1. Introduction
Pomegranate (
Punica granatum L.) is increasingly recognized as a highly valuable ingredient in cosmetic formulations, primarily due to its abundant array of bioactive compounds. These compounds confer significant antioxidant, anti-inflammatory, and anti-aging benefits, making pomegranate extracts particularly efficacious for dermatological applications [
1]. The fruit’s diverse components, notably its seeds and peel, are rich sources of diverse phytochemicals possessing considerable therapeutic potential for skin health. Pomegranate seed oil (PSO), typically obtained through cold-pressing, is distinguished by its unique fatty acid composition. Punicic acid, a conjugated linolenic acid, constitutes the major component, ranging from 65% to 83% of the oil’s total fatty acids [
2]. This unique fatty acid profile underscores the oil’s importance in drug delivery systems, where its lipophilic nature and stability enhance the encapsulation and controlled release of bioactive compounds, making it a promising carrier for pharmaceutical and cosmetic application This distinctive fatty acid profile, coupled with its lipophilic nature and inherent stability, positions PSO as an excellent candidate for advanced drug and cosmetic delivery systems, facilitating enhanced encapsulation and controlled release of active compounds [
3]. Furthermore, PSO contains important minor constituents such as linoleic acid (3–7%), oleic acid (4–9%), and various tocopherols (e.g.,
α- and
γ-tocopherol). These components collectively augment the oil’s potent antioxidant capabilities and provide notable photoprotective effects against ultraviolet (UV)-induced skin damage, further solidifying its role in comprehensive skincare formulations [
4]. Concurrently, pomegranate peel, often regarded as an underutilized agricultural by-product, represents a remarkably potent and sustainable source of diverse phenolic compounds significantly enhancing its biological activity. This rich composition includes a high concentration of hydrolysable tannins, notably punicalagins, which can constitute up to 20–25% of the peel’s dry weight. These punicalagins are metabolically converted into ellagic acid, a compound with well-documented health benefits, alongside other valuable phenolics such as gallic acid, catechin, and quercetin [
5]. These synergistic compounds collectively contribute to the peel’s strong antioxidant capacity by neutralizing free radicals, chelating metal ions, and inhibiting lipid peroxidation, thereby offering comprehensive protection to skin cells against oxidative stress and environmental damage [
6]. Beyond its formidable antioxidant effects, pomegranate peel extracts exhibit anti-inflammatory properties by suppressing pro-inflammatory cytokines (e.g., TNF-
α, IL-6) and antimicrobial activity against skin pathogens such as
Staphylococcus aureus, making it a multifunctional ingredient for acne-prone or sensitive skin [
7,
8]. The integration of these two components, seed oil and peel phenolics, offers a highly promising and holistic approach to developing advanced multifunctional cosmetic ingredients. This synergistic combination leverages the hydrating and barrier-repairing attributes of the oil with the comprehensive antioxidant, anti-inflammatory, and antimicrobial benefits derived from the peel, leading to enhanced overall bioactivity and efficacy in skincare formulations. Ultrasonication, an eco-friendly extraction technique, has garnered significant interest in its capacity to enhance the recovery of bioactive compounds. This method operates by inducing acoustic cavitation, which effectively disrupts plant cell walls, thereby facilitating the release of intracellular components [
9]. It proves particularly advantageous for enriching oils with valuable phenolic compounds, as it significantly improves mass transfer kinetics without the need for elevated temperatures that could potentially compromise the stability and integrity of heat-sensitive bioactives [
10]. Nevertheless, achieving optimal extraction efficiency and maximizing the phenolic enrichment and antioxidant activity through ultrasonication necessitates a meticulous optimization of various process parameters. These critical parameters include sonication time, the precise powder-to-oil ratio, and the applied sonication power. Consequently, systematic investigations employing robust experimental design methodologies, such as factorial designs or response surface methodology, are indispensable to thoroughly explore the parameter space and identify the most favorable conditions for superior extraction outcomes [
11].
Despite the bioactivity of pomegranate-derived ingredients, their direct incorporation into cosmetic formulations often presents significant challenges. These hurdles primarily stem from their inherent susceptibility to oxidative degradation and, in some cases, poor solubility, which can compromise their efficacy and shelf-life [
12,
13]. To overcome these limitations and fully exploit the potential of these valuable compounds, encapsulation within biocompatible carriers such as alginate nanobeads has proven to be a highly effective and promising strategy. This approach offers multifaceted advantages, including the provision of controlled release mechanisms, substantial improvements in ingredient stability, and enhanced bioavailability within the skin [
14]. Alginate, a naturally occurring polysaccharide extracted from brown algae, is particularly favored in cosmetic and pharmaceutical applications due to its excellent biocompatibility, biodegradability, and remarkable ability to form stable gel-like structures through ionic cross-linking with divalent cations like calcium ions [
15,
16]. This makes alginate an ideal material for creating protective nanocarriers that safeguard sensitive bioactive compounds while facilitating their targeted and sustained delivery.
In modern cosmetic science, hydrogels are increasingly recognized as effective carriers for sensitive bioactive compounds, thanks to their hydrophilic, porous structure and excellent skin compatibility. Nanoencapsulation represents a key strategy to protect fragile actives, improve their bioavailability, and extend their duration of action [
17]. Alginate nanobeads, made from a natural, biocompatible, and biodegradable polysaccharide, allow gentle encapsulation of thermosensitive compounds such as pomegranate oil, preserving their bioactive polyphenols and essential fatty acids. Thus, integrating pomegranate oil-loaded nanobeads into a simple hydrogel offers multiple benefits: alginate’s gentle nature ensures skin compatibility, the hydrogel protects the oil from degradation, and together they enable controlled, sustained release for improved skin efficacy [
18]. This innovative approach aligns with cosmetic market trends favoring natural, sustainable, and eco-friendly solutions. Using marine-derived alginate and food-industry byproduct pomegranate oil supports circular economy principles. Additionally, the minimalist formulation minimizes irritants, meeting the growing demand for clean beauty products [
19].
This study proposes a novel and sustainable strategy to develop multifunctional cosmetic ingredients by combining eco-friendly extraction, active oil enrichment, nanoencapsulation, and minimalist formulation. Specifically, it aims to: (1) optimize the enrichment of pomegranate seed oil with peel-derived polyphenols via ultrasonication using a Box–Behnken design; (2) characterize the enriched oil for TPC, TFC, fatty acid profile, and bioactivities (antioxidant, anti-inflammatory, and antimicrobial); (3) encapsulate the oil in alginate nanobeads to enhance stability and bioavailability; and (4) incorporate these nanobeads into a minimalist hydrogel designed to reduce irritation risk while ensuring effective delivery. This integrative approach, grounded in circular economy principles and aligned with clean beauty standards, supports the development of next-generation cosmetic products with enhanced efficacy and sustainability.
4. Discussion
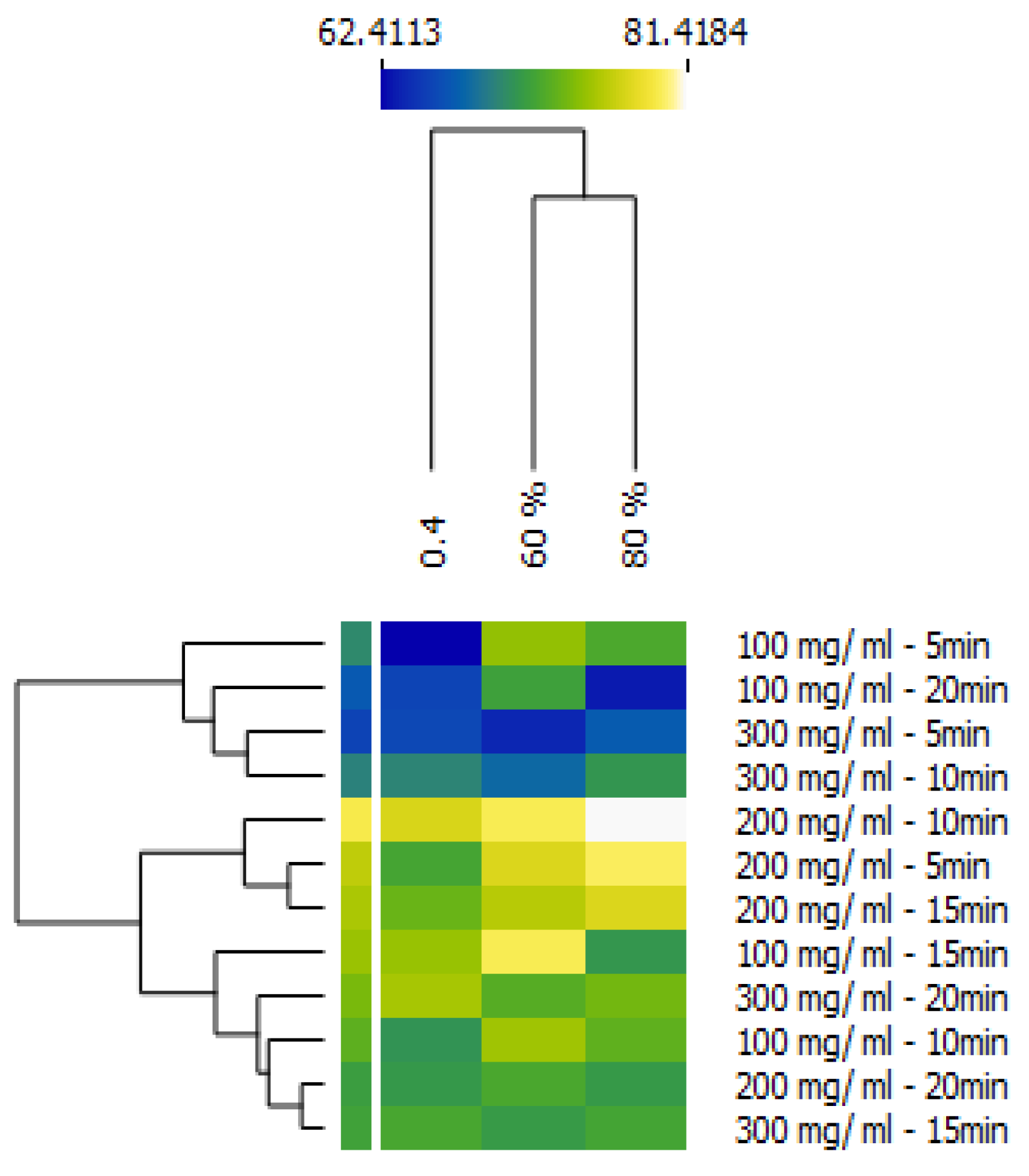
This study successfully developed an innovative cosmetic ingredient by enriching PSO with peel powder via ultrasonication, followed by encapsulation in alginate nanobeads and incorporation into a minimalist hydrogel. The comprehensive approach aimed to maximize the bioactive potential of pomegranate byproducts for advanced cosmeceutical applications. The results demonstrate significant improvements in bioactive content, antioxidant capacity, anti-inflammatory and antibacterial activities, and oxidative stability, positioning this formulation as a promising candidate for advanced cosmeceutical applications. The optimization of ultrasonication parameters through a Box–Behnken design was crucial in maximizing the extraction and enhancement of bioactive compounds. The identified optimal conditions (15 min, 90% power, 202 mg/mL powder-to-oil ratio), yielded a total phenolic content (YTPC) of 69.23 ± 1.66 mg GAE/g, total flavonoid content (YTFC) of 61.09 ± 1.66 mg QE/g, and DPPH antioxidant activity (YDPPH) of 78.63 ± 3.81%. These findings underscore the efficiency of ultrasonication as a green extraction technology for enhancing the phytochemical profile of natural extracts. The high R2 values (0.997–1.000) and non-significant lack-of-fit tests (p > 0.05) in our models confirm their robustness and predictive capability, indicating that the optimized parameters reliably lead to the desired bioactive enrichment.
Our results align with previous research demonstrating the effectiveness of ultrasound-assisted extraction and enrichment. For instance, the successful optimization of ultrasonication parameters for enhancing phenolic and flavonoid yields is consistent with the work of Hiranpradith et al. [
11], who optimized ultrasound-assisted extraction for
Centella asiatica. Their study similarly achieved high phenolic and flavonoid yields through meticulous parameter tuning, reinforcing the general applicability and efficacy of this technique across various plant matrices. Furthermore, the robustness of our experimental models, evidenced by high R
2 values and non-significant lack-of-fit tests, is comparable to findings reported by Roselló-Soto et al. [
29]. Their research also highlighted the effectiveness of ultrasound-assisted extraction in obtaining antioxidants from plant by-products, further validating our methodological approach and the reliability of our findings. These comparisons suggest that the developed process for pomegranate byproducts is competitive and scientifically sound within the broader context of natural product extraction and formulation.
The DPPH results highlight the efficacy of ultrasonication in enhancing antioxidant potential, with a peak Y
DPPH of 81.42% at 80% power, 10 min, and 200 mg/mL, reflecting optimal phenolic extraction without degradation. Lower values, such as 62.41% at 40% power, 5 min, and 100 mg/mL, suggest insufficient cavitation, while declines at extended times (e.g., 63.26% at 80% power, 20 min, 100 mg/mL) or higher ratios (e.g., 63.83% at 60% power, 5 min, 300 mg/mL) indicate potential thermal or oxidative degradation. This observation is consistent with findings by Chemat et al. [
9], who reported similar degradation effects of prolonged or excessive sonication on natural extracts. The non-linear effect of the powder-to-oil ratio is particularly noteworthy, with the 200–202 mg/mL range consistently outperforming both lower and higher extremes. This suggests an optimal phenolic-to-oil interface, where mass transfer is maximized without leading to under-extraction due to insufficient contact or oversaturation that could impede further extraction. This finding aligns with observations by Kumar et al. [
10], who emphasized the importance of an optimal solid-to-solvent ratio in extraction processes. The Box–Behnken design refined these findings, with time (b
2 = 69.05 for Y
TPC, 69.72 for Y
TFC,
p < 0.001) as the dominant factor, reflecting enhanced mass transfer via cell wall disruption [
9]. Negative quadratic terms (b
22 = −61.35, b
33 = −73.01 for Y
TPC) indicate phenolic degradation at extreme conditions, corroborating Yu et al. [
30].
The oxidative stability of the enriched pomegranate seed oil (PSO) was significantly enhanced. The peroxide value, a primary indicator of lipid oxidation, was dramatically reduced to 5.75 ± 0.30 meq O
2/kg in the enriched oil, compared to a substantially higher 50.95 ± 0.07 meq O
2/kg for the neutral oil. Concurrently, the acidity of the oil was also reduced from 2.06 ± 0.02 to 1.30 ± 0.02%, indicating a decrease in free fatty acids and an overall improvement in oil quality. These improvements in oxidative stability are primarily attributed to the incorporation of peel-derived phenolics, such as punicalagins and ellagic acid into the oil during the enrichment process. These compounds are potent antioxidants that scavenge free radicals and chelate metal ions, thereby interrupting the chain reactions of lipid peroxidation. Our findings are consistent with previous research, such as that by El-Hadary and Taha [
31], who reported that pomegranate peel extracts (400–600 ppm) outperformed TBHQ in stabilizing edible oils. This corroborates the efficacy of natural phenolic compounds from pomegranate byproducts as effective stabilizers.
The slight increase in K
232 value (3.44 ± 0.04 vs. 3.15 ± 0.07) in the enriched oil reflects conjugated phenolic structures. While K
232 is typically an indicator of primary oxidation products, in this context, the increase is likely due to the absorption characteristics of the newly introduced conjugated phenolic compounds, rather than an increase in undesirable oxidation products. This interpretation is supported by Drinić et al. [
32], who noted that the presence of certain conjugated compounds can influence K
232 values without necessarily indicating advanced oxidation, who noted that the presence of certain conjugated compounds can influence K
232 values without necessarily indicating advanced oxidation. Furthermore, while the fatty acid profile showed a minor reduction in punicic acid (from 86.39 ± 0.40% to 83.89 ± 0.45%), this was offset by an increase in saturated fatty acids, such as stearic (C18:0) and behenic (C22:0) acids. The higher proportion of saturated fatty acids contributes to enhanced oxidative stability, as they are less susceptible to peroxidation compared to unsaturated fatty acids. This observation is in line with the findings of Dimitrijevic et al. [
2], who demonstrated that a higher content of saturated fatty acids can improve the overall oxidative stability of oils.
The enriched pomegranate seed oil demonstrated markedly superior antioxidant activity (IC
50 = 105.06 µg/mL) compared to the neutral oil (IC
50 = 298.55 µg/mL), highlighting the synergistic contribution of phenolic and flavonoid compounds consistent with the findings of Benchagra et al. [
6]. This enhanced radical-scavenging capacity reflects the successful enrichment process and its ability to preserve thermosensitive bioactives. Furthermore, the enriched oil exhibited notable anti-inflammatory effects, with an IC
50 of 897.25 µg/mL for nitric oxide (NO) inhibition and a favorable selectivity index (SI = 1.07), indicating good biocompatibility and potential suitability for sensitive or inflamed skin. These results align with observations by Mandal et al. [
33], who noted pomegranate’s modulation of NF-κB and COX-2. In terms of antimicrobial performance, the enriched oil showed enhanced activity, particularly against
Klebsiella pneumoniae (12.00 ± 0.78 mm) and
Bacillus sp. (12.33 ± 0.22 mm), significantly outperforming the neutral oil, in agreement with Braga et al. [
7]. The observed biological activities can be attributed to the combined effects of punicic acid, tocopherols, and phenolic compounds, which together contribute to the oil’s protective role against oxidative stress, inflammation, and microbial imbalance. These properties reinforce its relevance in addressing skin conditions linked to inflammaging and photoaging, as previously supported by studies demonstrating reduced ROS generation and inhibition of matrix metalloproteinases (MMPs) [
2].
The development of alginate nanobeads encapsulating pomegranate seed oil and pomegranate peel polyphenols represents a significant advancement in dermatological science. This system synergistically combines the excellent biocompatibility and biodegradability of alginate with the lipid properties of PSO and the potent antioxidant and anti-inflammatory activities of pomegranate peel bioactives. Together, these components target key skin concerns such as oxidative stress, inflammation, barrier dysfunction, and premature aging. By enhancing the stability, bioavailability, and controlled release of encapsulated actives, this integrated delivery platform offers a comprehensive and multifunctional strategy for restoring and protecting skin health. Thus, Encapsulation in alginate nanobeads (mean size: 432.46 ± 12.59 nm, zeta potential: −30.74 ± 3.20 mV) ensured bioactivity preservation, with a DPPH inhibition of 70.22 ± 0.20%, consistent with Garbati et al. [
14]. The hydrogel maintained substantial bioactivity over 60 days under accelerated conditions (40 ± 2 °C, 75 ± 5% RH), with TPC and TFC decreasing by 19.5% and 20.1%, respectively, comparable to Yazidi et al. [
28]. Physicochemical stability was robust, with minimal pH (5.56 ± 0.02 to 5.48 ± 0.05) and viscosity (1520 ± 15 to 1495 ± 22 cP) changes, supporting Mitura et al. [
18]. Microbial stability remained within acceptable limits (<80 CFU/g), as noted by Ćorković et al. [
17], though slight increases by day 60 suggest potential preservative optimization [
19].
Alginate, a naturally derived polysaccharide, forms nanoscale hydrogels (50–500 nm) through ionic crosslinking with divalent cations (e.g., CaCl
2), providing a biocompatible matrix for precise delivery. These nanobeads ensure controlled release of labile compounds, such as PSO’s punicic acid and peel-derived ellagitannins, preserving their structural integrity and functional potency throughout application. their structural integrity and functional potency throughout application [
34,
35]. By penetrating the stratum corneum through both follicular and intercellular routes, the nanobead system enhances the epidermal retention of active compounds by 40–60% compared to non-encapsulated counterparts. Furthermore, in vitro assays on human keratinocytes demonstrate minimal cytotoxicity and negligible pro-inflammatory responses, supporting the system’s suitability for dermatological use, including on sensitive skin types [
36,
37,
38,
39].
Pomegranate seed oil (PSO), notably rich in punicic acid content (65–85%), strengthens the skin’s lipid matrix, reducing transepidermal water loss (TEWL) by 20–30% in dehydrated skin models, thus enhancing moisturization and barrier integrity [
40,
41]. Its anti-inflammatory properties, mediated through suppression of TNF-α, IL-6, and NF-κB signaling pathways, mitigate UV-induced erythema and photodamage, while its stimulation of fibroblast proliferation promotes collagen synthesis, improving dermal elasticity in aged skin [
39,
42,
43]. In synergy with PSO, pomegranate peel extract constituting nearly half of the fruit’s biomass (49–55%) offers a potent source of bioactive polyphenols including punicalagins, ellagic acid, and anthocyanins. These compounds exhibit strong radical-scavenging activities, with DPPH IC
50 values ranging from 8 to 12 μg/mL and effectively inhibit lipid peroxidation. Moreover, they exert skin-brightening effects by reducing tyrosinase activity and melanin synthesis by 40–60%, mimicking the mechanism of kojic acid and offering a natural alternative for the management of hyperpigmentation and uneven skin tone [
44,
45,
46].
The synergistic interplay of alginate nanobeads, PSO, and peel bioactives amplifies their therapeutic potential. Nanoencapsulation serves as a protective and controlled-release strategy that enhances the physicochemical stability of PSO, reducing oxidative degradation by approximately 50%, while also preserving the integrity and bioefficacy of sensitive polyphenolic compounds during storage [
34,
47]. This encapsulation approach ensures prolonged shelf-life and sustained biological activity of the formulation.
In vivo murine studies demonstrate that these composite nanobeads accelerate wound healing, increasing collagen deposition by 35% and epithelialization rates by 25% compared to controls, while topical application reduces UVB-induced DNA damage and matrix metalloproteinase-9 (MMP-9) expression by 70%, underscoring their photoprotective efficacy [
36,
38,
48]. These promising outcomes are corroborated by additional in vivo experiments, which further emphasize the improved therapeutic performance of the composite nanobeads. Topical application of these nanocarriers accelerates the wound healing process, as evidenced by a 35% increase in collagen deposition and a 25% improvement in epithelialization rate compared to untreated controls. Additionally, under UVB exposure, the nanobead system markedly reduces DNA damage and inhibits the expression of matrix metalloproteinase-9 (MMP-9) by up to 70%. These findings underscore the formulation’s robust photoprotective properties and its potential to mitigate photoaging and support skin regeneration.
Despite encouraging outcomes, certain limitations persist. Alginate’s sensitivity to low pH (<4) may limit its use in non-topical applications; however, this is not a concern for dermal formulations [
35]. Scalability requires advanced techniques, such as high-pressure homogenization or ultrasonication, to ensure uniform nanobead synthesis [
47]. Additionally, regulatory frameworks require standardized quantification of peel-derived polyphenols, particularly punicalagins A and B, to guarantee consistency and safety [
35,
36]. Future research should focus on integrating these nanobeads into microneedle systems to enhance skin penetration, particularly for anti-aging and photoprotection. Clinical trials are also needed to validate their potential in treating melasma, psoriasis, and atopic dermatitis.
The developed cosmetic formulation, incorporating pomegranate seed oil enriched with peel-derived polyphenols and encapsulated in alginate nanobeads within a minimalist hydrogel, aligns with the safety and biocompatibility requirements of established regulatory frameworks, such as the EU Cosmetic Regulation (EC No 1223/2009) [
49] and FDA guidelines for cosmetic ingredients [
50]. The use of ECOCERT-certified organic pomegranate materials ensures compliance with standards for natural and sustainable ingredients. However, to fully meet regulatory requirements, future studies will include comprehensive safety assessments, such as skin irritation and sensitization tests, to confirm compliance with these frameworks and ensure consumer safety. Additionally, standardized quantification of key bioactive compounds, particularly punicalagins, will be conducted to guarantee batch-to-batch consistency and regulatory approval for commercial cosmetic applications.