SA and alphaCD used in the permeation experiments (Table 1).
3.1.3. Validation of Skin Extraction
The extraction of sorbic acid was evaluated under different experimental conditions and the best result is reported in Table 3.

Table 3.
Experimental conditions and recovery % for sorbic acid extraction from SC and stripped skin.
In the case of ethysorbate, it was not possible to validate an extraction procedure, due to the volatility of this compound. The extraction was then performed using a mixture of MeOH: 0.1 M HCl: Transcutol (1:1:1). This vehicle guarantees a good solubilisation of ethylsorbate, freely miscible with Transcutol, and good penetration into the tissue due to the presence of the aqueous phase. Additionally, the recovery percentage of sorbic acid (that can be formed into the tissue following ethylsorbate hydrolysis) was evaluated with this method and resulted in quantitative data.
3.2. Transdermal Penetration and Skin Retention of Ethylsorbate and Sorbic Acid from Solutions
3.2.1. Ethylsorbate (ES)
Ethylsorbate (ES) is a liquid compound characterized by low MW and relatively high lipophilicity. These characteristics suggest high skin permeability. To evaluate ES permeation properties, experiments were performed using the pure liquid (d = 0.93 g/mL) and a 0.6 mg/mL water solution (clear, close to saturation). Being an ester, ES can be metabolized into the skin to form sorbic acid (SA), that was quantified with the same analytical method (Figure 1). The permeation data (Figure 3a) refers only to SA, because only this compound was recovered in the receptor solution. Since in both cases the saturation degree was equal to 1, in the first two hours the permeation profiles were comparable, regardless of the 1000-fold difference in concentration. The profile obtained with the water saturated solution tended then to flatten, probably due to ES depletion in the donor solution, linked both to permeation/skin retention and to the possible evaporation.
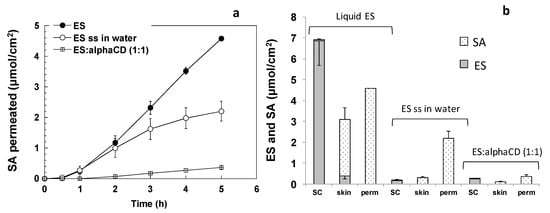
Figure 3.
Permeation and skin retention of sorbic acid and ethylsorbate starting from pure ES, from a water saturated solution of ES, and from a water solution containing a 1:1 molar ratio of ES:alphaCD (ES conc 2.5 mg/mL). (a) Permeation profiles (only SA was found in the receptor compartment), (b) ES and SA retention in SC and viable skin and amount permeated after 5 h (average ± sem).
Skin retention results are reported in Figure 3b. In this case, both ethylsorbate and sorbic acid were recovered. Ethylsorbate represented the majority of the amount accumulated in the SC, probably due to the low enzymatic activity of this layer. Some considerations could be done from the preliminary results obtained: (1) Liquid ES highly accumulated in the SC; (2) ES was almost quantitatively converted to SA in the viable skin; (3) the use of a saturated water solution drastically reduced the accumulation in the viable skin; (4) most of the sorbate that was recovered after 5 h was found in the receptor compartment. In order to reduce permeation and increase skin retention, ES was applied in a solution containing alpha cyclodextrins (ES:CD molar ratio 1:1); this approach determined a 5-fold reduction in the amount of ES permeated, while no statistically significant variations were found for the accumulated amount.
The use of ethylsorbate is complicated due to its volatility, its peculiar smell and the tendency to adsorb onto and be retained by several types of plastic materials. The use of the inclusion complex with alphaCD is a possible strategy to reduce this problem. A possible alternative to ethylsorbate is represented by sorbic acid. Indeed, this molecule also has demonstrated triplet carbonyl quenching activity [14]. Additionally, it is currently used in cosmetic formulations as a preservative.
3.2.2. Sorbic Acid (SA)
Figure 4 illustrates the permeation and the skin retention of sorbic acid after the application of a saturated solution in water (SA ss) (conc ≃2 mg/mL; pH ≈ 3). The permeability parameters indicate that sorbic acid has very good permeation properties, with a permeability coefficient of 3.15 ± 0.04 × 10−2 cm/h. Aiming to reduce the permeated amount, 1:1 molar ratio complexes (sorbic acid final concentration 2.5 mg/mL) using both alpha and HPbetaCDs were prepared and evaluated. Results highlight a comparable skin accumulation and permeation in the case of HPbetaCD (p > 0.05), while a significant (p < 0.001) reduction of SA permeation was found with alphaCD. The differences between the permeability coefficients obtained using HPbetaCD and alphaCD (2.00 ± 0.30 × 10−2 and 0.87 ± 0.25 × 10−2 cm/h, respectively, p < 0.05) could be ascribed to the different solubility –thus thermodynamic activity– of SA in the two vehicles (see Figure 2).
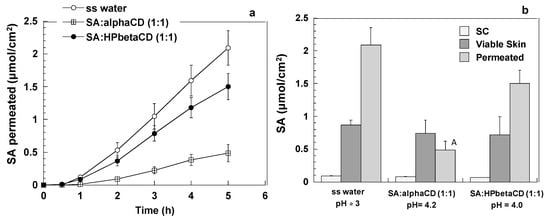
Figure 4.
Permeation and skin retention of sorbic acid starting from a water saturated solution (ss) of SA (SA conc. 2 mg/mL), and from water solutions containing a 1:1 molar ratio of SA:CD (SA conc. 2.5 mg/mL). (a) Permeation profiles, (b) accumulation in SC and viable skin and amount permeated after 5 h. A: p < 0.05 for comparison to the amount permeated in the other two conditions (average ± sem).
Overall, the application of the SA:alphaCD 1:1 molar ratio complex, with a SA concentration of 2.5 mg/mL and a pH of 4.2 attained a relatively high skin accumulation, and limited its permeation.
3.3. Formulation of Sorbic Acid: AlphaCD Complex in an After Sun Gel
The aim of the following studies was the formulation of a sorbic acid:alphaCD complex in a cosmetically acceptable after sun formulation. Thus, to increase the viscosity, hyaluronic acid was selected.
The addition of HA to a water solution of sorbic acid: alphaCD 1:1 complex could have multiple effects on SA permeability. Indeed, the viscosity increases which could modify drug diffusion and release from the formulation; additionally, any substance added to the sorbic acid: alphaCD 1:1 complex solution could, in principle, displace SA from the complex, thus modifying its behavior. All these factors should be taken into consideration, keeping in mind that the final goal is to reduce as much as possible SA permeation while sustaining the skin levels.
The impact of the presence of hyaluronic acid on sorbic acid permeation and skin retention was evaluated. The results are presented in Figure 5.
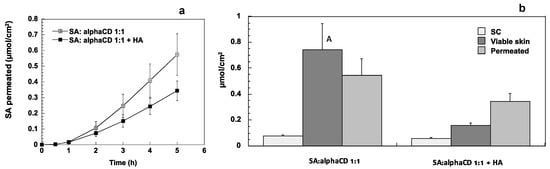
Figure 5.
Permeation profiles and skin retention of sorbic acid from solutions and gels (SA conc. 2.5 mg/mL). (a) Permeation profiles, (b) accumulation in SC and viable skin and amount permeated after 5 h. The detailed composition of the solutions and gels used is reported in Table 1. A: Statistically different from the amount accumulated in viable skin from the gel (average ± sem).
The addition of HA tended to reduce the amount retained and permeated, probably because the viscosity imparted by the presence of HA could reduce the overall diffusion of SA.
3.4. Relevance of the SA Skin Concentration Obtained
Sorbic acid is a preservative with antibacterial and antifungal properties listed in Annex V of the Regulation of the European Parliament and of the Council on Cosmetic Products (EC 1223/2009); it can be used for cosmetic preparations at a maximum concentration of 0.6% [15]. Due to the reduction in the use of parabens and thanks to its natural origin, sorbic acid use in cosmetic formulations has increased by 67% from 2009 to 2013 [16]. The use of “natural preservatives” is indeed considered appealing by many consumers and antimicrobials of natural origin are presently the object of intense cosmetic research [17]. Among them, sorbic acid demonstrated very good performance in the preservation of cosmetic emulsions [18] and is also frequently used in combination with other natural compounds to obtain a synergistic effect [19].
The reported activity as a triplet quenching agent with the possibility of reducing the formation of dark CDPs, could expand its use in broader applications, such as the strengthening of the protection against UV irradiation in after sun formulation [1,2]. In order to exert its protective activity, this molecule should accumulate in the epidermis (in particular in melanocytes) but, due to its physicochemical properties, a high percentage of sorbic acid permeates across the skin. In order to reduce the transport and improve the availability at the site of action, the use of a 1:1 molar ratio complex with alphacyclodextrin can be a simple and efficacious strategy (Figure 4).
It is not possible to establish with certainty if the skin levels obtained (Figure 5) can block the mutagenic activity of UV-induced melanin derivatives, however some calculations can be done. Literature data indicate that a 10 ng/mL (0.07 nmol/mL) concentration of ethylsorbate inhibits the production of dark CPDs in pigmented mouse melanocytes after UVA exposure [1]. By considering an SA accumulation in viable skin of 0.16 µmol/cm2, an average skin weight of 0.135 g/cm2 and assuming a skin density = 1, it is possible to calculate the concentration in the skin. The obtained value is 1.19 µmol/mL, i.e., approximately 17,000-fold higher than the one effective in vitro. This result suggests that the skin level found is more than enough to exert the quencher activity, even considering the different set-up (isolated cells vs organized tissues) and the probably higher difficulty of sorbic acid (ionized at physiological pH) to cross the cell membrane for comparison to ethyl sorbate.
To formulate the sorbic acid: alpha CD complex in a cosmetically acceptable vehicle, hyaluronic acid was selected. This polymer, together with the thickening property, is an excellent hydrating agent [20,21,22]. Additionally, some data suggest that HA-based cosmetic formulations may also protect the skin against ultraviolet irradiation due to the free radical scavenging properties of HA [23].
The developed formulation could be a valuable tool for a broad protection against the damage induced by UV radiation. However, the preservation of the antimicrobial activity of sorbic acid in the presence of cyclodextrins and its effective activity towards the formation of dark CPDs should be evaluated.
In principle, triple state quenchers could be used also during UV exposure, but in this case sorbic acid is not a good candidate, since it could increase CPDs production [3]. Analogs of sorbate having lower triplet energies seems more appropriate.
Author Contributions
Conceptualization, S.N. and C.P.; methodology, S.P., A.D.; validation, A.D., L.G.L.; investigation, S.P., A.D.; writing—original draft preparation, S.N. and C.P; writing—review and editing, S.N., P.S., S.P., C.P., L.G.L.; funding acquisition, P.S.
Funding
This research received no external funding.
Acknowledgments
The authors want to thank Dott. Pierugo Cavallini and Macello Annoni S.p.A. (Busseto, Parma, Italy) for providing porcine ears. Former undergraduate students Erika Breda, Noemi Frattini, Greta Bellini and Ludovica Nodari are gratefully acknowledged for the contribution in data collection.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A.; et al. Melanoma. Nat. Rev. Dis. Primers 2015, 1, 15003. [Google Scholar] [CrossRef] [PubMed]
- Ultraviolet Radiation and the INTERSUN Programme, FAQ Relating to Skin Cancers. Available online: https://www.who.int/uv/faq/skincancer/en/index1.html (accessed on 28 January 2019).
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.; Halaban, R.; Douki, T.; Brash, D.E. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Delinasios, G.J.; Karbaschi, M.; Cooke, M.S.; Young, A.R. Vitamin E inhibits the UVAI induction of “light” and “dark” cyclobutane pyrimidine dimers, and oxidatively generated DNA damage, in keratinocytes. Sci. Rep. 2018, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Vogensen, S.B.; Brewster, M.E.; Konradsdottir, F. Effects of cyclodextrins on drug delivery through biological membranes. J. Pharm. Sci. 2007, 96, 2532–2546. [Google Scholar] [CrossRef] [PubMed]
- CFR-Code of Federal Regulation, Title 21: Food and Drug, Part 172: Food Additives Permitted for Direct Addition to Food for Human Consumption. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172showFR=1 (accessed on 28 January 2019).
- Surber, C.; Wilhelm, K.P.; Hori, M.; Maibach, H.I.; Guy, R.H. Optimization of topical therapy: Partitioning of drugs into stratum corneum. Pharm. Res. 1990, 7, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Hoekman, D. Exploring QSAR—Hydrophobic, Electronic, and Steric Constants; ACS: Washington, DC, USA, 1995. [Google Scholar]
- Vaughan, C.D. Using solubility parameters in cosmetics formulation. J. Soc. Cosmet. Chem. 1985, 36, 319–333. [Google Scholar]
- Sloan, K.B.; Koch, S.A.M.; Siver, K.G.; Flowers, F.P. Use of Solubility Parameters of Drug and Vehicle to Predict Flux Through Skin. J. Investig. Dermatol. 1986, 87, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Yalkowsky, S.H.; Dannenfelser, R.M. Aquasol Database of Aqueous Solubility; College of Pharmacy, University of Arizona: Tucson, AZ, USA, 1992. [Google Scholar]
- Higuchi, T.; Connors, K.A. Phase-Solubility Techniques. Adv. Anal. Chem. Instr. 1965, 4, 117–122. [Google Scholar]
- Kuo, K.L.; Hsieh, Y.Z. Determination of preservatives in food products by cyclodextrin modified capillary electrophoresis with multiwavelength detection. J. Chromatogr. A 1997, 768, 334–341. [Google Scholar] [CrossRef]
- Velosa, A.C.; Baader, W.J.; Stevani, C.V.; Mano, C.M.; Bechara, E.J. 1,3-diene probes for detection of triplet carbonyls in biological systems. Chem. Res. Toxicol. 2007, 20, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R1223 (accessed on 28 January 2019).
- Malpede, A. Ingredienti Cosmetici: Le Classi Chimiche, Fisiche e Funzionali. In Manuale del Cosmetologo; D’Agostinis, G., Mignini, E., Eds.; Tecniche Nuove: Milano, Italy, 2015; pp. 7–136. [Google Scholar]
- Herman, A. Antimicrobial Ingredients as Preservative Booster and Components of Self-Preserving Cosmetic Products. Curr. Microbiol. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kocevar Glavac, N.; Lunder, M. Preservative efficacy of selected antimicrobials of natural origin in a cosmetic emulsion. Int. J. Cosmet. Sci. 2018, 40, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Saviuc, C.; Ciubuca, B.; Dinca, G.; Bleotu, C.; Drumea, V.; Chifiriuc, M.C.; Popa, M.; Pircalabioru, G.G.; Marutescu, L.; Lazar, V. Development and Sequential Analysis of a New Multi-Agent, Anti-Acne Formulation Based on Plant-Derived Antimicrobial and Anti-Inflammatory Compounds. Int. J. Mol. Sci. 2017, 18, 175. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Sparavigna, A. The 24-hour skin hydration and barrier function effects of a hyaluronic 1%, glycerin 5%, and Centella asiatica stem cells extract moisturizing fluid: An intra-subject, randomized, assessor-blinded study. Clin. Cosmet. Investig. Dermatol. 2017, 10, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Ferrillo, M.; Vastarella, M.; Cantelli, M.; Mazzella, C.; Fabbrocini, G. Instrumental, clinical and subjective evaluation of the efficacy of a cosmetic treatment for home use. J. Cosmet. Laser Ther. 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Wartewig, S.; Bottcher, R.; Poppl, A.; Hoentsch, J.; Ozegowski, J.H.; Neubert, R.H. The effects of hyaluronan and its fragments on lipid models exposed to UV irradiation. Int. J. Pharm. 2003, 254, 223–234. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).