1. Introduction
Plastics have become ubiquitous since the invention of synthetic polymers in 1907, prized for their versatility, durability, and low cost [
1,
2]. Over time, they have become indispensable in daily life, with consumption increasing exponentially. Consequently, an estimated 10 to 40 million tonnes of microplastics are released into the environment each year. However, beneath the convenience that plastics provide lies growing concern over their environmental impact and potential risks to human health [
3]. As plastic waste undergoes degradation, it fragments into microplastics (<5 mm) and nanoplastics (<1 μm) [
3,
4]. These microscopic particles have been found in marine environments, soil, air, and even human biological samples such as placental tissue, breast milk, and blood [
5].
While the long-term health effects of microplastics remain unclear, emerging evidence suggests that they may impact multiple human systems, including the digestive, respiratory, endocrine, reproductive, and immune systems [
6,
7]. Although ingestion and inhalation are considered the primary exposure routes, dermal absorption is increasingly recognized as a potential pathway [
7].
Microplastics are not only pervasive in the environment but are also intentionally added to personal care and cosmetic products (PCCPs) for exfoliation, texture enhancement, and product stabilization. This widespread use raises concerns about direct dermal exposure, inadvertent inhalation, and environmental contamination [
8,
9]. This review aims to examine the presence of microplastics in cosmetics, elucidate their potential routes of exposure, and evaluate their effects on skin health.
2. Methods
A search was conducted in the electronic databases PubMed and Cochrane Library, covering all available records from database inception to March 31, 2025, using search terms including “microplastics”, “nanoplastics”, “personal care products”, “microbeads”, “health effect”, “dermatologic effect”, “dermatology”, “moisturizers”, “emollients”, “cosmetics”, “skin absorption”, “cutaneous exposure”, “dermal toxicity”, “skin barrier”, “inflammation”, “oxidative stress”, “skin aging”, “senescence”, “cellular senescence”, and “skin cancer”. We included English-language articles that evaluated the presence, absorption, and dermatologic impact of microplastics and nanoplastics in cosmetic or personal care products. In vitro, in vivo, and clinical studies assessing skin-related outcomes were considered. Non-English articles were excluded unless relevant dermatological findings were reported. Preference was given to high-quality peer-reviewed articles, and reference lists were reviewed to include relevant studies.
This review has a limitation in that we did not perform a formal risk-of-bias or quality assessment of the included studies. Although we aimed to include peer-reviewed and relevant literature, the lack of standardized appraisal may limit the ability to evaluate potential bias and the methodological rigor of the reviewed studies.
3. Microplastics
3.1. Definition of Microplastics
Microplastics are synthetic polymer particles measuring less than 5 mm in diameter and are composed primarily of carbon and hydrogen atoms linked in polymer chains [
4,
10]. These particles are insoluble in water and have been detected across diverse environments, including marine ecosystems, soil, air, and even in food and drinking water [
3,
11].
Microplastics are generally classified into two categories based on their origin [
3]. Primary microplastics are deliberately manufactured at the microscopic scale for use in commercial and industrial applications, such as cosmetics, medical products, and toothpaste [
2,
10] Microbeads, commonly used in exfoliating products, are a typical example [
8]. In contrast, secondary microplastics originate from the breakdown of larger plastic debris through mechanical abrasion, thermal degradation, photolysis, or biological activity [
8,
10]. Nanoplastics, typically defined as plastic particles smaller than 1 μm [
4,
10,
11], pose particular concern due to their enhanced ability to penetrate biological tissues and interact with cells. Because of their persistence and potential toxicity, both microplastics and nanoplastics have become major subjects of environmental and biomedical research. Their widespread presence and potential for bioaccumulation underscore the urgent need for effective mitigation and regulatory strategies [
12].
3.2. Routes of Microplastic Exposure
Humans can be exposed to microplastics through multiple sources, including plastic packaging, textiles, degraded plastic materials, fishing gear, and personal hygiene products [
7]. The primary exposure pathways are ingestion, inhalation, and dermal contact [
12].
Widespread marine contamination has made seafood and the broader food chain significant conduits for microplastic ingestion [
13]. Due to their small size, microplastics consumed by marine organisms may bioaccumulate and ultimately be transferred to humans through dietary intake [
14,
15]. Studies have confirmed the presence of microplastics in aquatic animals, table salt, honey, and sugar [
13,
16,
17]. Alarmingly, drinking water is also a notable source: microplastics have been detected in both bottled and tap water [
18]. A global study found microplastic contamination in 81% of 159 tap water samples, primarily consisting of fibers smaller than 5 mm [
19]. Other investigations revealed that both glass and plastic bottles contain comparable levels of microplastic particles [
20,
21]. It is estimated that individuals who exclusively consume bottled water may ingest up to 90,000 microplastic particles annually, compared to approximately 4000 from tap water [
22].
Airborne microplastics represent an emerging exposure concern [
7,
23,
24]. Recent studies have identified microplastic particles in the air. Notably, one study involving European participants reported an average of 9.18 ± 2.45 microplastic particles per 100 mL of bronchoalveolar lavage fluid, with a mean particle size of 1.73 ± 0.15 mm [
25]. Moreover, research has demonstrated that inhaled microplastics can translocate to the placenta and fetal tissues [
26].
Although dermal exposure is generally considered a less significant route, several factors influence skin permeability. Particle size plays a key role—nanoplastics are more likely to penetrate, while larger particles may infiltrate through hair follicles, sweat glands, or abrasions [
27,
28]. In vitro studies with primary skin cells demonstrate that nano-sized plastics can penetrate and accumulate in skin tissues, with particles up to 6 μm absorbed by keratinocytes [
29]. Additional research has shown that nanoplastics smaller than 200 nm can migrate through skin furrows, lipid channels, and hair follicles, reaching the viable epidermis and even being internalized by skin cells [
30].
A quantitative study on skin exposure revealed that after 24 h, the highest concentrations of microplastics were detected in scalp hair, followed by facial and hand skin. The accumulation on the scalp may be attributed to its larger surface area and electrostatic properties, which facilitate microplastic retention [
31]. Additionally, higher humidity levels were associated with increased microplastic adhesion. It has also been reported that substances such as oleic acid and ethanol can alter the lipid fluidity of the stratum corneum, thereby enhancing the transdermal absorption of microplastics [
32].
4. Dermatologic Effects of Microplastics
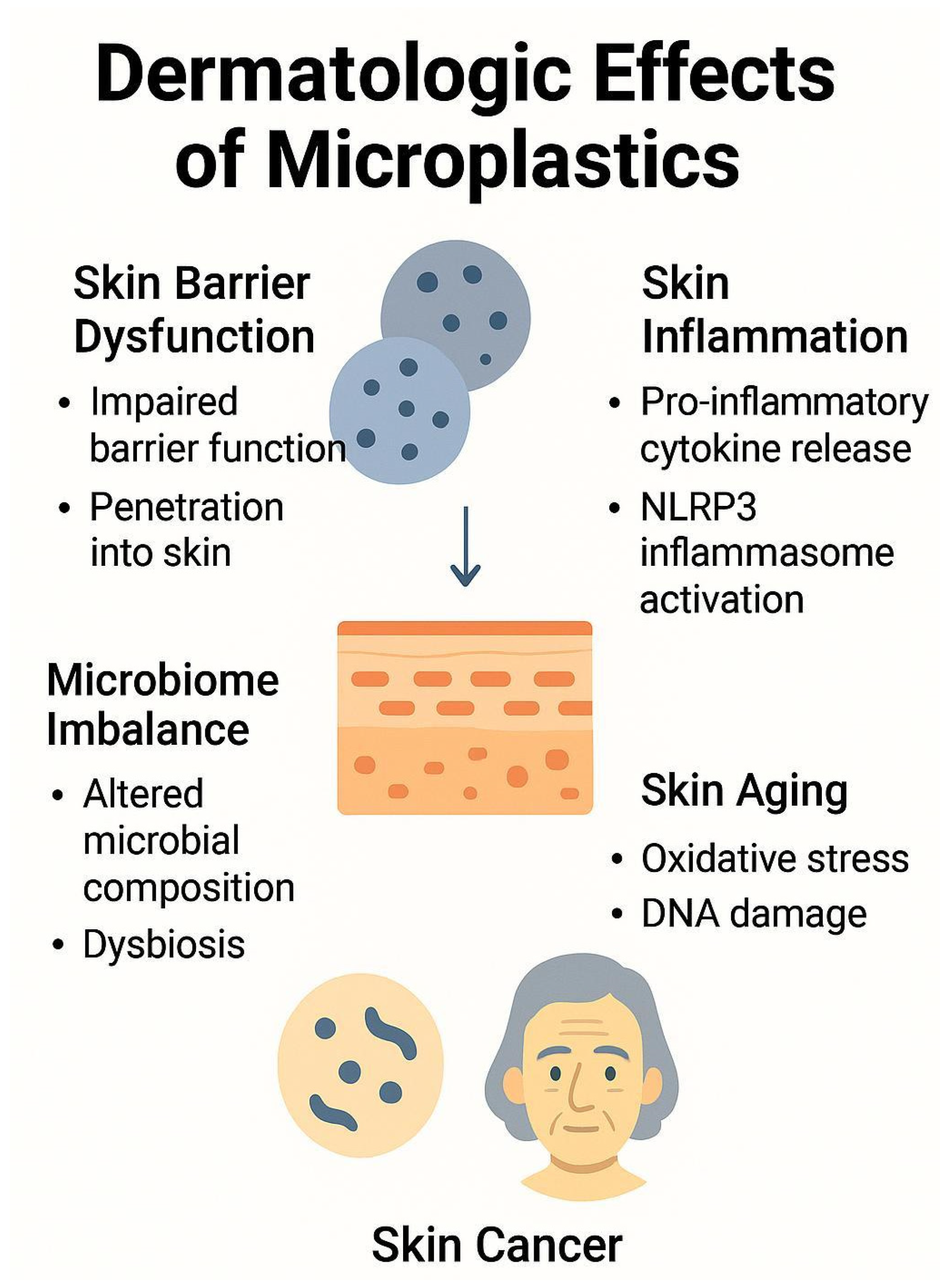
Microplastics and nanoplastics have been increasingly scrutinized for their potential effects on skin health (
Figure 1). Although much of the current evidence stems from in vitro and animal studies, emerging data suggest that these particles may disrupt skin barrier function, provoke inflammatory responses, alter the skin microbiome, accelerate aging processes, and possibly contribute to carcinogenesis (
Table 1) [
2]. While this review summarizes the current understanding of microplastic-induced dermatologic effects, it is important to note that most existing studies are based on in vitro experiments. Therefore, caution is warranted when interpreting these findings in the context of actual human health risks. Many of the experimental conditions do not fully reflect the complexity of real-life human exposure, including factors such as dermal absorption rates, skin barrier integrity, and chronic low-dose exposure. To accurately assess the potential health impacts of microplastics on human skin, further studies using in vivo models, human-relevant exposure conditions, and quantitative exposure assessments are needed.
4.1. Skin Barrier Dysfunction
A growing body of evidence indicates that microplastics and nanoplastics may compromise skin barrier integrity through both physical interaction and immune-mediated mechanisms [
2]. A recent study demonstrated that the introduction of nanoplastics into a stratum corneum membrane model significantly reduced the stability of the lipid layer. This effect was concentration-dependent, with nanoplastics tested at 0, 5, 10, 50, and 100 mg/L, and greater disruption was observed at higher concentrations, especially at 100 mg/L [
33]. Due to their minuscule size, microplastics and nanoplastics can penetrate the skin and interact with cells and structural biomolecules [
34,
48,
49].
Upon contact with the skin, these particles may trigger immune responses via the recognition of pathogen-associated and damage-associated molecular patterns by receptors expressed on keratinocytes, Langerhans cells, dendritic cells, melanocytes, macrophages, and T cells [
2]. The subsequent activation cascade results in the release of antimicrobial peptides and pro-inflammatory cytokines—including IL-1, IL-6, IL-10, IL-17, IL-18, IL-22, and TNF—which disrupt skin homeostasis, recruit additional immune cells, and weaken the structural integrity of the epidermis [
35,
50].
Furthermore, experimental models showed 5 nm sized nanoplastics can disrupt protein structure by inducing misfolding and denaturation and compromise lipid bilayers, thereby undermining cellular membrane integrity [
51,
52]. Prolonged exposure to nanoparticles (30–300 nm) may activate mitochondrial apoptotic pathways, increasing oxidative stress and inflammation, which in turn weakens the skin barrier and may predispose individuals to conditions such as atopic dermatitis [
34,
35,
36].
In vitro, microplastics and nanoplastics (200 nm to 6 µm) have also been shown to interfere with intercellular adhesion by activating the β-catenin signaling pathway, which reduces the expression of adhesion proteins such as E-cadherin and vinculin. This reduction compromises cell–cell junctions [
29], increases skin permeability, and exacerbates inflammatory responses [
49].
4.2. Skin Inflammation
Microplastics and nanoplastics have been detected in human blood and various organs, raising significant concern regarding their systemic and cutaneous effects. While much of the existing research has focused on internal organ systems—including the respiratory, digestive, reproductive, endocrine, nervous, and immune systems [
7,
12,
17]—an increasing number of studies are now highlighting their dermatological implications [
2]. Although the exact mechanisms by which microplastics and nanoplastics affect the skin remain to be fully elucidated, accumulating evidence suggests that they may trigger or exacerbate inflammatory skin conditions.
Experimental models have demonstrated that the internalization of polyethylene microplastics (8 μm, 1000 ng/L) by gill skin cells activates the NF-κB signaling pathway, a central mediator of inflammation and immune regulation. This activation leads to increased expression of pro-inflammatory cytokines, including TNF-α, IFN-γ, IL-2, IL-6, IL-8, and IL-1β, while concurrently suppressing anti-inflammatory cytokines such as IL-4 and IL-10 [
37]. This pro-inflammatory shift also promotes apoptosis via increased caspase-3 expression and activates the NLRP3 inflammasome, resulting in elevated IL-1β synthesis and immune overactivation [
37,
38]. The crosstalk between NF-κB signaling and the NLRP3 inflammasome appears to synergistically amplify inflammatory responses, contributing to the development or worsening of inflammatory skin conditions [
29,
53].
Notably, a study assessing the impact of fragmented polystyrene (fPS) ≤ 10 µm in size on human skin revealed that exposure of keratinocytes (HaCaT cells) and dermal fibroblasts to fPS resulted in cellular infiltration, increased cytotoxicity, and significant inflammatory responses. In a 3D human skin model, fPS particles penetrated the dermis within one hour, with particles smaller than 2 μm detected at an average maximum concentration of 4.7 μg. RNA sequencing identified marked upregulation of pro-inflammatory genes such as IL-1α, IL-1β, IL-18, IL-6, IL-8, ICAM-1, FOS, and JUN at both the mRNA and protein levels [
54].
Complementary findings from a zebrafish model demonstrated that the expression patterns of TNF-α and IL-1β varied depending on microplastic particle size (0.1 μm, 5 μm, and 200 μm). Similarly, primary skin cell studies showed that inflammatory responses were most significantly altered when exposed to nanoplastics and microplastics smaller than 6 μm. These results suggest that particle size is a critical determinant of immune reactivity, with smaller particles exhibiting a greater capacity to interact with and activate skin cells [
29].
4.3. Microbiome Imbalance
Microplastics have been implicated in disrupting the microbiome balance in various organ systems, including the skin. While most existing studies focus on the gastrointestinal microbiome, emerging research suggests that microplastics may also alter the composition and function of the skin microbiota.
Although direct studies on the skin microbiome remain limited, parallels can be drawn from evidence on the gut microbiome [
55,
56]. Ingestion of polyethylene microplastics has been shown to increase the abundance of potentially pathogenic bacterial groups such as
Firmicutes and
Bacteroidetes [
6,
40,
41] while reducing beneficial bacteria and butyrate production [
57]. Previous research using juvenile guppies exposed to polystyrene microplastics (32–40 μm, 100–1000 μg/L for 28 days) demonstrated that microplastics accumulated in the gut, reduced digestive enzyme activity, and triggered inflammatory immune responses. Moreover, gut microbiota diversity and functional capacity were significantly disrupted [
39]. Similar microbial imbalances—or dysbioses—are hypothesized to occur on the skin surface upon chronic exposure to microplastics.
Disruptions in the skin microbiome can compromise barrier integrity and immune modulation, potentially exacerbating inflammatory dermatoses such as atopic dermatitis, psoriasis, and acne [
58,
59,
60,
61,
62]. Moreover, changes in microbial diversity and the relative abundance of commensal vs. pathogenic species may influence cytokine production, sebum composition, and skin pH, further contributing to cutaneous inflammation [
58,
59,
60,
61,
62].
Cutibacterium acnes is widely recognized as a major contributor to acne development, with RT4 and RT5 strongly associated with acne-prone skin [
63,
64]. Notably, acne severity appears to be driven more by a loss of strain diversity—particularly the dominance of phylotype IA1—than by an overall increase in bacterial load [
61,
65]. Recent studies have identified notable differences in the skin microbiome between psoriasis patients and healthy individuals. In psoriasis, microbial diversity tends to be reduced, particularly in lesional skin [
66]. Psoriatic plaques often show an increased presence of
Staphylococcus aureus,
Staphylococcus epidermidis,
Bacillus spp.,
Corynebacterium spp., and
Klebsiella pneumoniae, while beneficial microbes like
Cutibacterium spp.,
Actinobacteria spp., and
Bacteroides spp. are decreased [
67,
68].
Emerging research highlights the bidirectional relationship between the gut and skin, known as the gut–skin axis [
69,
70]. Dysbiosis of the gut microbiome caused by microplastics can result in the systemic release of inflammatory mediators and metabolic byproducts that negatively affect skin health [
70]. Hence, microplastic-induced microbial dysregulation may have both local and systemic implications for dermatologic conditions [
69].
4.4. Skin Aging
Microplastics and nanoplastics have been shown to induce oxidative stress, contributing to tissue toxicity. In a mouse model, oral administration of microplastics and nanoplastics of various sizes (50 nm, 500 nm, and 5000 nm) led to increased particle distribution across multiple organs and elevated reactive oxygen species (ROS) production [
42]. Similarly, a study using primary gill epidermal cells demonstrated that these polyethylene microplastics (8 μm, 1000 ng/L) generate ROS and suppressed antioxidant enzyme activity, resulting in DNA damage and apoptosis [
37].
In in vitro studies using BEAS-2B bronchial epithelial cells, nanopolystyrene particles (~50 nm, 10 and 50 μg/mL) were internalized by cells, leading to increased ROS production, mitochondrial membrane depolarization, and early apoptosis. These exposures also induced autophagy and endoplasmic reticulum stress, accompanied by elevated levels of amino acids and TCA cycle intermediates, as well as upregulated expression of stress markers such as LC3-II, ATF6, DDIT3, and ERN1 [
43,
44]. When the body’s antioxidant defense mechanisms are overwhelmed and unable to effectively neutralize ROS, oxidative stress can accelerate skin cell aging [
71,
72]. An in vitro study using two skin cell lines showed that nano-sized microplastics were internalized in a time- and dose-dependent manner, leading to increased inflammation and cellular senescence via upregulation of p16 and p21. Mitochondrial oxidative stress and AIM2 inflammasome activation were identified as key mechanisms [
45].
These findings collectively suggest that microplastic exposure may contribute to oxidative stress-induced skin damage and premature aging.
4.5. Skin Cancer
Emerging evidence suggests that microplastics can infiltrate cells, disrupt key biological processes, and create a carcinogenic microenvironment [
5,
73]. These particles contribute to cancer development through multiple mechanisms, including the induction of DNA damage, oxidative stress, inflammation, and interference with cellular signaling pathways.
In vitro research has shown that microplastics (1 μm, 0.25 to 1.0 mg/mL) penetrate squamous cell carcinoma cell lines (SCL-1, A431), enhancing cell proliferation and accelerating cell cycle progression, as indicated by increased expression of Cyclin D1, c-Myc, Bcl-2, and Ki-67. This is associated with the induction of mitochondrial ROS, disruption of mitochondrial membrane potential, and opening of the mitochondrial permeability transition pore, which leads to the release of mitochondrial DNA into the cytoplasm. The cytosolic mitochondrial DNA subsequently activates the NLRP3 inflammasome, contributing to the formation of a pro-inflammatory microenvironment and further enhancing tumor cell proliferation [
46]. In addition, a previous report indicated that microplastics activate signaling pathways such as ERK, JNK/SAPK, p38, and MAPK, which are associated with tumorigenesis and uncontrolled cell proliferation [
73].
Moreover, microplastics trigger inflammatory responses by activating the NLRP3 inflammasome and upregulating pro-inflammatory mediators such as TNF-α, IFN-γ, TLR4, AP-1, IRFs, Th17, IL-6, and IL-1β [
37,
46,
74]. These inflammatory cascades promote tumor initiation, angiogenesis, invasion, and metastasis. Concurrently, microplastics impair immune surveillance by disrupting the immune system’s capacity to recognize and eliminate malignant cells, thereby facilitating immune evasion and cancer progression [
73,
75]
Additionally, microplastics may contribute to tumorigenesis through endocrine disruption and genetic and epigenetic alterations, including DNA methylation, histone modification, and microRNA dysregulation. In a study using fathead minnows (
Pimephales promelas), researchers examined DNA methylation changes induced by polyethylene microplastics (150–500 μm) at concentrations of 100 and 2000 particles/L. Persistent methylation changes were also observed in the F1 generation, indicating a transgenerational epigenetic effect. The differentially methylated genes were associated with hormone signaling, metabolism, inflammation, and tRNA function [
47]. Acting as vectors, microplastics can also carry and concentrate carcinogenic substances such as persistent organic pollutants and heavy metals, further amplifying their toxic effects. Notably, even at environmentally relevant concentrations, microplastics have been shown to induce cytotoxicity, compromise skin barrier integrity, and alter gene expression profiles [
73].
5. Microplastics and Cosmetics
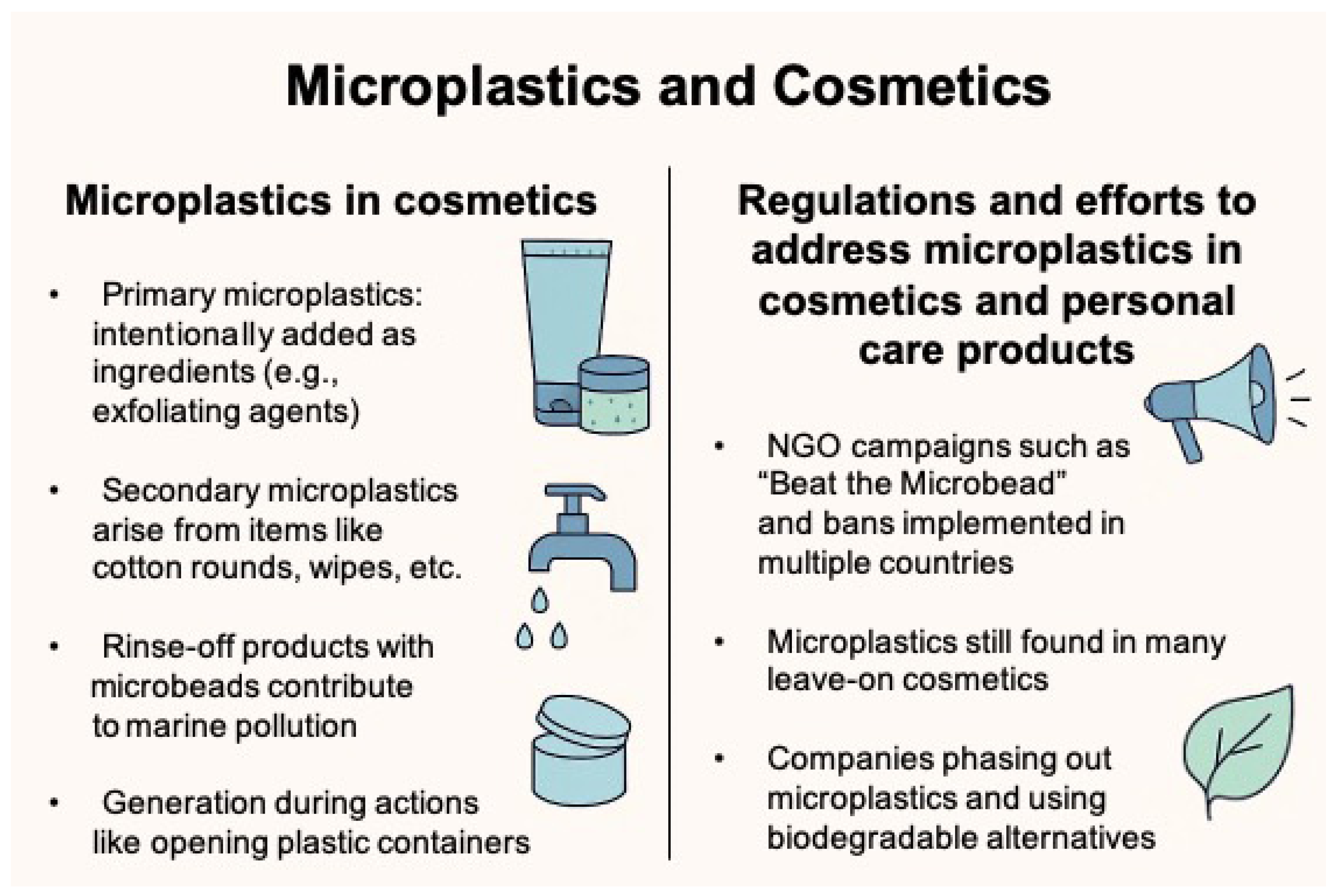
5.1. Microplastics in Cosmetics
Microplastics in cosmetics are broadly categorized into primary microplastics, which are intentionally added as ingredients, and secondary microplastics, which originate from the degradation or abrasion of cosmetic packaging materials (
Figure 2) [
8]. Primary microplastics are incorporated into cosmetic formulations to fulfill various functional roles, e.g., exfoliation, cleansing, opacity modulation, imparting a smooth or silky texture, and achieving an illuminating effect on the skin. They can be used in lipstick, loose or pressed powders, and liquid or thick emulsions with a powdery feel and may also be used as a carrier for other ingredients.
Among environmental plastic pollutants, those derived from personal care and cosmetic products (PCCPs) constitute a significant share, particularly due to their high particle count and widespread usage [
76,
77].
Microbeads, a common type of primary microplastic, are small plastic particles found in various PCCPs such as shampoos, toothpaste, exfoliants, nail art products, and glitter [
76,
78]. These particles vary in size and composition, often exhibiting irregular shapes and the ability to aggregate into larger masses [
78]. Microbeads used in facial scrubs or body exfoliants typically have rough, uneven surfaces, which confer a high surface-area-to-volume ratio, enhancing their capacity to adsorb and transport harmful substances [
79,
80].
Once used, rinse-off products containing microbeads enter household drainage systems and are not completely removed by conventional wastewater treatment facilities. As a result, these particles can be released into natural water systems and eventually accumulate in marine environments [
80,
81].
Beyond direct cosmetic ingredients, recent studies have revealed that microplastics can also originate from cosmetic packaging. For instance, microplastic fragments are generated during routine actions such as opening, cutting, or tearing plastic containers [
82]. This highlights packaging as an underrecognized source of microplastic pollution. While primary microplastics used in toxicology studies often have standardized shapes and sizes, secondary microplastics are highly variable, making risk assessment more complex [
40]. These particles can be cubic, spherical, rod-shaped, or even jagged, with sharp-edged microplastics posing a particular concern due to their potential to cause physical irritation or injury within biological tissues [
7].
5.2. Regulations and Efforts to Address Microplastics in Cosmetics and Personal Care Products
As awareness has grown regarding the environmental and health hazards associated with microplastics in personal care and cosmetic products (PCCPs), coordinated efforts by governments, industries, and environmental organizations have accelerated. Non-governmental organizations have played a pivotal role in raising public awareness and prompting regulatory action. One of the most influential campaigns, “Beat the Microbead”, was launched in 2012 by the Plastic Soup Foundation. This initiative identifies microplastic-containing products, provides consumers with an ingredient-scanning app, and advocates for global bans on microplastics in cosmetics (
https://www.beatthemicrobead.org/about-us/ (accessed on 1 June 2025)). In response to such advocacy and mounting scientific evidence, several countries have implemented regulatory measures (
Table 2). In 2015, the United States enacted the Microbead-Free Waters Act, banning the use of plastic microbeads in rinse-off cosmetics [
8]. Canada followed with a ban in 2016 targeting exfoliating and cleansing products, while South Korea introduced a similar ban in 2017 [
7]. Other countries, including Italy, Sweden, the United Kingdom, and New Zealand, have also enforced national prohibitions on the use of microplastics in certain cosmetic formulations. More recently, the European Union adopted Commission Regulation (EU) 2023/2055, which restricts the intentional addition of microplastics in a broad range of products.
However, regulatory coverage remains incomplete and inconsistently applied across product categories. While rinse-off products such as facial cleansers and scrubs are frequently targeted by legislation, microplastics are still commonly found in leave-on products, particularly color cosmetics like lipsticks and eye makeup. A 2022 analysis by the Plastic Soup Foundation reported that 87% of products from major European cosmetic brands contained microplastic ingredients, highlighting the limited scope of current regulations. Furthermore, definitions of what constitutes a microplastic vary across jurisdictions. The European Chemicals Agency [
83], for instance, does not currently classify engineered nanoplastics, water-soluble polymers, liquid and semi-solid polymers, or biodegradable polymers as microplastics, despite growing concerns about their similar ecological and toxicological impacts. Environmental groups and scientists have criticized this limited definition, warning that it may allow potentially harmful substances to evade regulation. The Plastic Soup Foundation has called for a more comprehensive and inclusive definition of microplastics within EU policy frameworks. In addition, current analytical techniques for microplastic identification and quantification are not yet sufficiently standardized or optimized for regulatory enforcement. While methods such as micro-FTIR and Raman spectroscopy, Py-GCMS, optical microscopy, scanning electron microscopy, and thermogravimetric analysis offer potential, they remain time-consuming and technically demanding, particularly when applied to complex formulations such as leave-on cosmetic products [
8,
84]. These products often contain emulsifiers, oils, and silicones, which complicate the separation and analysis of microplastic particles and may even degrade them during sample preparation [
85,
86]. As a result, establishing a rapid, reproducible, and robust testing framework across diverse product categories remains a significant challenge for regulatory bodies. In parallel with regulatory initiatives, the cosmetics industry has increasingly acknowledged the need to reduce its environmental footprint and has taken proactive steps to eliminate microplastics. Unilever, for example, phased out plastic scrub beads from its products as early as 2014. In South Korea, companies such as Amorepacific and LG Household & Health Care began reformulating exfoliating products in 2009, substituting microplastics with biodegradable natural alternatives like cellulose, walnut shell powder, perlite (volcanic ash), and silica. Similarly, Korean cosmetic manufacturers Cosmax and Kolmar Korea have explored innovative plant-based alternatives, such as pear cell-derived ingredients, to minimize microplastic content. In 2022, Kolmar Korea further advanced its sustainability efforts by developing a technology that replaces microplastics in color cosmetics with environmentally friendly silica particles.
Together, these regulatory actions and industry-led innovations represent meaningful progress toward reducing microplastic pollution from the cosmetic sector. However, significant challenges remain, particularly in harmonizing definitions, expanding regulatory scope, and ensuring the safety and efficacy of alternative materials.
6. Future Perspectives
6.1. Alternatives to Conventional Synthetic Polymers
In response to the environmental and health concerns associated with microplastics, there is a growing emphasis on developing biopolymers as sustainable alternatives to conventional synthetic polymers, such as polyethylene, in cosmetic packaging. Biopolymers are derived from natural sources, including plant biomass, cellulose, and microbial metabolites, and are gaining traction due to their biodegradability, biocompatibility, and lower ecological impact [
83].
Among these, poly (lactic acid) [
74]—a biodegradable aliphatic polyester synthesized through the fermentation of sugars—is widely recognized for its non-toxicity, high mechanical strength, and biocompatibility. PLA is already utilized in food packaging, tissue engineering, pharmaceuticals, and cosmetic packaging [
87]. Under natural conditions, particularly sunlight exposure, PLA can biodegrade completely within six months to one year, making it a viable alternative for single-use plastic applications [
83,
88].
Starch-based polymers represent another promising group of bioplastics. Composed primarily of amylose and amylopectin, starch is sourced from potatoes, corn, rice, and wheat [
89]. These polymers are low in toxicity, biocompatible, and highly biodegradable. Their functional properties can be enhanced through the incorporation of plasticizers such as citric acid, sorbitol, or glycerol, improving their flexibility and applicability in cosmetic packaging [
83,
90].
Polybutylene succinate (PBS), which is primarily used in packaging applications, is synthesized via the polycondensation of succinic acid and butanediol. It exhibits excellent thermal stability and mechanical performance. However, high production costs currently hinder its widespread commercial adoption [
91].
In parallel, innovative approaches are being explored to develop biomaterials from renewable and waste-derived sources, including seafood waste (e.g., shrimp and shellfish shells), fruit and agricultural byproducts, and marine plastic debris [
92,
93,
94]. Furthermore, bio-composite materials incorporating coconut fibers, jute fibers, and other lignocellulosic materials are under active investigation for cosmetic and packaging applications due to their enhanced sustainability and mechanical performance [
95,
96].
Within cosmetic formulations, carbohydrate-based biopolymers—such as cellulose beads and starch-based granules—as well as natural exfoliating agents, including salt, sugar, coffee grounds, bamboo powder, almond and apricot seed powders, walnut shell powder, and pumice (volcanic rock), offer effective and biodegradable alternatives to synthetic microbeads, supporting a shift toward eco-conscious product development.
However, most existing studies have evaluated the degradation kinetics of biodegradable polymers and microplastic additives under idealized laboratory or industrial composting conditions, which may not accurately reflect real-world environments such as wastewater systems or marine ecosystems where cosmetic products are ultimately discharged. This study has a limitation in that it does not provide comparative half-life data under realistic environmental conditions. However, future research should aim to generate such data and also address the potential for biodegradable alternatives to fragment into secondary nanoplastics during their use and degradation processes.
6.2. Approaches to Microplastic Removal
Several promising strategies have emerged for mitigating microplastic pollution through biological means. Studies have demonstrated the potential of microorganisms—including bacteria (e.g.,
Pseudomonas,
Bacillus,
Rhodococcus), fungi (e.g.,
Aspergillus,
Penicillium), and microalgae (e.g.,
Chlamydomonas reinhardtii)—to biodegrade microplastics, offering cost-effective and eco-friendly alternatives to conventional physical or chemical degradation processes [
97,
98,
99,
100,
101,
102,
103]. These microbial strategies harness specific enzymes, such as hydrolases and oxidases, to break down plastic polymers into smaller, non-toxic byproducts [
99,
104,
105]. Although the practical application of microplastic biodegradation remains limited, recent studies have reported the use of genetic engineering to enhance the microplastic degradation of microbial strains such as
Ideonella sakaiensis [
106].
In parallel, novel biomedical approaches are being explored to address internal microplastic accumulation. For instance, specific probiotic strains such as
Lactiplantibacillus plantarum DT88 and
Lacticaseibacillus paracasei DT66 have shown the ability to adsorb polystyrene particles within the gastrointestinal tract, promoting excretion and alleviating intestinal inflammation in mice [
107]. These findings suggest that strain-specific probiotics may serve as a future therapeutic option to reduce the health risks associated with microplastic ingestion.
7. Conclusions
Microplastics in cosmetics have emerged as a pressing concern for both human health and environmental sustainability. Although regulatory actions have been implemented in several countries—including the United States, South Korea, and the European Union—microplastics continue to be introduced into the environment through both direct use and packaging-related sources, indicating that current measures are insufficient. Evidence suggests that microplastic exposure may disrupt skin barrier integrity, trigger inflammation, induce oxidative stress, and accelerate skin aging, underscoring the need for safer alternatives.
To address these challenges, recent advancements in biopolymer technologies, sustainable packaging, and the use of natural exfoliants provide promising avenues for reducing microplastic pollution. However, further multidisciplinary research is essential to better understand the long-term health effects of microplastics and to guide the establishment of a comprehensive, globally harmonized regulatory framework.
As public concern and scientific evidence continue to mount, the cosmetics industry must take proactive steps to phase out microplastic ingredients, invest in eco-friendly innovations, and transition toward sustainable product development. Such efforts will not only help mitigate environmental damage but also safeguard consumer health and align with global sustainability goals.
Author Contributions
Conceptualization, J.H.H. and H.S.K.; writing—original draft preparation, J.H.H. and H.S.K.; writing—review and editing, J.H.H. and H.S.K.; supervision, H.S.K.; project administration, H.S.K.; funding acquisition, H.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant number: 2023R1A2C1007759) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2023-KH-136575 and RS-2025-02217860).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Williams, A.T.; Rangel-Buitrago, N. The past, present, and future of plastic pollution. Mar. Pollut. Bull. 2022, 176, 113429. [Google Scholar] [CrossRef]
- Aristizabal, M.; Jimenez-Orrego, K.V.; Caicedo-Leon, M.D.; Paez-Cardenas, L.S.; Castellanos-Garcia, I.; Villalba-Moreno, D.L.; Ramirez-Zuluaga, L.V.; Hsu, J.T.S.; Jaller, J.; Gold, M. Microplastics in dermatology: Potential effects on skin homeostasis. J. Cosmet. Dermatol. 2024, 23, 766–772. [Google Scholar] [CrossRef]
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty years of microplastic pollution research-what have we learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef]
- Ramsperger, A.; Bergamaschi, E.; Panizzolo, M.; Fenoglio, I.; Barbero, F.; Peters, R.; Undas, A.; Purker, S.; Giese, B.; Lalyer, C.R.; et al. Nano- and microplastics: A comprehensive review on their exposure routes, translocation, and fate in humans. NanoImpact 2023, 29, 100441. [Google Scholar] [CrossRef]
- Dzierzynski, E.; Gawlik, P.J.; Puzniak, D.; Flieger, W.; Jozwik, K.; Teresinski, G.; Forma, A.; Wdowiak, P.; Baj, J.; Flieger, J. Microplastics in the Human Body: Exposure, Detection, and Risk of Carcinogenesis: A State-of-the-Art Review. Cancers 2024, 16, 3703. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Cho, J.; Sohn, J.; Kim, C. Health Effects of Microplastic Exposures: Current Issues and Perspectives in South Korea. Yonsei Med. J. 2023, 64, 301–308. [Google Scholar] [CrossRef]
- Kukkola, A.; Chetwynd, A.J.; Krause, S.; Lynch, I. Beyond microbeads: Examining the role of cosmetics in microplastic pollution and spotlighting unanswered questions. J. Hazard. Mater. 2024, 476, 135053. [Google Scholar] [CrossRef]
- Anagnosti, L.; Varvaresou, A.; Pavlou, P.; Protopapa, E.; Carayanni, V. Worldwide actions against plastic pollution from microbeads and microplastics in cosmetics focusing on European policies. Has the issue been handled effectively? Mar. Pollut. Bull. 2021, 162, 111883. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef]
- Chandra, S.; Walsh, K.B. Microplastics in water: Occurrence, fate and removal. J. Contam. Hydrol. 2024, 264, 104360. [Google Scholar] [CrossRef]
- Zhao, B.; Rehati, P.; Yang, Z.; Cai, Z.; Guo, C.; Li, Y. The potential toxicity of microplastics on human health. Sci. Total Environ. 2024, 912, 168946. [Google Scholar] [CrossRef]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Response to Comment on “Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver”. Environ. Sci. Technol. 2016, 50, 12523–12524. [Google Scholar] [CrossRef]
- Winiarska, E.; Jutel, M.; Zemelka-Wiacek, M. The potential impact of nano- and microplastics on human health: Understanding human health risks. Environ. Res. 2024, 251, 118535. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Furst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ossmann, B.E.; Sarau, G.; Holtmannspotter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.C.; Saha, G. Effect of microplastics deposition on human lung airways: A review with computational benefits and challenges. Heliyon 2024, 10, e24355. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Wu, H.; Li, S.; Liu, Z.; Zhang, Y. Airborne micro- and nanoplastics: Emerging causes of respiratory diseases. Part. Fibre Toxicol. 2024, 21, 50. [Google Scholar] [CrossRef]
- Baeza-Martinez, C.; Olmos, S.; Gonzalez-Pleiter, M.; Lopez-Castellanos, J.; Garcia-Pachon, E.; Masia-Canuto, M.; Hernandez-Blasco, L.; Bayo, J. First evidence of microplastics isolated in European citizens’ lower airway. J. Hazard. Mater. 2022, 438, 129439. [Google Scholar] [CrossRef]
- Fournier, S.B.; D’Errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.J.; Fabris, L.; Yurkow, E.J.; Stapleton, P.A. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 2020, 17, 55. [Google Scholar] [CrossRef]
- Bastyans, S.; Jackson, S.; Fejer, G. Micro and nano-plastics, a threat to human health? Emerg. Top. Life Sci. 2022, 6, 411–422. [Google Scholar]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Derm.-Endocrinol. 2009, 1, 197–206. [Google Scholar] [CrossRef]
- Schmidt, A.; da Silva Brito, W.A.; Singer, D.; Muhl, M.; Berner, J.; Saadati, F.; Wolff, C.; Miebach, L.; Wende, K.; Bekeschus, S. Short- and long-term polystyrene nano- and microplastic exposure promotes oxidative stress and divergently affects skin cell architecture and Wnt/beta-catenin signaling. Part. Fibre Toxicol. 2023, 20, 3. [Google Scholar] [CrossRef]
- Doge, N.; Hadam, S.; Volz, P.; Wolf, A.; Schonborn, K.H.; Blume-Peytavi, U.; Alexiev, U.; Vogt, A. Identification of polystyrene nanoparticle penetration across intact skin barrier as rare event at sites of focal particle aggregations. J. Biophotonics 2018, 11, e201700169. [Google Scholar] [CrossRef]
- Abbasi, S.; Turner, A. Human exposure to microplastics: A study in Iran. J. Hazard. Mater. 2021, 403, 123799. [Google Scholar] [CrossRef]
- Kuo, T.R.; Wu, C.L.; Hsu, C.T.; Lo, W.; Chiang, S.J.; Lin, S.J.; Dong, C.Y.; Chen, C.C. Chemical enhancer induced changes in the mechanisms of transdermal delivery of zinc oxide nanoparticles. Biomaterials 2009, 30, 3002–3008. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Hu, J.; Guo, C.; Ye, Z.; Shang, Y.; Lian, C.; Liu, H. The effects of size and surface functionalization of polystyrene nanoplastics on stratum corneum model membranes: An experimental and computational study. J. Colloid. Interface Sci. 2023, 638, 778–787. [Google Scholar] [CrossRef]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef]
- Celebi Sozener, Z.; Cevhertas, L.; Nadeau, K.; Akdis, M.; Akdis, C.A. Environmental factors in epithelial barrier dysfunction. J. Allergy Clin. Immunol. 2020, 145, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.M.; Twayana, K.S.; Ravanan, P.; John, T.; Mukherjee, A.; Jenkins, D.F.; Chandrasekaran, N. Prospects on the nano-plastic particles internalization and induction of cellular response in human keratinocytes. Part. Fibre Toxicol. 2021, 18, 35. [Google Scholar] [CrossRef]
- Cao, J.; Xu, R.; Wang, F.; Geng, Y.; Xu, T.; Zhu, M.; Lv, H.; Xu, S.; Guo, M.Y. Polyethylene microplastics trigger cell apoptosis and inflammation via inducing oxidative stress and activation of the NLRP3 inflammasome in carp gills. Fish Shellfish. Immunol. 2023, 132, 108470. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-kappaB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Huang, J.N.; Wen, B.; Zhu, J.G.; Zhang, Y.S.; Gao, J.Z.; Chen, Z.Z. Exposure to microplastics impairs digestive performance, stimulates immune response and induces microbiota dysbiosis in the gut of juvenile guppy (Poecilia reticulata). Sci. Total Environ. 2020, 733, 138929. [Google Scholar] [CrossRef]
- Emenike, E.C.; Okorie, C.J.; Ojeyemi, T.; Egbemhenghe, A.; Iwuozor, K.O.; Saliu, O.D.; Okoro, H.K.; Adeniyi, A.G. From oceans to dinner plates: The impact of microplastics on human health. Heliyon 2023, 9, e20440. [Google Scholar] [CrossRef] [PubMed]
- Souza-Silva, T.G.; Oliveira, I.A.; Silva, G.G.D.; Giusti, F.C.V.; Novaes, R.D.; Paula, H.A.A. Impact of microplastics on the intestinal microbiota: A systematic review of preclinical evidence. Life Sci. 2022, 294, 120366. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Ng, C.T.; Zou, L.; Lu, Y.; Chen, J.; Bay, B.H.; Shen, H.M.; Ong, C.N. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells. Nanotoxicology 2019, 13, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Cui, J.; Sun, G.; Miao, X.; Pufang, Z.; Nannan, L. Nano-sized microplastics exposure induces skin cell senescence via triggering the mitochondrial localization of GSDMD. Environ. Pollut. 2024, 349, 123874. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Jiang, G. Microplastics exposure promotes the proliferation of skin cancer cells but inhibits the growth of normal skin cells by regulating the inflammatory process. Ecotoxicol. Environ. Saf. 2023, 267, 115636. [Google Scholar] [CrossRef]
- Wade, M.J.; Bucci, K.; Rochman, C.M.; Meek, M.H. Microplastic exposure is associated with epigenomic effects in the model organism Pimephales promelas (fathead minnow). J. Hered. 2025, 116, 113–125. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Yee, M.S.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Holloczki, O.; Gehrke, S. Can Nanoplastics Alter Cell Membranes? Chemphyschem 2020, 21, 9–12. [Google Scholar] [CrossRef]
- Holloczki, O.; Gehrke, S. Nanoplastics can change the secondary structure of proteins. Sci. Rep. 2019, 9, 16013. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, J.; Ferrara, F.; Vallese, A.; Guiotto, A.; Colella, S.; Pecorelli, A.; Valacchi, G. Comparison of Pollutant Effects on Cutaneous Inflammasomes Activation. Int. J. Mol. Sci. 2023, 24, 16674. [Google Scholar] [CrossRef] [PubMed]
- Song, G.B.; Nam, J.; Ji, S.; Woo, G.; Park, S.; Kim, B.; Hong, J.; Choi, M.G.; Kim, S.; Lee, C.; et al. Deciphering the links: Fragmented polystyrene as a driver of skin inflammation. J. Hazard. Mater. 2024, 480, 135815. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Chi, J.; Patterson, J.S.; Jin, Y.; Kim, K.J.; Lalime, N.; Hawley, D.; Lewis, F.; Li, L.; Wang, X.; Campen, M.J.; et al. Metabolic Reprogramming in Gut Microbiota Exposed to Polystyrene Microplastics. Biomedicines 2025, 13, 446. [Google Scholar] [CrossRef]
- Fournier, E.; Ratel, J.; Denis, S.; Leveque, M.; Ruiz, P.; Mazal, C.; Amiard, F.; Edely, M.; Bezirard, V.; Gaultier, E.; et al. Exposure to polyethylene microplastics alters immature gut microbiome in an infant in vitro gut model. J. Hazard. Mater. 2023, 443, 130383. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.S. Prurigo nodularis and the microbiome. Clin. Dermatol. 2025, in press. [Google Scholar] [CrossRef]
- Woo, Y.R.; Kim, H.S. Interaction between the microbiota and the skin barrier in aging skin: A comprehensive review. Front. Physiol. 2024, 15, 1322205. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.S. Microbiome of the Skin and Gut in Atopic Dermatitis (AD): Understanding the Pathophysiology and Finding Novel Management Strategies. J. Clin. Med. 2019, 8, 444. [Google Scholar] [CrossRef]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef]
- Kim, H.S.; Keum, H.L.; Chung, I.Y.; Nattkemper, L.; Head, C.R.; Koh, A.; Sul, W.J.; Pastar, I.; Yosipovitch, G. Characterization of a Perturbed Skin Microbiome in Prurigo Nodularis and Lichen Simplex Chronicus. J. Investig. Dermatol. 2023, 143, 2082–2085.e2085. [Google Scholar] [CrossRef]
- Dreno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21, 18–24. [Google Scholar] [CrossRef]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Pecastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32 (Suppl. S2), 5–14. [Google Scholar] [CrossRef]
- Alekseyenko, A.V.; Perez-Perez, G.I.; De Souza, A.; Strober, B.; Gao, Z.; Bihan, M.; Li, K.; Methe, B.A.; Blaser, M.J. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome 2013, 1, 31. [Google Scholar] [CrossRef]
- Radaschin, D.S.; Iancu, A.V.; Ionescu, A.M.; Gurau, G.; Niculet, E.; Bujoreanu, F.C.; Beiu, C.; Tatu, A.L.; Popa, L.G. Comparative Analysis of the Cutaneous Microbiome in Psoriasis Patients and Healthy Individuals-Insights into Microbial Dysbiosis: Final Results. Int. J. Mol. Sci. 2024, 25, 10583. [Google Scholar] [CrossRef] [PubMed]
- Fahlen, A.; Engstrand, L.; Baker, B.S.; Powles, A.; Fry, L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch. Dermatol. Res. 2012, 304, 15–22. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef] [PubMed]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Kumar, N.; Lamba, M.; Pachar, A.K.; Yadav, S.; Acharya, A. Microplastics—A Growing Concern as Carcinogens in Cancer Etiology: Emphasis on Biochemical and Molecular Mechanisms. Cell Biochem. Biophys. 2024, 82, 3109–3121. [Google Scholar] [CrossRef]
- Yurchenko, A.A.; Rajabi, F.; Braz-Petta, T.; Fassihi, H.; Lehmann, A.; Nishigori, C.; Wang, J.; Padioleau, I.; Gunbin, K.; Panunzi, L.; et al. Genomic mutation landscape of skin cancers from DNA repair-deficient xeroderma pigmentosum patients. Nat. Commun. 2023, 14, 2561. [Google Scholar] [CrossRef]
- Yang, W.; Jannatun, N.; Zeng, Y.; Liu, T.; Zhang, G.; Chen, C.; Li, Y. Impacts of microplastics on immunity. Front. Toxicol. 2022, 4, 956885. [Google Scholar] [CrossRef]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Deng, L.; Li, G.; Peng, S.; Wu, J.; Che, Y. Microplastics in personal care products: Exploring public intention of usage by extending the theory of planned behaviour. Sci. Total Environ. 2022, 848, 157782. [Google Scholar] [CrossRef]
- Cheung, P.K.; Fok, L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bostan, N.; Ilyas, N.; Akhtar, N.; Mehmood, S.; Saman, R.U.; Sayyed, R.Z.; Shatid, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Pandiaraj, S. Toxicity assessment of microplastic (MPs); A threat to the ecosystem. Environ. Res. 2023, 234, 116523. [Google Scholar] [CrossRef]
- Kan, C.; Wang, F.; Xiang, T.; Fan, Y.; Xu, W.; Liu, L.; Yang, S.; Cao, W. Wastewater treatment plant effluents increase the global warming potential in a subtropical urbanized river. Water Res. 2024, 266, 122349. [Google Scholar] [CrossRef] [PubMed]
- Engler, R.E. The complex interaction between marine debris and toxic chemicals in the ocean. Environ. Sci. Technol. 2012, 46, 12302–12315. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 4841. [Google Scholar] [CrossRef] [PubMed]
- Cubas, A.L.V.; Bianchet, R.T.; Reis, I.; Gouveia, I.C. Plastics and Microplastic in the Cosmetic Industry: Aggregating Sustainable Actions Aimed at Alignment and Interaction with UN Sustainable Development Goals. Polymers 2022, 14, 4576. [Google Scholar] [CrossRef]
- Aprea, A.; Mariani, D.; Trimigno, E.; Marcucci, C.; Cortella, R. Microplastics detection in some cosmetic samples by accelerated solvent extraction and Micro-FTIR. Talanta 2025, 283, 127190. [Google Scholar] [CrossRef]
- Savary, G.; Grisel, M.; Picard, C. Impact of emollients on the spreading properties of cosmetic products: A combined sensory and instrumental characterization. Colloids Surf. B Biointerfaces 2013, 102, 371–378. [Google Scholar] [CrossRef]
- Schrank, I.; Moller, J.N.; Imhof, H.K.; Hauenstein, O.; Zielke, F.; Agarwal, S.; Loder, M.G.J.; Greiner, A.; Laforsch, C. Microplastic sample purification methods—Assessing detrimental effects of purification procedures on specific plastic types. Sci. Total Environ. 2022, 833, 154824. [Google Scholar] [CrossRef]
- Swetha, T.A.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A review on biodegradable polylactic acid (PLA) production from fermentative food waste—Its applications and degradation. Int. J. Biol. Macromol. 2023, 234, 123703. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable Polylactic Acid and Its Composites: Characteristics, Processing, and Sustainable Applications in Sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, J.; Liu, Y.; Zou, S.Y.; Li, L. Hierarchical Structure and Thermal Property of Starch-Based Nanocomposites with Different Amylose/Amylopectin Ratio. Polymers 2019, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; Sreekala, M.S.; Kunaver, M.; Huskic, M.; Thomas, S. Morphology, transport characteristics and viscoelastic polymer chain confinement in nanocomposites based on thermoplastic potato starch and cellulose nanofibers from pineapple leaf. Carbohydr. Polym. 2017, 169, 176–188. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-Based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Wan, M.C.; Qin, W.; Lei, C.; Li, Q.H.; Meng, M.; Fang, M.; Song, W.; Chen, J.H.; Tay, F.; Niu, L.N. Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.; Lima, M.A.B.; Franco, L.O.; Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Mahmud, M.Z.A.; Mobarak, M.H.; Hossain, N. Emerging trends in biomaterials for sustainable food packaging: A comprehensive review. Heliyon 2024, 10, e24122. [Google Scholar] [CrossRef]
- Kuram, E. Natural Fiber-Based Polymer Composites for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2025, 36, 1027–1084. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Horvath, P.G.; Koczan, Z.; Alpar, T. Thermo-mechanical properties of pretreated coir fiber and fibrous chips reinforced multilayered composites. Sci. Rep. 2021, 11, 3618. [Google Scholar] [CrossRef]
- Moog, D.; Schmitt, J.; Senger, J.; Zarzycki, J.; Rexer, K.H.; Linne, U.; Erb, T.; Maier, U.G. Using a marine microalga as a chassis for polyethylene terephthalate (PET) degradation. Microb. Cell Fact. 2019, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.Y.; Ying Tang, D.Y.; Khoo, K.S.; Kay Lup, A.N.; Chew, K.W. Nature’s fight against plastic pollution: Algae for plastic biodegradation and bioplastics production. Environ. Sci. Ecotechnol 2020, 4, 100065. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Park, S.B.; Tran, Q.G.; Cho, D.H.; Choi, D.Y.; Lee, Y.J.; Kim, H.S. Functional expression of polyethylene terephthalate-degrading enzyme (PETase) in green microalgae. Microb. Cell Fact. 2020, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Barragan, J.; Dominguez-Malfavon, L.; Vargas-Suarez, M.; Gonzalez-Hernandez, R.; Aguilar-Osorio, G.; Loza-Tavera, H. Biodegradative Activities of Selected Environmental Fungi on a Polyester Polyurethane Varnish and Polyether Polyurethane Foams. Appl. Environ. Microbiol. 2016, 82, 5225–5235. [Google Scholar] [CrossRef]
- Cacciari, I.; Quatrini, P.; Zirletta, G.; Mincione, E.; Vinciguerra, V.; Lupattelli, P.; Giovannozzi Sermanni, G. Isotactic polypropylene biodegradation by a microbial community: Physicochemical characterization of metabolites produced. Appl. Environ. Microbiol. 1993, 59, 3695–3700. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef]
- Khan, S.; Nadir, S.; Shah, Z.U.; Shah, A.A.; Karunarathna, S.C.; Xu, J.; Khan, A.; Munir, S.; Hasan, F. Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ. Pollut. 2017, 225, 469–480. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic biodegradation: Frontline microbes and their enzymes. Sci. Total Environ. 2021, 759, 143536. [Google Scholar] [CrossRef]
- Sevilla, M.E.; Garcia, M.D.; Perez-Castillo, Y.; Armijos-Jaramillo, V.; Casado, S.; Vizuete, K.; Debut, A.; Cerda-Mejia, L. Degradation of PET Bottles by an Engineered Ideonella sakaiensis PETase. Polymers 2023, 15, 1779. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, T.; Rao, C. Novel probiotics adsorbing and excreting microplastics in vivo show potential gut health benefits. Front. Microbiol. 2024, 15, 1522794. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).