Abstract
In this study, 7-O-kojic acid monopalmitate (7-O-KAP) was synthesized using palmitic acid and kojic acid where the yield and biological activities were analyzed. The highest yield of 7-O-KAP (43%) can be obtained at molar ratio of 1:1, enzyme loading of 5% (w/v), temperature of 70 °C, using immobilized lipase N435 in solvent-free system. Stirred tank reactor (STR) provides better mixing of the substrates and biocatalyst with better yield of 7-O-KAP, compared to fluidized tank reactor (FTR) and packed bed reactor (PBR). The 7-O-KAP exhibited pseudoplastic behavior with flow behavior index (n) being less than 1. The 7-O-KAP showed better depigmenting activity with the reduction of melanin content in Danio rerio embryo to 18.70%, significantly lower than the positive control, kojic acid (60.39%) at highest concentration tested (250 μg/mL). Intracellular tyrosinase in Danio rerio embryo was also reduced when treated with 7-O-KAP (12.53%), compared to kojic acid (37.36%) at concentration of 250 μg/mL. In FRAP assay, 7-O-KAP had antioxidant activity of 8156 AAE/mL, which was higher than kojic acid (6794 AAE/mL) at concentration of 2 mg/mL. The 7-O-KAP also reduced peroxidation activity to 12.21%, which was better compared to kojic acid (31.68%) at 2 mg/mL. Moreover, it was found that lipid peroxidation activity of 7-O-KAP (12.21%) was comparable to BHT (11.56%) at 2 mg/mL. Based on this study, 7-O-KAP could be an alternative compound for whitening agent and antioxidant compared to kojic acid and BHT, respectively.
Keywords:
antioxidant; depigmenting; enzymatic; fluidized tank; G361 cells; packed bed; stirred tank 1. Introduction
Kojic acid is an organic acid and a secondary metabolite, mostly biosynthesized by Aspergillus species [1,2]. Kojic acid producing strains, A. flavus, can be improved by the monospore isolation and mutation methods to obtain a stable monokaryotic strain capable of producing a high amount of kojic acid [1]. In biosynthesis pathway of kojic acid, glucose was utilized and underwent multiple steps of conversion to gluconic acid lactone, 3-ketogluconic acid lactone, and 3-ketoglucose, before finally being converted to kojic acid. Until today, kojic acid is still being used as whitening agent in many cosmetic products, but it has low stability and can be difficult to be formulated in cream.
To overcome such limitations of kojic acid, its derivatives were created and synthesized. A number of writings have showed that kojic acid derivatives are best formulated in cream and better depigmenting effect compared to kojic acid. In industry, kojic acid derivatives were developed and produced via chemical process which were not environmentally friendly. A new synthesis approach using enzymatic process, where cost and the use of hazardous chemical can be reduced or minimized, was developed. Unlike chemical process using chemical catalyst, immobilized lipase is susceptible to inhibition, which therefore requires an appropriate bioreactor design, mode of operation, substrate supply, efficient product removal, reuse of immobilized lipase, scale-up, and process control.
Despite this, a very high yield of kojic acid derivatives (KAD) was studied and optimized in shake flask, however, there is no assurance that the same high yield could be achieved in the bioreactor, as the mechanism and design are different [1,2,3]. Moreover, enzymatic synthesis of kojic acid derivatives in bioreactor is interesting, as it is a next step to large scale production using environmental friendly processes [2]. Stirred tank reactor (STR) in batch operating mode was usually used for large scale enzymatic synthesis of several compounds with higher yield compared to continuous STR [1,2,3]. Studies on different types of impeller design and biocatalyst reusability in STR of some other compounds have also been reported [4]. Some other compounds had better performance and yield in the fluidized tank reactor (FTR) and packed bed reactor (PBR) [5,6]. Fluidization mode in FTR may minimize damage to biocatalysts and its immobilization supports. The continuous reaction mode in PBR also provides better biocatalyst protection and reduces the potential for mechanical damage [7]. Providing the advantages of FTR and PBR in other reported studies, it is interesting to illustrate possibility for KAD synthesis using some parameters in such bioreactors.
Kojic acid has two OHs, one connected to a carbon atom of benzene ring, and another connected to a saturated carbon. Previous reports have showed that OH at carbon 5 of kojic acid was preferably esterified, but OH at carbon 7 of kojic acid can be esterifed, probably depending on the type of substrates, biocatalysts, and the behavior of the OH group [8]. Lipases form Peudomonas cepacia and Rhizomucor miehei can result in secondary OH esterified of kojic acid to fatty acid [8]. However, the effect of lipases from other microorganisms such as Candida antarctica on molecular structure of kojic acid was not clearly described. The commercial value for KAD synthesis not only depends on the biomanufacturing aspect, but also related to its biological activities. Different kinds of KAD were synthesized using various acyl donors, which had specific biological activity and commercial value. For instance, kojic acid ester is an improved version of KAD that increases its hydrophobicity, and is suitable for cosmetic formulation.
Although it is known that some KAD has better whitening, methods of evaluation are different between researchers. In this study, we propose an alternative approach to evaluate KAD using zebrafish as in vivo model [9]. Further, antioxidant activity of KAD was also evaluated where antioxidant environment or system greatly influence its activity. Other types of KAD may have higher antioxidant activity than kojic acid, but in this study, antioxidant activity of KAD was also compared to other compounds, such as ascorbic acid, gallic acid, and butylated hydroxytoluene (BHT). The lipid peroxidation assay, which is more suitable for hydrophobic molecules such as KAD, was presented in this study.
Therefore, in this study, the effect of commercial biocatalysts in STR, FTR, and PBR on the performance of the synthesis of KAD was investigated. The chemical and rheological property of KAD was also evaluated. The biological activities of KAD related to its depigmenting and antioxidant was also studied using selected assays.
2. Materials and Methods
2.1. Materials
Magnesium sulphate (MgSO4), calcium chloride (CaCl2), sodium bicarbonate (NaHCO3), potassium chloride (KCl), palmitic acid, acetonitrile (MeCN), glucose, L-3,4-dihydroxyphenylalanine (L-DOPA), alpha-melanocyte stimulating hormone (α-MSH), MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), synthetic melanin, 1-phenyl-2-thiourea (PTU), dimethyl sulfoxide (DMSO), and 2,4,6-Tri(2-pyridyl)-s-triazine, (TPTZ) were purchased from Sigma (St. Louis, MO, USA), and all chemicals used were analytic reagent grade. Kojic acid was biosynthesisysed using a locally isolated strain, as previously mentioned [2].
2.2. Enzymatic Synthesis of KAD in STR
KAD was synthesized using immobilized lipase Novozyme (N435) in small scale 0.1 L STR. The configuration of the reactor was 5.2 cm (Dt, tank diameter), 10.4 cm (Ht, tank height), and 0.12 L (Vt, total volume). The reactor was equipped with a magnetic bar (2.5 cm × 0.5 cm) on a magnetic stirring hotplate (RCT basic IKAMAG® safety control) for agitation at 400 rpm. In this study, working liquid height (Hl), working volume (Vw), and ratio of Di/Dt was 2.9 cm, 0.03 L, and 0.48, respectively. The scale of the working volume (Vw) to total volume (Vt) was 1:4. The by-product water from esterification process was removed using a vacuum pump connected to the reactor. The temperature within the reactor vessel was controlled by a heating coil, which was a stainless tubing with hot water pumped through into it by the water bath. The enzymatic esterification experiments using STR were carried out at various operating parameters, such as temperature (ranged from 50 °C to 90 °C) and molar ratio of acyl donor to kojic acid (1:1 to 3:1). The yields obtained from each run were also compared to other immobilized lipases from Rhizomucor miehei (RMIM) and Thermomyces lanuginosus (TLIM). The fatty acids were initially liquefied at 70 °C for 30 min prior to the addition of lipase (0.5 to 5%, w/v) into the reaction mixture. The performance of N435 reusability after second and third cycles was compared to the first cycle where two different washing solvents (MeCN and DMSO) were used.
2.3. Fluidized Tank Reactor (FTR)
The synthesis of KAD was also conducted in FTR by comparing its productivity using two different biocatalysts (RMIM and N435). A similar glass vessel used for STR was also used as FTR. However, the vessel was not agitated with impeller, but fluidized with air at a flow rate of 5 L/min to create sufficient mixing to suspend all immobilized lipase particles in reaction liquid, a mixture of palmitic acid and kojic acid. The temperature within the bioreactor was controlled at 70 °C. The esterification was started by adding the lipase to the reaction mixture, and the reaction was continued for 240 min. All experiments were performed in triplicate. During the reaction, sample aliquots (200 μL) were taken from the reaction mixture for every 30 min for analysis. The molar mass ratios of fatty acid to kojic acid in the mixture were fixed at 1:1.
2.4. Packed Bed Reactor (PBR)
In this system, esterification of fatty acid and kojic acid with MeCN were performed in a column (inner diameter = 1.0 cm, length = 35 cm) equipped with jacketed hot water. Prior to insertion of fatty acid and kojic acid into the column, the substrates at molar ratios of 1:1 were mixed in mixing container at 60 °C to obtain homogenous mixture, and fed from to the top of the reactor with pumps. Esterification was started by adding reaction mixture into the column, and 10 mL of aliquot was collected continuously. For identification purposes using GC, 200 µL from 10 mL aliquot was used. All experiments were performed in triplicate. Immobilized lipase RMIM and N435 were used as biocatalyst, and were packed into the middle of the reactor. The amounts of packed RMIM were 2.0, 4.0, and 6.0 g, which were equivalent to bed heights of 2.0, 4.0, and 6.0 cm, respectively.
2.5. Conversion and Analysis
The product was analyzed on a gas chromatograph (GC) (Agilent Technologies Inc, Wilmington, NC, USA) using a ZB-5HT Inferno non-polar column with length, internal diameter, and film thickness of 15 m, 0.53 mm, and 0.15 μm, respectively with nitrogen (N2) as carrier gas. The oven initial temperature was maintained at 100 °C, before being increased to 225 °C at 15 °C min−1, and to 280 °C at 30 °C min−1 for the holding time of 1 min. The injector temperature was set at 340 °C in splitless mode, and flame ionization detectors (FID) temperature was set at 350 °C. The product component was quantified by an integrator with 1,2,3-tributyrylglycerol as internal standard (IS). The conversion was calculated using Equations (1) and (2):
Calculation for DRF’
DRF IS = AIS/CIS
DRF std = Astd/Cstd
DRF’ = DRF std/DRF IS
Calculation for the conversion (%)
where Acomp = peak area for each component; AIS = peak area for internal standard; CIS = concentration for internal standard; DIS = detector response factor of internal standard; DRF std = detector response factor of standard; DRF’ = relative detector response factor; and Ccomp = concentration for each component.
Ccomp = (Acomp/AIS) × (CIS/DRF’)
Conversion (%) = (Ccomp/mole of kojic acid) × dilution factor × 100
2.6. Purification and Molecular Structure
The reaction mixture was purified using column chromatography with silica gel (60F254) as its stationary phase and hexane/ethyl acetate (70:30, v/v) as its mobile phase. The eluents were collected at time interval and analyzed using GC. Single peak compound from GC analysis was further analyzed using GC-mass spectrometer (Perkin Elmer Clarus 600MS, PerkinElmer Inc., Waltham, MA, USA) for molecular weight identification. Then, 1D and 2D NMR analysis was carried out using Varian NMR Unity Inova 500 MHz (Varian Inc, Palo Alto, CA, USA) with Pulsed Field Gradient to obtain 1H-NMR, 13C-NMR, heteronuclear single-quantum correlation (HSQC) and heteronuclear multiple-bond correlation (HMBC) spectra.
2.7. Rheological Behaviour
The melt rheological properties of the reaction mixture were determined using a HAAKE™ MARS III Thermo Scientific Rheometer (Thermo Fisher Scientific Inc, Waltham, MA, USA). The measurements were performed in the rotational mode and 20 mm parallel cone-plate measuring geometry with the gap setting of about 0.5 mm. Each kind of rheological experiment was performed at its corresponding temperature and the temperature was controlled by a water bath connected to the peltier system in the bottom plate. An equilibration of 3 min was performed before each measurement. Shear stress-shear rate and viscosity-shear rate data were obtained at 70 °C and 90 °C on the samples of palmitic acid, mixture of palmitic acid and kojic acid, and purified KAD. The instrument was programmed for set temperature and equilibration, followed by one-cycle shear in which the shear rate was increased linearly from 50 to 1000 s−1 in 2 min. This process was repeated two more times for each sample. All measurements were carried out in a normal atmospheric conditions, and flow behavior of the reaction mixtures was determined using k and n utilizing the Power Law equation which the shear stress, τ, is given by
where:
τ = K × (γ)n
- K is the flow consistency index (SI units Pa·sn),
- γ is the shear rate or the velocity gradient perpendicular to the plane of shear (SI unit s−1), and
- n is the flow behavior index (dimensionless) represents an apparent or effective viscosity as a function of the shear rate (SI unit Pa·s).
In the case where flow behavior (n) is less than 1 (shows non-Newtonian behavior), the apparent viscosity and consistency index of samples were calculated and compared, using non-Newtonian models (Herschel-Bulkley) where yield stress were to be considered.
Herschel-Bulkley model
where τ is the shear stress (Pa), γ is the shear rate (s−1), τo is yield stress (Pa), K is the flow consistency index (Pa·sn), and n is the flow behavior index (dimensionless).
τ = (τo) + K × (γ)n
2.8. Cell Viability
McCoy’s 5A containing 10% v/v FBS, 2 mM glutamine and 1% v/v penicillin/streptomycin (100 IU/50 μg/mL) in humidified atmosphere containing 5% CO2 in air at 37 °C were used as culture condition for G361 cells. The KAD samples were prepared by being dissolved in 0.1% DMSO, and followed by the addition of the new medium. In MTT assay, 1 × 105 cells/well of G361 cells were seeded in a 96-well microtiter plate and wait overnight for the cell adhere to completely onto the plate. After 24 h incubation, the medium was removed and a new medium containing samples with concentration ranging from 62.5 to 500 μg/mL was added into the 96-well plate. After 48 h incubation, medium was removed and 50 μL of MTT solutions (1.0 mg/mL) was added to each well and the incubation continued for another 4 h. Then, formazon was solubilized in DMSO and was measured at absorbance of 450 nm.
2.9. Melanin Contents
Experimentation using Danio rerio embryo was performed according to the guidelines and approval given by local ethics community (UPM/IACUC/AUP No. R024/2014). Embryos were pretreated with 0.2 mM phenylthiourea (PTU) at 1 dpf (day-post fertilization) for 12 h, before the addition of fresh medium containing 100 µM α-MSH and KAD samples [10]. For the control, the same method was followed, but without the addition of samples. After 24 h incubation, the protein and melanin content was assayed and determined. The centrifuged pellet was dissolved in 1 mL of 1 M NaOH at 100 °C for 30 min and vortexed. The absorbance of the supernatant was calculated at 490 nm and the actual concentration of melanin was generated from the standard curve of known melanin concentrations (0–300 µg/mL). The melanin content was expressed as a percentage (%).
2.10. Tyrosinase Activity
The same method as in melanin assay was used for tyrosinase assay, except that after 24 h incubation, ten (10) zebrafish embryos were sonicated in cold lysis buffer made of 1% Triton X-100, 20 mM sodium phosphate at pH 6.8, 1 mM EDTA, 1 mM PMSF. The lysate was then centrifuged at 800 rpm for 5 min. Lysate with total protein of 250 µg was added in 100 µL of lysis buffer in 96-well plate. Then, 100 µL of L-DOPA (1 mM) was added and further incubated for 60 min at 37 °C. The absorbance was measured at 490 nm using the microplate reader where the final activity was expressed in percentage (%) [9]. KA was used as a positive control.
2.11. FRAP
In this assay, acetate buffer (300 mM, pH 3.6), 10 mM TPTZ and 20 mM FeCl3·6H2O were mixed in a ratio of 10:1:1 to be a working FRAP reagent. Then, 10 μL of samples (0.125 to 2 mg/mL) were mixed with 250 µL of FRAP reagent and incubated at 37 °C for 10 min. The absorbance at 630 nm was monitored using an MR-96A microplate reader (Mindray, Shenzhen, China). Ascorbic acid was used as a positive control. All reagents were freshly prepared before used. The standard curve for FRAP assay was generated by using the ascorbic acid as a reducing agent. The antioxidant activity is expressed as the number of gram equivalents of ascorbic acid per mL [11].
2.12. Lipid Peroxidation
The lipid solution was made of 10 mg/mL lecithin suspended in 0.01 M phosphate buffered saline at pH 7.4. The TCA–TBA–HCl solution was prepared with a mixture of 15 g of TCA, 0.37 g of TBA, and 2 mL of concentrated HCl in 100 mL distilled water. Then, 200 µL of lipid solution, 200 µL of 400 μM FeCl3, 200 µL of 400 μM ascorbic acid, and 200 µL of tested compound solution were mixed and incubated in a water bath at 37 °C in dark for 60 min, before the addition of 400 uL TCA–TBA–HCl solution. Then, the solution was heated at 100 °C for 15 min in a water bath and cooled down with ice. The pink solution formed was centrifuged at 3500 rpm for 10 min. The blank was made with 200 µL of distilled water substitution for 200 µL of sample. The absorbance of the supernatant was measured at 532 nm. Ascorbic acid, gallic acid and BHT were used as positive controls. The lipid peroxidation activity was calculated according to Equation (5) [12].
Activity (%) = (Absorbance of compounds/Absorbance of control) × 100%
2.13. Statistics
Data were collected as mean ± standard error (S.E.M) of at least three determinations (n = 3). Statistical analysis was performed using GraphPad Prism 5.0. The evaluation of statistical significance was determined by ANOVA with Bonferrroni’s Post Hoc test, where p values less than 0.05 (p < 0.05) were considered to be statistically significant.
3. Results
3.1. Bioreactors
3.1.1. Stirred Tank Reactor
In general, the conversion of KAD was increased with increasing reaction time using all biocatalysts. By using lipase from Rhizomucor miehei (RMIM), the conversion of KAD for 240 min reaction time at 70 °C, 80 °C, and 90 °C was 32%, 27%, and 18%, respectively (Figure 1a). Bonferroni’s Multiple Comparison Test shows that there is no significant difference conversion for 240 min reaction time at 70 °C and 80 °C but there is significant conversion of KAD at 70 °C and 90 °C. Very low yield of KAD was synthesized using lipase from Thermomyces lanuginosus (TLIM), which was less than 1% at all temperatures for 240 min reaction time (Figure 1b). The conversion of KAD was 38% using lipase from Candida antarctica (N435) at reaction temperature of 80 °C, which was significantly higher compared to reaction temperature of 70 °C (18%), but less significant at 90 °C (32%) for 240 min reaction time (Figure 1c). The conversion of KAD at 90 °C was initially higher for the first 90 min compared to reaction temperature of 80 °C, but gradually decreased until 240 min. Based on Figure 1, it was found that highest conversion of KAD can be obtained using N435 at 80 °C. Figure 2 shows the effect of molar ratio on the conversion of KAD using N435. Bonferroni’s Multiple Comparison Test shows that there was no significant difference between molar ratio of 3:1, 2:1, and 1:1. The effect of enzyme loading using N435 is illustrated in Figure 3. It was shown that 5% enzyme loading gave 43% conversion of KAD, significantly higher compared to 1% (14%) and 0.5% (7%). The highest yield of KAD can be obtained at substrate molar ratio of 3:1, enzyme loading of 5% (w/v), and temperature of 80 °C, using immobilized lipase N435 in solvent-free system. In the study of lipase reusability in STR, the conversion of KAD after the second and third cycle using N435 with acetonitrile as washing solvent was maintained at 33%. However, the conversion of KAD when using DMSO as washing solvent was slightly low, which was 25% and 16% for second and third cycle, respectively (Figure 4).
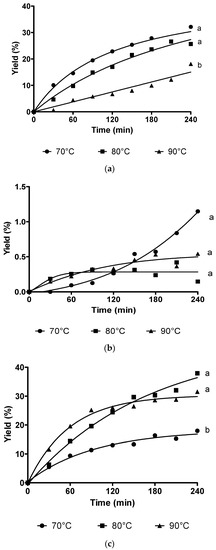
Figure 1.
The experimental data of solvent-free 7-O-kojic acid monopalmitate synthesis in stirred tank reactor (STR) for 240 min using RMIM (a), TLIM (b) and Novozyme 435 (c) at 70 °C, 80 °C, and 90 °C. Data are presented as means ± S.E.M, n = 3. The letters a and b indicate significant difference at p < 0.05 with Bonferroni’s Multiple Comparison Test.
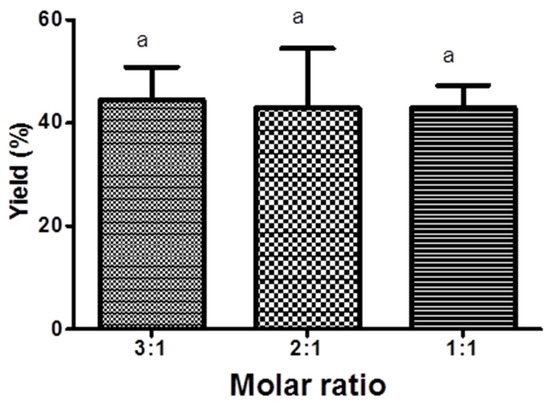
Figure 2.
The effect of different molar ratio (palmitic acid:kojic acid) on 7-O-kojic acid monopalmitate synthesis in solvent-free system at 240 min using Novozyme 435 at 70 °C. Data are presented as means ± S.E.M, n = 3. The letter a indicates significant difference at p < 0.05 with Bonferroni’s Multiple Comparison Test.
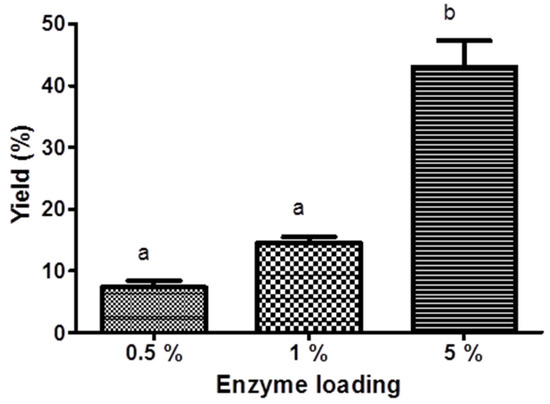
Figure 3.
The effect of enzyme loading on 7-O-kojic acid monopalmitate synthesis in solvent-free system at 240 min using Novozyme 435 at 70 °C. Data are presented as means ± S.E.M, n = 3. The letters a and b indicate significant difference at p < 0.05 with Bonferroni’s Multiple Comparison Test.
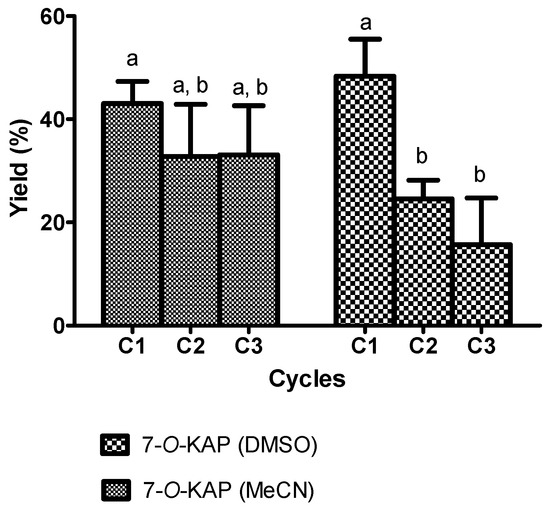
Figure 4.
The effect of Novozyme 435 reusability on 7-O-kojic acid monopalmitate (7-O-KAP) synthesis utilizing dimethyl sulfoxide (DMSO) and acetonitrile (MeCN) as washing solvent at 240 min using at 70 °C. Data are presented as means ± S.E.M, n = 3. The letter a indicates significant difference at p < 0.05 with Bonferroni’s Multiple Comparison Test. Note that C1, C2 and C3 represents first, second and third cycles respectively.
3.1.2. Fluidized Tank Reactor and Packed Bed Reactor
The enzymatic esterification performance of KAD in FTR is illustrated Figure 5. Overall, the conversion of KAD increased with increasing reaction time using N435 and RMIM. The conversion of KAD synthesized using N435 was 15%, slightly higher than those obtained using RMIM (13%) for reaction time of 240 min. The conversion of KAD in FTR was still lower than the one obtained in STR even after three cycles of lipase reusability. In PBR, the conversion of KAD at reaction temperature of 60 °C and 80 °C with substrate molar ratio of 1:1 using N435 and RMIM is shown in Table 1. Some conversion of KAD was less influenced by lipase bed length of 2 to 4 cm. However, significant yield of KAD was noted at lipase bed length of 6 cm. A very low yield (less than 4%) of KAD was synthesized using N435 and RMIM in PBR, compared to FTR and STR. Temperature has less effect on yield of KAD synthesis regardless of lipase used. Time may have an important role in KAD synthesis, as the batch operating system showed a better yield compared to the continuous process.
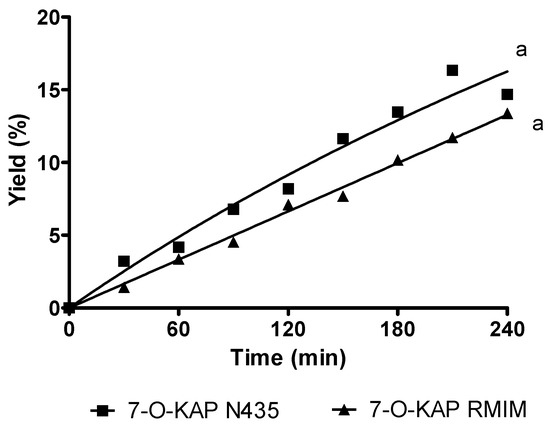
Figure 5.
The experimental data of solvent-free 7-O-kojic acid monopalmitate (7-O-KAP) synthesis in fluidized tank reactor (FTR) at 70 °C, 5% enzyme loading for 240 min using Novozyme 435 (N435) and RMIM. Data are presented as means ± S.E.M, n = 3. The letter a indicates significant difference at p < 0.05 with Bonferroni’s Multiple Comparison Test.

Table 1.
The yield of 7-O-KAP synthesized by enzymatic esterification using immobilized lipase (N435 and RMIM) in a continuous packed bed reactor. The substrate molar ratio used for this experiment was 1:1. The letters a, b, and c indicate significant difference at p < 0.05 with Bonferroni’s Multiple Comparison Test.
3.2. Characterization of KAD
The highest yield KAD synthesized in STR using N435 was purified and used for further characterization, which later identified as 7-O-kojic acid monopalmitate (7-O-KAP). The molecular weight of 7-O-KAP was 380 m/z, as determined using GCMS. Chemical structure of 7-O-KAP was obtained via 1D-NMR and 2D-NMR (Figure 6). The 1D and 2D-NMR revealed the chemical structure of 7-O-KAP (Table 2). The correlation between hydrogen atom and carbon atom in chemical structure of 7-O-KAP was confirmed using HSQC and HMBC. In the HSQC spectrum, the carbon atom value of 24 to 34 ppm were directly joined to its corresponding hydrogen atom value of 0.8 to 2.4 ppm. The carbon atom value of 24 to 34 ppm corresponds to CH2-CH2-CH3 chain (C1’ to C16’). On the other hand, carbon atom value of 61.1 ppm is directly joined to its corresponding hydrogen atom value of 4.93 ppm. The carbon value of 61.1 ppm corresponds to C7. The carbon atom value of 110 ppm (C3) directly joined to its corresponding hydrogen atom value of 6.61 ppm. The carbon atom value of 138.5 ppm (C6) directly joined to its corresponding hydrogen value of 7.85 ppm. In HMBC data, the spectrum shows neighboring carbon closely linked to the carbon atom, which is directly joined to its hydrogen atom. The hydrogen atom (value of 6.61 ppm) bind to C3 (110 ppm) near to C4 (165 ppm) and C5 (146 ppm). Meanwhile, hydrogen atom (7.85 ppm) bind to C6 (138.5 ppm) near to C2 (174.5 ppm), C4 (168) and C5 (146 ppm). It also shows that hydrogen atom (4.93 ppm) bind to C2 (61.13 ppm) near to C2 (172 ppm), C1’ (163) and C3 (111 ppm). These data show that OH at C7 of kojic acid was enzymatically esterified with palmitic, which results in 7-O-kojic acid monopalmitate synthesis.
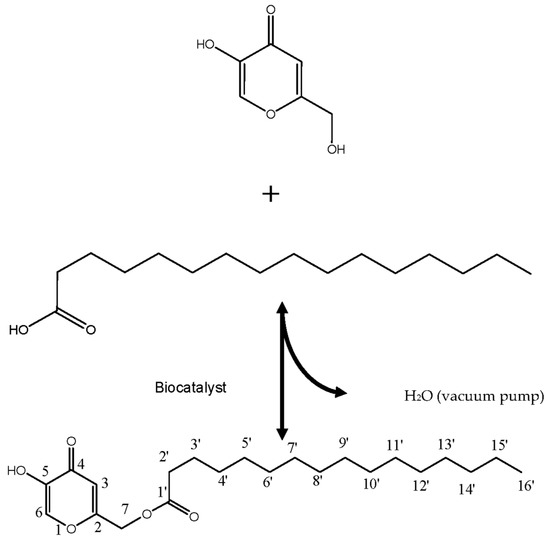
Figure 6.
Reaction scheme and molecular structure of 7-O-kojic acid monopalmitate (7-O-KAP).

Table 2.
The 1H-NMR, 13C-NMR with HSQC and HMBC analysis for 7-O-KAP.
3.3. Rheological Behaviour
The rheological behavior of fatty acid and KAD are summarized in Table 3. The smaller value of n indicates that the decline of viscosity with increasing shear rate is more rapid, which is the behavior of pseudoplasticity. The n values for palmitic acid were 0.877 and 0.853 measured at 70 °C and 90 °C, respectively. The flow behavior (n) was less than 1 even with the addition of fatty acid into kojic acid at molar ratio of 1:1 and 3:1. The viscosity of a suspension depends on several factors such as size of solid particles, particle size distribution, concentration, pressure, and temperature. In this study, the consistency index (K) for fatty acids decreased when the temperature increased. K, is a direct measure of viscosity at a given rate of shear. The larger the value of K, the greater the viscosity at a given shear rate. At temperatures of 70 °C and 90 °C, the K values for PA were 0.01821 and 0.01525 Pa·sn, respectively. The K value was significantly larger in the presence of kojic acid in the reaction mixture compared to the presence of fatty acid alone. The K values of mixture of kojic acid to palmitic acid at a ratio of 1:1 were 0.5586 Pa·sn, respectively. When the ratio of fatty acid to kojic acid was increased to a ratio of 3:1, the K values were decreased. The flow behavior index for purified KAD was less than 1, suggesting that it was also exhibited a pseudoplastic behavior.

Table 3.
The rheological behavior of fatty acid, reaction mixture (PA:KA) and purified 7-O-KAP. Data are presented as means ± S.E.M, n = 3.
The yield stress τo (Pa) of palmitic acid was smaller compared to the yield stress of the mixture of fatty acids and kojic acid. As the yield stress of fatty acids was small, there is a small difference between the consistency index, K, determined by the Herschel–Bulkley and Power law models. For instance, the K value determined by the Power law model for palmitic acid (at 90 °C) was 0.0152 Pa·sn where yield stress was equal to 0. This is compared to the K value determined by the Herschel–Bulkley model for palmitic acid, which was 0.0138 Pa·sn, where yield stress 0.2087 Pa is to be considered. On the other hand, the K value determined by the Power law model for palmitic acid to kojic acid mixture (ratio of 1:1) was 0.5586 Pa·sn. This is compared to the K value determined by the Herschel-Bulkley model, which was 0. 0.3476 Pa·sn where yield stress 5.9336 Pa is to be considered. The information of rheological properties gave an understanding in relation to the reaction mixture to its viscosity, mixing quality, and its effect on yield. For instance, the addition of fatty acids in the reaction mixture reduced viscosity of the reaction mixture (consistency index of ratio 1:1 (0.55) reduced to 0.03 at ratio of 3:1 (palmitic acid:kojic acid)). The present of yield stress showed that significant agitation speed required for better mixing quality.
3.4. Cell Viability
Cytotoxicity of kojic acid and 7-O-KAP are evaluated based on cell viability of G361 cells, and is shown in Figure 7. At lowest concentration (62.5 μg/mL) of kojic acid and 7-O-KAP, the cell viability was above 80%. There was no significant reduction in cell viability after incubation of G361 cells with kojic acid and 7-O-KAP at concentration of 62.5 μg/mL to 250 μg/mL. However, the number of viable cells treated with 7-O-KAP was slightly lower (below 80%) at concentration of 500 and 1000 μg/mL. The non-toxic concentration of kojic acid and 7-O-KAP was used to evaluate the depigmenting effect. Based on ANOVA analysis using Bonferroni’s Multiple Comparison Test, kojic acid and 7-O-KAP are not significantly difference. However, 7-O-KAP at 1000 μg/mL had significantly lower cell viability, as compared to control and kojic acid at low concentration (62.5 μg/mL and 125 μg/mL).
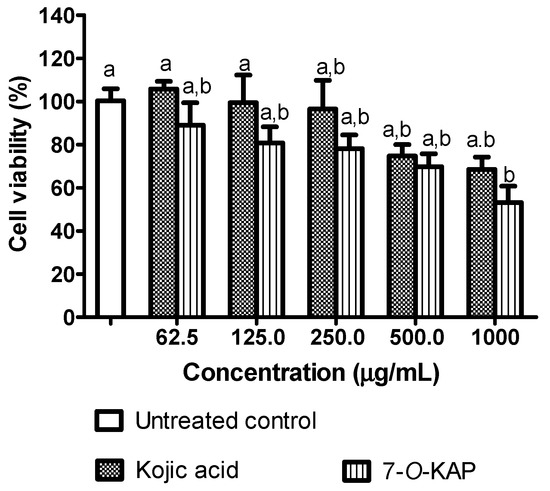
Figure 7.
The effect of kojic acid and 7-O-kojic acid monopalmitate (7-O-KAP) on the cell viability of G361 human melanoma skin cells. The cells were treated with various doses of kojic acid and 7-O-KAP (7.81–250 μg/mL) for 72 h and were examined by MTT assay. Data are presented as means ± S.E.M and expressed as % of untreated control, n = 3. The letters a and b indicate significant difference at p < 0.05 with Bonferroni’s Multiple Comparison Test.
3.5. Depigmenting Activity
The inhibitory effect of α-MSH stimulated melanin formation in Danio rerio embryo treated with kojic acid and 7-O-KAP is summarized in Figure 8. The inhibitory effect of kojic acid and 7-O-KAP was evaluated at 62.5 μg/mL, 125 μg/mL, and 250 μg/mL. The α-MSH stimulated melanin formation was significantly reduced when treated with 7-O-KAP (18.70 to 42.55%) at all concentrations tested compared to untreated control. The reduction of melanin formation in Danio embryo treated with 7-O-KAP (18.70%) was also significantly lower compared to the one treated with kojic acid (60.39%) at concentration of 250 μg/mL. Figure 9 describes the inhibitory effect of α-MSH stimulated tyrosinase activity in Danio rerio embryo treated with kojic acid and 7-O-KAP. The tyrosinase activity was significantly reduced when treated with kojic acid and 7-O-KAP at all concentrations tested (62.5 μg/mL to 250 μg/mL) compared to untreated control. There was slight difference of tyrosinase activity in Danio embryo when treated with kojic acid and 7-O-KAP. At the highest concentration tested, tyrosinase activity was reduced to 37.36% and 12.53% when treated with kojic acid and 7-O-KAP, respectively.
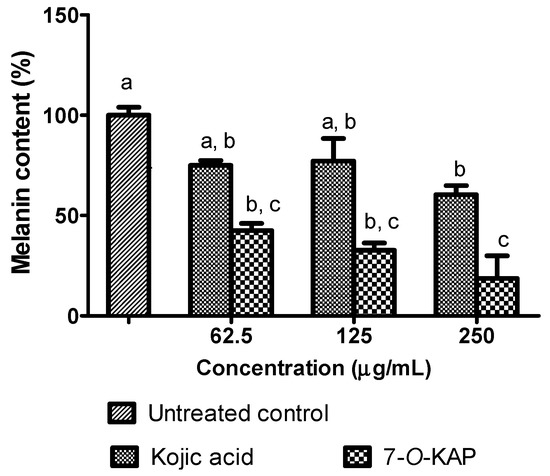
Figure 8.
Melanin content of Danio rerio embryo after 48 h treatment with kojic acid and 7-O-kojic acid monopalmitate (7-O-KAP). The results are expressed as means ± S.E.M of percentage (%) to untreated control, n = 3. Kojic acid was used as a positive control. The letters a, b, and c indicate significant difference at p < 0.05 with Bonferroni’s Multiple Comparison Test.
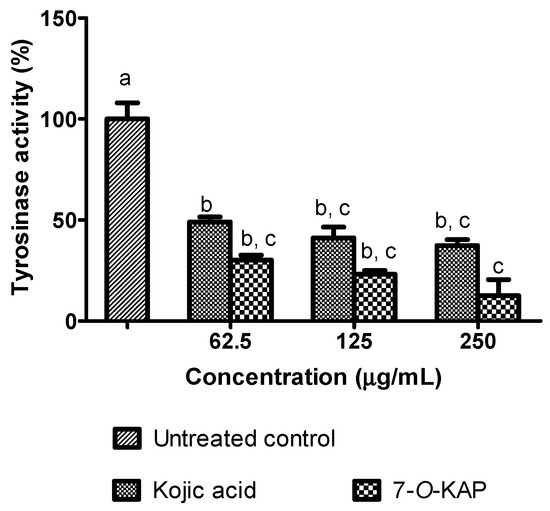
Figure 9.
Tyrosinase activity in Danio rerio embryo after 48 h treatment with kojic acid and 7-O-kojic acid monopalmitate (7-O-KAP). The results are expressed as means ± S.E.M of percentage (%) to untreated control, n = 3. Kojic acid was used as a positive control. The letters a, b, and c indicate significant difference at p < 0.05 with Bonferroni’s Multiple Comparison Test.
3.6. Antioxidant
Figure 10 shows FRAP assay of kojic acid and 7-O-KAP where it was calculated using ascorbic acid standard curve (Figure 11 and Table 4). In the FRAP assay, the antioxidant activity of 7-O-KAP at concentration of 0.125 to 1 mg/mL was slightly higher than kojic acid. At highest concentration of 2 mg/mL, 7-O-KAP showed significant antioxidant activity (8156 AAE/mL) compared to kojic acid (4986 AAE/mL). In Figure 12, kojic acid and 7-O-KAP significantly reduced lipid peroxidation activity at all concentrations tested (0.5 to 2 mg/mL). Lipid peroxidation activity was significantly reduced when treated with 7-O-KAP (12.21%) at concentration of 2 mg/mL compared to kojic acid (31.68%). Compared to other compounds, 7-O-KAP was better at reducing lipid peroxidation, as compared to ascorbic acid and gallic acid, and was almost comparable to BHT. For instance, at concentration of 2 mg/mL, lipid peroxidation activity of 7-O-KAP, ascorbic acid, gallic acid, and BHT were 12.21%, 37.06%, 23.10%, and 11.56%, respectively.
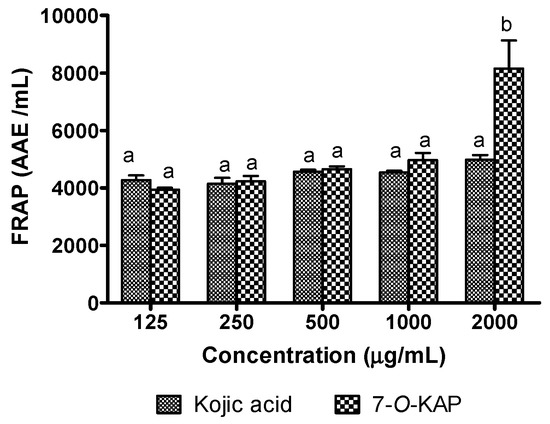
Figure 10.
Ferric ion antioxidant power (FRAP) of kojic acid and 7-O-kojic acid monopalmitate (7-O-KAP). Data are presented as means ± S.E.M, and expressed as number of gram equivalents of ascorbic acid per mL (AAE/mL), n = 3. The letters a and b indicate significant difference at p < 0.05 with Bonferroni’s Multiple Comparison Test.
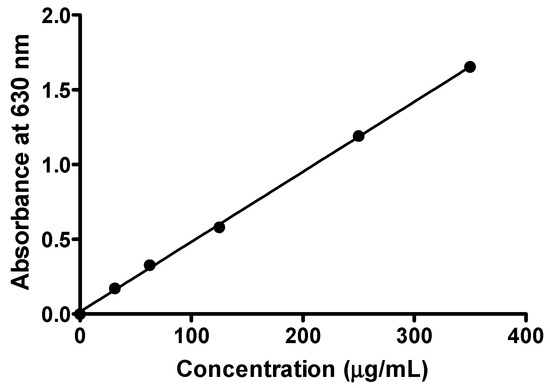
Figure 11.
The standard curve of ferric ion antioxidant power (FRAP) was obtained using 0–350 μg/mL of ascorbic acid at absorbance of 630 nm.

Table 4.
Linear regression of the FRAP standard curve obtained using 0–350 μg/mL of ascorbic acid.
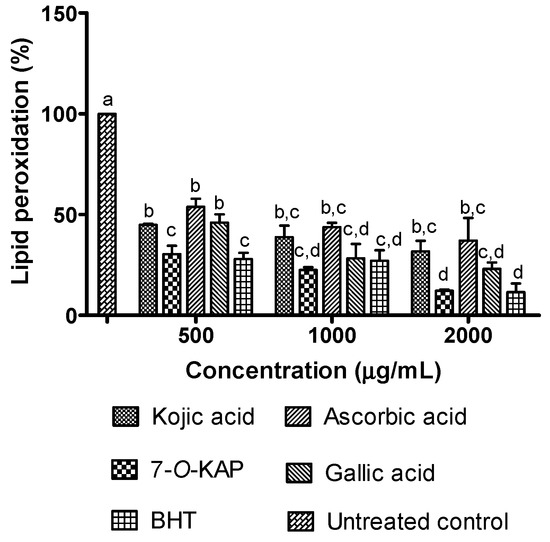
Figure 12.
The effect of kojic acid and 7-O-kojic acid monopalmitate (7-O-KAP), ascorbic acid, gallic acid and BHT on the activity of lipid peroxidation. The results are expressed as means ± S.E.M of percentage (%) to untreated control, n = 3. BHT was used as positive control. The letters a, b, c, and d indicate significant difference at p < 0.05 with Bonferroni’s Multiple Comparison Test.
4. Discussion
In the lipase-based esterification process, several factors greatly influenced and determined the yield of KAD, such as positional, substrate, and fatty acid specificity of an enzyme [13]. RMIM has different fatty acid specificities, and prefers saturated fatty acid as a substrate, while TLIM specificity for polyunsaturated fatty acid resulted in the increment of the ester and product conversion [14]. RMIM showed better fatty acid specificity to C12:0 (short saturated fatty acid) than C18:1, 18:2, 18:3, 20:4, 20:5, and 22:6, in which the fatty acid specificity pattern deceased with increasing number of carbon atom and double bond in the fatty acids chain [15]. In contrast, lipase from Candida antarctica was found to have fatty acid specificity to straight chain fatty acid with 10–18 carbon atoms. For instance, lower fatty acid such as hexanoic, octanoic, and unsaturated 9-cis octadecenoic acid, were found to be poor substrates for ethyl D-glucopyranoside esters [14,16].
Enzymatic synthesis of wax ester, adipate ester, hexyl laurate, pentyl octanoate, and ethyl butyrate have been conducted in STR using solvent and solvent-free system with significant high yield of up to 80% [6,16,17,18,19,20]. The combination of supercritical fluid and molecular sieves can improve the yield of sugar esters up to 67% [18,21]. In FTR and PBR systems, immobilized M. miehei lipase showed a better operational stability, higher yield, and better reusability performance than C. rugosa lipase after the successive batches of esterification [7,19,22]. In another study, kinetics in a fluidized system have a slightly better esterification profile (90.2% yields after 14 h) compared to a batch system [5]. Several esters such as amyl caprylate, ethyl cinnamate, and polyglycerol ester have been reported and synthesized in such reactors [7,23,24].
Mechanical mixing was applied to increase the contact between the reactants (kojic acid and fatty acid) and lipase enzyme, resulting in an increase of the mass transfer rate and yield. The viscosity of the mixture containing kojic acid and fatty acid was significantly higher than the fatty acid liquid alone. A viscous mixture can lower the chance of contact between substrates and lipase, which can result in low yield. The rheological property of the reaction mixture might be one of the factors that determine the selection of the reactors. Several Newtonian-like mixture compounds, such as methyl ester and palm esters, were successfully synthesized in stirred tank reactors [24,25]. For adipate ester and sugar ester synthesis, slightly high agitation speed (500–600 rpm) was need to achieved maximum yield, where it may have related to their mixing property and viscosity [25]. In the case of the synthesis of sucrose ester solutions, the changes in viscosity of sucrose ester solutions in dimethyl formamide (DMF) solvent were suggested due to basis of hydrogen-bond density, ester–ester/solvent–ester interactions, and mixed partial esters production [26]. The presence of yield stress (Pa) explain the reason that the synthesis of certain compounds required certain mixing or agitation speed to create significant mixing conditions, which lead to homogenization with significant increase in yield. The rheological behavior of KAD also provides some information on the characteristic of cosmetic formulation, and the right rheological properties for the different application may be needed. A new KAD can replace kojic acid and palmitic acid in formulation, but the subject of our current interest is whether this ingredient is adequate for formulation, or whether KAD has the desirable rheological characteristics for cosmetic product, or its effect on the product formulation.
Kojic acid has been used for many years as a depigmenting agent. However, the efficacy and penetration level of kojic acid into skin decreases when expose to extreme pH, temperature, and photon level, which lead to its degradation [27]. KAD had far better depigmenting activity compared to kojic acid. Improvement of KAD hydrophobic properties led to greater depigmenting activity and penetrability, as compared to hydrophilic nature of kojic acid [2]. Increases in absorption are also related to structure of the cell membrane which is made of a phospholipid bilayer [28]. A recent study showed that poly(carbonate-esters) and polyesters comprised of kojic acid were more stable [27]. The aliphatic lipophilic kojic acid dienols were significantly more efficient than kojic acid at inhibiting melanin synthesis in melanocytes [27]. The depigmenting activity may derive from the kojic acid part of the KAP molecule, but there are several factors that influence its activity than just comparing the amount of kojic acid part presence. Factors such as penetrability and hydrophobic-hydrophilic balance play significant roles in its activity [27,29]. Cytotoxicity using MTT assay measures damage to mitochondrion, so penetrability of compound or molecules into the cell membrane may play some role to its cytotoxic effect. However, KAD synthesized in this study showed no significant cytotoxic effect as compared to kojic acid at low concentration. Moreover, kojic acid, which is polar, may have some difficulty in penetrating into the cells, compared to uncharged and hydrophobic molecules [28].
KAD as a prospective anti-oxidant was also reported where the capability of KA-3,4-methylenedioxy cinnamic acid ester to inhibit lipid peroxidation in HaCaT keratinocytes is about 47% higher than tertbutylhydroperoxide, which was used as a positive control [30]. Several researchers have reported that saturated palmitic acid was more capable and effective at decreasing TBARS formation, compared to unsaturated palmitoleic acid—whereas, shorter chain length, such as capric and caprylic acids, did not show inhibition to lipid peroxidation [31]. Due to autoxidation, polyunsaturated fatty acids can give rise to hydroperoxides which involve an attack of oxygen at the allylic position [32]. In melanogenesis, superoxide anion was proposed to play a role in non-enyzmatic production of melanin. In this process, oxidation of L-dopa by oxygen molecules produces a dopa-semiquinone radical and superoxide. The dopa-semiquinone might be oxidized by superoxide, resulting in the formation of dopaquinone and hydrogen peroxide, respectively [33].
5. Conclusions
In this study, STR could be the best approach for enzymatic synthesis of KAD compared to other reactor systems. The highest yield of KAD could be synthesized using N435 at 80 °C for 4 h duration. The rheological properties of substrates and KAD could be the reason that STR is the best option for the synthesis of KAD, compared to FTR and PBR. The use of N435 has advantages over other lipases tested at high reaction temperature of 80 °C. At low temperatures, the reaction mixture tends to become more viscous than at high temperature, which may influence that yield of KAD. The addition of fatty acid and optimum reaction temperature can reduce its viscosity and may probably reduce the shear effect. This may help in minimizing damage to lipase and preserve high catalytic activity even after several cycles. The immobilization support of N435 is more stable compared to RMIM that gave brown pigment to the reaction mixture. The 7-O-KAP showed better depigmenting activity by lowering tyrosinase activity and melanin content of Danio rerio’s embryo. The improved melanin formation inhibition effect, and reduced tyrosinase activity of KAD compared to free kojic acid, was likely from the enhanced chemical stability of KAD, as well as increased permeability through cell membranes due to increased hydrophobicity. Therefore, 7-O-KAP showed to be a better depigmenting agent and it could be safer alternative compared to kojic acid. On the other hand, 7-O-KAP could also be safer option for antioxidant compared to BHT.
Acknowledgments
This project was previously supported by CRDF-MTDC (grant vote No. 6364704) and myPhD from Ministry of Higher Education (MOHE).
Author Contributions
A.F.B.L. was responsible for this research project, experimental design, and preparation of this manuscript. M.H. was a co-researcher in charge of animal cell culture experimentation and antioxidant assay. S.A. was a co-researcher in charge of Danio experimentation. A.B.A. was the principal researcher in charge of overall enzymatic synthesis of kojic acid derivative experimentation. All authors read and approved the final manuscript.
Conflicts of Interest
Authors claims that there is no conflict of interest in this article.
References
- El-Boulifi, N.; Ashari, S.E.; Serrano, M.; Aracil, J.; Martínez, M. Solvent-free lipase-catalyzed synthesis of a novel hydroxyl-fatty acid derivative of kojic acid. Enzym. Microb. Technol. 2014, 55, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Lajis, A.F.B.; Basri, M.; Mohamad, R.; Hamid, M.; Ashari, S.E.; Ishak, N.; Zookiflie, A.; Ariff, A.B. Enzymatic synthesis of kojic acid esters and their potential industrial applications. Chem. Pap. 2013, 67, 573–585. [Google Scholar]
- Adnani, A.; Basri, M.; Chaibakhsh, N.; Ahangar, H.A.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Abdul-Rahman, M.B. Chemometric analysis of lipase-catalyzed synthesis of xylitol esters in a solvent-free system. Carbohydr. Res. 2011, 346, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Chaibakhsh, N.; Abdul-Rahman, M.B.; Vahabzadeh, F.; Abd-Aziz, S.; Basri, M.; Salleh, A.B. Optimization of operational conditions for adipate ester synthesis in a stirred tank reactor. Biotechnol. Bioprocess Eng. 2010, 15, 846–853. [Google Scholar] [CrossRef]
- Saponjić, S.; Knežević-Jugović, Z.D.; Bezbradica, D.I.; Zuza, M.G.; Saied, O.A.; Bosković-Vragolović, N.; Mijin, D.Z. Use of Candida rugosa lipase immobilized on sepabeads for the amyl caprylate synthesis: Batch and fluidized bed reactor study. Electron. J. Biotechnol. 2010, 13, 1–15. [Google Scholar] [CrossRef]
- Skoronski, E.; Padoin, N.; Soares, C.; Furigo, A., Jr. Stability of immobilized Rhizomucor miehei lipase for the synthesis of pentyl octanoate in a continuous packed bed bioreactor. Braz. J. Chem. Eng. 2014, 31, 633–641. [Google Scholar] [CrossRef]
- Jakovetic, S.M.; Lukovic, N.D.; Boskovic-Vragolovic, N.M.; Bezbradica, D.I.; Picazo-Espinosa, R.; Knezevic-Jugovic, Z.D. Comparative study of batch and fluidized bed bioreactors for lipase-catalyzed ethyl cinnamate synthesis. Ind. Eng. Chem. Res. 2013, 52, 16689–16697. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Hamid, M.; Ariff, A.B. Depigmenting effect of Kojic acid esters in hyperpigmented B16F1 melanoma cells. J. Biomed. Biotechnol. 2012, 2012, 952452. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.C.H.; Ding, H.Y.; Kuo, S.Y.; Chin, L.W.; Wu, J.Y.; Chang, T.S. Evaluation of In Vitro and In Vivo depigmenting activity of raspberry ketone from Rheum officinale. Int. J. Mol. Sci. 2011, 12, 4819–4835. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Song, K.; Jung, P.; Kim, J.; Ro, H. Inhibitory effect of arctigenin from fructus arctii extract on melanin synthesis via repression of tyrosinase expression. Evidence-Based Complement. Altern. Med. 2013, 2013, 965312. [Google Scholar] [CrossRef] [PubMed]
- Jonfia-Essien, W.A.; West, G.; Alderson, P.G.; Tucker, G. Phenolic content and antioxidant capacity of hybrid variety cocoa beans. Food Chem. 2008, 108, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Kim, M.J.; Hayat, K.; Xia, S.; Feng, B.; Zhang, X. Characteristics and antioxidant activity of ultrafiltrated Maillard reaction products from a caseinglucose model system. Food Chem. 2009, 117, 48–54. [Google Scholar] [CrossRef]
- Sharma, S.; Kanwar, S.S. Organic solvent tolerant lipases and applications. Sci. World J. 2014, 2014, 625258. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ayub, M.A.Z. Effects of the combined use of Thermomyces lanuginosus and Rhizomucor miehei lipases for the transesterification and hydrolysis of soybean oil. Process Biochem. 2011, 46, 682–688. [Google Scholar] [CrossRef]
- Ray, S. Applications of extracellular microbial lipase—A review. Int. J. Res. Biotechnol. Biochem. 2015, 5, 6–12. [Google Scholar]
- Martins, A.B.; Graebin, N.G.; Lorenzoni, A.S.G.; Roberto, F.L.; Ayub, M.A.Z.; Rodriguesa, R.C. Rapid and high yields of synthesis of butyl acetate catalyzed by Novozym 435: Reaction optimization by response surface methodology. Process Biochem. 2011, 46, 2311–2316. [Google Scholar] [CrossRef]
- Hlavsov’a, K.; Wimmer, Z.; Xanthakis, E.; Bernasek, P.; Sovova, H.; Zarevuckae, M. Lipase activity enhancement by SC-CO2 treatment. Z. Naturforsch. 2008, 63, 779–784. [Google Scholar]
- Gandhi, N.N.; Vijayalakshmi, V.; Sawant, S.B.; Joshi, J.B. Immobilization of Mucor miehei lipase on ion exchange resins. Chem. Eng. J. 1996, 61, 149–156. [Google Scholar] [CrossRef]
- Ishak, N.; Lajis, A.F.B.; Mohamad, R.; Ariff, A.B.; Halim, M.; Wasoh, H. Kojic acid esters: Comparative review on its methods of synthesis. J. Biochem. Microbiol. Biotechnol. 2016, 4, 7–15. [Google Scholar]
- Kuo, C.H.; Chen, H.H.; Chen, J.H.; Liu, Y.C.; Shieh, C.J. High yield of wax ester synthesized from cetyl alcohol and octanoic acid by lipozyme RMIM and Novozym 435. Int. J. Mol. Sci. 2012, 13, 11694–11704. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.M.; de Lima Filho, J.L.; de Castro, H.F. Comparative performance of microbial lipases immobilized on magnetic polysiloxane polyvinyl alcohol particles. Braz. Arch. Biol. Technol. 2008, 51, 889–896. [Google Scholar] [CrossRef]
- Sabeder, S.; Habulin, M.; Knez, Z. The lipase-catalyzed synthesis of fatty acid fructose esters in organic media and in supercritical carbon dioxide. Chem. Ind. Chem. Eng. Q. 2006, 12, 147–151. [Google Scholar] [CrossRef]
- Hilterhaus, L.; Minow, B.; Müller, J.; Berheide, M.; Quitmann, H.; Katzer, M.; Thum, O.; Antranikian, G.; Zeng, A.P.; Liese, A. Practical application of different enzymes immobilized on sepabeads. Bioprocess Biosyst. Eng. 2008, 31, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, X.J.; Nie, K.L.; Wang, F.; Liu, J.F.; Wang, P.; Tan, T.W. Synthesis of wax esters by lipase-catalyzed esterification with immobilized lipase from Candida sp. 99–125. Chin. J. Chem. Eng. 2011, 19, 978–982. [Google Scholar] [CrossRef]
- Keng, P.S.; Basri, M.; Ariff, A.B.; Abdul-Rahman, M.B.; Rahman, R.N.Z.A.; Salleh, A.B. Scale-up synthesis of lipase-catalyzed palm esters in stirred-tank reactor. Bioresour. Technol. 2008, 99, 6097–6104. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.S.; Deshpande, T.D.; Kulkarni, R.D.; Mahulikar, P.P. Synthesis of sucrose–coconut fatty acids esters: Reaction kinetics and rheological analysis. Ind. Eng. Chem. Res. 2013, 52, 5024–15033. [Google Scholar] [CrossRef]
- Faig, J.J.; Moretti, A.; Joseph, L.B.; Zhang, Y.; Nova, M.J.; Smith, K.; Uhrich, K.E. Biodegradable kojic acid-based polymers: Controlled delivery of bioactives for melanogenesis inhibition. Biomacromolecules 2017, 18, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Reece, J.B.; Urry, L.A.; Cain, M.L.; Wasserman, S.A.; Minorsky, P.V.; Jackson, R.B. Chapter 7: Membrane structure and function. In Campbell Biology, 9th ed.; Pearson: Boston, MA, USA, 2011; pp. 125–138. [Google Scholar]
- Rho, H.S.; Baek, H.S.; Ann, S.M.; Kim, D.H.; Chang, I.S. Synthesis of new melanogenic compounds containing two molecules of kojic acid. Bull. Korean Chem. Soc. 2008, 29, 1569–1571. [Google Scholar]
- Rho, H.S.; Baek, H.S.; You, J.W.; Kim, S.; Lee, J.Y.; Kim, D.H.; Chang, I.S. New 5-Hydroxy-2-(hydroxymethyl)-4H-pyran-4-one derivative has both tyrosinase inhibitory and antioxidant properties. Bull. Korean Chem. Soc. 2017, 28, 471–473. [Google Scholar] [CrossRef]
- Ismail, M.; Mariod, A.; Bagalkotkar, G.; Ling, H.S. Fatty acid composition and antioxidant activity of oils from two cultivars of Cantaloupe extracted by supercritical fluid extraction. Grasas Aceites 2009, 61, 37–44. [Google Scholar] [CrossRef]
- Ndhlala, R.; Moyo, M.; Staden, J.V. Natural antioxidants: Fascinating or mythical? Biomolecules 2010, 15, 6905–6930. [Google Scholar]
- Vavricka, J.; Christensen, B.M.; Li, J. Melanization in living organisms: A perspective of species evolution. Protein Cell 2010, 1, 830–841. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).