Use of Vegetable Oils to Improve the Sun Protection Factor of Sunscreen Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oil-Based Vehicle Preparation
2.3. Determination of Required Hydrophilic Lipophlic Balance (rHLB)
2.4. Spreadability
2.5. Occlusive Properties
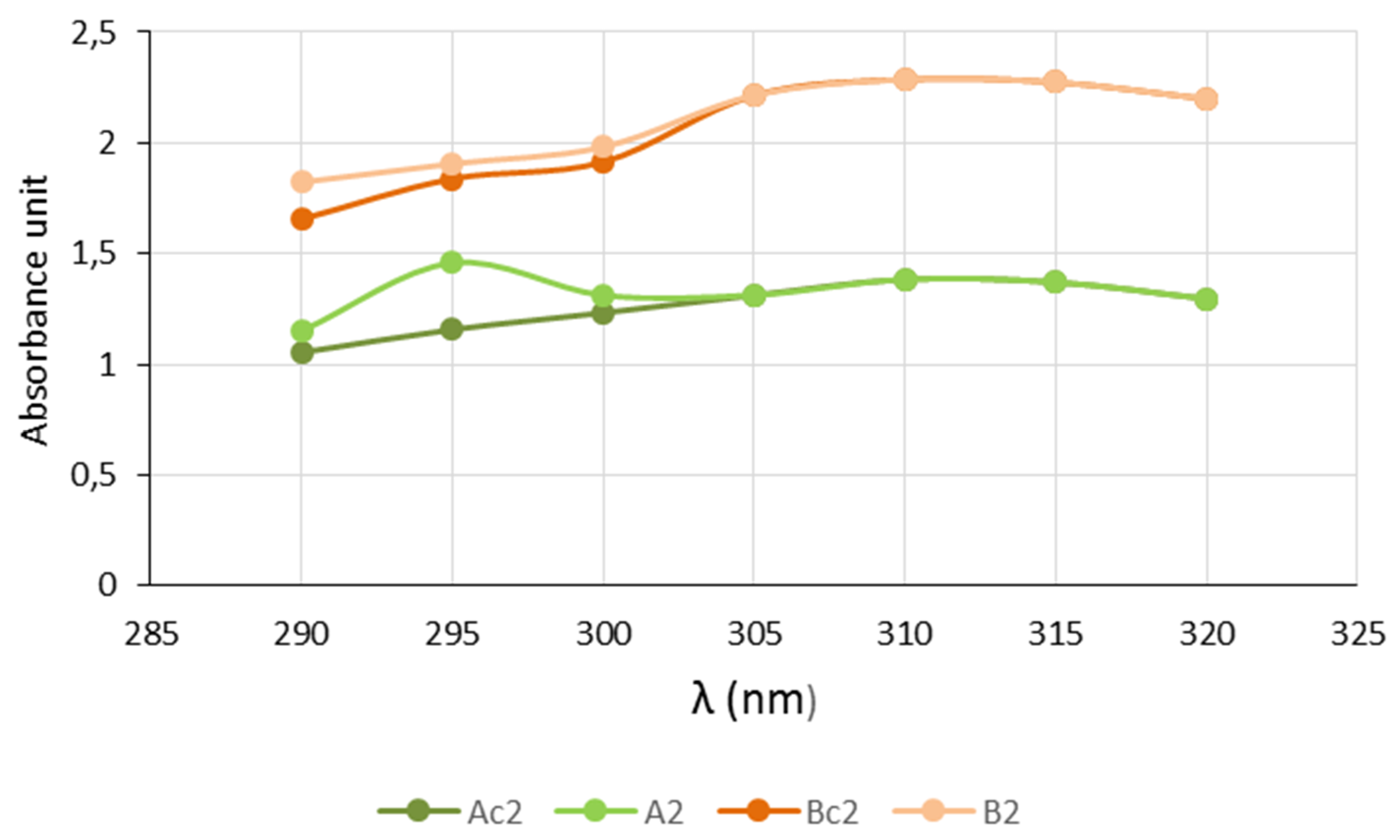
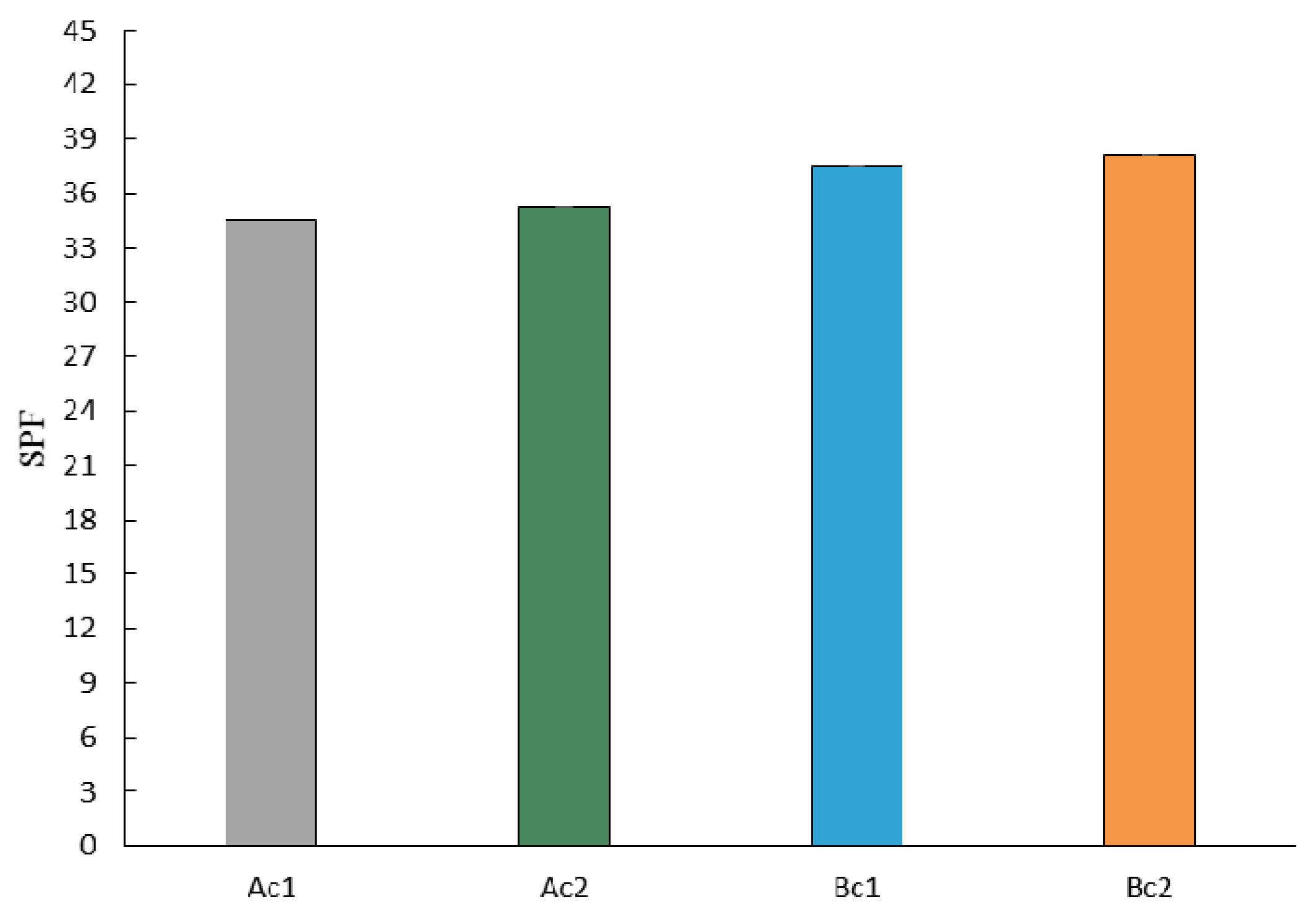
2.6. In Silico and In Vitro Sun Protection Factor (SPF) Determination
3. Results and Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef]
- Burnett, C.L.; Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Plant-Derived Fatty Acid Oils. Int. J. Toxicol. 2017, 36, 51S–129S. [Google Scholar] [CrossRef] [PubMed]
- Chanchal, D.; Swarnlata, S. Novel approaches in herbal cosmetics. J. Cosmet. Dermatol. 2008, 7, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.D.; Saraf, S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacogn. Res. 2010, 2, 22–25. [Google Scholar] [CrossRef]
- Kale, S.; Sonawane, A.; Ansari, A.; Ghoge, P.; Waje, A. Formulation and in-vitro determination of sun protection factor of Ocimum basilicum, linn. leaf oils sunscreen cream. Int. J. Pharm. Pharm. Sci. 2010, 2, 147–149. [Google Scholar]
- Andréo-Filho, N.; Bim, A.V.K.; Kaneko, T.M.; Kitice, N.A.; Haridass, I.N.; Abd, E.; Santos Lopes, P.; Thakur, S.S.; Parekh, H.S.; Roberts, M.S.; et al. Development and evaluation of lipid nanoparticles containing natural botanical oil for sun protection: Characterization and in vitro and in vivo human skin permeation and toxicity. Skin Pharmacol. Physiol. 2018, 31, 1–9. [Google Scholar] [CrossRef]
- Varvaresou, A. Percutaneous absorption of organic sunscreens. J. Cosmet. Dermatol. 2006, 5, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Puglisi, G. Evaluation of sunscreen safety by in vitro skin permeation studies: Effects of vehicle composition. Die Pharmazie 2013, 68, 34–40. [Google Scholar] [CrossRef]
- Montenegro, L.; Turnaturi, R.; Parenti, C.; Pasquinucci, L. In Vitro Evaluation of Sunscreen Safety: Effects of the Vehicle and Repeated Applications on Skin Permeation from Topical Formulations. Pharmaceutics 2018, 10, 27. [Google Scholar] [CrossRef]
- Nash, J.F. Human safety and efficacy of ultraviolet filters and sunscreen products. Dermatol. Clin. 2006, 24, 35–51. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Schaefer, H. Benefit and risk of organic ultraviolet filters. Regul. Toxicol. Pharmacol. 2001, 33, 285–299. [Google Scholar] [CrossRef]
- Hayden, C.G.J.; Cross, S.E.; Anderson, C.; Saunders, N.A.; Roberts, M.S. Sunscreen penetration of human skin and related keratinocyte toxicity after topical application. Skin Pharmacol. Physiol. 2005, 18, 170–174. [Google Scholar] [CrossRef]
- Montenegro, L. Nanocarriers for skin delivery of cosmetic antioxidants. J. Pharm. Pharmacogn. Res. 2014, 2, 73–92. [Google Scholar]
- Korać, R.R.; Khambholja, K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharm. Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef]
- Montenegro, L. Lipid-based nanoparticles as carriers for dermal delivery of antioxidants. Curr. Drug Metab. 2017, 18, 469–480. [Google Scholar] [CrossRef]
- Bassiri-Jahromi, S. Punica granatum (Pomegranate) activity in health promotion and cancer prevention. Oncol. Rev. 2018, 12, 345. [Google Scholar] [CrossRef]
- Afaq, F.; Malik, A.; Syed, D.; Maes, D.; Matsui, M.S.; Mukhtar, H. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes paragraph sign. Photochem. Photobiol. 2005, 81, 38–45. [Google Scholar] [CrossRef]
- Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Afaq, F.; Mukhtar, H. Photochemopreventive effect of pomegranate fruit extract on UVA-mediated activation of cellular pathways in normal human epidermal keratinocytes. Photochem. Photobiol. 2006, 82, 398–405. [Google Scholar] [CrossRef]
- Alander, J. Shea Butter—A multi-functional ingredient for food and cosmetics. Lipid Technol. 2004, 16, 202–205. [Google Scholar]
- Israel, M.O. Effects of topical and dietary use of shea butter on animals. Am. J. Life Sci. 2014, 2, 303–307. [Google Scholar] [CrossRef]
- Otto, A.; du Plessis, J.; Wiechers, J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef]
- Montenegro, L.; Paolino, D.; Puglisi, G. Effects of silicone emulsifiers on in vitro skin permeation of sunscreens from cosmetic emulsions. J. Cosmet. Sci. 2004, 55, 509–518. [Google Scholar]
- Chatelain, E.; Gabard, B.; Surber, C. Skin penetration and sun protection factor of five UV filters: Effect of the vehicle. Skin Pharmacol. Physiol. 2003, 16, 28–35. [Google Scholar] [CrossRef]
- Kurul, E.; Hekimoğlu, S. Skin permeation of two different benzophenone derivatives from various vehicles. Int. J. Cosmet. Sci. 2001, 23, 211–218. [Google Scholar] [CrossRef]
- Hilton, J.; Woollen, B.H.; Scott, R.C.; Auton, T.R.; Trebilcock, K.L.; Wilks, M.F. Vehicle effects on in vitro percutaneous absorption through rat and human skin. Pharm. Res. 1994, 11, 1396–1400. [Google Scholar] [CrossRef]
- Montenegro, L.; Carbone, C.; Paolino, D.; Drago, R.; Stancampiano, A.H.; Puglisi, G. In vitro skin permeation of sunscreen agents from O/W emulsions. Int. J. Cosmet. Sci. 2008, 30, 57–65. [Google Scholar] [CrossRef]
- Chaudhary, B.; Verma, S. Preparation and evaluation of novel in situ gels containing acyclovir for the treatment of oral herpes simplex virus infections. Sci. World J. 2014, 2014, 280928. [Google Scholar] [CrossRef]
- Garg, A.; Aggarwal, D.; Garg, S.; Singla, A.K. Spreading of semisolid formulations: An update. Pharm. Technol. 2002, 26, 84–105. [Google Scholar]
- Montenegro, L.; Rapisarda, L.; Ministeri, C.; Puglisi, G. Effects of lipids and emulsifiers on the physicochemical and sensory properties of cosmetic emulsions containing Vitamin E. Cosmetics 2015, 2, 35–47. [Google Scholar] [CrossRef]
- Wissing, S.; Lippacher, A.; Müller, R. Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J. Cosmet. Sci. 2001, 52, 313–324. [Google Scholar]
- Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect. Pharmaceutics 2017, 10, 58. [Google Scholar] [CrossRef]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E.A. Comparison of in vivo and in vitro testing of sunscreen formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Allen, J.M.; Gosset, C.J.; Allen, S.F. Photochemical formation of singlet molecular oxygen (1O2) in illuminated aqueous solutions of several commercially available sunscreen active ingredients. Chem. Res. Toxicol. 1996, 9, 605–609. [Google Scholar] [CrossRef]
- Afonso, S.; Horita, K.; Sousa e Silva, J.P.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; Esteves da Silva, J.C.; Sousa Lobo, J.M. Photodegradation of avobenzone: Stabilization effect of antioxidants. J. Photochem. Photobiol. B Biol. 2014, 140, 36–40. [Google Scholar] [CrossRef]
- Sayre, R.M.; Dowdy, J.C.; Gerwig, A.J.; Shields, W.J.; Lloyd, R.V. Unexpected photolysis of the sunscreen octinoxate in the presence of the sunscreen avobenzone. Photochem. Photobiol. 2005, 81, 452–456. [Google Scholar] [CrossRef]
- Chatelain, E.; Gabard, B. Photostabilization of butyl methoxydibenzoylmethane (Avobenzone) and ethylhexyl methoxycinnamate by bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter. Photochem. Photobiol. 2001, 74, 401–406. [Google Scholar] [CrossRef]
- Almeida, I.F.; Gaio, A.R.; Bahia, M.F. Hedonic and descriptive skin feel analysis of two oleogels: Comparison with other topical formulations. J. Sens. Stud. 2008, 23, 92–113. [Google Scholar] [CrossRef]
- Yamashita, Y.; Miyahara, R.; Sakamoto, K. Emulsion and emulsification technology. In Cosmetic Science and Technology—Theoretical Principles and Applications; Sakamoto, K., Lochhead, R., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 489–506. ISBN 978-0-12-802005-0. [Google Scholar]
- Fonseca, A.P.; Rafaela, N. Determination of sun protection factor by UV-Vis spectrophotometry. Health Care Curr. Rev. 2013, 1, 108. [Google Scholar] [CrossRef]
- Yang, S.I.; Liu, S.; Brooks, G.J.; Lanctot, Y.; Gruber, J.V. Reliable and simple spectrophotometric determination of sun protection factor: A case study using organic UV filter-based sunscreen products. J. Cosmet. Dermatol. 2018, 17, 518–522. [Google Scholar] [CrossRef]
- Korkina, L.; Kostyuk, V.; Potapovich, A.; Maye, W.; Talib, N.; De Luca, C. Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation. Cosmetics 2018, 5, 32. [Google Scholar] [CrossRef]
- Dutra, E.A.; Oliveira, D.A.G.C.; Kedor-Hackman, E.R.; Santoro, M.I.R.M. Determination of Sun Protection Factor (SPF) of sunscreens by ultraviolet spectrophotometry. Braz. J. Pharm. Sci. 2004, 3, 381–385. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef]
- Moghimipour, E.; Ramezani, Z.; Handali, S. Solid lipid nanoparticles as a delivery system for Zataria multiflora essential oil: Formulation and characterization. Curr. Drug Deliv. 2013, 10, 151–157. [Google Scholar] [CrossRef]
- Montenegro, L.; Pasquinucci, L.; Zappalà, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles. Pharmaceutics 2017, 9, 48. [Google Scholar] [CrossRef]
- Alhaj, N.A.; Shamsudin, M.N.; Alipiah, N.M.; Zamri, H.F.; Bustamam, A.; Ibrahim, S.; Abdullah, R. Characterization of Nigella sativa L. essential oil-loaded solid lipid nanoparticles. Am. J. Pharm. Toxicol. 2010, 5, 52–57. [Google Scholar] [CrossRef]
| Ingredient | % (w/w) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formulation Code | ||||||||||||
| Ac1 | A1 | Ac2 | A2 | Bc1 | B1 | Bc2 | B2 | Cc1 | C1 | Cc2 | C2 | |
| Acemoll TN | 17 | 16 | 16 | 15 | 16 | 16 | 16 | 14 | 17 | 16 | 16 | 15 |
| Cetiol C5 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| Cetiol Sensoft | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Vegelight | 20 | 19 | 19 | 18 | 20 | 18 | 18 | 18 | 18 | 17 | 18 | 17 |
| Cetiol OE | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 13 | 13 |
| EHS | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| TA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Argan oil | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| WGO | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| BPO | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 |
| PMG | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 |
| BEMT | 5 | 5 | 5 | 5 | 6 | 6 | 6 | 6 | 7 | 7 | 7 | 7 |
| BMBM | 3 | 3 | 5 | 5 | 3 | 3 | 5 | 5 | 3 | 3 | 5 | 5 |
| OMC | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 9 | 9 | 10 | 10 |
| λ | EE × I |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0839 |
| 320 | 0.0180 |
| Total | 1 |
| Formulation | pH | rHLB | S% | F |
|---|---|---|---|---|
| Ac1 | 6.08 | 10 | 35.63 ± 2.45 | 26.55 ± 14.38 |
| A1 | 6.90 | 10 | 40.91 ± 5.02 | 26.84 ± 13.45 |
| Ac2 | 6.80 | 12 | 36.17 ± 2.84 | 21.94 ± 0.40 |
| A2 | 6.27 | 12 | 34.16 ± 0.10 | 24.39 ± 3.59 |
| Bc1 | 6.84 | 10 | 42.58 ± 2.66 | 34.94 ± 1.20 |
| B1 | 6.86 | 10 | 26.96 ± 1.14 | 27.14 ± 2.43 |
| Bc2 | 6.50 | 12 | 31.76 ± 2.93 | 28.82 ± 11.45 |
| B2 | 6.10 | 12 | 39.17 ± 2.56 | 16.95 ± 4.00 |
| λ | BPO | PMG |
|---|---|---|
| 290 | 1.486 | 3.198 |
| 295 | 1.001 | 2.161 |
| 300 | 0.620 | 1.837 |
| 305 | 0.166 | 0.403 |
| 310 | 0.000 | 0.000 |
| 315 | 0.000 | 0.000 |
| 320 | 0.000 | 0.000 |
| Formulation | SPF | E% |
|---|---|---|
| Ac2 | 12.91 | --- |
| A2 | 14.39 | 10.3 |
| Bc2 | 21.08 | --- |
| B2 | 21.35 | 1.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, L.; Santagati, L.M. Use of Vegetable Oils to Improve the Sun Protection Factor of Sunscreen Formulations. Cosmetics 2019, 6, 25. https://doi.org/10.3390/cosmetics6020025
Montenegro L, Santagati LM. Use of Vegetable Oils to Improve the Sun Protection Factor of Sunscreen Formulations. Cosmetics. 2019; 6(2):25. https://doi.org/10.3390/cosmetics6020025
Chicago/Turabian StyleMontenegro, Lucia, and Ludovica Maria Santagati. 2019. "Use of Vegetable Oils to Improve the Sun Protection Factor of Sunscreen Formulations" Cosmetics 6, no. 2: 25. https://doi.org/10.3390/cosmetics6020025
APA StyleMontenegro, L., & Santagati, L. M. (2019). Use of Vegetable Oils to Improve the Sun Protection Factor of Sunscreen Formulations. Cosmetics, 6(2), 25. https://doi.org/10.3390/cosmetics6020025






