Evaluation of Genotoxic and DNA Photo-Protective Activity of Bryothamnion triquetrum and Halimeda incrassata Seaweeds Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aqueous Extracts Preparation
2.2. Experimental Biological System and Procedures
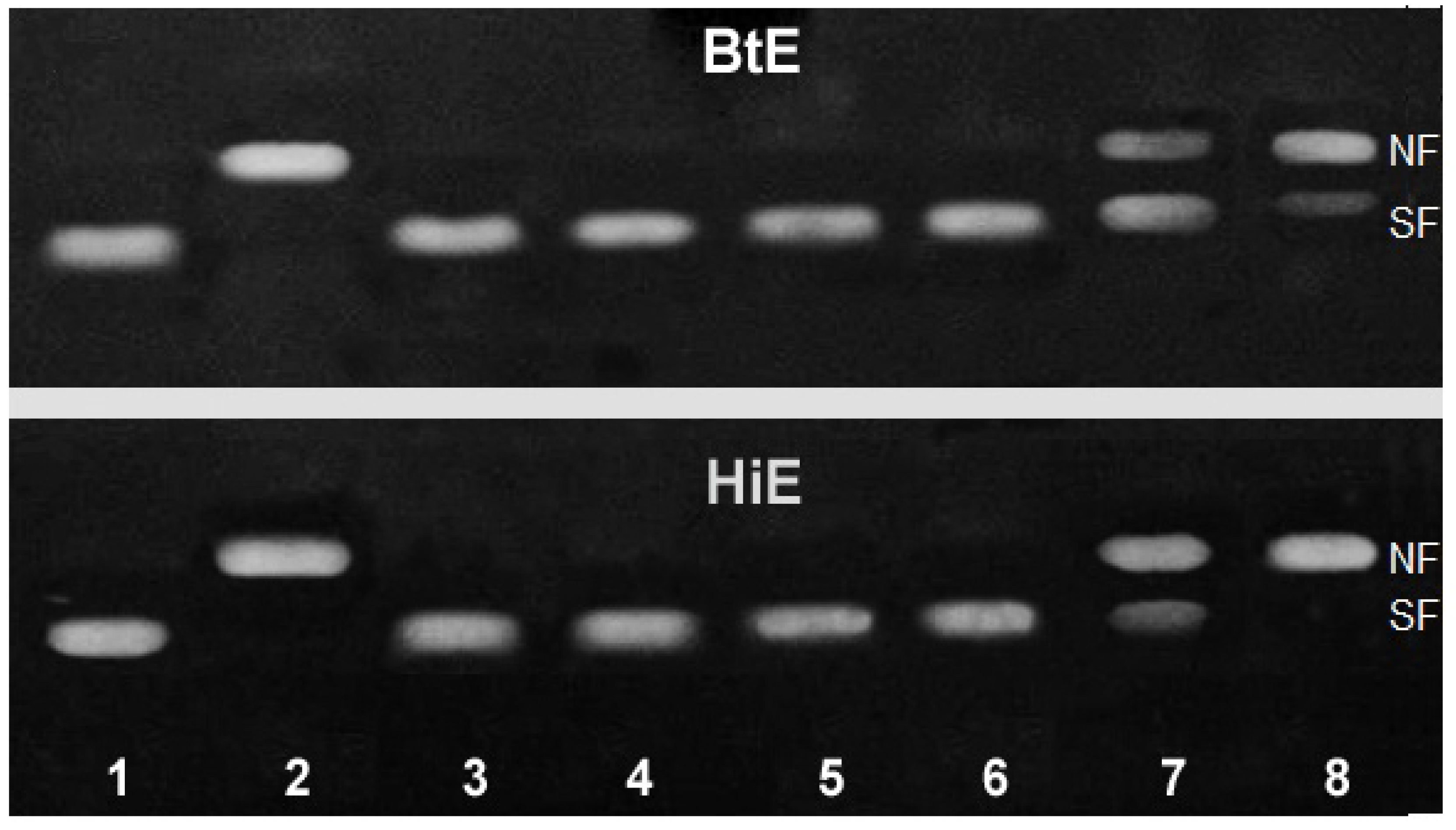
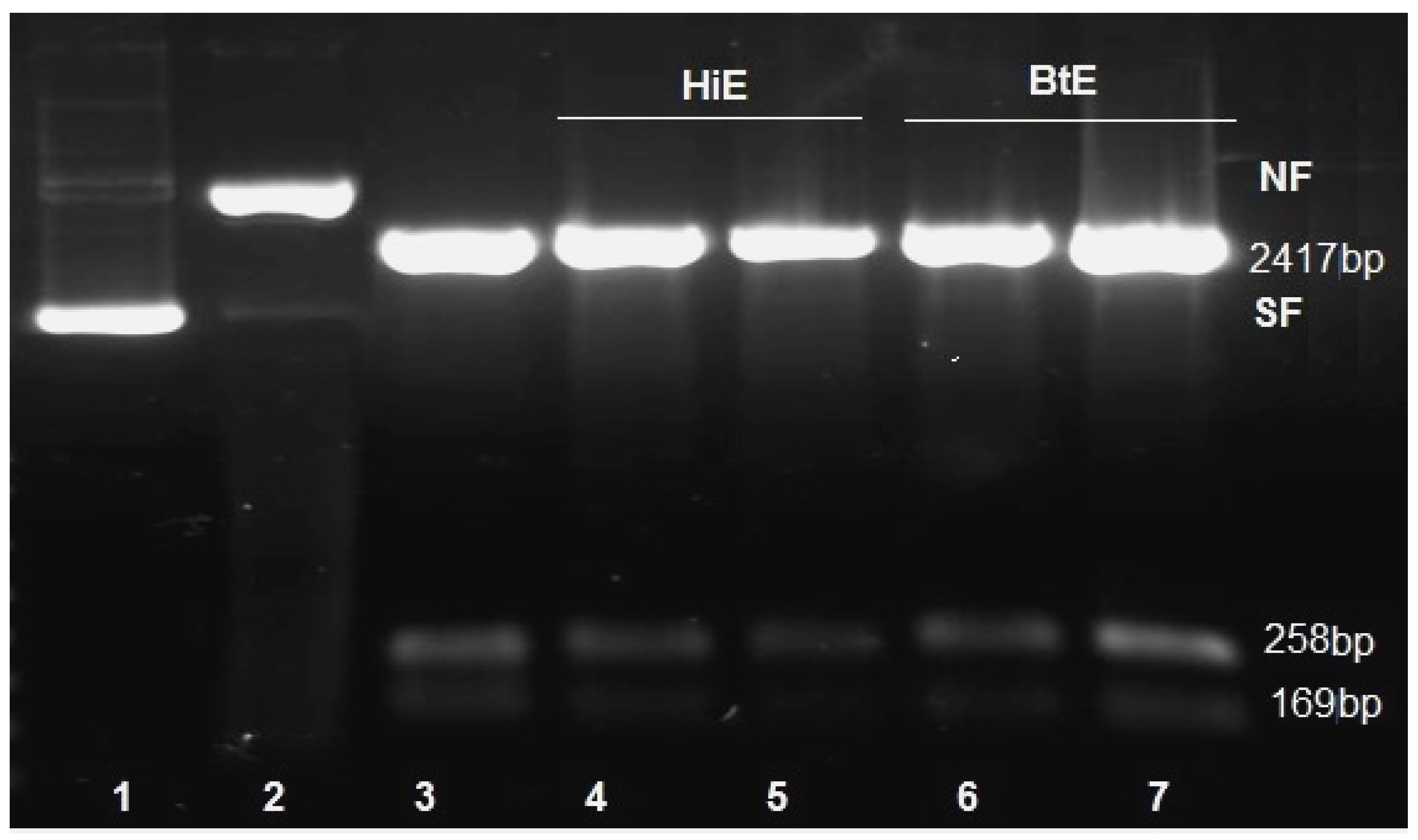
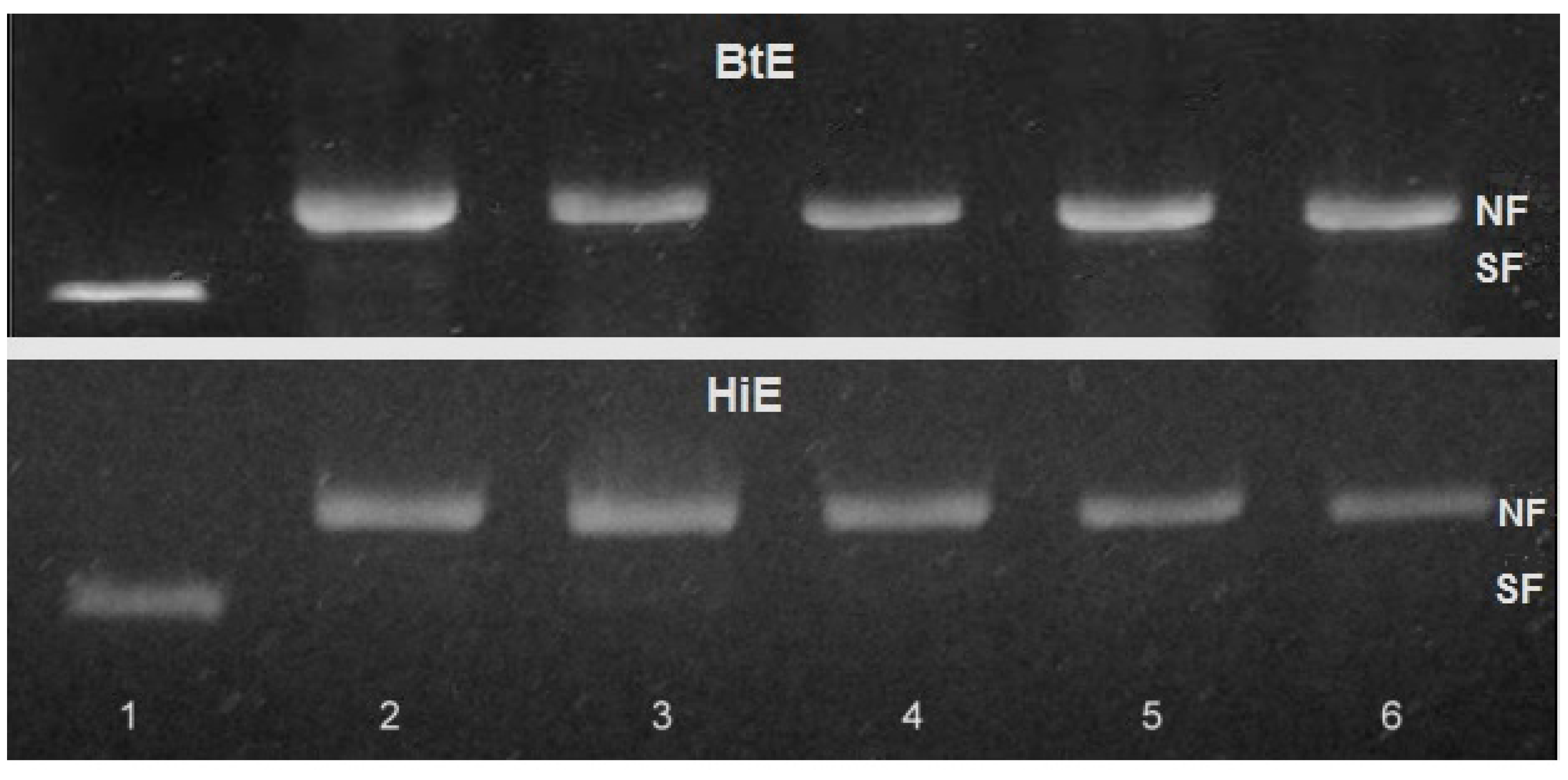
2.2.1. Genotoxicity of Seaweeds Extract
- (1)
- Eco RI, Bam HI and Pvu II enzyme mix (3 μL) were incubated with 1.0 mg/mL of seaweeds extracts (5 μL) in multi-core buffer (2 μL), BSA (2 μL) and deionized water (3 μL) during 30 min. After, the mixes were complemented with plasmid DNA (500 ng).
- (2)
- Plasmid DNA (500 ng) was incubated with 1.0 mg/mL of seaweed extracts (5 μL) during 30 min. Afterwards, the mixes were complemented with multi-core buffer (2 μL), BSA (2 μL), deionized water (3 μL) and Eco RI, Bam HI and Pvu II enzyme mix (3 μL).
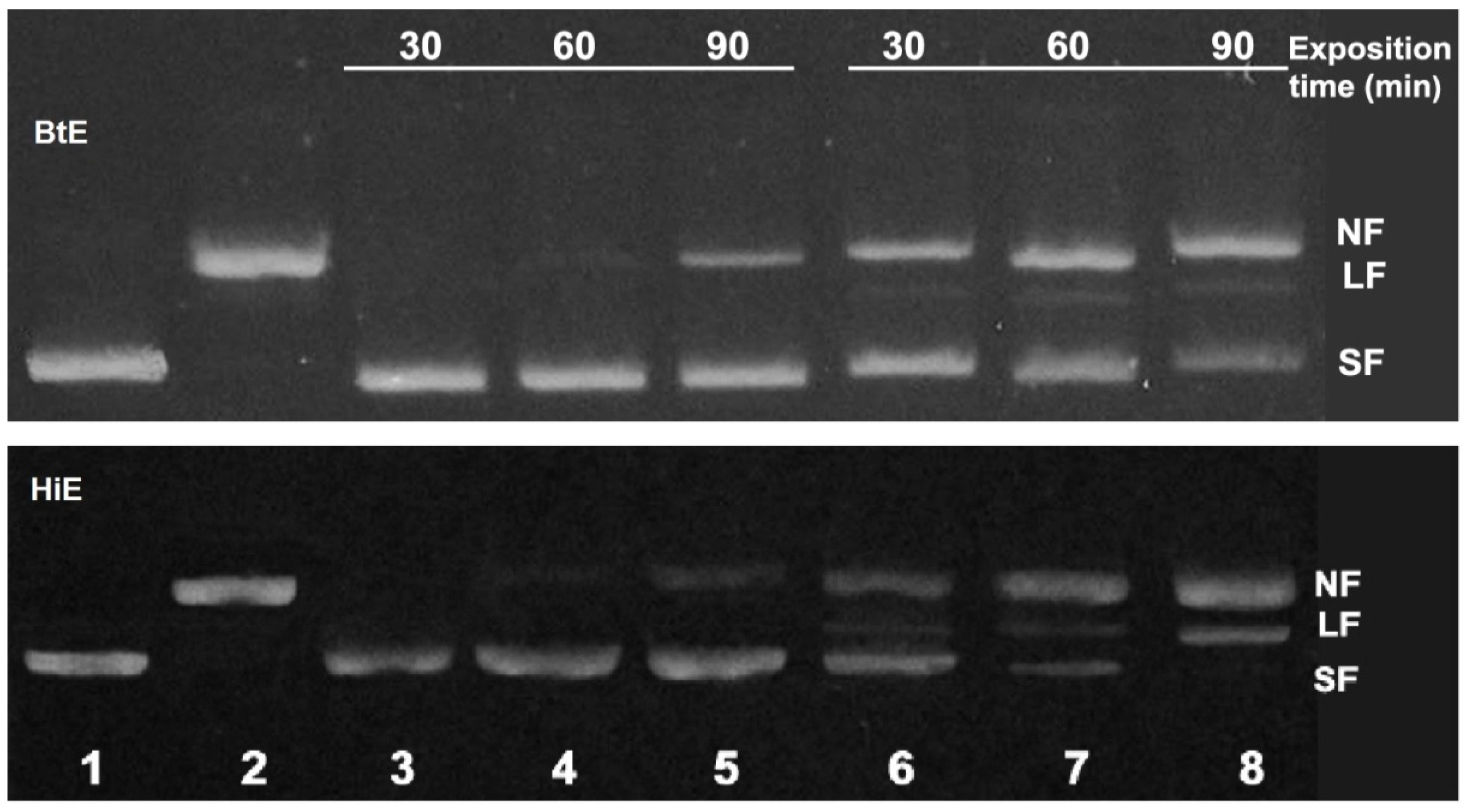
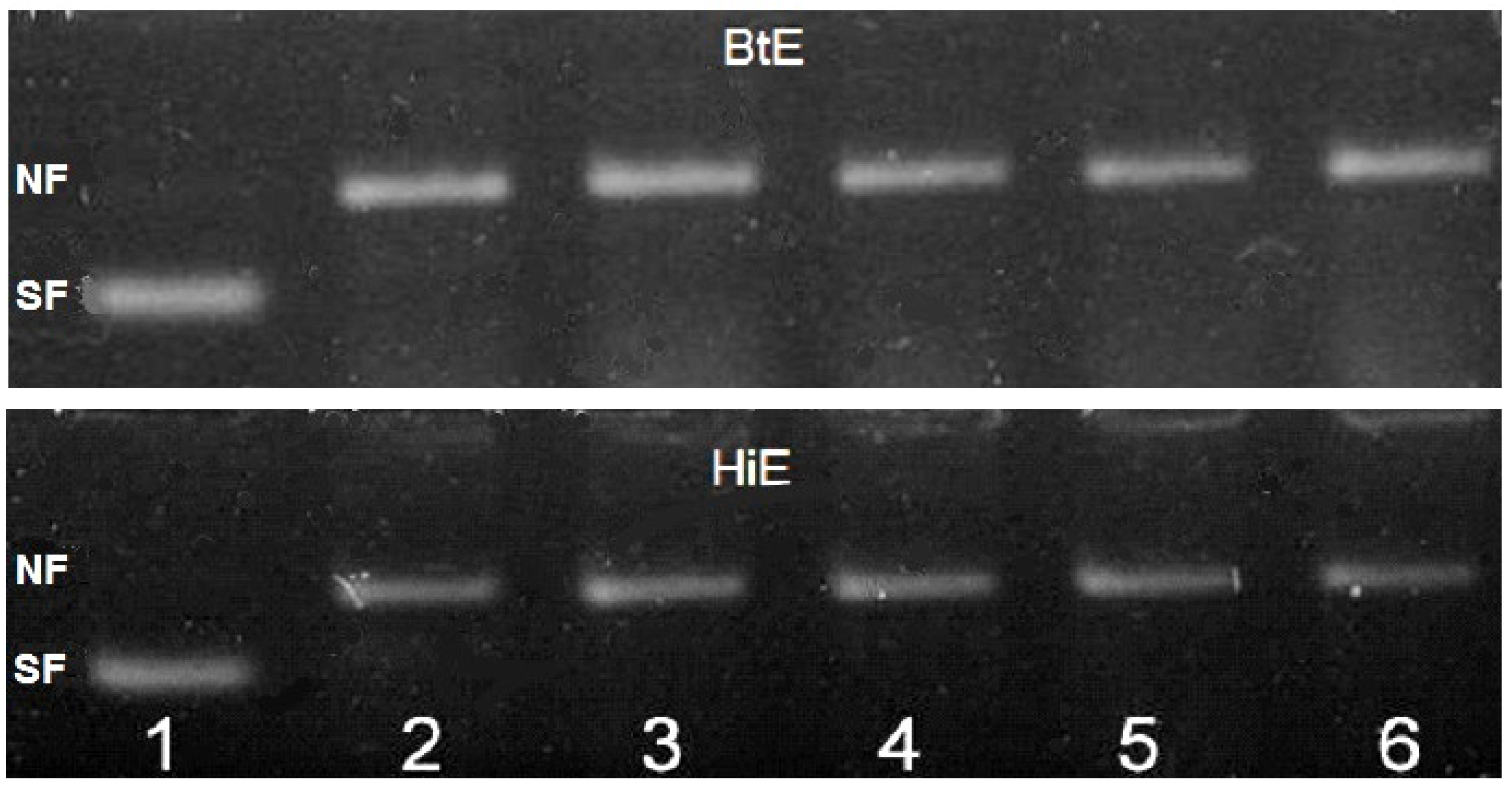
2.2.2. Antigenotoxicity of Seaweed Extracts
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Megna, M.; Lembo, S.; Balato, N.; Monfrecola, G. “Active” photoprotection sunscreens with DNA repair enzymes G. Ital. Dermatol. Venereol. 2017, 152, 302–307. [Google Scholar]
- Batista-González, A.E.; Charles, M.B.; Mancini-Filho, J.; Vidal-Novoa, A. Las algas marinas como fuentes de fitofármacos antioxidantes. Rev. Cuba. Plantas Med. 2009, 14, 1–18. [Google Scholar]
- Gutiérrez, R.; González, K.L.; Valdés, O.D.; Hernández, Y.; Acosta, Y. Seaweeds as sources of bioactive compounds in the benefit of human health: A review. Rev. Cienc. Biol. Salud 2016, 18, 20–27. [Google Scholar]
- Cardozo, K.H.M.; Marques, L.G.; Carvalho, V.M.; Carignan, M.O.; Pinto, E.; Marinho-Soriano, E.; Colepicolo, P. Analyses of photoprotective compounds in red algae from the Brazilian coast. Braz. J. Pharmacogn. 2011, 21, 202–208. [Google Scholar] [CrossRef]
- Gamal, A.A.E. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.; Luca, C.D.; Pastore, S. Plant polyphenols and human skin: Friends or foes. Ann. N. Y. Acad. Sci. 2012, 1259, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mugica, A.; Batista-González, A.E.; Mondejar, D.; Soto, Y.; Brito, V.; Vázquez, A.; Brömme, D.; Zaldívar-Muñoz, C.; Mancini-Filho, J.; Vidal-Novoa, A. In vitro antiatherogenicity of extracts from Halimeda incrassata seaweed antioxidant activity and smooth muscle cell migration studies. Ars Pharm. 2013, 54, 4–11. [Google Scholar]
- Viana, G.S.B.; Freitas, A.L.P.; Lima, M.M.L.; Vieira, L.A.P.; Andrade, M.C.H.; Benevides, N.M.B. Antinociceptive activity of sulfated carbohydrates from the red algae Bryothamnion seaforthii (Turner) Kütz. and B. triquetrum (S.G. Gmel.) M. Howe. Braz. J. Med. Biol. Res. 2002, 35, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante-Silva, L.H.; Matta, C.B.; Vital, M.; Barbosa-Filho, J.M.; Pereira, D.; de Oliveira Santos, B.V.; de Miranda, G.E.; Alexandre-Moreira, M.S. Antinociceptive and Anti-Inflammatory Activities of Crude Methanolic Extract of Red Alga Bryothamnion triquetrum. Mar. Drugs 2012, 10, 1977–1992. [Google Scholar] [CrossRef] [PubMed]
- Linares, A.F. Actividad Antioxidante y Neuroprotectora In Vitro del Extracto Acuoso del Alga Bryothamnion triquetrum (Ceramiales, Rhodomelaceae). Ph.D. Thesis, Universidad de La Habana, Havana, Cuba, 2005. (In Spanish). [Google Scholar]
- Costa-Mugica, A.; Batista-Gonzalez, A.E.; Mondejar, D.; Soto-López, Y.; Brito-Navarro, V.; Vázquez, A.M.; Brömme, D.; Zaldívar-Muñoz, C.; Vidal-Novoa, A.; de Oliveira Silva, A.M.; et al. Inhibition of LDL-oxidation and antioxidant properties related to polyphenol content of hydrophilic fractions from seaweed Halimeda incrassata (Ellis) Lamouroux. Braz. J. Pharm. Sci. 2012, 48, 31–37. [Google Scholar] [CrossRef]
- Díaz-Gutierrez, D.; Méndez-Ortega, W.; Oliveira Silva, A.M.; Zaldívar-Muñoz, C.; Mancini-Filho, J.; Vidal-Novoa, A. Comparación de las propiedades antioxidantes y contenido de polifenoles de extractos acuosos de las algas marinas Bryothamnion triquetrum y Halimeda opuntia. Ars Pharm. 2015, 56, 89–99. [Google Scholar] [CrossRef]
- Sánchez-Lamar, A.; González, J.; Cozzi, R.; Fiore, M.; Ricordy, R.; De Salvia, R. El extracto acuoso del alga verde Halimeda incrassata y su capacidad de proteger a los cromosomas del daño causado por estrés oxidativo. Rev. CENIC Cienc. Biol. 2001, 3, 14–15. [Google Scholar]
- González-Pumariega, M.; Vernhes, M.; Menck, C.F.M.; Sánchez-Lamar, Á. Evaluación de la toxicidad del extracto acuoso de Bryothamnion triquetrum en células humanas. Rev. CENIC Cienc. Biol. 2010, 41, 1–7. [Google Scholar]
- Vidal-Novoa, A.; Fallarero-Linares, A.; Labañino, M.; Sánchez-Lamar, A.; Batista-Gonzalez, A.; Amo, S.; Mancini-Filho, J. Evaluaciones toxicológicas de un extracto acuoso del alga marina Bryothamnion triquetrum (Gmelin) M.A.Howe en estudios in vitro y modelos animales. Ars Pharm. 2012, 53, 15–20. [Google Scholar]
- Vidal-Novoa, A.; Motidome, M.; Mancini-Filho, J.; Linares, A.F.; Tanae, M.M.; Torres, L.M.B.; Lapa, A.J. Actividad antioxidante y ácidos fenólicos del alga marina Bryothamnion triquetrum (S.G.Gmelim) Howe. Braz. J. Pharm. Sci. 2001, 37, 373–382. [Google Scholar]
- Moreno, S.R.F.; Freitas, R.S.; Rocha, E.K.; Lima-Filho, G.L.; Bernardo-Filho, A.M. Protection of plasmid DNA by a Ginkgo biloba extract from the effects of stannous chloride and the action on the labeling of blood elements with technetium-99m. Braz. J. Med. Biol. Res. 2004, 37, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Schuch, A.P.; Garcia, C.C.M.; Makita, K.; Menck, C.F.M. DNA damage as a biological sensor for environmental sunlight. Photochem. Photobiol. Sci. 2013, 12, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- González-Pumariega, M.; Fuentes-León, F.; Vernhes, M.; Schuch, A.P.; Menck, C.F.M.; Sánchez-Lamar, Á. El extracto acuoso de Cymbopogon citratus protege al ADN plasmídico del daño inducido por radiación UVC. Ars Pharm. 2016, 57, 193–199. [Google Scholar]
- Vidal-Novoa, A.; Andrade-Wartha, E.R.S.; Batista-González, A.E.; Fallarero-Linares, A.; Oliveira Silva, A.M.; Genovese, M.I.; Vuorela, P.; Costa-Mugica, A.; Mancini-Filho, J. Antioxidant activity and possible bioactive components in hydrophilic and lipophilic fractions from the seaweed Halimeda incrassata. Braz. J. Pharmacogn. 2011, 21, 53–57. [Google Scholar] [CrossRef]
- Aboudoulatif, D.; Selva, D.; Gunasekaren, V.; Jagadeesan, S.G.; Kwashie, E.-G.; Muthiah, R.; Edmond, C.E. In vitro and in vivo Genotoxicity Assessment of Total Alkaloids of Ageratum conyzoides L. Leaves (Asteraceae) by Alkaline Comet Assay. Int. J. Pharm. Sci. Res. 2015, 6, 2748–2754. [Google Scholar]
- Retz de Carvalho, L.; Roque, N.F. Fenóis Halogenados Eou Sulfatados de Macroalgas Marinhas. Química Nova 2000, 23, 757–764. [Google Scholar] [CrossRef]
- Vidal-Novoa, A.; Fallarero, A.; Andrade-Wartha, E.R.; Oliveira Silva, A.M.; Lima, A.D.; Torres, R.P.; Vuorela, P.; Mancini-Filho, J. Composición química y actividad antioxidante del alga marina roja Bryothamnion triquetrum (S.G.Gmelin) Howe. Braz. J. Pharm. Sci. 2006, 42, 549–600. [Google Scholar]
- Hanawalt, P.C. Historical perspective on the DNA damage response. DNA Repair 2015, 36, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Kejnovský, E.; Nejedlý, K.; Kypr, J. Factors influencing resistance of UV-irradiated DNA to the restriction endonuclease cleavage. Int. J. Biol. Macromol. 2004, 34, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, G. Bryothamnion triquetrum: Su Capacidad Citotóxica, Genotóxica y Antigenotóxica en Células CHO. Ph.D. Thesis, Universidad de La Habana, Havana, Cuba, 2003. (In Spanish). [Google Scholar]
- Vernhes, M. Evaluación de la Capacidad Fotoprotectora de Phyllanthus orbicularis, Pinus caribeae y Halimeda monile en DNA Plasmídico. Mater’s Thesis, Universidad de La Habana, Havana, Cuba, 2008. (In Spanish). [Google Scholar]
- Bandyopadhyay, N.; Gautam, S.; Sharma, A. Suppression of SOS repair in E. coli: Possible mechanism of antimutagenicity and protective effects of common vegetables. Int. J. Food Sci. Nutr. 2014, 65, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Lamar, Á.; González-Pumariega, M.; Fuentes-León, F.; Vernhes Tamayo, M.; Schuch, A.P.; Menck, C.F.M. Evaluation of Genotoxic and DNA Photo-Protective Activity of Bryothamnion triquetrum and Halimeda incrassata Seaweeds Extracts. Cosmetics 2017, 4, 23. https://doi.org/10.3390/cosmetics4030023
Sánchez-Lamar Á, González-Pumariega M, Fuentes-León F, Vernhes Tamayo M, Schuch AP, Menck CFM. Evaluation of Genotoxic and DNA Photo-Protective Activity of Bryothamnion triquetrum and Halimeda incrassata Seaweeds Extracts. Cosmetics. 2017; 4(3):23. https://doi.org/10.3390/cosmetics4030023
Chicago/Turabian StyleSánchez-Lamar, Ángel, Maribel González-Pumariega, Fabiana Fuentes-León, Marioly Vernhes Tamayo, André P. Schuch, and Carlos F. M. Menck. 2017. "Evaluation of Genotoxic and DNA Photo-Protective Activity of Bryothamnion triquetrum and Halimeda incrassata Seaweeds Extracts" Cosmetics 4, no. 3: 23. https://doi.org/10.3390/cosmetics4030023
APA StyleSánchez-Lamar, Á., González-Pumariega, M., Fuentes-León, F., Vernhes Tamayo, M., Schuch, A. P., & Menck, C. F. M. (2017). Evaluation of Genotoxic and DNA Photo-Protective Activity of Bryothamnion triquetrum and Halimeda incrassata Seaweeds Extracts. Cosmetics, 4(3), 23. https://doi.org/10.3390/cosmetics4030023




