Abstract
Background: Silkworm cocoons are rich in bioactive compounds beneficial for cosmetic applications. This study presented a novel approach by comparing microwave and ultrasonic pretreatments to enhance silk protein extraction efficiency. The aim was to evaluate the effects of pretreatment methods and extraction solvents on the bioactive components, physicochemical properties, and biological activities of silkworm cocoon extracts for cosmetic applications. Methods: Cocoons of Bombyx mori (Nang Lai) were pretreated using conventional soaking (12 h), microwave (3 min), or ultrasonication (30 min), and then subjected to aqueous or enzymatic extraction. The extracts were analyzed for protein, phenolic, and flavonoid content. Structural and thermal properties were characterized using infrared spectroscopy, X-ray diffraction, differential scanning calorimetry, and thermogravimetric analysis. Antioxidant and anti-aging properties were assessed by measuring the inhibition of nitric oxide, 2,2-diphenyl-1-picrylhydrazyl (DPPH•), and collagenase. Skin moisturizing effects and irritation potential were tested. Results: Silkworm cocoons pretreated with microwave (ALM) and ultrasonication (ALS), followed by enzymatic extraction, had the highest yields (21.6 ± 0.5% and 21.7 ± 0.4%, respectively). Despite their slightly lower protein contents, these extracts showed elevated phenolic and flavonoid content. ALM and ALS demonstrated strong antioxidant activities, with DPPH• scavenging of 65.9 ± 0.2% and 65.2 ± 0.3%, collagenase inhibition of 60.3 ± 0.8% and 59.7 ± 1.7%, and nitric oxide inhibition of 13.5 ± 0.4% and 12.9 ± 0.2%, respectively. Skin moisturizing effects increased by 63.6 ± 2.1% for ALM and 61.2 ± 1.5% for ALS, compared to 1.3 ± 0.6% in the control. All extracts were found to be non-irritating for topical application, indicating their safety for skincare formulations. Conclusions: Microwave and ultrasonication pretreatments, in combination with enzymatic extraction, provide an effective, time-efficient, and sustainable method for producing silkworm cocoon extracts with promising cosmetic applications.
1. Introduction
Silkworm cocoons are primarily composed of two principal proteins: fibroin, which serves as the structural protein contributing to the strength and durability of the silk fiber, and sericin, a sticky and adhesive protein that coats the fibroin fibers and facilitates their binding together [1]. In the silk industry, sericin, a globular protein that accounts for approximately 20–30% of silk fibers [2,3], is removed from fibroin and discarded as waste [4,5]. Large volumes of wastewater are generated during degumming, releasing water-soluble sericin protein as a significant and often underutilized byproduct, with global silk processing producing about 50,000 tons of sericin annually [6]. Traditionally, fibroin has been viewed as the more valuable material, leading to the routine removal and disposal of sericin during the degumming process [4,5]. However, recovering sericin from the degumming wastewater can help reduce environmental pollution and reclaim a useful biopolymer with significant potential for various beneficial applications [7].
In recent years, sericin and fibroin have attracted considerable attention due to their favorable physicochemical properties, excellent biocompatibility, and diverse biological activities. Sericin has been reported to have various beneficial properties, such as antioxidation, anti-inflammatory effects, and the ability to accelerate cell proliferation and collagen synthesis stimulation [8,9,10]. Moreover, sericin has moisturizing and anti-aging abilities [10]. Aside from sericin and fibroin, a variety of amino acids, mainly glutamic acid, threonine, glycine, aspartic acid, and serine, were also detected in the silkworm cocoons [11,12]. Amino acids are vital for skin health by supporting moisture retention and pH balance, protecting against UV damage, and maintaining healthy skin [13]. Moreover, amino acids serve as the building blocks of structural proteins like keratin, collagen, and elastin, making them essential for skin health and anti-aging [13]. On the other hand, silkworm cocoons are reported to contain polyphenols and flavonoids, which possess antioxidant properties that help protect the skin from oxidative stress and premature aging [7]. These properties are highly valued in skincare formulations aimed at reducing oxidative stress, delaying the signs of aging, and improving skin hydration. Therefore, silkworm cocoons are of interest to be used as an active ingredient in cosmetic products.
A previous study reported that the beneficial amino acids were successfully extracted from silkworm cocoon using boiling water for 6 h [7]. Aside from using the water for the silkworm cocoon extraction, various chemicals, such as sodium bicarbonate, ammonia, organic solvents, organic acids (tartaric and citric acid), and soap, were also used [14]. However, the extraction methods using these chemicals are not environmentally friendly and may produce high levels of organic ions [10,15]. Enzymatic extraction would be an alternative and non-hazardous method [16,17]. The silkworm cocoon extract from an enzymatic extraction using protease has been used as an ingredient in food and cosmetics [18]. Different commercial protease enzymes, including Alcalase®, Neutrase®, and Flavourzyme®, have been used in the silkworm cocoon enzymatic extraction due to the major components of proteins and amino acids [19]. Among these enzymes, the one exhibiting the highest degree of hydrolysis was Alcalase® [19], a commercially available alkaline serine endopeptidase derived from Bacillus licheniformis. Alcalase® possesses broad substrate specificity and remains active and stable under alkaline conditions (pH 9.5–12.0) and elevated temperatures (55–60 °C) [20]. Due to its strong proteolytic activity, Alcalase® is particularly effective in hydrolyzing silk proteins into low molecular weight peptides, which are desirable for cosmetic applications owing to their enhanced skin permeability and bioactivity [7]. Moreover, the effectiveness of enzymatic extraction for silkworm cocoons has been compared with water extraction, revealing that enzymatic extraction yielded higher levels of protein, amino acids, total phenolic content, and total flavonoid content, as well as exhibited superior biological activities [7].
Although there have been various methods used for extracting the bioactive and beneficial components from silkworm cocoons, the effectiveness of these methods can be significantly influenced by the pretreatment processes applied prior to extraction, which may affect the yield, purity, and functional properties of the extracts. Traditional extraction protocols for silkworm cocoon bioactives typically involve prolonged pretreatment steps, such as overnight soaking [7], which are time-consuming. To address these limitations, the integration of rapid pretreatment techniques, specifically ultrasonication and microwave-assisted processing, was considered a promising approach prior to aqueous or enzymatic extraction. Therefore, the present study aimed to investigate the effects of pretreatment processes prior to extraction on the physical, chemical, and biological properties related to the cosmetic applications of silkworm cocoons.
2. Materials and Methods
2.1. Silkworm Cocoon Materials
Yellow silkworm cocoons of the B. mori L. (var. Nang Lai) strain from Saraburi were kindly provided by the Queen Sirikit Sericulture Center in Chiang Mai, Thailand.
2.2. Chemical Materials
Bicinchoninic acid (BCA) protein assay kit, cupric sulfate, Alcalase® enzyme from B. licheniformis, sodium hydroxide (NaOH), and griess reagent were purchased from Merk KGaA (Darmstadt, Germany). Sodium carbonate (Na2CO3), calcium chloride (CaCl2), and sodium chloride (NaCl) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Folin–Ciocalteu reagent, ammonium persulfate ((NH4)2S2O8), aluminum chloride (AlCl3), potassium acetate (CH3COOK), ferric chloride (FeCl3), ferrous sulfate (FeSO4), monosodium phosphate (NaH2PO4), disodium phosphate (Na2HPO4), sodium acetate (CH3COONa), and sodium lauryl sulfate (SLS) were purchased from Loba Chemie (Mumbai, India). Sericin standard from Bombyx mori, L-ascorbic acid (purity ≥ 99.0%), gallic acid, quercetin, 2,2-diphenyl-1-Picrylhydrazyl (DPPH), 2,4,6-tripyridyl-S-triazine (TPTZ), sodium nitroprusside (SNP), collagenase from Clostridium histolyticum, N-[3-(2-furyl)acryloyl]-Leu-Gly-Pro-Ala (FALGPA), epigallocatechin gallate (EGCG), hyaluronidase from bovine testes, hyaluronic acid sodium salt from Streptococcus equi, bovine serum albumin (BSA), and oleanolic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetic acid, methanol, and hydrochloric acid (HCl) were analytical-grade and purchased from RCI Labscan Co., Ltd. (Bangkok, Thailand).
2.3. Pretreatment Process of Silkworm Cocoon
Yellow silkworm cocoons were cut into small pieces using scissors and subjected to different pretreatment methods, including conventional soaking, microwave-assisted, and ultrasound-assisted pretreatment methods.
2.3.1. Conventional Soaking Pretreatment Method
The yellow silkworm cocoons were pretreated through the conventional soaking method following the method of Bungthong et al. (2021) with some modifications [7]. Small pieces of yellow silkworm cocoons were immersed in DI water at a ratio of 1:25 w/v and soaked at an ambient temperature for 12 h. The 1:25 ratio was selected based on the bulk volume of finely cut cocoons to ensure complete submersion of the samples during the pretreatment process. This ratio is consistent with that used by Kim et al. (2023) in the degumming process of cocoons from various silkworm strains, supporting its suitability for an effective process [21]. Subsequently, the soaking water was decanted, and the remaining water was further removed by filtering through a fine muslin cloth to extract as much residual water as possible, yielding moist silkworm cocoon material for subsequent extraction.
2.3.2. Microwave-Assisted Pretreatment Method
The yellow silkworm cocoons were pretreated through the microwave-assisted method following the method of Pan et al. (2024) with some modifications [22]. Small pieces of yellow silkworm cocoons were immersed in DI water at a ratio of 1:25 w/v and subjected to the microwave oven (UltimateTaste300, Electrolux Co., Ltd., Zhongshan, China) with an operating voltage of 220 V/50 Hz and a power level of 1000 W for 3 min. The microwave treatment duration of 3 min was chosen based on our primary analyses to maximize extraction efficiency while minimizing protein degradation. Subsequently, the soaking water was decanted, and the remaining water was further removed by filtering through a fine muslin cloth to extract as much residual water as possible, yielding moist silkworm cocoon material for subsequent extraction.
2.3.3. Ultrasound-Assisted Pretreatment Method
The yellow silkworm cocoons were pretreated through the microwave-assisted method following the method of Lai et al. (2024) with some modifications [23]. Small pieces of yellow silkworm cocoons were immersed in DI water at a ratio of 1:25 w/v and subjected to an ultrasonication bath (Multifunctional Ultrasonic Cleaner, Bioevopeak, Jinan, China) for 30 min. The ultrasonication treatment duration of 30 min was chosen based on our primary analyses to maximize extraction efficiency while minimizing protein degradation. Subsequently, the soaking water was decanted, and the remaining water was further removed by filtering through a fine muslin cloth to extract as much residual water as possible, yielding moist silkworm cocoon material for subsequent extraction.
2.4. Extraction Process of Silkworm Cocoon
2.4.1. Aqueous Extraction
The moistened silkworm cocoons, pretreated by various methods including conventional soaking, microwave-assisted treatment, and ultrasonication, were extracted by boiling in DI water at 100 °C for 2 h [12]. After extraction, the solution was allowed to cool to room temperature and subsequently strained through a fine muslin cloth to remove the silkworm cocoon residues. The filtrate was collected and aliquoted into 50 mL centrifuge tubes, which were pre-cooled to −20 °C prior to lyophilization. The DI water was removed by freeze-drying using a freeze dryer (LyoQuest Arctic, Telstar, Barcelona, Spain). Before starting the lyophilization process, the condenser was pre-cooled to −40 °C for 30 min. The centrifuge tubes containing the frozen samples were placed into lyophilization flasks, which were then inserted into the freeze dryer. A vacuum was applied until the pressure reached 0.12 mbar, and the samples were freeze-dried for 24 h. After drying, the vacuum was gradually released to stabilize the pressure. The dried samples were removed from the flasks and stored at 4 °C until further analysis. The yields of silkworm cocoon extracts were calculated using the following equation:
where A is the weight of the yellow silkworm cocoon extract from aqueous extraction, and B is the weight of the yellow silkworm cocoon material used for extraction. All experiments were conducted in triplicate.
Yield (%) = (A/B) × 100,
2.4.2. Enzymatic Assisted Extraction
The moistened silkworm cocoons, pretreated by various methods including conventional soaking, microwave-assisted treatment, and ultrasonication, were extracted using 0.5% (v/v) Alcalase® enzyme at pH 8.0 and 50 °C for 2 h [24]. After extraction, the solution was allowed to cool to room temperature and subsequently strained through a fine muslin cloth to remove the silkworm cocoon residues. The filtrate was then subjected to a freeze dryer (LyoQuest Arctic, Telstar, Barcelona, Spain) as described in Section 2.4.1. The yields of silkworm cocoon extracts were calculated using the following equation:
where A is the weight of the yellow silkworm cocoon extract from enzymatic assisted extraction, and B is the weight of the yellow silkworm cocoon material used for extraction. All experiments were conducted in triplicate.
Yield (%) = (A/B) × 100,
2.5. Scanning Electron Microscopy (SEM) Analysis of Yellow Silkworm Cocoon Extracts
The morphological characteristics of yellow silkworm cocoon extracts were examined using SEM. Due to the hygroscopic nature of the enzymatically extracted samples, a dehydration step was necessary to prevent structural collapse under vacuum. Dehydration was performed following a modified protocol based on Koon et al. (2019) [25]. Briefly, the samples were sequentially immersed in 25%, 50%, 75%, 95%, and 99.7% v/v ethanol for 15 min at each concentration. This step was repeated three times to ensure thorough dehydration. Following ethanol treatment, samples were transferred to a hot-air oven (UM500, Memmert, Schwabach, Germany) and dried at 80 °C for 1 h to remove residual solvent and obtain a solid form. The dried samples were then mounted on aluminum stubs using carbon adhesive tape and coated with a thin layer of gold (10 nm thickness) using a sputter coater under vacuum (10−4 mbar) in the presence of argon gas. The coating was achieved with a current of 20 mA for 40 s to enhance conductivity and image resolution. Morphological imaging was conducted using a scanning electron microscope (SU3800, Hitachi, Tokyo, Japan) at magnifications of 500× and 2000× to observe surface texture and microstructural features of the extracts.
2.6. Chemical Composition Determination of Silkworm Cocoon Extract
2.6.1. Total Protein Content
The total protein content of the silkworm cocoon extracts was quantified using a protein analyzer (Dumatherm N Pro, C. Gerhardt GmbH & Co. KG, Königswinter, Germany) [26]. Briefly, 20 g of silkworm cocoon extract wrapped in tin foil was placed in the sample tray. The nitrogen content was determined by combustion at high temperatures in a pure oxygen atmosphere. During combustion, nitrogen was separated from other combustion products and quantified using a thermal conductivity detector [27]. Protein content was calculated based on nitrogen content using a conversion factor of 6.25. All assays were conducted in triplicate with independent replicates.
2.6.2. Analysis of Protein Molecular Weight Distribution by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The protein molecular weight (MW) distribution profiles in each yellow silkworm cocoon extract were analyzed by SDS-PAGE [28]. In brief, 15 µL of a 10 mg/mL aqueous solution of each extract was mixed with 5 µL of Laemmli sample buffer containing 2-mercaptoethanol as a reducing agent. The resulting mixtures were then heated at 95 °C for 5 min and centrifuged at 5000 rpm for 1 min using a microcentrifuge (MINI-10K+, Hangzhou Miu Instruments Co., Ltd., Hangzhou, China). Subsequently, 10 µL of the supernatant was loaded into individual wells of a 12% SDS-PAGE gel. Electrophoresis was carried out at a constant voltage of 120 V and a current of 90 mA for 1 h and 40 min. Precision Plus Protein™ Dual Xtra Standards (Bio-Rad Laboratories, Hercules, CA, USA) were used as molecular weight markers. Following electrophoresis, gels were fixed in a solution containing 10% v/v acetic acid and 40% v/v methanol for 30 min with gentle shaking at 10 rpm and a rocking angle of ±20° using a decolorizing shaker (Magic Mixer TMM-PL, Topscien®, Ningbo, China). Gels were then stained with Coomassie Brilliant Blue G-250 for 1 h under the same shaking conditions. Distaining was performed using 10% v/v acetic acid with continuous shaking until protein bands became clearly visible against a transparent background. Gel images were captured using a gel documentation system (Gel Doc™ EZ Imager, Bio-Rad Laboratories, Hercules, CA, USA). Sericin was used as a reference protein for comparison of molecular weight distribution profiles. All experiments were performed independently in duplicate.
2.6.3. Total Phenolic Content
The total phenolic content of the silkworm cocoon extracts was quantified utilizing a Folin–Ciocalteu assay [29]. Briefly, an aqueous solution of each extract was prepared at a concentration of 1 mg/mL. An aliquot of 20 μL of this solution was combined with 100 μL of tenfold diluted Folin–Ciocalteu reagent and incubated at ambient temperature for 4 min. Subsequently, 80 μL of 75 g/L sodium carbonate solution was applied, and the reaction mixture was incubated for an additional 2 h at room temperature. The absorbance was measured at 750 nm using a microplate spectrophotometer (SPECTROstar Nano, BMG Labtech, Ortenberg, Germany). The results were expressed as milligrams of gallic acid equivalents (GAE) per gram of silkworm cocoon extract, calculated using the calibration curve generated from gallic acid standards at concentrations ranging from 1.56 to 200 μg/mL. All assays were conducted in triplicate with independent replicates.
2.6.4. Total Flavonoids Content
The total flavonoid content of the silkworm cocoon extracts was quantified utilizing a modified aluminum chloride colorimetric method [30]. Briefly, an aqueous solution of each extract was prepared at a concentration of 1 mg/mL. An aliquot of 20 μL of this solution was combined with 80 μL of 10% w/v aluminum chloride (AlCl3) solution and 100 µL of 1.0 M potassium acetate (CH3COOK) solution. Subsequently, the reaction mixture was incubated in the dark for an additional 30 min at room temperature. The absorbance was measured at 415 nm using a microplate spectrophotometer (SPECTROstar Nano, BMG Labtech, Ortenberg, Germany). The results were expressed as milligrams of quercetin equivalents (QE) per gram of silkworm cocoon extract, calculated using the calibration curve generated from gallic acid standards at concentrations ranging from 1.56 to 200 μg/mL. All assays were conducted in triplicate with independent replicates.
2.7. Characterization of Silkworm Cocoon Extracts
2.7.1. Fourier Transform Infrared (FT-IR) Spectroscopy
The functional groups and chemical compositions of the yellow silkworm cocoon extracts were analyzed using FT-IR spectroscopy. Measurements were conducted in transmittance mode with a single reflection diamond attenuated total reflectance (ATR) module (ALPHA II, Bruker, Karlsruhe, Germany), following the procedure described by Thewanjutiwong et al. (2023) [31]. For each measurement, the sample was placed directly onto the diamond crystal of the ATR accessory. A pressure arm was then used to ensure firm contact between the sample and the crystal surface, allowing optimal interaction with the IR beam. Spectral data were collected over the range of 400–4000 cm−1, with transmittance (%) plotted on the Y-axis and wavenumber (cm−1) on the X-axis. This analysis enabled the identification of characteristic functional groups present in the samples by examining the absorption peaks corresponding to molecular vibrations. The FT-IR spectra were used to compare the biochemical compositions across the different silkworm cocoon extracts, the enzyme, and the sericin standard.
2.7.2. X-Ray Diffraction (XRD)
The crystallinity of the yellow silkworm cocoon extracts was analyzed using an X-ray diffractometer (D2 PHASER, Bruker, Karlsruhe, Germany), equipped with a Cu Kα radiation source (λ = 1.54 Å) operating at 30 kV and 10 mA, as described by Thewanjutiwong et al. (2023) [31]. Each sample was carefully packed into a 25 mm specimen ring and mounted onto the XRD sample holder. The holder was then positioned on the sample stage within the XRD chamber. Scanning was conducted over a 2θ range of 5° to 80°, with a step size of 0.2°·s−1. The resulting XRD diffractograms were plotted with intensity (arbitrary units, a.u.) on the Y-axis and 2θ angle (degrees) on the X-axis. The diffraction patterns were analyzed using Bruker DIFFRAC Measurement XRD software (Version 8.6.3.0, Bruker AXS GmbH, Karlsruhe, Germany) to assess the degree of crystallinity and to compare structural differences among silkworm cocoon extracts, the enzyme, and the sericin standard.
2.7.3. Differential Scanning Calorimetry (DSC)
Thermal properties of the yellow silkworm cocoon extracts were analyzed using a DSC (DSC25, TA Instruments, New Castle, DE, USA) under an inert nitrogen atmosphere with a flow rate of 50 mL/min, following standard thermal analysis protocols. Approximately 8–9 mg of each sample was accurately weighed, sealed in aluminum pans, and subjected to a controlled heating program. The samples were heated from 50 °C to 500 °C at a constant rate of 10 °C/min. The resulting thermograms were recorded with heat flow (W/g) on the Y-axis and temperature (°C) on the X-axis. These thermograms were used to assess thermal transitions such as denaturation, degradation, or crystallization behavior, allowing comparison of the thermal stability among silkworm cocoon extracts, the enzyme, and the sericin standard.
2.7.4. Thermogravimetric Analysis (TGA)
The thermal stability and decomposition behavior of the yellow silkworm cocoon extracts were evaluated using TGA (TGA550, TA Instruments, New Castle, DE, USA). The analysis was conducted under an inert nitrogen atmosphere with a flow rate of 60 mL/min, ensuring a stable, oxygen-free environment throughout the heating process. Approximately 10 mg of each sample was weighed and placed in a platinum pan. The samples were then heated from 50 °C to 800 °C at a constant heating rate of 10 °C/min. Thermograms were plotted with percent weight (%) on the Y-axis and temperature (°C) on the X-axis. The resulting weight loss profiles were used to assess thermal degradation stages and to compare the thermal stability of the silkworm cocoon extracts, the enzyme, and the sericin standard.
2.8. Determination of Biological Activities of Silkworm Cocoon Extracts
2.8.1. NO• Inhibition
The NO• inhibition of the yellow silkworm cocoon extracts was assessed using the Griess assay, modified from the method of Sumanont et al. (2004) [32]. Briefly, 80 µL of 10 mM sodium nitroprusside in PBS pH 7.4 was mixed with 20 µL of sample solution (1 mg/mL in DI water). The mixture was incubated at room temperature for 150 min. Following incubation, 100 µL of Griess reagent, prepared by combining 1% (w/w) sulfanilamide and 0.1% (w/w) N-naphthyl ethylenediamine dihydrochloride (NED) in 2% (w/w) phosphoric acid, was added in equal volumes. The resulting mixture was incubated at room temperature for 10 min in the dark. Absorbance was measured at 540 nm using a microplate reader (SPECTROstar Nano, BMG Labtech, Ortenberg, Germany). The NO• inhibition was calculated using the following equation:
where A represents the absorbance of the reaction mixture without the sample (control group), indicating the maximum NO• production, and B represents the absorbance of the reaction mixture in the presence of the sample solution, which reflects the degree of NO• scavenging. Gallic acid was used as a positive control, while sericin was included as a reference standard for comparison at the same concentration as the samples (1 mg/mL). All experiments were performed independently in triplicate.
NO• inhibition (%) = [(A − B)/A] × 100,
2.8.2. DPPH• Radical Scavenging Activity
The free radical scavenging activity of the yellow silkworm cocoon extracts was evaluated using the DPPH assay, with modifications based on the methods of Brem et al. (2004) [33]. Briefly, 20 μL of each sample solution (10 mg/mL in DI water) was mixed with 180 μL of 167 μM DPPH solution prepared in methanol. The mixtures were incubated at room temperature in the dark for 30 min to allow the reaction to occur. Following incubation, the absorbance was measured at 520 nm using a microplate reader (SPECTROstar Nano, BMG Labtech, Ortenberg, Germany) [34]. The DPPH• radical scavenging activity was calculated using the following equation:
where A represents the absorbance of the reaction mixture without the sample (control group), indicating the maximum concentration of DPPH• radicals, and B represents the absorbance of the reaction mixture containing the sample, reflecting the extent of DPPH• radical scavenging activity. Ascorbic acid and gallic acid were used as a positive control, while sericin was included as a reference standard for comparison at the same concentration as the samples (10 mg/mL). All experiments were performed independently in triplicate.
DPPH• radical scavenging activity (%) = [(A − B)/A] × 100,
2.8.3. Collagenase Inhibition
The collagenase inhibitory activity of the yellow silkworm cocoon extracts was evaluated using a modified enzymatic substrate method based on Thring et al. (2009) [35]. The enzyme reaction system was prepared using 50 mM Tricine buffer pH 7.5, containing 400 mM sodium chloride (NaCl) and 10 mM calcium chloride (CaCl2). Briefly, 10 μL of each sample solution (10 mg/mL in Tricine buffer pH 7.5) was mixed with 20 μL of collagenase solution (5 units/mL) and incubated at room temperature for 15 min. Following pre-incubation, 80 μL of Tricine buffer pH 7.5 and 40 μL of 2 mM FALGPA in Tricine buffer pH 7.5 were added to initiate the reaction. The decrease in absorbance was measured at 340 nm in kinetic mode for 20 min using a microplate reader (SPECTROstar Nano, BMG Labtech, Ortenberg, Germany). Collagenase inhibitory activity was calculated using the following equation:
where A represents the reaction rate of the enzyme-substrate mixture without the sample, and B represents the reaction rate of the mixture containing the sample solution. EGCG was used as the positive control. EGCG and gallic acid were used as a positive control, while sericin was included as a reference standard for comparison at the same concentration as the samples (10 mg/mL). All experiments were performed independently in triplicate.
Collagenase inhibition (%) = [(A − B)/A] × 100,
2.8.4. Skin Moisture Enhancement
Skin moisture enhancement of the yellow silkworm cocoon extracts was assessed using an ex vivo model with pig’s ear skin. Extract solutions were prepared by dissolving the silkworm cocoon extracts in DI water at concentrations of 1% w/w. For each test, 20 μL of the sample solution was applied to a 2 × 2 cm area of pig’s ear skin. Untreated pig’s ear skin served as the control. Skin hydration levels were measured using a Corneometer® CM 825 (Courage + Khazaka electronic, Köln, Germany), following the method of Heinrich et al. (2003) [36]. Baseline skin moisture was recorded prior to application, and a second measurement was taken 30 min after application. The moisturizing efficacy was calculated using the following equation:
where A represents the skin moisture value measured after the sample application for 30 min, and B represents the skin moisture value measured before the application. Sericin was used as a reference standard for comparison at the same concentration as the samples (1% w/w). All experiments were performed independently in triplicate.
Skin moisture enhancement (%) = [(A − B)/B] × 100,
2.9. Determination of Irritation Potential of Silkworm Cocoon Extracts
The potential irritation of the yellow silkworm cocoon extracts was evaluated using the hen’s egg test on the chorioallantoic membrane (HET-CAM), adapted from the methods of Somwongin et al. (2018) [37]. Fertilized hen’s eggs, aged 7–10 days, were obtained from the Faculty of Agriculture, Chiang Mai University, Thailand. First, the blunt end of the eggshell was carefully opened. A few drops of 0.9% w/v sodium chloride (NaCl) solution were applied to moisten the internal membrane, which was then gently peeled away using forceps, avoiding contact with the underlying blood vessels. Next, 30 μL of the test sample solution (1% w/w in NaCl) was applied directly onto the chorioallantoic membrane (CAM). The CAM was immediately observed for 5 min under a stereoscope (NS80, Ningbo Yongxin Optics, Ningbo, China) to detect early signs of irritation, including hemorrhage, vascular lysis, and coagulation. The onset time (in seconds) of each irritation response was recorded, and the irritation score (IS) was calculated using the following equation:
where t(h) represents the time in seconds at which hemorrhage was first observed, t(l) represents the time in seconds at which vascular lysis was first observed, and t(c) represents the time in seconds at which coagulation was first observed. IS were classified as follows: 0.0–0.9 as no irritation, 1.0–4.9 as mild irritation, 5.0–8.9 as moderate irritation, and 9.0–21.0 as severe irritation [38]. A solution of 1% w/v sodium lauryl sulfate (SLS) served as the positive control, and a 0.9% w/v NaCl solution as the negative control. The experiment was performed in duplicate independently.
IS = [(301 − t(h))/300 × 5] + [(301 − t(l))/300 × 7] + [(301 − t(c))/300 × 9],
2.10. Statistical Analysis
Data were expressed as mean ± standard deviation (SD). Differences among samples were evaluated using one-way ANOVA followed by Tukey’s post hoc test, performed using IBM SPSS Statistics version 23.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at p < 0.05. To evaluate the relationship between the chemical constituents of yellow silk cocoon extracts and their biological activities, both simple linear regression and Pearson correlation analyses were performed. The strength of the correlations was interpreted following the guideline proposed by Mukaka (2012), where correlation coefficients ranging from 0.90 to 1.00 (or −0.90 to −1.00) were classified as very high, 0.70 to 0.90 (or −0.70 to −0.90) as high, 0.50 to 0.70 (or −0.50 to −0.70) as moderate, 0.30 to 0.50 (or −0.30 to −0.50) as low, and 0.00 to 0.30 (or −0.00 to −0.30) as negligible [39].
3. Results and Discussion
3.1. Silkworm Cocoon Extracts and Their Chemical Compositions
The visual appearance of yellow silkworm cocoons of B. mori (var. Nang Lai) and their extracts obtained via different pretreatment and extraction techniques is shown in Figure 1. The yellow silkworm cocoons in their intact, unprocessed form exhibited a vibrant golden-yellow hue, characteristic of the Nang Lai variety. The weight of each cocoon was 110.8 ± 0.1 mg, which corresponded well with a previous study that reported the weight of the cocoon shell to be 115.3 ± 0.2 mg [40]. The cocoons have an ellipsoidal form and smooth, densely woven surfaces, indicating the presence of sericin, a natural gum protein that holds fibroin fibers together [41,42]. The outer layer appears slightly fuzzy due to fine silkworm fibers that extend from the main body of the cocoon. Their firm and compact structure reflects the dense, protective environment spun by the silkworm during its pupal stage [43]. On the other hand, the visual appearance of the aqueous extracts and Alcalase® enzyme-assisted extracts from yellow silkworm cocoons differed markedly depending on the extracting solvents. The aqueous extracts appeared lightly turbid to cloudy, with pale yellow coloration and visible suspended fibrous particles. In contrast, the Alcalase® enzyme-assisted extracts exhibited a more uniform and denser appearance.
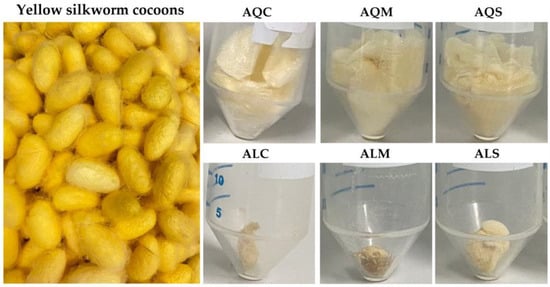
Figure 1.
The external appearance of yellow silkworm cocoons of the B. mori (var. Nang Lai) and its extracts derived from aqueous extraction after pretreatment with conventional soaking for 12 h (AQC), microwave treatment for 3 min (AQM), and ultrasonication for 30 min (AQS), along with its extracts from Alcalase® enzyme-assisted extraction after pretreatment with conventional soaking for 12 h (ALC), microwave treatment for 3 min (ALM), and ultrasonication for 30 min (ALS).
The surface morphology of yellow silkworm cocoon extracts, as visualized by SEM, revealed distinct structural differences depending on the pretreatment technique and extraction method, as shown in Figure 2. The findings demonstrated that enzyme-assisted extraction significantly enhanced the disintegration of silkworm cocoon structures compared to aqueous methods alone. Furthermore, pretreatment techniques such as microwave and ultrasonication amplify this effect by disrupting the silk matrix and improving enzyme-substrate interaction. The observed changes in surface morphology and porosity were likely to contribute to improved extractability, solubility, and potential bioavailability in subsequent cosmetic or pharmaceutical applications.
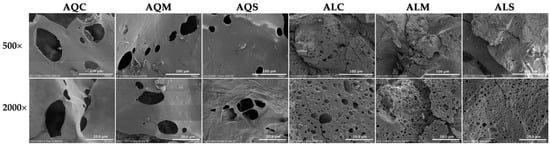
Figure 2.
SEM micrographs of the extracts from yellow silkworm cocoons of the B. mori derived from aqueous extraction after pretreatment with conventional soaking for 12 h (AQC), microwave treatment for 3 min (AQM), and ultrasonication for 30 min (AQS), along with its extracts from Alcalase® enzyme-assisted extraction after pretreatment with conventional soaking for 12 h (ALC), microwave treatment for 3 min (ALM), and ultrasonication for 30 min (ALS). Images were captured using SEM at magnifications of 500× (top row) and 2000× (bottom row) to visualize surface morphology and microstructural differences among the treatment groups.
Microwave-assisted pretreatment resulted in visibly smoother surfaces with signs of partial melting and fewer, irregular pores. The internal heating effect of microwave energy likely induced localized thermal stress, leading to partial collapse of the fibrous matrix and the formation of dense and consolidated regions. This structural compaction limits porosity, suggesting moderate disruption but without complete breakdown of the protein architecture. In contrast, the pretreatment with ultrasound exhibited the extract with moderate surface fragmentation with roughened textures and enlarged uneven pores. Ultrasonication is known to generate acoustic cavitation, leading to microjet formation and shear forces that partially degrade structural integrity [44]. The observed morphology confirms enhanced mechanical disruption compared to conventional or microwave-assisted treatments.
On the other hand, enzyme-assisted extraction using Alcalase® after conventional soaking markedly altered the microstructure, producing irregular and fractured particle surfaces with a significantly reduced presence of intact sheets. This suggested that enzymatic hydrolysis effectively cleaved peptide bonds within the cocoon matrix, leading to a granular morphology and breakdown of structural continuity [45]. Combining microwave pretreatment with Alcalase® resulted in severe morphological disintegration, with the appearance of amorphous and sponge-like structures densely packed with fine pores. This highly porous, fragmented texture indicates a synergistic effect between microwave-induced thermal stress and enzymatic degradation, promoting extensive protein denaturation and hydrolysis [22]. Similarly, the extract from ultrasound pretreatment displayed the most extensive surface disruption. A highly porous, foam-like morphology was observed, with densely packed small pores and a homogenous distribution of fine fragments. The pronounced microstructural breakdown suggested that ultrasonic cavitation improved enzyme accessibility and penetration, leading to efficient matrix degradation and a finer particle distribution [45,46].
The yields of each extract, as shown in Table 1, also noted the significance of extracting solvents. The Alcalase® enzyme-assisted extracts yielded distinctly higher extraction yields compared to the aqueous extracts (p < 0.05). The extraction yield of enzyme-assisted methods was nearly three times higher than that of aqueous extractions. This indicated that enzymatic hydrolysis facilitated the breakdown of the cocoon structure, leading to more efficient extraction. The findings were consistent with a previous study that reported an approximately twofold increase in the yield of enzyme-assisted hydrolysis compared to water extraction [7]. Alcalase®, a protease primarily composed of the serine protease subtilisin, has been extensively applied in silk degumming processes [47]. In addition to breaking down sericin, it is also capable of degrading fibroin [47,48], which contributes to a higher extraction yield due to the release of fibroin-derived components, including peptides and amino acids.

Table 1.
Extraction yields and chemical constituents of yellow silkworm cocoon extracts from aqueous and enzymatic extraction with various pretreatment methods.
However, the pretreatment method was found to have no effect on the extraction yields. The conventional soaking pretreatment, which involved soaking silkworm cocoons in water overnight (12 h), hydrated and softened the fibers, allowing the extracting solvent to penetrate more effectively and enabling more efficient subsequent extraction processes. On the other hand, microwave irradiation leveraged hot spot generation from entrapped water molecules to break disulfide bonds and facilitate extraction efficiency [49], while ultrasonication pretreatment enhanced extraction through cavitation, where the continuous expansion and collapse of microbubbles in the liquid released localized energy, disrupting the material matrix and promoting the release of bioactive compounds [50]. Although similar extraction yields were obtained across all pretreatment methods, the significantly shorter processing times associated with ultrasonication (30 min) and microwave irradiation (3 min) present clear advantages over conventional soaking, which requires prolonged treatment (12 h) to achieve comparable results. Both microwave and ultrasonication pretreatments offer physical enhancements that accelerate molecular disruption, achieving comparable fiber accessibility within a significantly shorter duration, whereas conventional soaking relies on extended hydration and softening over time to reach a similar effect. These findings remarked on the potential of microwave and ultrasonic pretreatments as more time-efficient and energy-saving alternatives, without compromising extraction efficiency.
3.2. Chemical Compositions of Silkworm Cocoon Extracts
The total protein, phenolic, and flavonoid contents of yellow silkworm cocoon extracts are shown in Table 1. Although the protein concentration was higher in the aqueous extracts, the total protein content calculated on a dry weight basis was significantly greater in the enzymatic extracts (14.0 ± 0.3 to 14.3 ± 0.1% w/w) compared to the aqueous extracts (6.9 ± 0.1 to 7.7 ± 0.5% w/w), demonstrating the superior efficiency of enzymatic extraction for protein recovery from silkworm cocoons. Additionally, the higher extraction yields obtained through enzymatic extraction can be attributed to the broad substrate specificity of the enzyme, which effectively hydrolyzed sericin and partially degraded fibroin and other matrix components [47,48]. This enzymatic action facilitated the release of additional peptides, amino acids, and associated bioactive compounds, including phenolics and flavonoids. This broader extraction profile may have diluted the protein concentration in the enzymatic extracts, leading to a lower relative protein content compared to aqueous extracts. Therefore, despite the lower apparent protein concentration, enzyme-assisted extraction yielded a higher absolute amount of protein, emphasizing its effectiveness for protein recovery from silkworm cocoons.
In silkworm cocoons, peptides and amino acids are primarily derived from the two major protein components, including fibroin and sericin [51]. Sericin, the hydrophilic adhesive protein that coats fibroin fibers, contains a broad range of amino acids and can be readily extracted using both aqueous and enzymatic methods [52]. In contrast, fibroin is a structural core protein composed mainly of repetitive sequences of glycine, alanine, and serine, which form β-sheet structures that confer mechanical strength and stability [53]. Due to its highly ordered and insoluble nature, fibroin is not easily extracted with water alone but can be partially degraded and solubilized using proteolytic enzymes such as Alcalase® [47]. This differential extractability explains the higher yield and protein content observed in the enzymatic extracts compared to the aqueous ones.
Additionally, the molecular weight distribution of protein components in yellow silkworm cocoon extracts was evaluated by SDS-PAGE, and the resulting protein banding patterns are shown in Figure 3. Sericin was hence used as a reference protein. Aqueous extracts obtained after different pretreatment methods exhibited prominent bands across a broad molecular weight range, particularly between ~10–75 kDa. This suggested that the aqueous extractions were able to solubilize relatively intact or partially hydrolyzed sericin and other soluble protein fractions. In contrast, samples extracted using Alcalase® enzyme following the same pretreatments exhibited significantly lighter bands, primarily in the low molecular weight region below 20 kDa. This shift toward lower molecular weight bands indicates that enzymatic hydrolysis effectively degraded higher molecular weight proteins into smaller peptides. Therefore, the SDS-PAGE profiles confirmed that enzymatic extraction yields peptides of lower molecular weight compared to aqueous extraction. Furthermore, the pretreatment using both microwave and ultrasonication appeared to be the most effective approach for the extraction of low molecular weight proteins, which would be desirable for further applications as low-molecular-weight peptides are beneficial, such as in cosmetics for improved skin penetration and bioactivity [54].
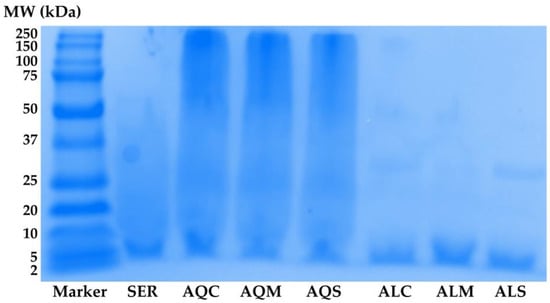
Figure 3.
Protein molecular weight distribution profiles analyzed by SDS-PAGE of sericin (SER) and yellow silkworm cocoon extracts (B. mori var. Nang Lai). Aqueous extracts were obtained after pretreatment by conventional soaking for 12 h (AQC), microwave treatment for 3 min (AQM), and ultrasonication for 30 min (AQS). Enzyme-assisted extracts using Alcalase® were obtained after the same respective pretreatments: conventional soaking (ALC), microwave (ALM), and ultrasonication (ALS).
Silkworm cocoons contain not only fibroin and sericin but also secondary metabolites such as phenolics and flavonoids, which are derived from the silkworm’s diet and contribute to the coloration of the silk [55]. The current study demonstrated that both phenolic and flavonoid compounds were successfully extracted from silkworm cocoons using both aqueous and enzymatic extractions. While enzymatic extraction alone resulted in only a modest increase in these bioactive compounds, the application of physical pretreatment, both microwave irradiation and ultrasonication, significantly enhanced their extraction (p < 0.05). These pretreatments facilitate structural disruption through localized heating and cavitation effects, respectively [50,56], thereby increasing matrix permeability and promoting the release of phenolics and flavonoids that are otherwise tightly bound. Consequently, both aqueous and enzymatic extracts showed a marked improvement in phenolic and flavonoid content following pretreatment. Previous research has identified significant quantities of phenolic compounds, including (+)-catechin and quercetin, in silk cocoons [55]. These bioactive compounds are well-known for their potent biological properties, which could contribute to the functional benefits of silk-derived products.
All the pretreatment processes were conducted at room temperature; therefore, the extraction of bioactive components during this step was considered negligible. This aligns with the well-established understanding that effective degumming of silk cocoons, particularly for the release of sericin and associated bioactive compounds, requires elevated temperatures, typically above 90 °C [7]. At such high temperatures, the disruption of hydrogen bonds and hydrophobic interactions within the silk protein matrix facilitates the solubilization of sericin and other water-soluble constituents [7]. In contrast, room temperature conditions are insufficient to break these molecular interactions, resulting in minimal diffusion of bioactive compounds into the soaking medium. Although the pretreatment processes involving microwave and ultrasonication may have caused a slight elevation in temperature, the short durations of 30 min and 3 min, respectively, were insufficient to reach the high temperatures required for effective degumming or significant extraction of bioactive compounds. Consequently, the pretreatment water was not expected to contain appreciable levels of these compounds. However, the enzymatic extraction following these pretreatment processes resulted in extracts with higher bioactive contents. This suggests that the integration of enzymatic extraction with physical pretreatments offers a promising strategy to enhance the recovery of valuable bioactive compounds from silk cocoons.
Although microwave and ultrasonication are widely employed as effective extraction techniques for various bioactive compounds from natural sources, their combined use with enzymatic extraction remains limited. This is primarily due to the fact that microwave irradiation generates high temperatures that are often incompatible with the optimal activity range of enzymes and can result in enzyme denaturation [49]. Similarly, ultrasonic waves can produce localized high temperatures and intense shear forces, which may inactivate enzymes such as proteases commonly used in extraction processes [56]. To preserve enzymatic activity and ensure maximum extraction efficiency, ultrasound in this study was applied only as a pretreatment step rather than during enzymatic extraction. This strategy allowed physical disruption of the silk cocoon matrix and improved material permeability, while maintaining the integrity and activity of the enzymes used in the subsequent extraction phase.
3.3. Characteristics of Silkworm Cocoon Extracts
The physicochemical characteristics of silkworm cocoon extracts, sericin, and Alcalase® were analyzed using FTIR, XRD, DSC, and TGA, as shown in Figure 4.
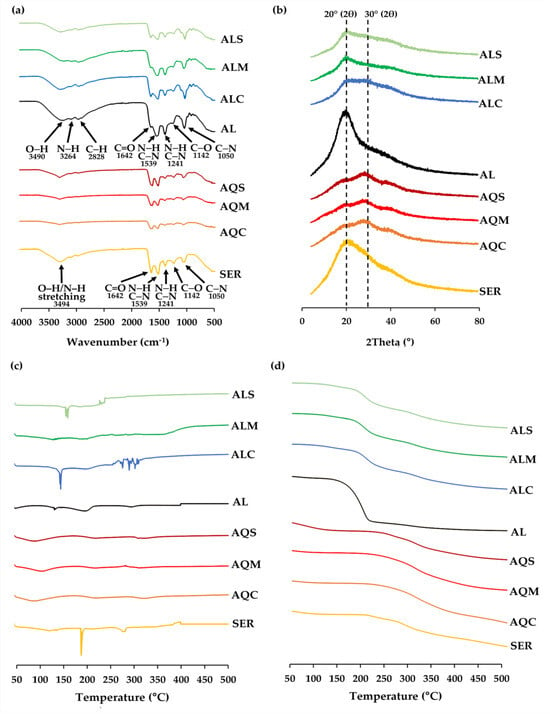
Figure 4.
FTIR spectra (a), XRD spectra (b), DSC thermogram (c), and TGA thermogram (d) of sericin (SER), Alcalase® (AL), and extracts from yellow silkworm cocoons of the B. mori (var. Nang Lai) from aqueous extraction after pretreatment with conventional soaking for 12 h (AQC), microwave treatment for 3 min (AQM), and ultrasonication for 30 min (AQS), along with its extracts from Alcalase® enzyme-assisted extraction after pretreatment with conventional soaking for 12 h (ALC), microwave treatment for 3 min (ALM), and ultrasonication for 30 min (ALS).
In the FTIR spectra (Figure 4a), sericin exhibited strong absorption bands corresponding to a broad amide A band (~3494 cm−1), amide I (C=O stretching, ~1642 cm−1), amide II (N–H bending and C–N stretching, ~1539 cm−1), and amide III (C–N stretching and N–H bending, ~1241 cm−1), confirming its proteinaceous nature [57]. The amide I peak at 1642 cm−1 typically indicates a combination of β-sheet and random coil structures, which are known to contribute to the stable conformation of sericin [58]. The results are consistent with previous reports, which revealed that sericin exhibited a broad amide A band at 3270 cm−1 and amide I and II peaks at 1650 and 1530 cm−1, respectively [59]. Moreover, there are additional characteristic peaks for sericin at 1142 cm−1 and 1050 cm−1, which can be attributed to C–O and C–N stretching vibrations, respectively, further supporting the presence of protein components. The findings were in line with a previous study that suggested a signature peak for sericin at 1070 cm−1 [60].
Notably, these FTIR signature bands were also present in all silkworm cocoon extracts, including those obtained via aqueous and enzymatic extraction, though with slight shifts and intensity variations. This consistent presence of amide I, II, and III bands across all extract samples confirmed the successful extraction of sericin protein components, regardless of the method used. Additionally, the broad O–H/N–H band in the region around 3260–3490 cm−1 in all samples further supports the proteinaceous and hydrophilic nature of the extracts. The observed similarity in spectral profiles suggests that both aqueous and enzymatic extraction methods preserve the key structural features of sericin. This highlights their potential for applications where the functional groups of sericin (e.g., amide and hydroxyl groups) contribute to bioactivity.
On the other hand, the FTIR spectrum of Alcalase® showed characteristic absorption bands that differ from those of sericin and silk cocoon extracts. A broad absorption around 3490 cm−1, corresponding to O–H stretching, and a shoulder at 3264 cm−1, assigned to N–H stretching, were observed. These bands are typically associated with the amide A region, indicating the presence of hydrogen-bonded hydroxyl and amino groups. Additionally, a peak at 2828 cm−1 was attributed to C–H stretching vibrations, reflecting aliphatic chains or side groups in the enzyme structure. The C–H stretching peaks were more prominent in Alcalase® and all the enzymatic extracts than in sericin or aqueous extracts, suggesting the presence of residual enzyme components or enhanced exposure of aliphatic side chains due to enzymatic hydrolysis. Additionally, the key protein backbone vibrations were also identified, including amide I (C=O stretching, ~1642 cm−1), amide II (N–H bending and C–N stretching, ~1539 cm−1), and amide III (C–N stretching and N–H bending, ~1241 cm−1). In addition, peaks at 1142 cm−1 and 1050 cm−1 may be attributed to C–O and C–N stretching vibrations, respectively, likely arising from side chains or glycosylated residues in the enzyme. However, the enzymatic extracts retained amide-related bands with shifts and reduced intensities, suggesting possible protein hydrolysis or structural modification during Alcalase® treatment [61].
The XRD profiles (Figure 4b) gave insight into the crystallinity of the silkworm cocoon extracts. Sericin, Alcalase®, and all enzymatic extracts exhibited their highest diffraction peaks at approximately 20° (2θ), whereas all aqueous extracts showed their highest peaks at approximately 30° (2θ). The broad diffraction peak observed near 20° (2θ) reflected the largely amorphous character of sericin, highlighting the presence of disordered or partially formed β-sheet arrangements typical of silk proteins [10]. Similar observations were made in earlier research where XRD patterns of sericin exhibited broad peaks at 18.86° [10]. and 19.2° [62], demonstrating consistency with the present findings. In the case of enzymatic extracts, the peak at 20° (2θ) reflected the presence of shorter peptide fragments resulting from Alcalase® hydrolysis, which disrupted any potential higher-order structure and reinforced an amorphous profile. In contrast, the aqueous extracts exhibited their highest peaks at 30° (2θ). This shift indicated a different structural arrangement, likely arising from aggregation or partial reorganization of the intact sericin chains during the pretreatment and drying processes. Without enzymatic cleavage, these longer chains realigned and formed medium-range ordered domains, giving rise to the more intense 30° (2θ) peak. The results suggested that enzymatic hydrolysis prevents such reordering by reducing the molecular weight and increasing structural disorder, while aqueous extraction promoted aggregation of intact protein chains into semi-ordered structures.
DSC thermograms (Figure 4c) revealed distinct thermal transitions among the tested samples. Sericin exhibited multiple thermal transitions, including a dehydration event around 190.2 °C, and significant endothermic peaks at 282.5 °C and 395.6 °C, corresponding to sericin denaturation and decomposition. The findings were in line with a previous study, which reported prominent endothermic transitions at 220 °C (attributed to thermally induced molecular motion and melting of sericin peptides) and 312 °C (thermal decomposition) [63]. In contrast, Alcalase® enzymatic extract displayed distinct transitions at 134.2, 201.7, 296.5, and 400.1 °C. All aqueous extracts showed similar DSC patterns, indicating that pretreatment processes had minimal effect on their thermal behavior. However, the aqueous extracts exhibited lower-temperature endothermic events (100.2–115.7 °C), reflecting earlier thermal responses when compared to enzymatic extracts, which exhibited higher thermal transitions (128.2–158.9 °C). This suggested that enzymatic extraction provided thermally more stable and potentially more complex bioactive profiles, likely due to the selective hydrolysis of protein structures and release of functional peptides. Interestingly, the pretreatment methods significantly influenced the thermal behavior of enzymatic extracts. Among them, conventional soaking produced the most thermally complex extract, with an initial endothermic peak at ~147.1 °C (moisture loss), a broad transition at ~201.5 °C (glass transition or thermal degradation onset), and multiple peaks between 250–300 °C, indicative of degradation of diverse bioactive compounds. Microwave pretreatment exhibited broader transitions at 128.2 °C and 200.6 °C, along with a higher-temperature peak at 368.5 °C, suggesting partial degradation or structural rearrangement due to microwave exposure. Meanwhile, ultrasonication resulted in a sharper, well-defined peak at ~158.9 °C, suggesting enhanced extraction and potential protein denaturation. Therefore, enzymatic extracts offered superior thermal stability and bioactive complexity compared to aqueous extracts.
TGA profiles (Figure 4d) supported the findings of DSC by demonstrating weight loss trends across temperature ranges. All samples exhibited an initial weight loss below 150 °C, consistent with moisture evaporation. Sericin showed a pronounced multi-step degradation pattern, with major weight losses occurring near 250–400 °C. Enzymatic extracts exhibited enhanced thermal stability compared to aqueous extracts, showing gradual multi-step weight loss that suggested the presence of diverse, thermally stable bioactive molecules. Aqueous extracts exhibited steeper weight reductions, indicating lower thermal resistance. Therefore, enzymatic hydrolysis yielded the silkworm cocoon extracts with improved thermal stability and complex degradation profiles, while aqueous extracts demonstrated earlier thermal decomposition and simpler component composition.
3.4. Cosmeceutical Properties of Silkworm Cocoon Extracts
The cosmeceutical properties of silkworm cocoon extracts, including antioxidant activity (via NO• inhibition and DPPH• scavenging), anti-collagenase activity, and skin moisturizing effects, are presented in Table 2.

Table 2.
Antioxidant, anti-skin ageing, and moisturizing enhancement properties of silkworm cocoon extracts from aqueous and enzymatic extraction with various pretreatment methods.
Excess nitric oxide (NO), especially from inducible nitric oxide synthase (iNOS), contributes to skin aging by generating oxidative stress, sustaining chronic inflammation, and degrading collagen and elastin through increased matrix metalloproteinases (MMPs) [64,65]. These processes lead to wrinkles, skin thinning, and loss of elasticity. Inhibiting NO production could help reduce oxidative damage, suppress inflammation, and preserve the skin’s structural integrity, making it a promising strategy in anti-aging skincare. In this study, silkworm cocoon extracts showed limited NO• inhibition overall, though results varied depending on the extraction solvents and pretreatment methods. Therefore, optimizing the extraction process, particularly through enzymatic extraction combined with extended pretreatment using microwave or ultrasound techniques, may enhance the biological activities of the extract [18]. However, prolonged pretreatment can cause heat-induced denaturation [66]. Notably, the combination of ultrasonic pretreatment with microwave has been reported to improve extraction efficacy, resulting in higher levels of bioactive compounds and antioxidant activities [67]. Thus, careful optimization of pretreatment duration is essential to maximize extract quality and functionality.
Although the silkworm cocoon extracts exhibited low NO• inhibition, they showed promising DPPH• radical scavenging activity. The enzymatic extracts demonstrated significantly greater DPPH• radical scavenging potency compared to the aqueous extracts prepared with the same pretreatment method (p < 0.05), while both microwave and ultrasonication pretreatments had considerably greater effects than conventional soaking (p < 0.05). These findings suggested that combining enzymatic extraction with advanced pretreatment techniques, such as microwave or ultrasonication, could effectively enhance the antioxidant potential, particularly the DPPH• radical scavenging, of silkworm cocoon extracts. Phenolics and flavonoids were suggested to be the bioactive components responsible for the antioxidant activity, while sericin, a major protein in the extract, exhibited little effect. As DPPH• inhibition reflects strong antioxidant activity, which is highly beneficial in cosmetic applications [68,69], the silkworm cocoon extracts were of interest as a potential natural source of antioxidants for use in anti-aging and skin-protective formulations. Since antioxidants help neutralize the free radicals generated by environmental stressors such as UV radiation and pollution, which can otherwise lead to oxidative stress, cellular damage, and premature skin aging [70]. By reducing oxidative damage, DPPH• scavenging compounds can help prevent wrinkles, loss of elasticity, and dullness, while supporting overall skin health. Additionally, antioxidants may soothe inflammation and strengthen the skin barrier, making them valuable ingredients in anti-aging, soothing, and protective skincare formulations [71].
Aside from their antioxidant properties, the silkworm cocoon extracts demonstrated anti-skin aging effects through collagenase inhibition. The same trends were observed, with the greatest potency found in the enzymatic extracts subjected to microwave and ultrasonication pretreatments. Their collagenase inhibitory activities were 65.9 ± 0.2% and 65.2 ± 0.3%, respectively, indicating stronger potential for functional anti-aging applications compared to the other extracts, which exhibited inhibition below 60%. The collagenase inhibitory activity is attributed primarily to the presence of phenolic compounds and sericin within the extracts [72,73]. As collagenase is an enzyme that breaks down collagen, a key structural protein responsible for skin strength, firmness, and elasticity [74,75], the collagenase inhibition is highly beneficial in cosmetics and cosmeceutical applications. Excessive collagenase activity leads to collagen degradation, resulting in wrinkles, sagging, and loss of skin resilience, the hallmarks of aging skin [76]. By inhibiting collagenase, cosmetic ingredients help preserve collagen levels, maintain skin’s structural integrity, and slow down visible signs of aging [77]. This makes collagenase inhibitors valuable in anti-aging skincare formulations aimed at promoting more youthful-looking skin. Additionally, these findings highlighted their potential in the development of cosmeceuticals or nutricosmetics aimed at promoting skin health and youthfulness. Further clinical evaluations would be required to substantiate their efficacy and safety, particularly for applications such as anti-aging skincare products or dietary supplements, and to support their future commercial viability.
Skin moisture is a key factor in maintaining youthful-looking skin because well-hydrated skin stays plump, smooth, and elastic [78]. When skin is properly hydrated, fine lines and wrinkles appear less pronounced, and the overall texture looks healthier and more radiant [79]. Conversely, loss of hydration in the stratum corneum leads to fine wrinkling [80]. Therefore, preserving skin moisture is essential for a youthful-looking skin. And suggested one of the anti-wrinkle strategies. The current study noted that silkworm cocoon extracts significantly enhanced skin moisture (p < 0.05), with sericin suggested as the major component responsible for this effect. The enzymatic extracts showed particularly strong moisturizing activity, which could likely be attributed to the enhanced release of hydrophilic peptides and amino acids during enzymatic hydrolysis, improving skin hydration and moisture retention [81].
Correlation analysis between the chemical constituents and the biological activities related to cosmetic applications was evaluated using simple linear regression (Figure 5) and Pearson correlation (Table 3). The findings, as shown in Figure 5, revealed strong positive associations between total phenolic and flavonoid content with various biological activities. Total phenolic content exhibited significant correlations with NO• inhibition (R2 = 0.7409), DPPH• scavenging activity (R2 = 0.7028), collagenase inhibition (R2 = 0.7585), and skin moisture enhancement (R2 = 0.77). Similarly, total flavonoid content showed strong correlations with NO• inhibition (R2 = 0.7136), DPPH• scavenging activity (R2 = 0.5028), and collagenase inhibition (R2 = 0.9075), but a weak correlation with skin moisture enhancement (R2 = 0.243).
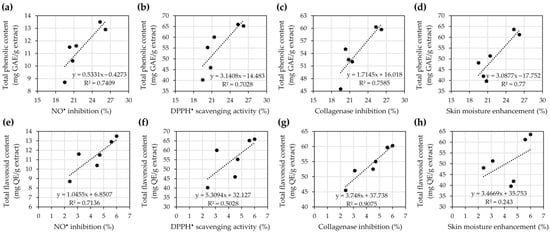
Figure 5.
Correlation analysis between total phenolic content (a–d) and total flavonoid content (e–h) with biological activities of yellow silk cocoon extracts, including NO• inhibition (a,e), DPPH• scavenging activity (b,f), collagenase inhibition (c,g), and skin moisture enhancement (d,h). Each panel includes the linear regression equation and coefficient of determination (R2), indicating the strength of the relationship between chemical content and biological activity.

Table 3.
The relationship between the chemical constituents and antioxidant activities of yellow silk cocoon extracts.
On the other hand, Pearson correlation analysis, as shown in Table 3, revealed that the total phenolic content demonstrated high positive correlations with all tested biological activities: NO• inhibition (r = 0.861), DPPH• scavenging (r = 0.838), collagenase inhibition (r = 0.871), and skin moisture enhancement (r = 0.878). Although none of these correlations were statistically significant (p > 0.05), the strength of association suggests that phenolic compounds may play a vital role in antioxidant and cosmetic-related bioactivities. These results were consistent with the simple linear regression analysis, which revealed relatively high R2 values across the same activities (R2 = 0.7028–0.770), indicating that phenolic content could explain a substantial proportion of the variation in biological responses.
Similarly, total flavonoid content displayed very high correlation with collagenase inhibition (r = 0.953) and high correlation with NO• inhibition (r = 0.845), supported by strong R2 values from the regression analysis (R2 = 0.7136 and 0.9075, respectively). However, the correlation with DPPH• scavenging was moderate (r = 0.709, R2 = 0.5028), while the correlation with skin moisture enhancement was low (r = 0.493, R2 = 0.243). These findings suggested that flavonoids contributed less to skin hydration than to enzymatic or antioxidant effects. Despite the absence of statistically significant p-values, these trends point to a strong relationship between the chemical content, particularly phenolics and flavonoids, and the biological performance of the yellow silk cocoon extracts. The high to very high correlation levels, especially for collagenase inhibition, highlight the potential of these natural compounds in cosmetic formulations targeting skin aging and oxidative stress.
Overall, silkworm cocoon extracts demonstrate great potential for cosmetic applications due to their antioxidant, anti-aging, and moisturizing properties. Pretreatment methods and extraction solvents are key factors influencing the efficacy and benefits of these extracts. Under identical pretreatment conditions, enzymatic extracts demonstrated significantly greater potency compared to their aqueous counterparts in all cosmeceutical properties (p < 0.05). Notably, microwave and ultrasonication pretreatments demonstrated superior efficacy compared to conventional soaking, in both aqueous and enzymatic extraction processes (p < 0.05). Further determination of the dose–response curves and IC50 values of the potent silkworm cocoon extracts is recommended to fully understand and quantify their effective concentrations for future application in cosmetic formulations. Additionally, future studies on their water retention and swelling capacity, along with their degradation behavior, would be recommended to better evaluate their functional performance and stability in the cosmetic formulations.
3.5. Irritation Profiles of Silkworm Cocoon Extracts
Irritation testing is another crucial assessment to confirm the safety of topical products. The HET-CAM test has been widely used as a suitable in vitro test in the cosmetic industry worldwide over the past decades [82]. The findings from the HET-CAM test were used to evaluate the irritation potential of silk cocoon extracts. Exposure of the CAM to silkworm cocoon extracts is illustrated in Figure 6, with corresponding irritation scores presented in Table 4. The positive control, 1% w/v sodium lauryl sulfate solution, induced severe irritation, reflected by an irritation score of 15.1 ± 0.6. Signs of irritation, including blood vessel hemorrhage, lysis, and coagulation, were observed within 5 min of exposure. These results align with previous findings by Somwongin et al. (2023), where 1% w/v SLS caused severe irritation with an irritation score of 11.94 ± 0.01 [37]. Conversely, the negative control, 0.9% w/v sodium chloride (NaCl) solution, showed no signs of irritation. Importantly, all silkworm cocoon extracts were non-irritating in the HET-CAM assay, supporting the conclusion that these extracts are safe and non-irritating for skin applications.
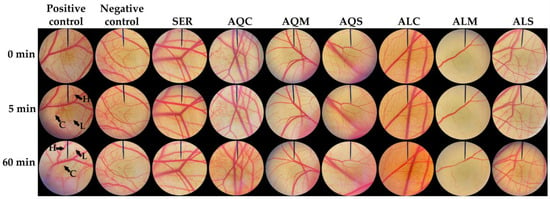
Figure 6.
CAM treated with 1% w/v sodium lauryl sulfate solution (positive control), 0.9% w/v sodium chloride solution (negative control), sericin (SER), and extracts from yellow silkworm cocoons of the B. mori (var. Nang Lai) from aqueous extraction after pretreatment with conventional soaking for 12 h (AQC), microwave treatment for 3 min (AQM), and ultrasonication for 30 min (AQS), along with its extracts from Alcalase® enzyme-assisted extraction after pretreatment with conventional soaking for 12 h (ALC), microwave treatment for 3 min (ALM), and ultrasonication for 30 min (ALS). H indicates hemorrhage, L indicates vascular lysis, and C indicates coagulation.

Table 4.
Irritation score and irritation potential of yellow silkworm cocoons of the B. mori (var. Nang Lai).
4. Conclusions
Microwave and ultrasonication pretreatments, followed by enzymatic extraction, significantly enhanced the extraction efficiency and bioactivities of silkworm cocoon extracts. These methods yielded 21.6 ± 0.5% and 21.7 ± 0.4%, respectively, compared to yields below 9% from aqueous extractions. Although the protein content per 100 g of extract was lower, the total protein yield relative to raw cocoon weight was approximately 14.0 ± 0.3% w/w, double that of aqueous extractions at approximately 7% w/w. The extracts also contained high levels of phenolic and flavonoid compounds, with total phenolic contents of 25.3 ± 0.3 and 26.2 ± 0.6 mg gallic acid equivalent per g extract and flavonoid contents of 6.0 ± 0.7 and 5.6 ± 0.4 mg quercetin equivalent per g extract. These corresponded to strong biological effects, including DPPH• radical scavenging activity of 66.1 ± 1.1% and 65.4 ± 1.3%, collagenase inhibition of 59.6 ± 1.2% and 60.3 ± 0.8%, and NO• inhibition of 12.9 ± 0.5% and 13.5 ± 0.4%, for microwave and ultrasonication extracts, respectively. Skin moisture content increased significantly by 63.6 ± 2.1% and 61.2 ± 1.5%, while the controls showed only 1.3 ± 0.6% improvement. Strong correlations were observed between total phenolic content and antioxidant or anti-inflammatory activities (r > 0.83), and between flavonoid content and collagenase inhibition (r = 0.953). Additionally, all extracts were confirmed to be non-irritating upon topical application through the HET-CAM test, supporting their safety for further topical use. Therefore, microwave and ultrasonication are recommended as sustainable and efficient pretreatment methods for obtaining high-quality cosmetic extracts from silkworm cocoons as active ingredients in anti-aging and moisturizing cosmetic products. The promising bioactivities of silkworm cocoon extracts, combined with sustainable and efficient extraction methods, demonstrate their industrial feasibility for large-scale production of high-quality cosmetic ingredients. Future research should focus on product formulation, long-term stability, safety testing, in vivo studies, preclinical trials, and clinical validation.
Author Contributions
Conceptualization, W.C.; methodology, S.S., J.I. and S.J.; validation, W.C.; formal analysis, S.C. and S.J.; investigation, S.C. and S.J.; resources, W.K. and W.C.; data curation, W.C.; writing—original draft preparation, S.C. and W.C.; writing—review and editing, S.C. and W.C.; visualization, S.C. and W.C.; supervision, W.K. and W.C.; project administration, W.C.; funding acquisition, S.C. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Fund 2025, Chiang Mai University, grant number 4772159. Sarocha Chareegun expresses sincere gratitude for the Teaching Assistant and Research Assistant (TA/RA) scholarship provided by Chiang Mai University. The APC was funded by the Center of Excellence in Pharmaceutical Nanotechnology, Faculty of Pharmacy, Chiang Mai University.
Data Availability Statement
All relevant data are included within the article.
Acknowledgments
The authors would like to acknowledge the Queen Sirikit Sericulture Center, Chiang Mai, Thailand, for their generous support in providing the silkworm cocoon materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ATR | Attenuated total reflectance |
| ALC | Yellow silkworm cocoon extract from Alcalase® enzyme-assisted extraction after pretreatment with conventional soaking for 12 h |
| ALM | Yellow silkworm cocoon extract from Alcalase® enzyme-assisted extraction after pretreatment with microwave treatment for 3 min |
| ALS | Yellow silkworm cocoon extract from Alcalase® enzyme-assisted extraction after pretreatment with ultrasonication for 30 min |
| AQC | Yellow silkworm cocoon extract from aqueous extraction after pretreatment with conventional soaking for 12 h |
| AQM | Yellow silkworm cocoon extract from aqueous extraction after pretreatment with microwave treatment for 3 min |
| AQS | Yellow silkworm cocoon extract from aqueous extraction after pretreatment with ultrasonication for 30 min |
| BCA | Bicinchoninic acid |
| BSA | Bovine serum albumin |
| CAM | Chorioallantoic membrane |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| DSC | Differential scanning calorimetry |
| EGCG | Epigallocatechin gallate |
| FALGPA | N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala |
| FT-IR | Fourier transform infrared |
| GAE | Gallic acid equivalents |
| HET-CAM | Hen’s egg test on the chorioallantoic membrane |
| IS | Irritation score |
| MW | Molecular weight |
| NED | N-naphthyl ethylenediamine dihydrochloride |
| NO• | Nitric oxide |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SEM | Scanning electron microscopy |
| SLS | Sodium lauryl sulfate |
| SNP | sodium nitroprusside |
| TGA | Thermogravimetric analysis |
| TPTZ | 2,4,6-Tripyridyl-S-triazine |
| XRD | X-ray diffraction |
References
- Yukuhiro, K.; Sezutsu, H.; Tsubota, T.; Takasu, Y.; Kameda, T.; Yonemura, N. Insect Silks and Cocoons: Structural and Molecular Aspects. In Extracellular Composite Matrices in Arthropods; Cohen, E., Moussian, B., Eds.; Springer: Cham, Switzerland, 2016; pp. 515–555. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Srichana, T. Potential applications of silkworm sericin, a natural protein from textile industry by-products. Waste Manag. Res. 2012, 30, 217–224. [Google Scholar] [CrossRef]
- Aghaz, F.; Hajarian, H.; Shabankareh, H.K.; Abdolmohammadi, A. Effect of sericin supplementation in maturation medium on cumulus cell expansion, oocyte nuclear maturation, and subsequent embryo development in Sanjabi ewes during the breeding season. Theriogenology 2015, 84, 1631–1635. [Google Scholar] [CrossRef]
- Chuang, C.C.; Prasannan, A.; Hong, P.D.; Chiang, M.Y. Silkworm-sericin degummed wastewater solution-derived and nitrogen enriched porous carbon nanosheets for robust biological imaging of stem cells. Int. J. Biol. Macromol. 2018, 107, 2122–2130. [Google Scholar] [CrossRef]
- Sothornvit, R.; Chollakup, R.; Suwanruji, P. Extracted sericin from silkworm waste for film formation. Songklanakarin J. Sci. Technol. 2010, 32, 17–22. [Google Scholar]
- Wu, J.H.; Wang, Z.; Xu, S.Y. Preparation and characterization of sericin powder extracted from silk industry wastewater. Food Chem. 2007, 103, 1255–1262. [Google Scholar] [CrossRef]
- Bungthong, C.; Siriamornpun, S. Changes in amino acid profiles and bioactive compounds of Thai silk cocoons as affected by water extraction. Molecules 2021, 26, 2033. [Google Scholar] [CrossRef] [PubMed]
- Prommuak, C.; De-Eknamkul, W.; Shotipruk, A. Extraction of flavonoids and carotenoids from Thai silkworm waste and antioxidant activity of extracts. Sep. Purif. Technol. 2008, 62, 444–448. [Google Scholar] [CrossRef]
- Rangi, A.; Jajpura, L. The biopolymer sericin: Extraction and applications. J. Text Sci. Eng. 2015, 5, 1000188. [Google Scholar] [CrossRef]
- Saha, J.; Mondal, M.I.; Sheikh, M.K.; Habib, M.A. Extraction, structural and functional properties of silk sericin biopolymer from Bombyx mori silk cocoon waste. J. Text. Sci. Eng. 2019, 9, 1000390. [Google Scholar] [CrossRef]
- Kumar, J.P.; Mandal, B.B. Antioxidant potential of mulberry and non-mulberry silkworm sericin and its implications in biomedicine. Free Radic. Biol. Med. 2017, 108, 803–818. [Google Scholar] [CrossRef]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm sericin: Properties and biomedical applications. Biomed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive compounds for skin health: A review. Nutrients. 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Lin, W.S.; Shih, C.H.; Chen, C.Y.; Kuo, S.H.; Li, W.L.; Lin, Y.S. Functionality of silkworm cocoon (Bombyx mori L.) sericin extracts obtained through high-temperature hydrothermal method. Materials. 2021, 14, 5314. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Shi, Z.; Jiang, W.; Liu, X.; Ni, Q.Q. Degumming of raw silkworm via steam treatment. J. Clean. Prod. 2018, 203, 492–497. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C. Enzyme-assisted extractions of polyphenols–A comprehensive review. Trends Food Sci. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Muniglia, L.; Claisse, N.; Baudelet, P.H.; Ricochon, G. Enzymatic Aqueous Extraction (EAE). In Alternative Solvents for Natural Products Extraction. Green Chemistry and Sustainable Technology; Chemat, F., Vian, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 167–204. [Google Scholar] [CrossRef]
- Sangwong, G.; Sumida, M.; Sutthikhum, V. Antioxidant activity of chemically and enzymatically modified sericin extracted from cocoons of Bombyx mori. Biocatal. Agric. Biotechnol. 2016, 5, 155–161. [Google Scholar] [CrossRef]
- Liaset, B.; Nortvedt, R.; Lied, E.; Espe, M. Studies on the nitrogen recovery in enzymic hydrolysis of Atlantic salmon (Salmo salar, L.) frames by Protamex™ protease. Process. Biochem. 2002, 37, 1263–1269. [Google Scholar] [CrossRef]
- Yakul, K.; Takenaka, S.; Nakamura, K.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Watanabe, M.; Chaiyaso, T. Characterization of thermostable alkaline protease from Bacillus halodurans SE5 and its application in degumming coupled with sericin hydrolysate production from yellow cocoon. Process Biochem. 2019, 78, 63–70. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, S.W.; Kim, K.Y.; Ki, C.S.; Um, I.C. Structural characteristics and properties of cocoon and regenerated silk fibroin from different silkworm strains. Int. J. Mol. Sci. 2023, 24, 4965. [Google Scholar] [CrossRef]
- Pan, M.; Jin, Y.; Ye, Y.; Jiang, W.; Zhu, L.; Lu, W. An efficient and eco-friendly method for removing sericin using microwave-assisted steam degumming. Environ. Technol. Inno. 2024, 35, 103674. [Google Scholar] [CrossRef]
- Lai, E.; Zhu, Y.; Lin, C.; Wang, J.; Lin, H. Effects of ultrasonic pre-treatment on physicochemical properties of sericin poteins extracted from cassava silkworm cocoon. J. Environ. Polym. Degrad. 2024, 32, 3835–3844. [Google Scholar] [CrossRef]
- Vaithanomsat, P.; Punyasawon, C. Process optimization for the production of Philosamia ricini (Eri Silk) pupae hydrolysate. Agr. Nat. Resour. 2008, 42, 341–352. [Google Scholar]
- Koon, M.A.; Ali, K.A.; Speaker, R.M.; McGrath, J.P.; Linton, E.W.; Steinhilb, M.L. Preparation of prokaryotic and eukaryotic organisms using chemical drying for morphological analysis in scanning electron microscopy (SEM). J. Vis. Exp. 2019, 143, e58761. [Google Scholar] [CrossRef]
- Alou, I.N.; van der Laan, M.; Annandale, J.G.; Steyn, J.M. Water and nitrogen (N) use efficiency of upland rice (Oryza sativa L.× Oryza glaberrima Steud) under varying N application rates. Nitrogen 2020, 1, 151–166. [Google Scholar] [CrossRef]
- Weng, Y.; Qin, J.; Eaton, S.; Yang, Y.; Ravelombola, W.S.; Shi, A. Evaluation of seed protein content in USDA cowpea germplasm. HortScience 2019, 54, 814–817. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Zheng, C.; Hu, L.; Wang, C.; Jiang, J.; He, B.; Jiang, G. Analysis of silver-associated proteins in pathogen via combination of native SDS-PAGE, fluorescent staining, and inductively coupled plasma mass spectrometry. J. Chromatogr. A 2019, 1607, 460393. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Punyoyai, C.; Somwongin, S.; Leelapornpisid, P.; Ingkaninan, K.; Waranuch, N.; Srivilai, J.; Thitipramote, N.; Wisuitiprot, W.; Schuster, R.; et al. Inhibition of 5α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex vaucher extract as functional food and nutraceuticals ingredients. Nutrients 2017, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Sriyab, S.; Laosirisathian, N.; Punyoyai, C.; Anuchapreeda, S.; Tima, S.; Chiampanichayakul, S.; Chaiyana, W. Nutricosmetic effects of Asparagus officinalis: A potent matrix metalloproteinase-1 inhibitor. Sci. Rep. 2021, 11, 8772. [Google Scholar] [CrossRef] [PubMed]
- Thewanjutiwong, S.; Phokasem, P.; Disayathanoowat, T.; Juntrapirom, S.; Kanjanakawinkul, W.; Chaiyana, W. Development of film-forming gel formulations containing royal jelly and honey aromatic water for cosmetic applications. Gels 2023, 9, 816. [Google Scholar] [CrossRef]
- Sumanont, Y.; Murakami, Y.; Tohda, M.; Vajragupta, O.; Matsumoto, K.; Watanabe, H. Evaluation of the nitric oxide radical scavenging activity of manganese complexes of curcumin and its derivative. Biol. Pharm. Bull. 2004, 27, 170–173. [Google Scholar] [CrossRef]
- Brem, B.; Seger, C.; Pacher, T.; Hartl, M.; Hadacek, F.; Hofer, O.; Vajrodaya, S.; Greger, H. Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochemistry 2004, 65, 2719–2729. [Google Scholar] [CrossRef]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-based DPPH Assay for Antioxidant Activity Analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.; Koop, U.; Leneveu-Duchemin, M.C.; Osterrieder, K.; Bielfeldt, S.; Chkarnat, C.; Degwert, J.; Häntschel, D.; Jaspers, S.; Nissen, H.P.; et al. Multicentre comparison of skin hydration in terms of physical-, physiological-and product-dependent parameters by the capacitive method (Corneometer CM 825). Int. J. Cosmet. Sci. 2003, 25, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Somwongin, S.; Sirilun, S.; Chantawannakul, P.; Anuchapreeda, S.; Yawootti, A.; Chaiyana, W. Ultrasound-assisted green extraction methods: An approach for cosmeceutical compounds isolation from Macadamia integrifolia pericarp. Ultrason. Sonochem. 2023, 92, 106266. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; de Souza Leão, V.L.X.; Guerra, J.M.C.; Sarubbo, L.A. Cookies and muffins containing biosurfactant: Textural, physicochemical and sensory analyses. J. Food Sci. Technol. 2023, 60, 2180–2192. [Google Scholar] [CrossRef]
- Mukaka, M.M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Homhuk, P.; Nonsrirach, T.; Promma, S.; Sumida, M.; Sutthikhum, V. Cocoon characters and antioxidant activity of sericin among Thai polyvoltine strains of the silkworm, Bombyx mori. Int. J. Wild Silkmoth Silk. 2017, 20, 25–36. [Google Scholar] [CrossRef]
- Morin, A.; Alam, P. Comparing the properties of Bombyx mori silkworm cocoons against sericin-fibroin regummed biocomposite sheets. Mater. Sci. Eng. C 2016, 65, 215–220. [Google Scholar] [CrossRef]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Chen, F.; Porter, D.; Vollrath, F. Structure and physical properties of silkworm cocoons. J. R. Soc. Interface. 2012, 9, 2299–2308. [Google Scholar] [CrossRef]
- Wei, L.; Liu, S.; Dong, F. Mechanism study of micro-jet generation induced by acoustic cavitation. J. Hydrodyn. 2024, 36, 1104–1117. [Google Scholar] [CrossRef]
- Joyjamras, K.; Netcharoensirisuk, P.; Roytrakul, S.; Chanvorachote, P.; Chaotham, C. Recycled sericin hydrolysates modified by Alcalase® suppress melanogenesis in human melanin-producing cells via modulating MITF. Int. J. Mol. Sci. 2022, 23, 3925. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Y.; Gong, K.; Zhou, Q.; Zhang, T.; Li, Q. A comparative study of ultrasonic degumming of silk sericin using citric acid, sodium carbonate and papain. Color Technol. 2019, 135, 195–201. [Google Scholar] [CrossRef]
- Rodbumrer, P.; Arthan, D.; Uyen, U.; Yuvaniyama, J.; Svasti, J.; Wongsaengchantra, P.Y. Functional expression of a Bombyx mori cocoonase: Potential application for silk degumming. Acta Biochim. Biophys. Sin. 2012, 44, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.D.; Cho, M.S.; Kim, J.S.; Kim, M.S.; Kim, C.H.; Kang, J.Y. Identification and characterization of a cocoon degradable enzyme from the isolated strain Bacillus subtilis Bs5C. Biotechnol. Bioprocess Eng. 2020, 25, 442–449. [Google Scholar] [CrossRef]
- Prakash, N.J.; Shanmugarajan, D.; Wang, X.; Kandasubramanian, B. Enhancement of mechano-structural characteristics of silk fibroin using microwave assisted degumming. Sustain. Chem. Pharm. 2022, 30, 100902. [Google Scholar] [CrossRef]
- Eom, S.J.; Lee, N.H.; Kang, M.C.; Kim, Y.H.; Lim, T.G.; Song, K.M. Silk peptide production from whole silkworm cocoon using ultrasound and enzymatic treatment and its suppression of solar ultraviolet-induced skin inflammation. Ultrason. Sonochem. 2020, 61, 104803. [Google Scholar] [CrossRef]
- Biganeh, H.; Kabiri, M.; Zeynalpourfattahi, Y.; Brancalhão, R.M.C.; Karimi, M.; Ardekani, M.R.S.; Rahimi, R. Bombyx mori cocoon as a promising pharmacological agent: A review of ethnopharmacology, chemistry, and biological activities. Heliyon 2022, 8, e10496. [Google Scholar] [CrossRef]
- Biswal, B.; Dan, A.K.; Sengupta, A.; Das, M.; Bindhani, B.K.; Das, D.; Parhi, P.K. Extraction of silk fibroin with several sericin removal processes and its importance in tissue engineering: A review. J. Polym. Environ. 2022, 30, 2222–2253. [Google Scholar] [CrossRef]
- Koh, L.D.; Cheng, Y.; Teng, C.P.; Khin, Y.W.; Loh, X.J.; Tee, S.Y.; Low, M.; Ye, E.; Yu, H.D.; Zhang, Y.W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Butkhup, L.; Jeenphakdee, M.; Jorjong, S.; Samappito, S.; Samappito, W.; Butimal, J. Phenolic composition and antioxidant activity of Thai and Eri silk sericins. Food Sci. Biotechnol. 2012, 21, 389–398. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Moon, J.Y.; Lee, Y.C. Insights into bioactive peptides in cosmetics. Cosmetics 2023, 10, 111. [Google Scholar] [CrossRef]
- Rejasse, B.; Lamare, S.; Legoy, M.D.; Besson, T. Influence of microwave irradiation on enzymatic properties: Applications in enzyme chemistry. J. Enzyme Inhib. Med. Chem. 2007, 22, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Manesa, K.C.; Kebede, T.G.; Dube, S.; Nindi, M.M. Profiling of silk sericin from cocoons of three southern African wild silk moths with a focus on their antimicrobial and antioxidant properties. Materials 2020, 13, 5706. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Zhang, M.; Adhikari, B. The inactivation of enzymes by ultrasound—A review of potential mechanisms. Food Rev. Int. 2014, 30, 1–21. [Google Scholar] [CrossRef]
- Punjataewakupt, A.; Reddy, N.; Aramwit, P. Enhancing clinical applications of PVA hydrogel by blending with collagen hydrolysate and silk sericin. J. Polym. Res. 2022, 29, 110. [Google Scholar] [CrossRef]
- Zhang, X.; Wyeth, P. Using FTIR spectroscopy to detect sericin on historic silk. Sci. China Chem. 2010, 53, 626–631. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Yarmand, M.S.; Ghanbarzadeh, B. The effects of combined enzymatic and physical modifications of lentil protein applying Alcalase, Flavourzyme, microbial transglutaminase, and ultrasound: Antioxidant, antihypertension, and antidiabetic activities. J. Food Meas. Charact. 2022, 16, 3743–3759. [Google Scholar] [CrossRef]
- Silva, V.R.; Hamerski, F.; Weschenfelder, T.A.; Ribani, M.; Gimenes, M.L.; Scheer, A.P. Equilibrium, kinetic, and thermodynamic studies on the biosorption of Bordeaux S dye by sericin powder derived from cocoons of the silkworm Bombyx mori. Desalin. Water Treat. 2016, 57, 5119–5129. [Google Scholar] [CrossRef]
- Caringella, R.; Bhavsar, P.; Dalla Fontana, G.; Patrucco, A.; Tonin, C.; Pozzo, P.D.; Zoccola, M. Fabrication and properties of keratoses/sericin blend films. Polym. Bull. 2022, 79, 2189–2204. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Pourbagher-Shahri, A.M.; Farkhondeh, T.; Talebi, M.; Kopustinskiene, D.M.; Samarghandian, S.; Bernatoniene, J. An overview of NO signaling pathways in aging. Molecules 2021, 26, 4533. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Almagro, N.; Morales-Soriano, E.; Villamiel, M.; Condezo-Hoyos, L. Hybrid high-intensity ultrasound and microwave treatment: A review on its effect on quality and bioactivity of foods. Ultrason. Sonochemistry 2021, 80, 105835. [Google Scholar] [CrossRef]
- Li, Q.; Cui, M.; She, J.; Sun, S.; Zhou, L.; Tang, F.; Guo, Y.; Liu, Y. Preparation high quality camellia oil by combining ultrasound pre-treatment and microwave as drying method: Interactive effect on drying kinetics, metabolite profile and antioxidant ability. Ultrason. Sonochemistry 2025, 117, 107338. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, J.H.; Kim, H.P.; Heo, M.Y. Biological screening of 100 plant extracts for cosmetic use (II): Anti-oxidative activity and free radical scavenging activity. Int. J. Cosmet. Sci. 1997, 19, 299–307. [Google Scholar] [CrossRef]
- Kusumawati, I.; Indrayanto, G. Natural antioxidants in cosmetics. Stud. Nat. Prod. Chem. 2013, 40, 485–505. [Google Scholar] [CrossRef]
- Bosebabu, B.; Cheruku, S.P.; Chamallamudi, M.R.; Nampoothiri, M.; Shenoy, R.R.; Nandakumar, K.; Parihar, V.K.; Kumar, N. An appraisal of current pharmacological perspectives of sesamol: A review. Mini-Rev. Med. Chem. 2020, 20, 988–1000. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, Y.J.; Kim, C.M.; Lee, Y.M. Revolutionizing cosmetic ingredients: Harnessing the power of antioxidants, probiotics, plant extracts, and peptides in personal and skin care products. Cosmetics 2024, 11, 157. [Google Scholar] [CrossRef]
- Jena, K.; Pandey, J.P.; Kumari, R.; Sinha, A.K.; Gupta, V.P.; Singh, G.P. Tasar silk fiber waste sericin: New source for anti-elastase, anti-tyrosinase and anti-oxidant compounds. Int. J. Biol. Macromol. 2018, 114, 1102–1108. [Google Scholar] [CrossRef]
- Fongsodsri, K.; Tiyasatkulkovit, W.; Chaisri, U.; Reamtong, O.; Adisakwattana, P.; Supasai, S.; Kanjanapruthipong, T.; Sukphopetch, P.; Aramwit, P.; Ampawong, S. Sericin promotes chondrogenic proliferation and differentiation via glycolysis and Smad2/3 TGF-β signaling inductions and alleviates inflammation in three-dimensional models. Sci. Rep. 2024, 14, 11553. [Google Scholar] [CrossRef]
- Jadach, B.; Mielcarek, Z.; Osmałek, T. Use of collagen in cosmetic products. Curr. Issues Mol. Biol. 2024, 46, 2043–2070. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A.K. Skin aging & modern age anti-aging strategies. Int. J. Clin. Dermatol. Res. 2019, 7, 209–240. [Google Scholar] [CrossRef]
- Porwal, M.; Rastogi, V.; Chandra, P.; Shukla, S. An Updated Review on the Role of Phytoconstituents in Modulating Signalling Pathways to Combat Skin Ageing: Nature’s Own Weapons and Approaches. J. Nat. Prod. 2024, 14, 55–71. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.S.H. Natural anti-aging skincare: Role and potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef]
- Parikh, S.A.; Kelsey, A.; Finch, J.; Grant-Kels, J.M. Skin Health and Healthy Aging: Skin Cosmetics. In Healthy Aging; Coll, P., Ed.; Springer: Cham, Switzerland, 2019; pp. 105–113. [Google Scholar] [CrossRef]
- Hashizume, H. Skin aging and dry skin. J. Dermatol. 2004, 31, 603–609. [Google Scholar] [CrossRef]
- Sjerobabski-Masnec, I.; Situm, M. Skin aging. Acta Clin. Croat. 2010, 49, 515–518. [Google Scholar]
- Harding, C.R.; Watkinson, A.; Rawlings, A.V. Dry skin, moisturization and corneodesmolysis. Int. J. Cosmet. Sci. 2000, 22, 21–52. [Google Scholar] [CrossRef]
- Steiling, W.; Bracher, M.; Courtellemont, P.; De Silva, O. The HET–CAM, a useful in vitro assay for assessing the eye irritation properties of cosmetic formulations and ingredients. Toxicol. Vitr. 1999, 13, 375–384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).