1. Introduction
The global hair care products market is estimated to reach approximately USD 97.00 billion by 2025 with a CAGR (Compound Average Growth Rate) of about 2.7% from 2025 to 2030 [
1].
Consumers are increasingly looking for products that improve the health and appearance of their hair while meeting ethical and eco-friendly standards.
Modern hair care requires high-performance formulations, which are aimed at satisfying the specific needs of each hair type and scalp health. Multifunctionality, skinification, and sustainability are the current trends in hair care.
The demand for effective and environmentally friendly products that provide hydration, nourishment, protection, damage repair, and restructuring for both hair and scalp is constantly growing. Together with the rise in hair skinification, referring to the inclusion of the same active ingredients used in skin care products in hair products, this demand fuels research on natural, biodegradable, and sustainable ingredients.
Therefore, the aim of this study was the evaluation of the possible beneficial effects of the rice derivatives used in skin care products on hair.
Despite being endowed with good mechanical properties, every day, hair experiences stress that can make it dull and brittle. The frequent use of straighteners, chemical treatments (dyeing and bleaching), and exposure to environmental conditions can cause significant damage. Some treatments that can cause reversible or irreversible damage to hair are as follows: permanent waving, bleaching, heat treatment, coloring, UV exposure, drying, brushing, etc.
In this work, the protective and restructuring efficacy of virgin hair and hair damaged by bleaching, UV irradiation, and heat treatment were studied.
Bleaching is a process that refers to the lightening of the natural color of the hair and sometimes it is also utilized to ready the hair for the subsequent coloring step. Using specified products [
2], this procedure is based on the oxidation of the melanin contained in the granules present in the cortical area of the hair. Depolymerization may occur, which leads to the presence of carboxylated derivatives that are soluble in an alkaline environment, which are then removed via washing. Melanin granules are associated with keratin via polypeptide residues [
2].
The bleaching technique is possible thanks to the use of alkaline oxidative treatments. As the first step, the reaction in this technique involves the solubilization of melanin granules through the oxidative breakage of the cross-linked disulfide bonds of the protein [
3]. The products employed make use of an oxidizing agent since melanin is highly resistant to treatments with reducing agents. Thus, the use of hydrogen peroxide in an alkaline environment (for example ammonia) ensures that the bonds break much more easily [
2,
3].
These oxidative treatments can act on the keratin itself, thus causing a series of changes in the properties of the hair [
2,
3]. After bleaching, the hair is dry, weakened, and fragile to the touch and it becomes knotty more easily. It is highly porous and therefore more prone to absorbing water with a consequent increase in drying times. It can absorb and retain a considerable quantity of cationic species due to the notable increase in anionic sites in the hair fiber. Bleached hair lends itself more easily than virgin hair to treatment with permanent lotions, but it requires the use of specific formulations that have lower concentrations of active ingredients. Bleached hair can also absorb various types of substances more easily and quickly [
2,
3].
Thermal treatment is part of the hair straightening process and permanent straightening requires chemical processes that determine an alteration in the chemical composition of the cortex [
4].
The effect of temperature can lead to temporary straightening involving electrostatic interactions and the breakdown of hydrogen bonds through the combination of mechanical stress (combing or brushing) and the use of a heat source (blow-dryer and/or other devices). This process results in a new temporary configuration of the hair that will return to its original state after some time [
4,
5].
Even though heat treatment is a temporary process, the damage to the hair is long-lasting; in fact, exposure to high temperatures leads to the irreversible denaturation of polypeptide chains and a consequent decrease in the structural integrity of the hair fiber. Damage occurs at the cuticle level, and it then extends to the innermost layers, involving the cortex, with a progressive structural loss of the cuticle which becomes irregular until the hair shaft breaks [
6,
7].
The damage that sunlight causes on hair is well documented [
8]. It leads to a change in the protein structures of the cuticle and cortex, cell membrane lipids, and melanin [
8,
9,
10]. Sun radiation and UV radiation are, together with coloring, the most common agents that provoke a superficial alteration in hair, thus causing a loss of color and combability [
11]. The damage induced by UV radiation (UV-B irradiation) involves changes in the mechanical properties of the hair fiber determined by photo-oxidation reactions, with the consequent breaking of R-S and S-S disulfide bonds, decomposition of lipids, decrease in melanin content, and degradation of protein-producing carbonyl and amino groups [
12,
13]. The production of reactive oxygen species (ROS) is also a consequence of exposure to UV rays. ROS are mainly generated via UVA radiation through the oxidation of some amino acids, like tryptophan and cysteine [
8].
Among the ingredients used in the formulation of hair products, oils play an important role. They constitute the basis for hair oils, and they are also included in other products that come into brief contact with hair and are rinsed out, such as conditioners and sometimes even shampoo [
14]. Currently, the focus is on vegetable oils that can act as a sustainable alternative to synthetic ingredients and are preferred by consumers. Alongside the classic coconut oil, commonly used oils include olive, argan, avocado, jojoba, and sunflower oils [
14,
15,
16,
17,
18].
Oils can penetrate the hair through the cuticle layers until they reach the cortex and/or are absorbed onto the surface of the fiber, thus increasing the hydrophobicity of both the surface and cortex [
15,
16]. Oils can provide a variety of benefits to hair, such as increased shine and softness; reduced friction, dryness and color loss; and nourishment and strengthening [
14,
15,
16,
17,
18]. Furthermore, oils have a protective effect on both undamaged hair and hair damaged by chemical agents (surfactants, oxidants, dyes, etc.), UV radiation, and thermal stress, thus aiding their repair [
14,
17,
18].
Our attention was focused on rice, as rice products have long been used for dermatological and cosmetic purposes, and on the use of rice water, which was recovered from rinsing rice used for hair care [
19]. In this work, two rice derivatives were selected: rice germ oil GX-N (INCI name: Rice Germ Oil) and ferulic acid (4-hydroxy-3-methoxycinnamic acid).
Rice germ oil GX-N appears as a reddish-brown paste at room temperature. It is characterized by a high content of γ-oryzanol (about 30%) [
20] in comparison with normal rice germ oil. It has antioxidant activity, and it has shown good efficacy in improving the appearance of particularly dry skin (unpublished data). Furthermore, it protects hair against UV rays, as demonstrated in a previous study [
21].
Ferulic acid is a phenolic derivative that appears in an odorless crystalline form and is white or pale yellow in color. It is poorly soluble in oil and in the aqueous phase, while it is soluble in ethanol. The main natural sources in which ferulic acid is present are rice bran, whole grain, barley, broccoli, banana, and other natural products [
22]. It has antioxidant activity due to its phenolic structure which can form a resonance-stabilized phenoxy radical responsible for its scavenging effect [
22,
23]. It also has anti-inflammatory activity [
24], the capability to absorb UV rays [
25], and a whitening and anti-wrinkle effect [
26]. This active ingredient is mainly utilized in skin care products [
27], and its protective capacity in terms of the scalp and the follicle is known [
28], but to our knowledge, there are no studies regarding its action on hair.
The two active ingredients studied were included in an O/W emulsion that can be used as a scalp-pack and hair mask. The goal of this study was to assess the protective and restructuring effectiveness of the hair mask using various methods [
21].
Thus, products containing rice germ oil GX-N, both alone and associated with ferulic acid, in concentrations of 1% and 0.1%, respectively, were evaluated in terms of their capacity to protect against heat and irradiation and the restructuring effect in bleached hair in comparison to a reference (control).
The methods applied for this evaluation are as follows: Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), a stress–strain test, polarized light microscopy analysis, and protein loss determination. These techniques are readily available and some of the most utilized for highlighting any of the superficial and structural characteristics of hair [
29,
30,
31,
32,
33,
34]. Determining the protein loss is another test used in the assessment of hair damage caused by various types of treatments and the protective capacity of the products applied [
18,
35,
36].
2. Materials and Methods
2.1. Materials
Natural black hair locks (length of 33 cm) were provided by Azienda Tricologica Italiana Srl (Rome, Italy), and rice germ oil GX-N, ferulic acid, and hair masks were provided by Tsuno Rice Fine Chemicals Co., Ltd. (Wakayama, Japan).
2.2. Preparation of the Samples
Caucasian virgin hair was chosen, as it is free of chemical and physical of damage. Before the treatment, the hair tresses were washed to remove any residues, as previously described [
21].
2.2.1. Hair Treatment
Four locks of hair weighing 20.00 g each were arranged: one strand represents the reference (untreated hair), while the other locks were treated with three masks, applying approximately 2.00 g of the product. The masks were carefully applied along the entire length of the hair, from the top portion to the tip. Subsequently, the masks were left on the hair for 3 min and a half without any further handling. The hair treated with the three hair masks was then rinsed with warm tap water for approximately 1 min and, after the hair was air-dried and kept, enveloped in aluminum foil, until the next analyses.
2.2.2. Thermal Treatment
The previously prepared hair locks and the untreated hair were subsequently divided into three groups: two were exposed to short heating cycles (5 and 10 slow steps of 12 s each) using a hot flat iron at 200 °C (50–60 Hz, 45 W, Imetec, Azzano San Paolo, Bergamo, Italy), while the third represents the reference not subjected to thermal stress.
2.2.3. Bleaching Process
The bleaching process was performed at room temperature using the following procedure: four locks of hair weighing 10.00 g each were prepared; the bleaching mixture was obtained by combining a bleaching cream and a 30% oxidant (Monacelli Italy Srl, Gubbio, Italy) in a 1:2 ratio.
The application of the treatment was then carried out on the strands of hair using a brush to ensure that the product was applied evenly throughout; the hair locks were enveloped in aluminum foil and left for about 60 min at room temperature. After that, the locks were washed under running warm water (about 30 °C) with approximately 1 g of shampoo. Three locks were treated with the three hair masks, as described, and the other strand represents the untreated bleached hair.
2.2.4. Hair Irradiation
Irradiation was performed in compliance with the International Standard Organization (ISO) 105B02 [
37]. The instrument employed was a Xenon Test Chamber Q-LAB, Q-SUN Mod. Xe-2 (Q-LAB, Westlake, OH (Ohio)). Every hair fiber was placed on the instrument support, and it was firmed at one end with an adhesive. The total irradiation of the hair fiber was achieved through a window present on the support. We used the following operating conditions: irradiation time, 40 h; power of the xenon lamp, 1800 watts; temperature, 47 °C; and relative humidity, 40%.
Irradiation was carried out on both the untreated hair and the hair treated with the three hair masks.
2.3. FT-IR Analysis
FT-IR spectra were registered using an Agilent Technologies Cary 630 FT-IR spectrometer (Agilent Technologies Italia Spa, Milan, Italy). Data were processed with the MicroLab program. The samples to be analyzed were housed in a suitable support (Diamond ATR Sampling Accessory) and acquired using Agilent MicroLab PC software B.05.3 (Agilent Technologies Italia Spa, Milan, Italy).
The FT-IR spectra were acquired on three portions of the hair: the portion close to the root (upper part), the middle part, and the distal portion (lower part, tip). Analyses were performed on the virgin, irradiated, bleached, and thermal-stressed hair, both untreated and treated with the three hair masks. In addition, an FT-IR analysis was carried out on the three hair masks and active ingredients.
2.4. SEM Analysis
A ESEM Quanta 400 (Hillsboro, OR, USA) was used to obtain SEM images. The sample was prepared by cutting the hair into 1 cm long pieces with a scalpel. It was attached to stubs using a copper conductive tape, and then coated with a layer of gold before the analysis.
The analysis was carried out on the virgin, irradiated, bleached, and thermal-stressed hair, both untreated and treated with the three hair masks.
SEM images were acquired on three parts of the hair (upper, middle, and lower), and on the knotted hair.
2.5. Stress–Strain Test
Tensile properties were evaluated on the virgin and irradiated hair, both untreated and treated with the three hair masks.
A dynamometer (Hounsfield Test Equipment, Dynamometer type CRE (Constant Rate of Extension); machine serial number: H10KS-0074), was used to attain the stress–strain curve and conduct a tenacity evaluation. This instrument can work at different elongation times constantly increasing from 5 mm/min to 20 mm/min, and it is equipped with software for drawing the force/elongation curve. An optical microscope (PZO MP3, Mod. 2563, PZO, Warsaw, Poland) was used to measure the hair fiber diameter. For the test, 40 hair fibers that were at least 10 cm long were used. The linear mass (LM) of the hair fibers was determined as previously described [
21].
2.6. HPF (Hair Protection Factor)
The HPF value was evaluated on the untreated hair and the hair treated with the masks containing active ingredients, on both non-irradiated and irradiated samples.
As previously described [
21], we measured the yield slopes between 15 and 30% of extension from the stress/strain curve acquired for each hair type in the previous test. The yield point at 15% extension represents a reference point for evaluating the changes in the tensile properties of the hair after the disulfide bonds were broken. When the hair is stretched, modifications in the yield slope of the stress–strain curve are related to the dose of UV radiation to which the hair is exposed.
The HPF was calculated using the following formula, as described by Natch [
38]:
where unprotected hair: untreated hair; protected hair: treated with mask.
2.7. Protein Loss
Protein loss was evaluated on the virgin, bleached, and thermal-stressed hair, both untreated and treated with hair masks. The appropriately modified Bradford colorimetric assay was used [
36,
39]. This method represents a valid alternative to the Lowry assay, and, through a defined analytical procedure, it allows for the identification of particularly small amounts of proteins [
36]. This test is based on the formation of a complex between the Coomassie Brillant Blue G-250 dye and the main protein present in hair, keratin [
36]. This complex forms quite quickly, and the interferences that could occur are fewer than those present in the Lowry method [
36]. In this analysis, the dye is present in its anionic form (pH 1.3), and it has an absorption maximum at about 590 nm [
35].
The samples were prepared according to the following procedure: first, 250.00 mg of hair was placed inside a glass tube with 4.0 mL of deionized water. Twice a day (morning and afternoon), each tube was placed in an ultrasonic bath for 15 min. After sonication, the samples were stored in a thermostat at 40 °C. This procedure was repeated for five consecutive days. This process allows for the solubilization of proteins and peptides from the surface of the hair.
To ensure the high sensitivity of the assay, a ratio of 1:1 between the sample and reagent volumes was used [
39]. Thus, a volume of 0.5 mL of the aqueous solution of the sample was added to 0.5 mL of Bradford reagent [constituted of 10 mg of Coomassie Brilliant Blue G-250 C.I. 42655, 10 mL of 85% H
3PO
4 (Merck KGaA, 64271 Darmstadt, Germany) and 5 mL of 96%
v/
v ethanol (Galeno srl, 59015 Carmignano-Prato, Italy) in 100 mL of distilled water].
The protein amount in the aqueous solutions was determined using an Agilent Cary 60 UV-Vis spectrophotometer (Agilent Technologies Italia Spa, Milan, Italy). The Cary Win UV 5.1.3 was used for data processing. Absorbance was determined at 590 nm, 10 min after sample incubation at room temperature. To calculate the amount of protein present, a calibration curve using bovine serum albumin (BSA) as a standard [lyophilized powder, crystallized, ≥98.0% (Merck KGaA, 64271 Darmstadt, Germany)] was employed.
To exclude any interference, blank tests were carried out. In particular, the analysis was performed on the water used for sample preparation and on the aqueous solutions containing the residual amount of the mask that remained on the hair after rinsing (approximately 10% of the applied quantity).
All analyses were performed in triplicate.
2.8. Polarized Light Microscopy Analysis
The analysis was carried out on virgin and damaged hair, both untreated and treated with the three hair masks.
A Optika B-383PLi trinocular biological polarized light microscope (BEL Engineering, Monza, Italy) with a 40x objective was used for the test. For each sample, three hair fibers with a length of approximately 7/8 cm were evaluated. The fragment of the hair placed in a cover glass was immersed in mineral oil. This operation is necessary to reduce the polarization aberrations caused by air–glass interfaces among the sample and the lenses [
40]. The diameter was measured based on the proximal portion. The results were interpreted by comparing the observed colors with the theoretical optimal colors, which were correlated to their respective diameters, as previously described [
21].
2.9. Statistical Analysis
The data were analyzed using an ANOVA and Student’s t-test. Statistical significance was accepted at a level of p < 0.05.
3. Results and Discussion
3.1. FT-IR Analysis
FT-IR is an analytical technique widely used for the evaluation of healthy and damaged hair [
30]. Combined with other investigation techniques, it can provide useful information for evaluating the effectiveness of hair care products.
3.1.1. Virgin Hair
The FT-IR spectra recorded on the three parts (upper, central, and lower parts) of the untreated hair did not differ from each other.
The assignment of the characteristic bands agreed with that reported in the literature and previously described [
21,
30].
Figure S1 shows the FT-IR spectra of the two hair masks utilized for the treatment compared to those of the hair mask without actives (control) and to those of the pure active.
Figure S2 shows the FT-IR spectra of the virgin hair treated with the three masks. As can be observed from these figures, it is not easy to detect the specific peaks relative to product traces due to the high absorption capacity of hair. We can only observe the overlap of the peaks.
3.1.2. Bleaching Hair
The bleaching process involves the oxidation of melanin [
2,
3]. This treatment causes secondary effects and damage to the morphological structure of the hair, both at the surface and internal structure levels [
41]. In fact, when the bleaching agent reacts with keratin, the cysteine contained in the hair is converted into cysteic acid (cystenylsulfonic acid, 1040 cm
−1) through the breakdown of S-S disulfide bonds. Chemical treatment also results in the formation of other cysteine oxidation products, such as cystine monoxide (1072 cm
−1) [
41,
42].
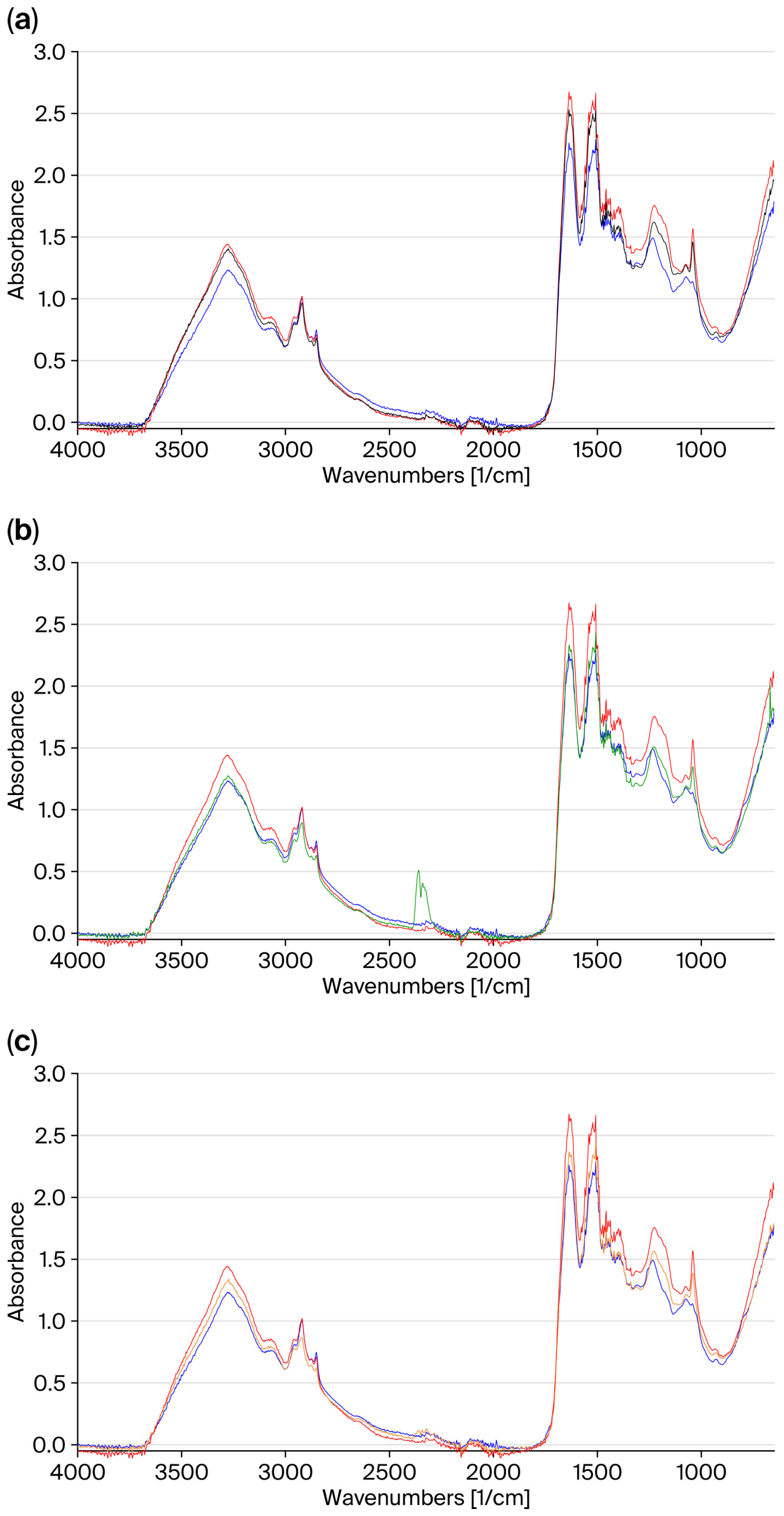
Figure 1 shows the FT-IR spectra recorded on the central part of the untreated hair (virgin and bleached) compared to those of bleached hair treated with the three masks.
In the spectrum of bleached hair, the peak of cysteic acid at 1041 cm
−1, the peak of cystine monoxide at 1075 cm
−1 (characteristic of the S = O absorption band), and a variation in absorption in the 1070–1150 cm
−1 area [
41,
42] are visible.
Furthermore, an increase in the intensity of the absorption bands of Amides I, II and III and the band at 3274 cm−1 is observed. The peak at 1230 cm−1 shows a change in shape, with the appearance of a shoulder at about 1170 cm−1 attributed to the asymmetric stretching of cysteic acid.
The comparison with the spectra of the hair treated with the masks shows a reduced intensity of the cysteic acid peak at 1040 cm
−1. This effect is greater for hair treated with the GX-N mask (
Figure 1b), and it is lesser for the control mask (
Figure 1a).
The spectral profile of the hair treated with the GX-N mask is superimposable to that of unbleached hair. The band at 1230 cm−1 almost recovers its original shape. With the GX-N+FA mask, a smaller overlap is observed. With the control mask, the recovery is low, and the spectrum is almost superimposable to that of the untreated bleached hair.
These results highlight that treatment with masks containing active ingredients leads to a restructuring effect on the hair, which is greater for the GX-N mask.
3.1.3. Heat–Treated Hair
High temperature causes damage to the cuticle and cortex of hair fibers; in fact, heat exposure can denature the microfibrils of the cortex [
7]. The hair layer of the cuticle has extended protein chains. A modification to the conformation of the proteins affects the hydrogen bonds, which fixes the helical structure and, consequently, modifies the access of water into the hair fibers. After thermal stress, the hair fiber presents damage in its structure and therefore in the protein composition [
7].
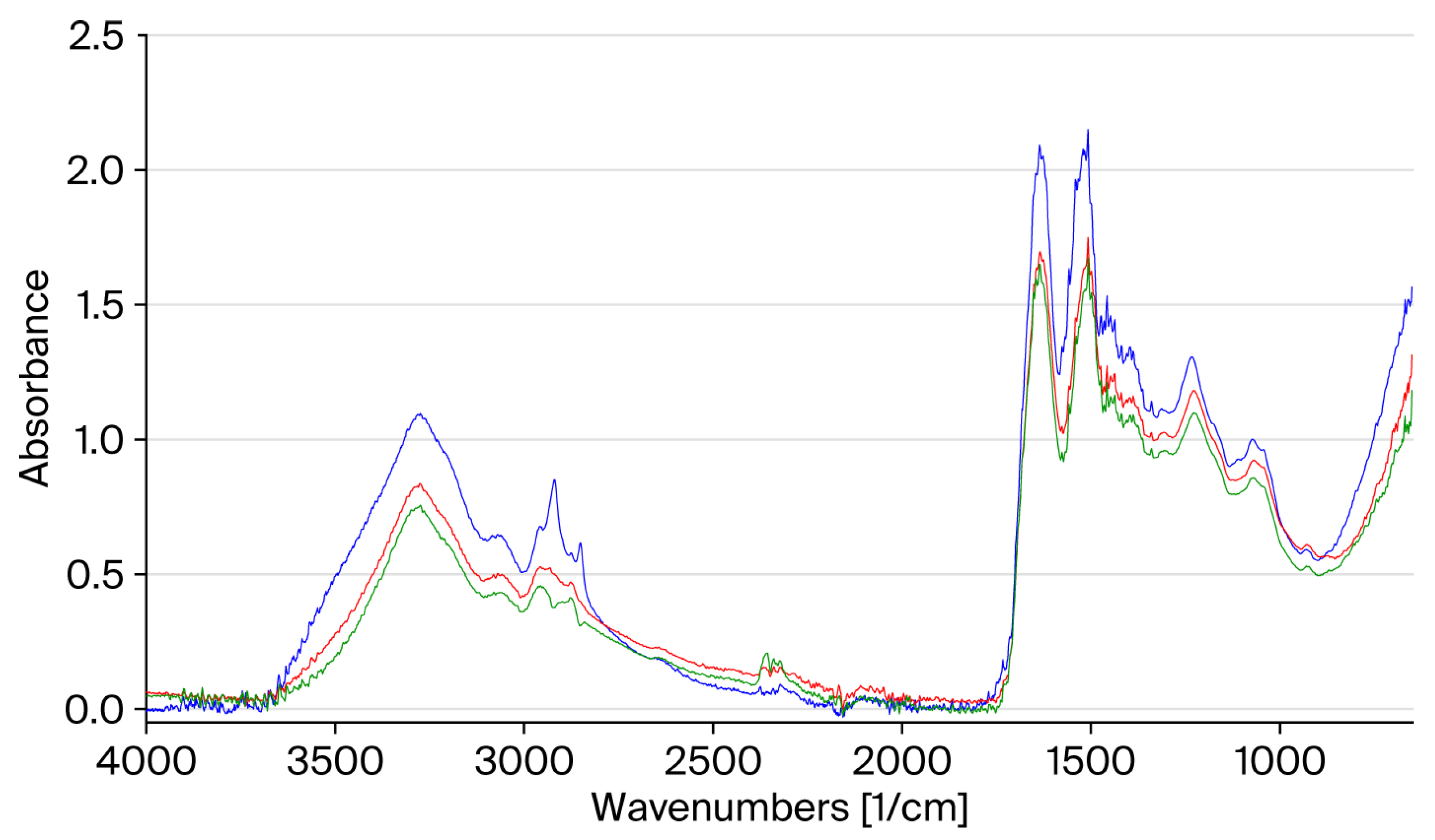
Figure 2 shows a comparison of the FT-IR spectra of the central part of the untreated and heat-treated hair. The thermal treatment leads to a reduction in the intensity of the absorption bands, which is more pronounced for hair subjected to a greater number of cycles.
The main changes are observed for the bands at 2920 cm
−1 and 2852 cm
−1, which are related to the stretching of the CH
3 and CH
2 groups, which may indicate a loss of proteins and lipids as a result of the damage to the cuticle [
7].
This is also confirmed by the reduction in the bands at 1450 cm
−1 and 1391 cm
−1 related to the bending of the CH
2 and CH
3 groups of the side chains of amino acids [
7]. The Amide I band at 1636 cm
−1 has a lower intensity. In the Amide II band, in addition to a reduction in intensity, a shift is observed from 1520 cm
−1 to 1500 cm
−1, which may indicate a change in the conformation of keratin from an α-helical structure to a β-sheet structure [
43]. We can also observe a reduction in the Amide III band at 1235 cm
−1 and its shift at 1220 cm
−1, which always indicates a change in conformation towards the β structure [
43].
Figures S3 and S4 show the FT-IR spectra acquired on the central part of untreated virgin hair and hair treated with the hair mask products compared to the hair subjected to heat treatment (5 and 10 cycles). The application of the reference product does not change the structure of the hair: the spectrum is comparable with that of virgin hair (
Figure S3a). The control mask does not show a protective effect against thermal stress. This is highlighted by the superposition of the FT-IR spectra of untreated and heat-treated hair (
Figure S4a).
The treatment with the GX-N hair mask highlights a protective effect against thermal stress. The FT-IR spectra of the hair treated with the mask and subjected to both 5 and 10 thermal cycles are comparable to those of the untreated virgin hair (
Figures S3b and S4b). We observed a recovery in the absorption intensity of the bands at 2920 cm
−1 and 2852 cm
−1, which underwent intensity reduction after thermal stress and were not restored by the control mask.
The same results were obtained with the treatment with the mask that also contained ferulic acid (hair mask GX-N+FA) (
Figures S3c and S4c).
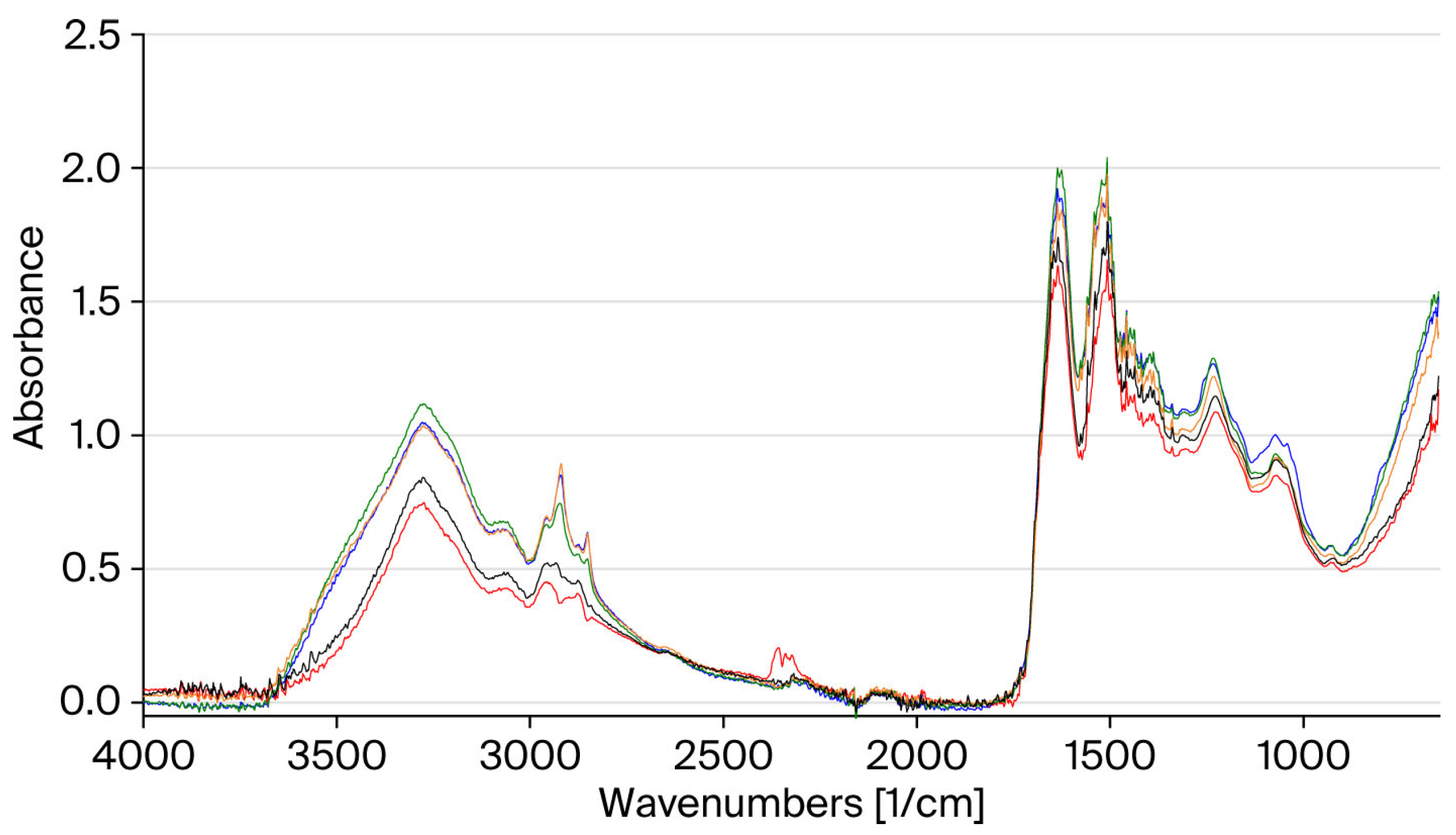
Figure 3 summarizes the results obtained. It shows the FT-IR spectra acquired on untreated virgin hair and on hair treated with the three masks and subjected to 10 thermal cycles. These results confirm the protective effect of the two active ingredients on the hair against thermal stress.
3.1.4. Irradiated Hair
FT-IR spectra were acquired on three portions of the hair, before and after irradiation, on both the untreated hair and the hair treated with the three masks.
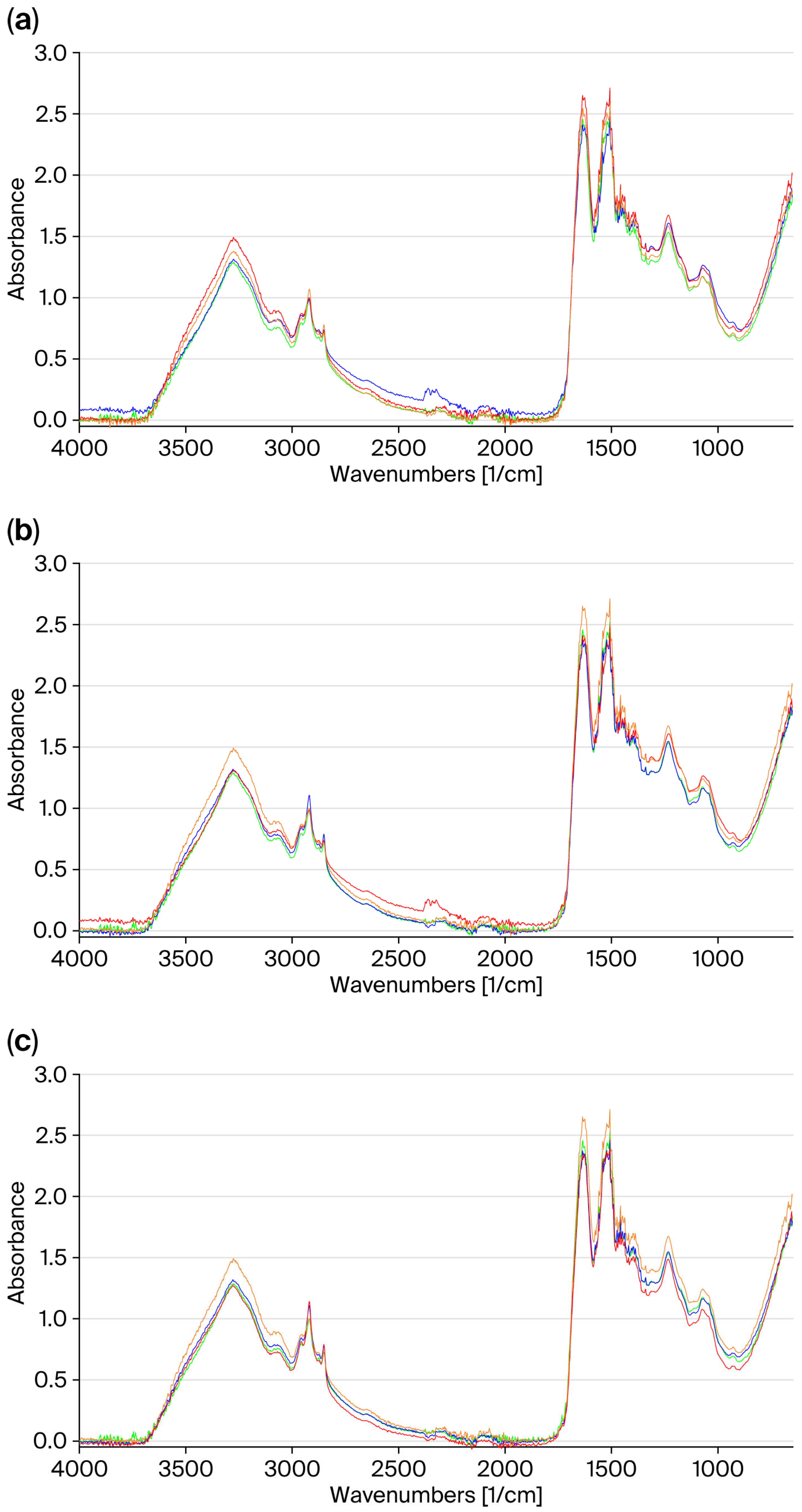
Figure 4 shows a comparison of the FT-IR spectra acquired on the central part of the untreated hair, both non-irradiated and irradiated, and irradiated treated hair.
Exposure to UV radiation causes a photo-oxidation of the hair; therefore, for bleached hair, the changes observed on the FT-IR spectrum imply an increase in the intensity of the band at 3274 cm−1 and the amide bands (I, II, and III). No significant changes in the area between 1000 and 1200 cm−1, which is characteristic of cysteine oxidation products, were observed.
The comparison of the spectra shows that the treatment with the control mask has no protective effect: in the amide area, the spectrum overlaps with that of the untreated irradiated hair (
Figure 4a).
The FT-IR spectra of the hair treated with the hair masks containing the active ingredients (GX-N and GX-N+FA), taken before and after irradiation, show a similar profile, and they are comparable to those of untreated non-irradiated hair (
Figure 4b,c). The bands in the amide region have similar absorption capacities. This finding highlights the protective efficacy of the two active ingredients on the hair against UV radiation.
3.2. SEM Analysis
The alteration in the keratin structure is produced by physical and chemical stress such as that from detergents, dyes, lightening, brushing, UV light, etc. Scanning electron microscope analysis is a technique that allows for the identification of the morphological features of hair, and thus the evaluation of the damage and the state of hair health [
31,
32,
44]. This technique also enables the identification of the particles deposited on the surface and their affinity with the ingredients included in hair care products [
29,
45].
This analysis was performed on three portions of the hair (upper, central, and lower). The images acquired on the central part of virgin and damaged hair (irradiated, bleached, heated), both untreated and treated with various hair masks, are shown in
Figure 5,
Figure 6,
Figure 7 and
Figure 8.
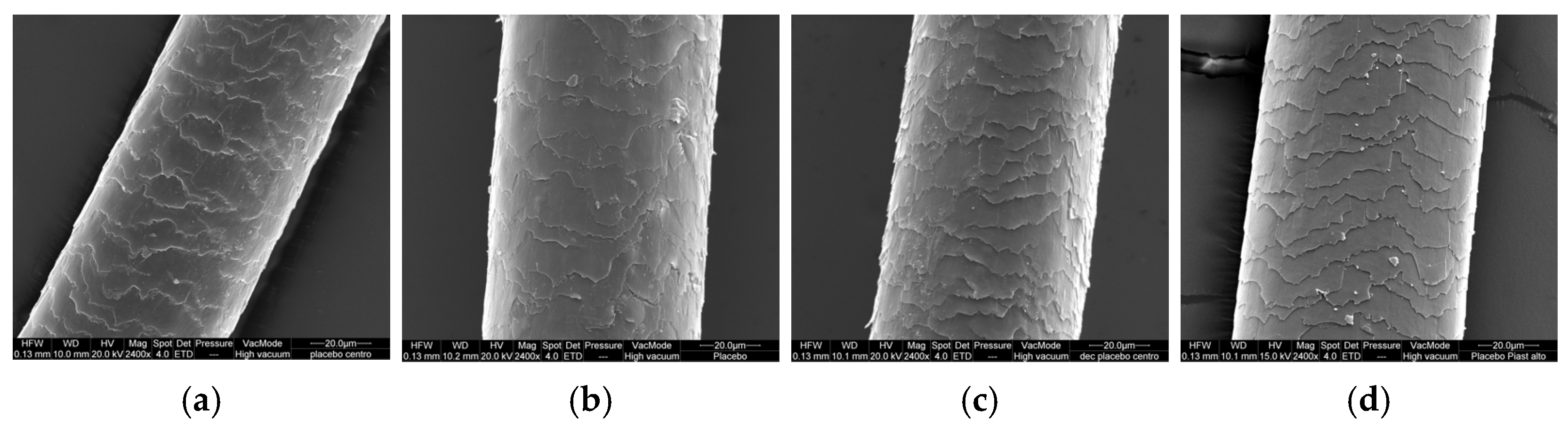
In
Figure 5, we can see that the various treatments lead to damage to the surface of the hair; in fact, the untreated virgin hair showed a smooth surface with normally intact cuticles, well-defined scales, and well-adherent plates (
Figure 5a). The irradiated hair had irregular and lightly raised cuticle edges with a less regular overlay (
Figure 5b). The SEM image of the bleached hair showed rougher and bulkier scales, with some lifting of the cuticles on the surface of the hair, and consequent superficial roughness and loss of shine (
Figure 5c). The heat treatment modified the hair cuticle: the SEM images showed rougher scales and raised cuticles, with some lifting of the cuticles on the surface of the hair (
Figure 5d). The same behavior was visible in both the upper and lower parts of the hair.
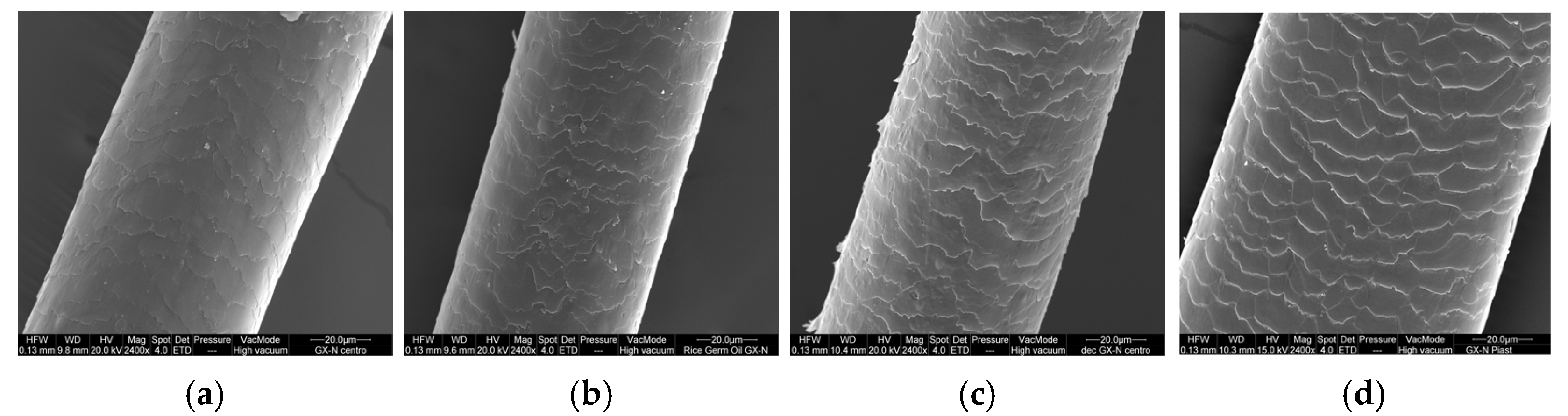
Figure 6,
Figure 7 and
Figure 8 show the SEM images of the hair (central part) treated with different hair masks (control and that with the active ingredients). The application of the hair masks leads to a modification in the surface characteristics of the hair.
The virgin hair, treated with the control hair mask, is less compact in all three parts, and the edge of the plates is more pronounced with respect to untreated hair (
Figure 6a). No improvement is observed on the hair irradiated and subjected to thermal stress (
Figure 6b,d), while on the bleached hair, the edges of the cuticles are less raised even if the surface still appears rough (
Figure 6c).
The treatment with the mask containing GX-N improves the surface characteristics of the hair compared to the hair untreated and treated with the control hair mask. The cuticle appears smooth and uniform (
Figure 7a). The same results are observed for irradiated hair: the treatment with the GX-N mask shows an improvement compared to untreated irradiated hair and irradiated hair treated with the control (
Figure 7b). The application of the mask on bleached and heat-treated hair restores its characteristics: the hair appears similar to untreated virgin hair (
Figure 7c,d).
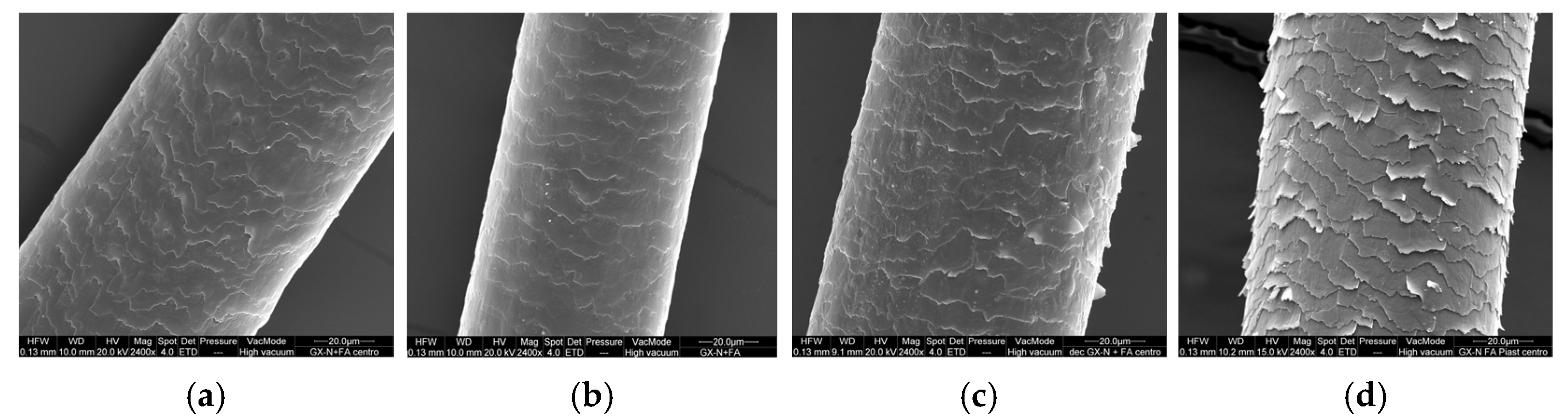
The treatment with the mask containing ferulic acid shows similar results on virgin and irradiated hair to those of the mask containing GX-N alone (
Figure 8a,b), while on bleached and thermal-treated hair the surface of the hair appears less hydrated and rougher due to the edges of the cuticle being raised more (
Figure 8c,d).
This result could be a consequence of the interaction of ferulic acid with hair proteins. Organic acids can bind to protein chains, replacing water molecules at the same binding sites. This causes a reduction in the water content in hair and its plasticizing effect with a possible strengthening of the hair structure [
46]. Ferulic acid can create hydrogen bonds through the carboxyl group and the phenolic hydroxyl. At the pH value of the mask (5.0), ferulic acid is partially dissociated (pKa~4.6), and the anionic form of the carboxyl group forms stronger hydrogen bonds than water molecules.
The data in the literature indicate that the penetration capacity of organic acids is slow. Therefore, in our case, considering the short contact time of the mask with the hair, the interaction mainly concerns the surface of the hair, which undergoes a decrease in hydration. This effect is more evident in thermal-stressed hair, in which the loss of water is greater due to the evaporation caused by temperature. In bleached hair, water absorption is greater as a consequence of the increased hydrophilicity determined by the degradation of proteins following the oxidation process.
Similar results can also be observed from the images acquired on the knotted hair (
Figures S5–S8). The treatment with masks containing active ingredients improves the characteristics of virgin and irradiated hair in comparison with untreated and control-treated hair (
Figures S5 and S6).
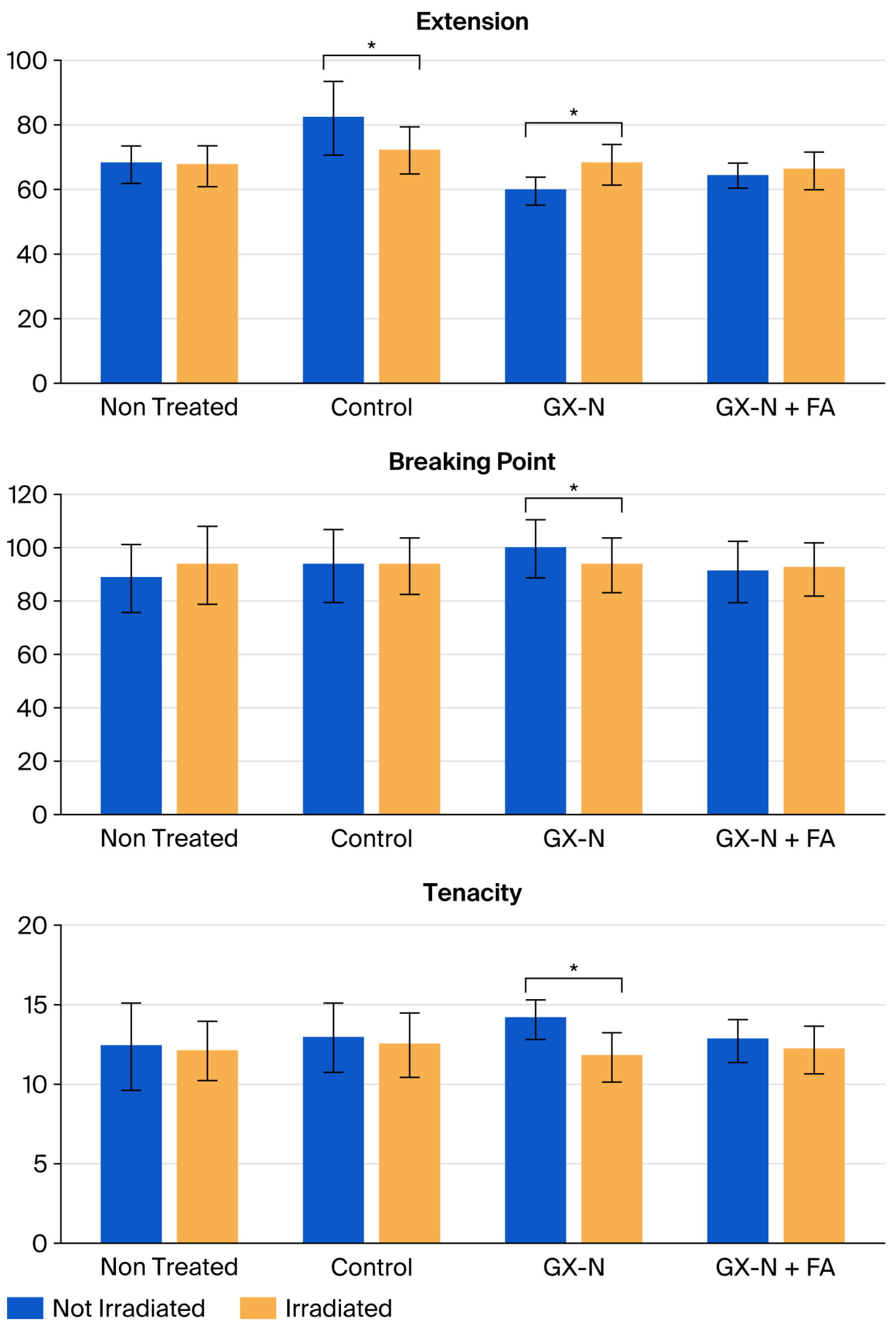
3.3. Stress–Strain Test
The stress–strain test involves stretching a fiber of known length at an established rate in diverse environments. When the hair fiber is straightened, a stress–strain curve is obtained. The curve shows three specific regions in which the hair’s response to the applied stress differs [
21]. The results obtained are shown in
Table 1 and
Figure 9. In all cases, the standard deviations measured were very likely due to variations in the diameter of the hair used.
The untreated hair, after irradiation, did not display any significant modification in elongation. The use of the control hair mask showed a significant difference (p = 1.65 × 10−9) in elongation (81.28%) with respect to untreated hair (67.77%). The same behavior was found for irradiated hair (72.06% treated vs. 67.44% virgin); in this case, we also found a significant difference (p = 0.0024). Before and after irradiation, the elongation of the hair treated with the control showed a significant difference (p = 3.06 × 10−5).
The use of the GX-N hair mask, on the other hand, results in less elongation than that for untreated hair (59.49% GX-N vs. 67.77% untreated, p = 1.42 × 10−10), while irradiation does not involve changes with respect to untreated hair (the elongation values are approximately the same) but shows significant differences before and after irradiation (p = 4.90 × 10−10). Additionally, in the presence of ferulic acid (GX-N+FA hair mask), the degree of elongation is reduced compared to untreated hair, and the difference is significant (p = 0.0017). However, the differences are not significant for the irradiated hair (untreated and treated) and the non-irradiated and irradiated hair treated with GX-N+FA hair mask.
The application of the GX-N hair mask leads to an increase in the breaking point force with respect to the untreated hair, and this increase is significant (100.00 cN GX-N vs. 88.43 cN untreated,
p = 2.40 × 10
−5). Thus, the active ingredients strengthen the hair and lead to an increase in the resistance to breakage before irradiation. The hair treated with the GX-N hair mask requires a greater breaking force, and its degree of elongation is less than that without the mask. Therefore, the hair is stiffer than untreated hair. This could be due to the penetration of oil into the hair, which, through the oil partition into the CMC, has a strengthening effect [
14,
16]. This effect is reduced with the GX-N+FA mask; the extension of the hair is higher, and its breaking point is lower. Ferulic acid seems to influence GX-N diffusion, and this fact could be attributed to its interaction with the protein component described above.
On the other hand, the hair treated with the GX-N+FA hair mask had the same breaking force value as untreated hair (91.10 cN vs. 88.43 cN, not significant difference). Irradiation did not produce statistically detectable modifications in the samples treated with both ingredients; the sample maintained the same values found in untreated hair. Regarding tenacity, no significant differences were noticed between untreated hair and the hair treated with the GX-N+FA hair mask, but they were found in the treatment with GX-N alone before irradiation (p = 0.0015). As for the breaking point force, the tenacity changed according to the hair diameter. The irradiation caused a statistically significant decrease in the tenacity value in the samples treated with the GX-N hair mask (p = 5.24 × 10−8), while the other treatments maintained the same results.
The results obtained show that only the treatment with the GX-N mask improves the mechanical properties of the hair before irradiation. After irradiation, the resistance of the hair is comparable to that of untreated hair and hair treated with the control. The addition of ferulic acid did not lead to any improvement. Again, the mechanical properties of the hair were comparable to those of untreated and control mask-treated hair, both before and after irradiation.
A comparison with the results obtained from the previous analyses shows that the protection against UV radiation of the two active ingredients concerns the surface of the hair but not its mechanical properties. In fact, the images acquired with the SEM show a smooth surface in both cases.
3.4. Hair Protection Factor
Ultraviolet radiation is one of the factors responsible for damaging the hair fiber [
8,
47]. First, the protection of the hair is enabled by the melanin content of the fiber; this molecule promotes the photochemical protection of proteins, present within the cortex, via its capability to absorb energy due to the presence of carbonyl groups and conjugated double bonds. Furthermore, this molecule immobilizes many ROS generated during exposure to UV radiation [
8]. However, melanin is degraded during this process; therefore, it is very important, as it is for the skin, to protect hair from this radiation. One way to protect hair from UV radiation is to use photoprotective substances, which, when deposited on the hair surface, protect the hair fiber [
8,
48,
49].
The protective capacity of these substances against radiation was evaluated by determining the HPF value. The HPF values calculated using the Natch formula were as follows:
On an arbitrary scale of HPF values from 0 to 15 corresponding to a degree of protection from 0 to 100 (%), the HPF values found correspond to about 2.72% protection in the hair treated with the GX-N hair mask and 8.65% protection in the case of hair treated with the GX-N+FA hair mask in reference to untreated hair, as can be extrapolated from the graphic proposed by Nacht [
38].
From the results obtained, we can conclude that the calculated HPF values indicate a low level of protection. However, in the formulation, there are no photoprotective substances (sunscreen), and the low value obtained is also due to the low concentration of the active ingredients. We can observe that the addition of 0.1% ferulic acid in the formulation leads to an increase in the HPF value of three times the value obtained with GX-N alone. This is likely due to its greater UV absorbing properties compared to the gamma oryzanol contained in the GX-N rice germ oil.
The results obtained show a lower protective capacity of the GX-N ingredient than that deduced from the data obtained in a previous study [
21]. The product containing the GX-N ingredient alone shows an HPF value (0.403) ten times lower than the value determined in a previous work (4.06) [
21]. This result highlights the influence of the vehicle on photoprotective efficacy. Indeed, the products tested in this and previous studies have a different composition, but they have the same concentration of active ingredient (1%). This observation agrees with the results obtained from the stress–strain test.
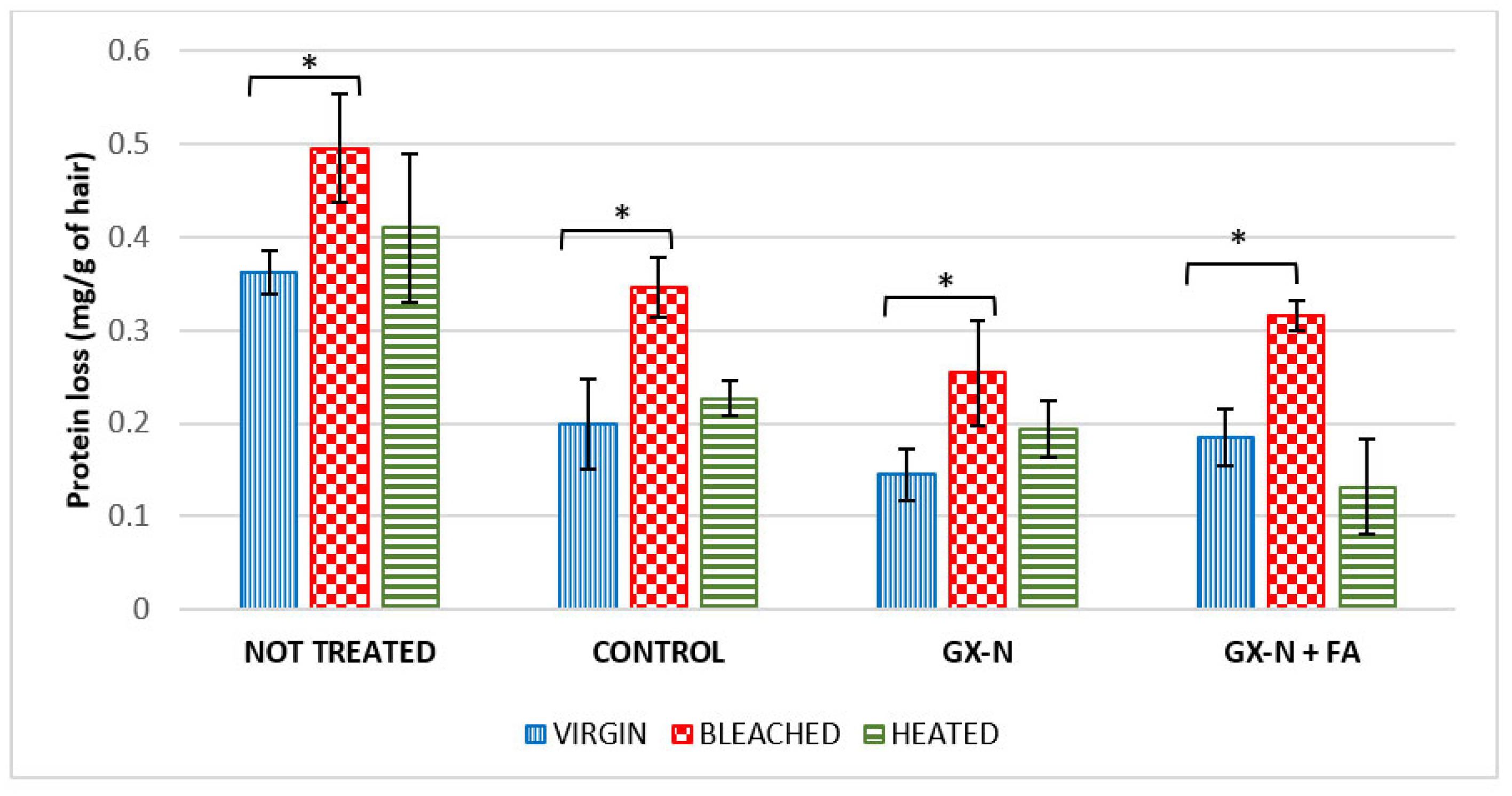
3.5. Protein Loss
The damage to the hair, caused by chemical agents (i.e., bleaching agent) and physical processes (i.e., UV radiation and thermal treatment), leads to an increase in protein loss [
18,
35,
36].
Protein degradation in the hair sample was measured using the appropriately modified Bradford assay [
39]. This degradation was then expressed using a ratio between the quantity of proteins released (expressed in milligrams) and the quantity of hair weighed initially (reported in grams).
The results obtained for the various hair samples analyzed are shown in
Table 2 and
Figure 10.
The results obtained from the blank test confirmed the absence of any interference due to the presence of traces of the product.
The data collected showed that, in untreated hair, physical or chemical damage caused a greater loss of proteins compared to virgin hair (0.362 mg/g virgin vs. 0.495 mg/g bleached vs. 0.410 mg/g heated hair). This may be related to the fact that the various damages (bleaching and heat damage) caused a reduction in the integrity of the hair strand, which led to a structural loss of the cuticle, which became irregular until the hair shaft broke. The bleaching treatment makes the hair more porous and hydrophilic and therefore more susceptible to water absorption and protein solubilization [
50]. This could confirm the results obtained since, in this treatment, the loss of proteins was greater than that obtained with thermal stress. The results also showed a significant difference between virgin hair and bleached hair (
p = 0.034).
By treating the hair with the hair mask without active ingredients (control hair mask), the loss of proteins is lower compared to that obtained for untreated hair, but even in this case, the loss of proteins is higher for the bleached and thermal-treated hair than for virgin hair. The reduction in protein loss compared to untreated hair is 45% for virgin hair, 30% for bleached hair, and 44.6% for heated hair. Furthermore, the data show a significant difference between virgin and bleached hair (p = 0.012).
The addition of rice germ oil in the formulation (GX-N hair mask) causes a further reduction in protein loss compared to hair treated with the reference (27% virgin hair, 27.5% bleached hair, 14.5% heated hair) and untreated hair (60% virgin hair, 48.6% bleached hair, and 52.7% heated hair). Additionally, in this case, the loss of proteins is greater in damaged hair with respect to virgin hair. The results also show a significant difference between virgin and bleached hair (p = 0.028). These results highlight and confirm the protective/restructuring effect of GX-N rice germ oil on both virgin and damaged hair. Its penetration brings both a strengthening and an increase in the hydrophobic characteristics of the hair.
The addition of ferulic acid in the cosmetic formulation (GX-N+FA hair mask) also determines, in this case, a reduction in protein loss with respect to untreated hair of 48.9% in virgin hair, 36.16% in bleached hair, and 67.8% in heated hair. The results show a significant difference between virgin hair and bleached hair (p = 0.035).
The results obtained with the two masks containing active ingredients are not significantly different, but it is possible to notice some differences on damaged hair. The application of the GX-N+FA mask on the hair damaged by chemical treatment leads to an increase of 24.41% in protein loss with respect to the hair mask containing GX-N alone (0.316 mg/g GX-N+FA hair mask vs. 0.254 mg/g GX-N hair mask). Instead, in the sample of hair damaged by physical treatment, we can observe a reduction in protein loss by 32% with respect to GX-N alone. These differences could be a consequence of the interaction of ferulic acid with hair proteins. In the bleached hair, ferulic acid decreases the diffusion of GX-N, thus reducing the strengthening effect and increasing protein loss. In the thermal-stressed hair, it decreases the interaction between proteins and water molecules, thus reducing their solubilization. Therefore, the addition of ferulic acid seems to increase hair protection when hair is subjected to heat treatment. Unfortunately, this beneficial effect coincides with a worsening of hair surface characteristics, as highlighted with the SEM analysis.
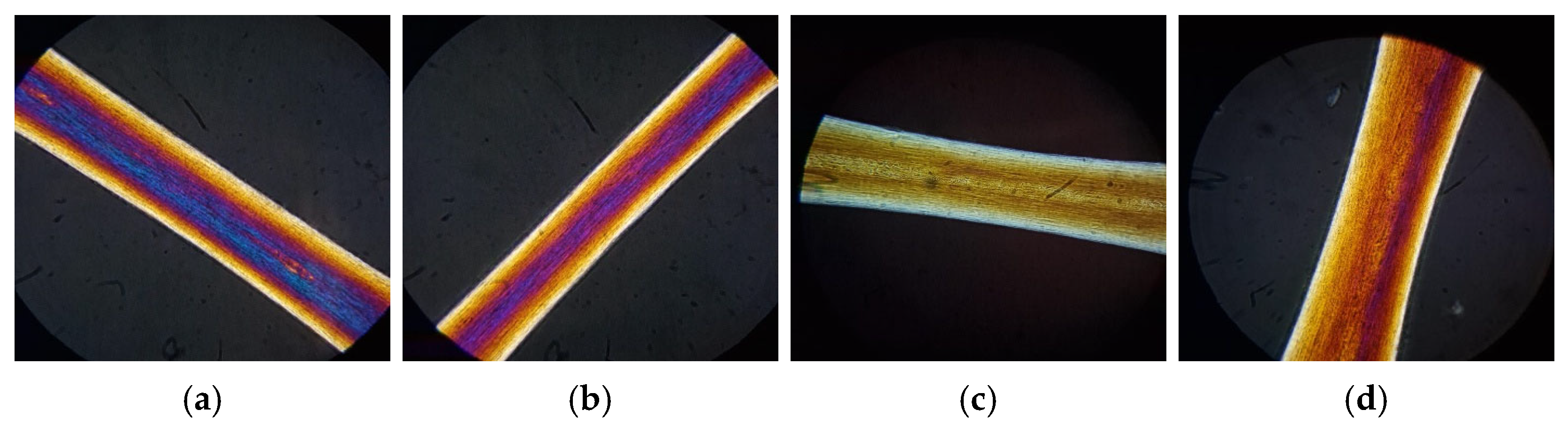
3.6. Polarized Light Microscopy Analysis
Polarized light microscopic analysis is a non-invasive, cheap, and easy optical technique capable of providing information on the hair fiber (structure, life cycle, and disorders) and scalp [
34,
51]. This technique visualizes the hair as bright and colored on a black background, since the keratin can delay the wave of polarized light that passes through it. The colors displayed are due to variations in diameter and the conditions of the keratin structure [
34].
The analysis was performed on three portions of the virgin hair and the hair damaged by UV radiation, bleaching, and heating, both untreated and treated with various masks (control and mask containing the active ingredients). The images acquired on the central part are shown in
Figure 11,
Figure 12,
Figure 13 and
Figure 14.
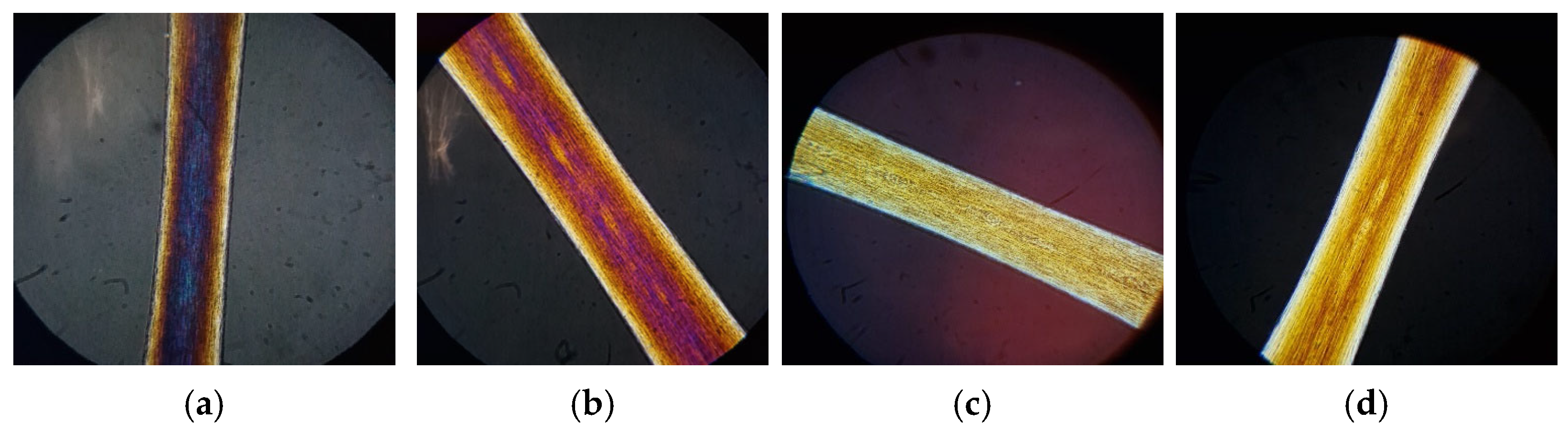
From the images shown in
Figure 11, we can see that untreated virgin hair has good keratin organization. In fact, it is possible to notice the light yellow color of the outer layer of the cuticle (
Figure 11a). After irradiation, the hair loses its optimal keratin structure: from
Figure 11b, it is possible to notice an increase in the yellow band in the external part of the hair fiber (index of deterioration of the cuticle) and a loss of polarization colors (index of damage of the cortical portion).
After chemical or thermal treatment, a non-optimal organization of keratin with the loss of polarization colors (red–violet) is also observed. For bleached hair (
Figure 11c), only the presence of the light yellow color is visible. In the case of heat treatment (
Figure 11d), it is possible to notice an intense yellow-brown color along the entire length of the hair. These colors on the hair fiber indicate and confirm that both treatments lead to damage to the hair structure.
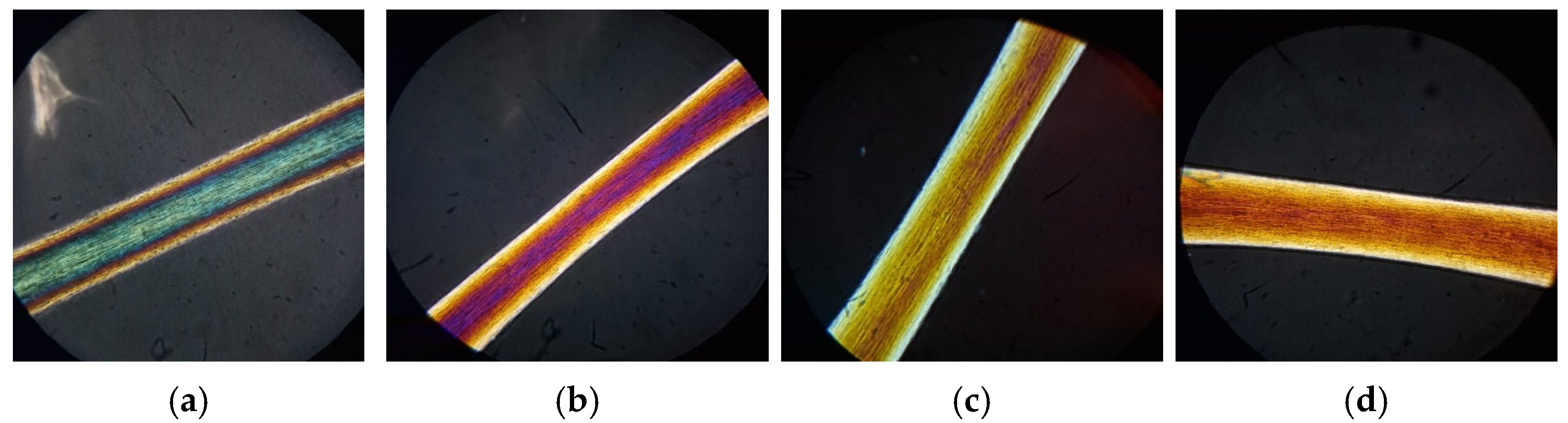
When treating hair with the mask without active ingredients (control), it is possible to notice that in virgin hair, there is a discrete keratin organization with slightly thickened yellow bands (
Figure 12a).
The analysis confirms that the formulation does not protect hair from various types of damages; in fact, from the images we can see that UV irradiation caused a variation in the polarization colors (red-purple coloration—an index of mild keratin disorder) and thicker lateral yellow bands in comparison to virgin hair (
Figure 12b). In the image of the bleached hair, we can observe a pronounced decay of keratin, which leads to a yellow-brown coloration (
Figure 12c). Additionally, in the case of the thermal treatment, we can observe a keratin disorder that is less pronounced than it is in bleached hair (orange-yellow color) (
Figure 12d).
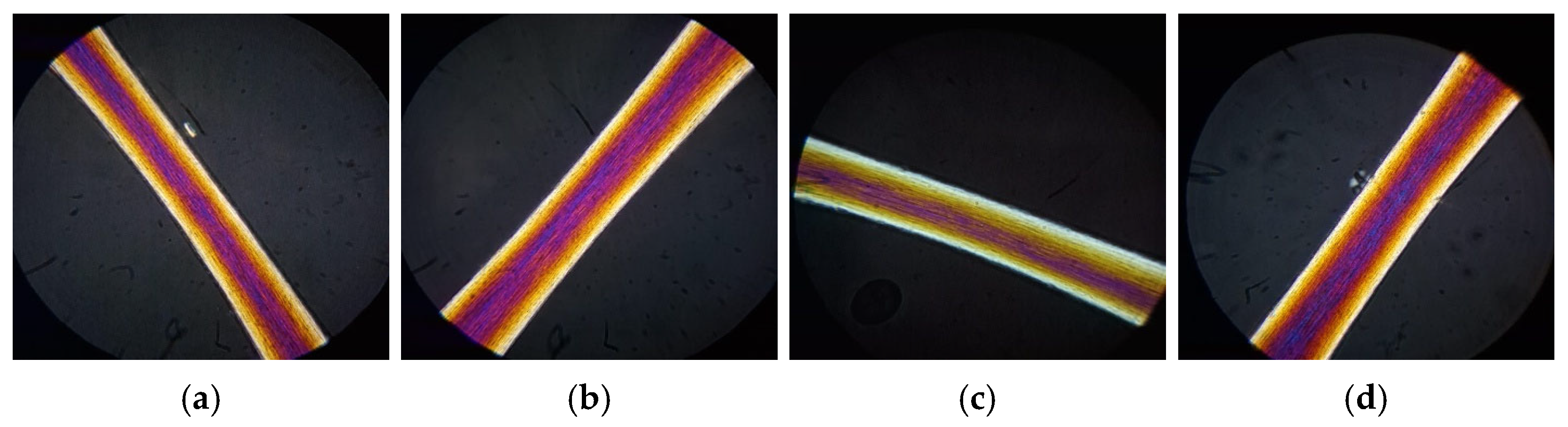
The virgin hair treated with the GX-N mask has a good keratin organization with regular yellow bands (improvement in polarization colors compared to untreated hair and hair treated with the control) (
Figure 13a).
After UV irradiation, no changes were noted in the cuticle or cortical area (
Figure 13b), thus confirming the protective effect of this rice derivative on hair. The bleached hair treated with this active ingredient has a red-purple color (color unchanged). The GX-N mask improves the hair fiber in comparison to untreated hair and hair treated with the control mask (
Figure 13c). After thermal treatment, the hair has a regular keratin organization along its entire length with regular yellow bands (
Figure 13d).
The addition of ferulic acid within the cosmetic formulation (GX-N+FA mask) does not lead to significant changes with respect to hair treated with GX-N alone (
Figure 14a). In irradiated hair, however, ferulic acid amplifies the protection against UV radiation (regular keratin and yellow bands) (
Figure 14b) in comparison to hair treated with GX-N alone. In bleached hair, on the other hand, there was a notable loss of polarization colors compared to hair treated with GX-N alone (comparable to hair treated with the control) (
Figure 14c): this behavior confirms the results obtained by SEM analysis. The thermal-treated hair, on the other hand, was better than the hair treated with the control mask but worse than the hair treated with the GX-N hair mask (
Figure 14d).
The images clearly show that the treatment with the masks containing rice derivatives improves the quality of hair in comparison to the control and untreated hair. The results obtained are in agreement and confirm those obtained in previous analyses.