Abstract
Chamomile essential oils (EOs) are widely used in cosmetics for their antioxidant, anti-inflammatory, and antimicrobial properties. Bulgaria, with its long-standing tradition in EO production, provides an ideal setting to examine the influence of species and cultivation practices on oil quality. This study compares the chemical composition and biological activity of EOs from German chamomile (Matricaria recutita L.) and Roman chamomile (Chamaemelum nobile L.), sourced from two major Bulgarian producers—Bulgarska Bilka Ltd. and Kateko Ltd. (Plovdiv, Bulgaria). Gas chromatography–mass spectrometry (GC–MS) profiling revealed species- and producer-dependent differences. German chamomile EOs were rich in β-farnesene, chamazulene, and bisabolol oxides, whereas Roman chamomile EOs were dominated by isobutyl angelate and related esters. Antioxidant activity, assessed via the ABTS assay, was higher in German chamomile EOs, especially from Bulgarska Bilka Ltd. The oils also showed photoprotective potential, with SPF values of 26–27 for German and 9–16 for Roman chamomile. Anti-inflammatory activity, evaluated by inhibition of albumin denaturation, was highest in Roman chamomile oils and comparable to that of prednisolone, while German chamomile also showed strong effects. Antimicrobial activity was generally low, with moderate effects observed only against Penicillium chrysogenum and Aspergillus flavus. These findings support the targeted use of chamomile EOs in cosmetics—German chamomile for antioxidant-rich, UV-protective, and microbiome-supportive care, and Roman chamomile for soothing, anti-inflammatory, and fragrance-enhancing applications.
1. Introduction
Chamomile (Asteraceae) is among the most widely used plants in both traditional and modern herbal medicine, renowned for its soothing, anti-inflammatory, and antimicrobial properties. Of particular interest today are the two main commercial species—German chamomile (M. recutita L.) and Roman chamomile (C. nobile L.)—which are valued for their essential oils (EOs), extensively incorporated into numerous therapeutic and cosmetic products. Native to Europe and western Asia, both species are now cultivated globally to satisfy the growing demand for natural bioactive compounds [1,2,3].
Bulgaria has a long-standing tradition in the cultivation of medicinal and aromatic plants and is a recognized producer of high-quality chamomile oils. Favorable agro-climatic conditions, fertile soils, and advanced cultivation practices have contributed to consistent EO production with strong commercial relevance [4].
Traditionally, preparations made from chamomile flower heads have been used to treat skin conditions such as dermatitis, eczema, and wounds, as well as various forms of pain, gastrointestinal, and respiratory discomfort [5,6,7]. These long-established applications have been increasingly supported by modern pharmacological research, particularly in relation to the skin-soothing, anti-inflammatory, and antimicrobial properties of chamomile EOs. Consequently, chamomile oils are now widely incorporated into cosmetic and personal care formulations, including creams, lotions, serums, baby care products, and shampoos designed for sensitive or irritated skin [7,8,9,10,11].
The chemical composition and yield of chamomile EOs are influenced by a complex interplay of genetic, environmental, and agronomic factors. Key determinants include species, genotype, geographical origin, harvest time, and the extraction method employed [6,12,13,14,15,16]. Abiotic stressors such as salinity, drought, temperature extremes, soil type, and nutrient availability further modulate EO biosynthesis [17,18,19,20,21]. Agronomic interventions—such as salicylic acid pre-treatment [22] or controlled heat exposure [23]—and post-harvest handling strategies (e.g., flower separation, controlled drying) also play a crucial role in optimizing oil quality and yield.
Chamomile EOs are most commonly obtained via steam distillation or hydrodistillation of fresh or dried floral material. However, advanced techniques such as supercritical CO2 extraction are gaining increasing attention for their enhanced efficiency and superior preservation of thermolabile constituents [24,25]. Beyond extraction, modern technological approaches—including oil fractionation, synergistic co-formulation, and emulsification—are increasingly applied to improve the bioavailability and functional performance of volatile constituents in cosmetic and therapeutic formulations [26,27,28,29].
The principal constituents of chamomile EOs—namely α-bisabolol, chamazulene, bisabolol oxides A and B, spiroethers, and esters such as isobutyl angelate—are primarily recognized for their anti-inflammatory, antioxidant, and antimicrobial activities. Growing evidence suggests that the therapeutic efficacy of chamomile oil is not solely attributable to its major compounds but rather emerges from synergistic interactions among multiple volatile constituents [1,5,6,7,30].
To ensure consistent therapeutic and cosmetic performance, chamomile EOs must meet established quality standards. The European Pharmacopoeia (10th Edition) defines specifications exclusively for the α-bisabolol chemotype of M. recutita oil, requiring a minimum content of 20% of this terpene [31]. ISO 19332:2020 further specifies compositional ranges for α-bisabolol (15–40%) and other key constituents, including α-bisabolol oxide A (2–27%), α-bisabolol oxide B (2–21%), chamazulene (5–22%), bisabolone oxide A (1–6.5%), and trans-β-farnesene (15–51%) [32].
For C. nobile, no official monograph exists in the European Pharmacopoeia, but its EO is standardized under ISO/FDIS 24600:2025 [33]. This includes 9–25% isobutyl angelate, 3–15% isoamyl angelate, 1–6% methallyl angelate, and 0.5–5% methallyl tiglate, alongside minor components such as pinocarvone and 1,8-cineole. Chamazulene is typically absent from C. nobile oil due to inherent biosynthetic differences and specific distillation conditions [33].
While both chamomile oils are generally recognized as safe for dermal use, they may provoke allergic responses in individuals sensitive to Asteraceae species. Regulatory authorities, including the European Medicines Agency (EMA) and the Scientific Committee on Consumer Safety (SCCS), permit their use in cosmetic formulations when allergenicity and skin tolerance are adequately addressed [34,35,36].
In addition to their therapeutic value, chamomile EOs—like many other essential oils—have demonstrated UV-absorbing properties, supporting their potential use as natural ingredients in sunscreen formulations [37,38,39]. This underscores their multifunctional relevance in cosmetic applications.
Given the growing demand for safe, botanically derived cosmetic ingredients—and the inherent variability in essential oil composition—there is a clear need to assess species- and producer-dependent differences in chamomile essential oils. This study presents a comparative analysis of M. recutita L. and C. nobile L. oils sourced from two leading Bulgarian producers: Bulgarska Bilka Ltd. and Kateko Ltd. (Plovdiv, Bulgaria). The oils are chemically profiled by GC/MS, and their antioxidant and antimicrobial activities are evaluated using ABTS radical scavenging and agar diffusion assays. In addition, UV absorption was measured spectrophotometrically, and the sun protection factor (SPF) was subsequently calculated. The findings are interpreted in the context of their potential application in natural cosmetic formulations targeting sensitive, inflamed, or UV-exposed skin. To our knowledge, this is the first comparative evaluation of M. recutita and C. nobile essential oils cultivated in Bulgaria, comparing two producers and linking chemotype-specific profiles to functional bioactivities for cosmetic use.
2. Materials and Methods
2.1. Essential Oil Samples and Their Sources
Essential oils of German chamomile (M. recutita L.) and Roman chamomile (C. nobile L.) were obtained from two Bulgarian manufacturers: Balgarska Bilka Ltd. and Kateko Ltd., both based in Plovdiv. The samples were delivered in their original, opaque vials, accompanied by quality certificates specifying botanical origin, extraction method, and production date. Upon receipt, oils were transferred to amber glass bottles and stored at 4 °C until analysis.
2.1.1. Balgarska Bilka Ltd.
Balgarska Bilka Ltd. cultivates both chamomile species in the Starosel and Razevo Konare regions. The M. recutita oil is derived from a standard commercial cultivar, while the C. nobile oil originates from the organically certified “Flore Pleno” variety, which has been cultivated under organic conditions since 2012. Essential oils from both species are obtained via steam distillation of the aerial parts—including fresh flower heads and stems—using in-house protocols optimized for high yield and retention of volatiles. However, specific yield data for Balgarska Bilka oils were not provided.
2.1.2. Kateko Ltd.
Kateko Ltd. sources raw materials of German (M. recutita L.) and Roman chamomile (C. nobile L.) from agricultural producers certified for species authenticity at the time of seed purchase from approved suppliers. German chamomile is primarily obtained from producers in Central Northern Bulgaria (Pleven, Veliko Tarnovo), the Stanke Dimitrov region, Northeastern Bulgaria (Shumen, Dobrich, Silistra), and the Haskovo and Plovdiv regions. Roman chamomile is supplied by growers located in Plovdiv, the Kazanlak Valley, Shumen, and Dobrich. The essential oil yield for German chamomile is approximately 0.96%, while for Roman chamomile it ranges from 0.3% to 1%.
The extraction of both oils at Kateko Ltd. is carried out via steam distillation. For M. recutita, 220–250 kg of fresh plant material is loaded per 1 m3 distillation vessel. The process lasts 8–12 h, starting at ~50 °C, with a gradual increase to 55–65 °C to optimize extraction. A 1% cooking salt solution is added to the Florentine vessel before distillation, with an additional 1 g of salt added every 10 min to maintain concentration. Due to the oil’s high viscosity, it is filtered at 60 °C post-extraction.
For C. nobile, the same amount of raw material is used per distillation cycle (220–250 kg/m3), but the process is shorter—typically 2–2.5 h—at a lower temperature range (35–40 °C), with a distillation rate of approximately 10%.
2.2. GC–MS Analysis of Chemical Composition
The chemical profiles of the EOs were determined by gas chromatography–mass spectrometry (GC–MS) with an Agilent 7890A GC system coupled to a 5975C inert XL EI/CI MSD detector (Agilent Technologies, Santa Clara, CA, USA). A 20 µL aliquot of each EO was diluted in 380 µL of hexane, and 1 µL of this solution was injected into an HP-5MS capillary column (30 m × 0.32 mm × 0.25 µm film thickness, Agilent Technologies, Santa Clara, CA, USA). The oven temperature program was initiated at 40 °C, increased to 300 °C at 5 °C/min, and held isothermally for 10 min. The injector temperature was maintained at 250 °C with a split ratio of 100:1. Volatile compounds were identified by comparing retention indices and mass spectra against the NIST 08 library [40]. Representative total ion chromatograms (TICs) for each essential oil sample are provided in the Supplementary Information (Figures S1–S4), illustrating the separation and reproducibility of detected volatiles. Only compounds with a spectral match of ≥90% were accepted. The method’s LOD and LOQ were 0.008% and 0.024%, respectively, based on S/N ≥3 and S/N ≥10.
2.3. Antioxidant Activity: ABTS Radical Scavenging Assay
The antioxidant activity was assessed using the ABTS•+ radical scavenging assay as described by Ivanov et al. [41]. The ABTS•+ radical cation was generated by mixing 7.0 mM ABTS (Sigma-Aldrich, Merck, Germany) with 2.45 mM potassium persulfate (Merck, Germany) and allowing the solution to react in the dark at room temperature for 16 h. Prior to analysis, the solution was diluted with methanol (1:30 v/v) to achieve an absorbance of 1.0–1.1 at 734 nm.
For the assay, 2.85 mL of the ABTS•+ solution was mixed with 0.15 mL of EO dissolved in methanol. The mixture was incubated at 37 °C for 15 min in the dark, and absorbance was recorded at 734 nm against a methanol blank. Antioxidant capacity was expressed as millimoles of Trolox equivalents per gram of essential oil (mM TE/g EO), based on a calibration curve generated with Trolox (0.05–0.5 mM). The IC50 value—the EO concentration required to inhibit 50% of ABTS•+ radicals—was also calculated.
2.4. Anti-Inflammatory Activity
The anti-inflammatory potential of the essential oils was assessed using the albumin denaturation assay. Each test sample was prepared by mixing 0.5 mL of a 5% aqueous bovine serum albumin (BSA) solution with 0.2 mL of essential oil diluted to 1 mg/mL in DMSO. The mixture was incubated at 37 °C for 15 min, followed by the addition of 2.5 mL phosphate-buffered saline (pH 6.3). The tubes were then heated at 80 °C for 30 min and allowed to cool for 5 min at room temperature. Turbidity was measured spectrophotometrically at 660 nm.
A blank sample consisted of 2.5 mL of buffer and 0.2 mL of DMSO. A product control was prepared using 0.5 mL BSA and 2.5 mL buffer without essential oil. The percentage inhibition of protein denaturation was calculated using the formula:
The control represents 100% protein denaturation. Commercial anti-inflammatory drugs, including prednisolone (corticosteroid) and acetylsalicylic acid (aspirin), were tested under the same conditions as reference standards.
2.5. UV Absorption and Calculation of Sun Protection Factor (SPF)
2.5.1. SPF Calculation
The absorbance of the EO solutions (prepared in ethanol) was measured spectrophotometrically in the wavelength range of 290–320 nm at 5 nm intervals. The SPF was calculated using the Mansur equation [42,43]:
where
- CF is the correction factor (10);
- EE(λ) is the erythemal effect spectrum at each wavelength λ;
- I(λ) is the solar intensity spectrum at each wavelength λ;
- Abs(λ) is the absorbance of the sample at each wavelength λ.
The product EE(λ) × I(λ) is considered a constant set of values across the 290–320 nm range and has been normalized based on data originally reported by Kaur (2010) and Malsawmtluangi et al. (2013) [42,43].
2.5.2. Critical Wavelength and UVA/UVB Ratio Determination
The critical wavelength λc, an indicator of UVA protection, was calculated using the following equation [44]:
where Aλ is the absorbance at wavelength λ. For each absorption spectrum, the area under the absorbance curve was estimated using trapezoidal integration, with absorbance values recorded at 5 nm intervals.
To evaluate UVA effectiveness, the UVA/UVB ratio was calculated as the ratio of total absorption in the UVA range (320–400 nm) to that in the UVB range (290–320 nm), according to Springsteen et al. (1999) [45]:
Absorbance values were measured in 5 nm increments, and the integrals were computed using Simpson’s rule for numerical approximation [46].
Based on the calculated UVA/UVB ratio, the EO samples were categorized using the Boots Star Rating System (Table 1).

Table 1.
UVA/UVB ratio classification according to the Boots Star Rating System.
2.6. Antimicrobial Activity: Agar Diffusion Assay
The antimicrobial activity of the chamomile EOs, pre-diluted in methanol to a concentration of 10 mg/mL, was evaluated using the agar well diffusion method as described by Tumbarski et al. [46].
2.7. Statistical Analysis
All experiments were conducted in triplicate. Results are expressed as mean values ± standard deviation (SD). Statistical comparisons among sample groups were performed using Duncan’s multiple range test, with significance set at p < 0.01. Data analyses were conducted using SPSS statistical software, version 27.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Chemical Composition of Chamomile Essential Oils (GC/MS Analysis)
The chemical profiles of EOs from German (M. recutita L.) and Roman chamomile (C. nobile L.), obtained from two Bulgarian producers, are summarized in Table 2 and Table 3. GC–MS analysis revealed significant interspecies and inter-producer variations in the qualitative and quantitative composition of volatile constituents.

Table 2.
Phytochemical profile of German chamomile (M. recutita L.) essential oil as analyzed by GC-MS.

Table 3.
Phytochemical profile of Roman chamomile (C. nobile L.) essential oil as analyzed by GC–MS. For abbreviations, refer to Table 1.
3.1.1. German Chamomile (M. recutita L.) Essential Oil
The essential oil derived from M. recutita exhibited producer-dependent differences (Table 2). The oil from Balgarska Bilka Ltd. was dominated by β-farnesene (30.0 ± 0.3%) and chamazulene (12.3 ± 0.3%), followed by sesquiterpenoids such as α-bisabolol oxide B (10.8 ± 0.5%), α-bisabolone oxide A (8.7 ± 0.2%), and α-bisabolol oxide A (6.1 ± 0.2%). Other notable compounds included bicyclogermacrene (5.4 ± 0.1%), germacrene D (4.9 ± 0.1%), and (Z)-spiroether (5.4 ± 0.2%). These constituents collectively accounted for approximately 82% of the total oil composition, contributing substantially to its biological and sensory attributes.
In contrast, the oil from Kateko Ltd. contained comparable levels of β-farnesene (31.8 ± 0.8%) but lower amounts of chamazulene (5.9 ± 0.1%). This sample exhibited higher concentrations of α-bisabolol oxide B (13.7 ± 0.8%), α-farnesene (9.7 ± 0.0%), and germacrene D (6.2 ± 0.1%). The sum of the major components constituted approximately 83% of the total profile, indicating consistency in the dominance of sesquiterpenes across producers, despite variations in specific compounds.
3.1.2. Roman Chamomile (C. nobile L.) Essential Oil
Marked compositional divergence was also evident in the C. nobile oils (Table 3). The EO from Balgarska Bilka Ltd. was characterized by isobutyl angelate (15.7 ± 0.2%), β-farnesene (11.3 ± 0.1%), germacrene D (10.5 ± 0.1%), limonene (10.4 ± 0.1%), isoamyl methacrylate (8.3 ± 0.2%), and methylallyl angelate (6.9 ± 0.2%), together comprising approximately 63% of the total volatile fraction.
The Kateko Ltd. EO, however, demonstrated a distinctly ester-rich profile. Major constituents included isobutyl angelate (28.6 ± 0.7%), 2-methylbutyl angelate (16.6 ± 0.3%), methylallyl angelate (8.5 ± 0.2%), isobutyl isobutyrate (7.7 ± 0.3%), and α-pinene (6.9 ± 0.0%). These components represented around 73% of the total composition. Notably, the oil lacked significant amounts of β-farnesene, germacrene D, and limonene, which were prominent in the sample from Balgarska Bilka Ltd.
3.2. Biological Activities of Chamomile Essential Oils
3.2.1. Antioxidant Activity (ABTS Assay)
The antioxidant capacities of the EOs were quantified via the ABTS radical scavenging assay, with results shown in Table 4 and visualized in Figure 1.

Table 4.
Antioxidant activity of German chamomile (M. recutita L.) and Roman chamomile (C. nobile L.) EOs by the ABTS method.

Figure 1.
The IC50 values of chamomile EOs were determined by the ABTS method. (a) Roman chamomile (C. nobile L.) Kateko Ltd.; (b) Roman chamomile (C. nobile L.) Balgarska Bilka Ltd.; (c) German chamomile (M. recutita L.) Kateko Ltd.; (d) German chamomile (M. recutita L.) Balgarska Bilka Ltd.
The antioxidant capacities of the EOs were quantified via the ABTS radical scavenging assay, with results shown in Table 3 and visualized in Figure 1.
German Chamomile EOs:
- Balgarska Bilka Ltd.: Displayed potent antioxidant activity, with 95.2% inhibition at 3.125 μg/mL and an IC50 of 0.64 ± 0.01 μg/mL Trolox equivalent values reached 205.00 ± 0.01 mM TE/g EO.
- Kateko Ltd.: Exhibited 97.2% inhibition at 3.125 μg/mL, with an IC50 of 1.13 ± 0.01 μg/mL and a Trolox equivalent of 209.77 ± 0.58 mM TE/g EO.
Roman Chamomile EOs:
- Balgarska Bilka Ltd.: Demonstrated greater antioxidant activity than Kateko’s oil, with 74.1% inhibition at 25 μg/mL and an IC50 of 6.20 ± 0.01 μg/mL (19.93 ± 0.42 mM TE/g EO).
- Kateko Ltd.: Showed lower activity, with 45.7% inhibition at 200 μg/mL and an IC50 of 210.07 ± 0.02 μg/mL (1.53 ± 0.01 mM TE/g EO).
These results highlight the superior antioxidant potential of M. recutita EOs, particularly from Balgarska Bilka Ltd., and underscore the influence of chemical composition on bioactivity.
3.2.2. Anti-Inflammatory Activity of the Essential Oils
The in vitro anti-inflammatory activity of the essential oils was evaluated based on their ability to inhibit thermally induced denaturation of bovine serum albumin. The results are expressed as the percentage inhibition of protein denaturation (Figure 2).
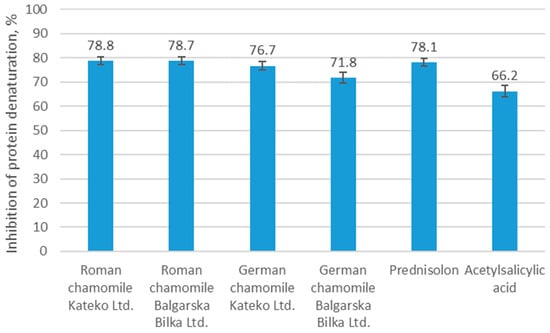
Figure 2.
In vitro anti-inflammatory activity of chamomile EOs and the controls (prednisolone and acetylsalicylic acid) expressed as inhibition of albumin denaturation (%) at a concentration of 1.00 mg/mL. Values represent mean ± SD (n = 3). Statistical significance was assessed using Duncan’s multiple range test (p < 0.01).
Both Roman chamomile essential oils demonstrated the highest anti-denaturation effects, with inhibition rates of 78.8% (Kateko Ltd.) and 78.7% (Balgarska Bilka Ltd.), closely matching the activity of the reference drug prednisolone (78.1%). German chamomile oils showed slightly lower values, with 76.7% inhibition for Kateko Ltd. and 71.8% for Balgarska Bilka Ltd. In comparison, acetylsalicylic acid exhibited a weaker protective effect, achieving 66.2% inhibition. These values were statistically comparable to prednisolone and significantly higher than acetylsalicylic acid, clearly demonstrating the anti-inflammatory strength of Roman chamomile EO in this model.
Overall, all chamomile essential oils at a concentration of 1 mg/mL showed substantial protective activity against albumin denaturation, with inhibition values comparable to those of standard anti-inflammatory drugs tested under identical conditions.
3.2.3. Ultraviolet Protection of Chamomile Essential Oils
The sun protection factor (SPF) values of the chamomile essential oils were calculated using the Mansur equation based on UV absorbance measurements. The absorbance spectra, SPF values, critical wavelengths, and UVA/UVB ratios are presented in Table 5. The UV absorption spectra of the essential oils at 1.00 mg/mL are shown in Figure 3, highlighting differences in absorbance between German and Roman chamomile EOs in both the UV-A and UV-B regions.

Table 5.
SPF value, critical wavelength value (λc), and UVA/UVB ratio at different concentrations of EOs from German chamomile (M. recutita L.) and Roman chamomile (C. nobile L.).
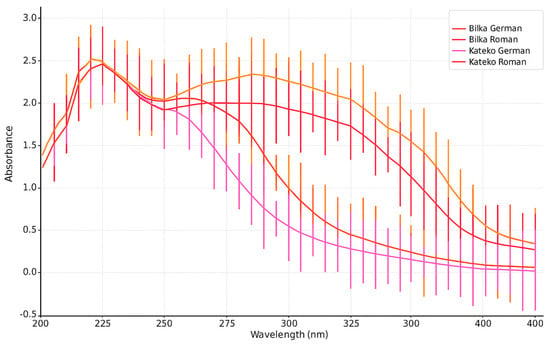
Figure 3.
UV absorption spectra (UV-A and UV-B range) of EOs from German (M. recutita L.) and Roman chamomile (C. nobile L.) at a concentration of 1.00 mg/mL. Each curve represents the mean of three replicates (n = 3), and error bars indicate standard deviation (±SD).
German chamomile oils showed SPF values ranging from 6.55 to 26.56, while Roman chamomile oils ranged from 1.11 to 15.65. In all samples, SPF values increased proportionally with essential oil concentration.
The critical wavelength, defined as the wavelength at which 90% of the total absorbance area from 290 to 400 nm is reached, ranged from 365 to 375 nm for German chamomile and was consistently 360 nm for Roman chamomile, indicating greater UVA absorption in the former. These values meet the European Commission’s broad-spectrum criteria and are within the range of SPF performance observed in marketed natural products containing UV-filtering plant extracts such as peppermint, lavender, and olive oil.
According to the Boots Star Rating System, German chamomile essential oils exceeded the threshold of 0.8, corresponding to the **** maximum protection category. Roman chamomile oils fell within the range of 0.6 to <0.8, corresponding to *** superior protection.
3.2.4. Antimicrobial Activity
Antimicrobial activity was assessed using the agar well diffusion method against a broad panel of microorganisms. As shown in Table 6, all essential oil samples exhibited low but measurable inhibitory effects against several bacterial and fungal strains, with only slight differences observed between species and producers. These findings suggest that chamomile essential oils may contribute to cosmetic formulations aimed at supporting skin microbiome balance or maintaining hygienic conditions.

Table 6.
Antimicrobial activity of German chamomile (M. recutita L.) EOs from Bulgarska Bilka Ltd. (1) and Kateko Ltd. (2) and Roman chamomile (C. nobile L.) EOs from Bulgarska Bilka Ltd. (3) and Kateko Ltd. (4), compared to control (methanol—MeOH) (5).
4. Discussion
This study demonstrates that essential oils from M. recutita L. (German chamomile) and C. nobile L. (Roman chamomile), cultivated in Bulgaria, exhibit distinct, species-specific chemical profiles. Additional compositional differences observed between producers are attributable to variations in cultivar, environmental conditions, and agricultural practices. Differences in steam distillation parameters likely also influenced the retention and relative abundance of key bioactive constituents.
The German chamomile EOs analyzed in this study correspond to the β-farnesene chemotype, a relatively rare profile in Europe but consistent with tetraploid cultivars reported from neighboring Serbia [47,48,49]. These oils were dominated by β-farnesene and bisabolane-type sesquiterpenoids, compounds recognized for their significant antimicrobial and aromatic properties [50,51]. The oil from Balgarska Bilka Ltd. contained notably higher levels of chamazulene—a compound responsible for the characteristic blue color of M. recutita EO and associated with anti-inflammatory activity—while the Kateko Ltd. sample showed elevated levels of bisabolol derivatives. This compositional divergence may reflect underlying differences in gene expression (e.g., cytochrome P450 reductase activity), environmental stressors, or cultivation conditions [22,52,53].
Roman chamomile oils were predominantly composed of angelic acid esters, particularly isobutyl angelate and methylallyl angelate. The oil from Kateko Ltd. contained significantly higher levels of these esters, aligning closely with profiles previously reported for Bulgarian chemotypes [54]. These low-volatility compounds are appreciated for their long-lasting aroma and soothing effects on the skin, supporting their application in cosmetic formulations designed for sensitive or irritated skin. Interestingly, despite the use of the entire aerial part during distillation, the chemical profile remained typical of flower-derived oils, highlighting the plant’s abundance of volatiles with cosmetic relevance [55].
The observed variations in chemical composition were reflected in the antioxidant activity of the EOs. German chamomile oils exhibited markedly higher antioxidant capacity than their Roman counterparts. The oil from Balgarska Bilka Ltd. showed the strongest effect, with an IC50 of 0.64 µg/mL and a maximum Trolox equivalent of 642.83 mM TE/g EO at the lowest tested concentration (0.097 µg/mL). This result is consistent with β-farnesene-rich profiles previously reported in Iranian accessions [56]. In contrast, Roman chamomile oils demonstrated lower antioxidant potential, with the highest value observed for the Balgarska Bilka sample (137.46 mM TE/g EO). The reduced activity of Roman chamomile is likely attributable to its lower content of oxygenated sesquiterpenes and the predominance of non-phenolic esters, which are less effective radical scavengers. Notably, German chamomile oils from European cultivars typically exhibit Trolox equivalents in the range of 350–600 mM TE/g EO, placing the Bulgarian samples at the upper end of this spectrum and confirming their potential for high-performance cosmetic applications, such as anti-aging and post-sun care formulations [9].
In parallel with antioxidant potential, the essential oils were evaluated for their ability to inhibit thermal denaturation of bovine serum albumin—a widely accepted in vitro model of anti-inflammatory activity. Roman chamomile oils exhibited the strongest inhibition (79%), comparable to the reference corticosteroid prednisolone (78%). German chamomile oils showed slightly lower values (72–77%) but still surpassed acetylsalicylic acid (66%) at the same concentration. These results suggest that both species possess notable anti-inflammatory properties, likely attributable to compounds such as chamazulene, bisabolol oxides, and angelate esters, which have been previously reported to modulate inflammatory pathways.
In addition to their antioxidant and anti-inflammatory properties, the tested chamomile EOs demonstrated notable potential for UV protection. At a concentration of 1.00 mg/mL, the SPF values of German chamomile oils were approximately 26–27, markedly higher than those of Roman chamomile oils, which ranged from about 9 to 16. These values exceed those reported for commonly used plant oils such as olive (SPF ~8), coconut (SPF ~8), peppermint (SPF ~7), and lavender (SPF ~5) [43,57]. Although β-farnesene (30–32%) and chamazulene (6–13%) are both abundant in German chamomile oil, only chamazulene has been shown to absorb UV radiation, contributing directly to the observed SPF values [38]. In contrast, β-farnesene and bisabolol oxides—particularly bisabolol oxide A and B—may support photoprotection indirectly through their antioxidant properties, which can help counteract UV-induced oxidative stress [56,58]. Chamazulene, however, is prone to photooxidation under UV exposure, forming degradation products that may alter its color and potentially its bioactivity. This photoinstability can be mitigated by co-formulating with antioxidants such as hydroxytyrosol, α-tocopherol, or TEMPO (2,2,6,6-tetramethylpiperidine 1-oxyl), a selective radical scavenger shown to enhance chamazulene’s stability in topical applications [59].
The antimicrobial testing revealed that both chamomile species exhibited low to moderate activity against a broad panel of microorganisms. For German chamomile, the strongest inhibition was observed against P. chrysogenum (13 mm for Balgarska Bilka Ltd.; 11 mm for Kateko Ltd.), while Roman chamomile oils were most effective against A. flavus (12 mm for both producers). These results are consistent with previous studies on the antimicrobial potential of chamomile [3] and support its application in formulations intended for acne-prone or microbiome-sensitive skin. Although moderate, the observed antimicrobial activity may contribute to skin barrier defense and offers promise as a natural alternative to synthetic preservatives in topical cosmetic products.
Collectively, these findings highlight the phytochemical richness and multifunctional bioactivity of chamomile essential oils sourced from Bulgarian cultivars. German chamomile oils, particularly those from Balgarska Bilka Ltd., stand out for their potent antioxidant capacity and distinctive therapeutic chemotype, while Roman chamomile oils present a gentler profile, enriched with esters known for their soothing and anti-inflammatory effects. These results provide a scientific basis for the rational selection of chamomile oils in cosmetic formulations tailored to specific skin concerns, including inflammation, oxidative stress, and microbial imbalance.
5. Conclusions
This study demonstrated that EOs from German and Roman chamomile cultivars grown in Bulgaria exhibit distinct, species-dependent chemical profiles influenced by genetic, environmental, and production-related factors. German chamomile oils, belonging to the β-farnesene chemotype, were rich in chamazulene and bisabolol-type sesquiterpenoids and showed superior antioxidant capacity, notable anti-inflammatory activity, strong UV protection, and moderate antimicrobial effects. In contrast, Roman chamomile oils were dominated by angelic acid esters, offering milder antioxidant activity but exhibiting the most pronounced anti-inflammatory effect, along with well-documented soothing and fragrance-enhancing properties. These compositional and functional differences support the targeted use of chamomile EOs in natural cosmetic formulations: German chamomile for antioxidant-rich, UV-protective, and microbiome-supportive skincare, and Roman chamomile for calming, anti-inflammatory, and skin-sensitive applications. The results highlight the added value of chemotype and producer selection in formulating next-generation botanical skincare products.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cosmetics12030123/s1, Figure S1: Total ion chromatogram (TIC) of German chamomile (M. recutita L.) EO (Balgarska Bilka Ltd.); Figure S2: Total ion chromatogram (TIC) of German chamomile (M. recutita L.) EO (Kateko Ltd.); Figure S3: Total ion chromatogram (TIC) of Roman chamomile (C. nobile L.) EO (Balgarska Bilka Ltd.); Figure S4: Total ion chromatogram (TIC) of Roman chamomile (C. nobile L.) EO (Kateko Ltd.).
Author Contributions
Conceptualization, K.N. and D.B.; methodology, I.I., I.D., Y.T. and I.Y. software, A.G. and N.P.; validation, N.P. and D.B.; formal analysis, K.N. and G.G.; investigation, N.P. and I.Y.; data curation, Y.T. and I.D.; writing—original draft preparation, I.I., D.B. and N.P.; writing—review and editing, K.N., D.B., G.G. and Y.T.; visualization, A.G.; supervision, K.N.; project administration, K.N.; funding acquisition, K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union—Next Generation EU, through the National Recovery and Resilience Plan of the Republic of Bulgaria (Project No. BG-RRP-2.004-0009-C02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are also available on request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge the support of the Medical University—Varna, for facilitating the publication of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EO(s) | Essential oil(s) |
| SPF | Sun Protection Factor |
| RT | Retention time, min |
| RI | Retention index |
| TIC | Total Ion Current % |
| SD | Standard Deviation |
| Nd | not detected |
| IZ | Inhibition zone |
References
- Dai, Y.L.; Li, Y.; Wang, Q.; Niu, F.J.; Li, K.W.; Wang, Y.Y.; Wang, J.; Zhou, C.Z.; Gao, L.N. Chamomile: A review of its traditional uses, chemical constituents, pharmacological activities and quality control studies. Molecules 2022, 28, 133. [Google Scholar] [CrossRef]
- Morillas-Cruz, A.Y.; Miranda-Huaman, M.J.; Moreno-Agustin, E.M.; Ganoza-Yupanqui, M.L. Chamaemelum nobile: A review of traditional uses, phytochemistry and pharmacology. Rev. Peru. Med. Integr. 2022, 7. Available online: https://rpmi.pe/index.php/rpmi/article/view/8 (accessed on 2 April 2025).
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A comprehensive study of therapeutic applications of chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef]
- Petkova, M.; Tahsin, N.; Yancheva, S.; Yancheva, H. Development of the Production of Aromatic Oil Crops in Bulgaria. In Proceedings of the China-Bulgaria Rural Revitalisation Development Cooperation Forum; Bulgaria, S., Ed.; Institute of Agricultural Economics: Sofia, Bulgaria, 2018; p. 71. [Google Scholar]
- Chauhan, E.S.; Jaya, A. Chamomile an Ancient Aromatic Plant—A Review. J. Ayurveda Med. Sci. 2017, 2, 251–255. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Castillo, M.E.C.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, N.; Nyczka, A.; Piwowarski, J.P.; Granica, S. Traditional Use of Chamomile Flowers (Matricariae flos) in Inflammatory-Associated Skin Disorders. Prospect. Pharm. Sci. 2024, 22, 59–73. [Google Scholar] [CrossRef]
- Chen, G.; Lv, C.; Nie, Q.; Li, X.; Lv, Y.; Liao, G.; Liu, S.; Ge, W.; Chen, J.; Du, Y. Essential Oil of Matricaria chamomilla Alleviates Psoriatic-Like Skin Inflammation by Inhibiting PI3K/Akt/mTOR and p38MAPK Signaling Pathway. Clin. Cosmet. Investig. Dermatol. 2024, 17, 59–77. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Dos Santos, D.S.; Barreto, R.S.S.; Serafini, M.R.; Gouveia, D.N.; Marques, R.S.; Nascimento, L.C.; Nascimento, J.C.; Guimarães, A.G. Phytomedicines Containing Matricaria Species for the Treatment of Skin Diseases: A Biotechnological Approach. Fitoterapia 2019, 138, 104267. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Kazeminava, F.; Abdollahi, B.; Gholizadeh, P.; Heydari, A.; Elmi, F.; Abbaszadeh, M.; Samadi Kafil, H. Matricaria chamomilla Essential Oil-Loaded Hybrid Electrospun Nanofibers Based on Polycaprolactone/Sulfonated Chitosan/ZIF-8 Nanoparticles for Wound Healing Acceleration. Int. J. Biol. Macromol. 2023, 247, 125718. [Google Scholar] [CrossRef]
- Höferl, M.; Wanner, J.; Tabanca, N.; Ali, A.; Gochev, V.; Schmidt, E.; Kaul, V.K.; Singh, V.; Jirovetz, L. Biological Activity of Matricaria chamomilla Essential Oils of Various Chemotypes. Planta Med. Int. Open 2020, 7, e114–e121. [Google Scholar] [CrossRef]
- Salehi, A.; Hazrati, S.; Behzadi, Y. Comparison of Essential Oil Content and Composition in Two German Chamomile (Matricaria chamomilla L.) Genotypes. J. Hortic. Sci. 2023, 18, 506–509. [Google Scholar] [CrossRef]
- Yadav, N.; Shakya, P.; Kumar, A.; Guatam, R.D.; Chauhan, R.; Kumar, D.; Kumar, A.; Singh, S.; Singh, S. Investigation on Pollination Approaches, Reproductive Biology and Essential Oil Variation during Floral Development in German Chamomile (Matricaria chamomilla L.). Sci. Rep. 2022, 12, 15285. [Google Scholar] [CrossRef]
- Shakya, P.; Thakur, R.; Sharan, H.; Yadav, N.; Kumar, M.; Chauhan, R.; Kumar, D.; Kumar, A.; Singh, S.; Singh, S. GGE Biplot and Regression-Based Multi-Environment Investigations for Higher Yield and Essential Oil Content in German Chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2023, 193, 116145. [Google Scholar] [CrossRef]
- Filipović, V.; Marković, T.; Dimitrijević, S.; Song, A.; Prijić, Ž.; Mikić, S.; Čutović, N.; Ugrenović, V. The First Study on Cultivating Roman Chamomile (Chamaemelum nobile (L.) All.) for Its Flower and Essential Oil in Southeast Serbia. Horticulturae 2024, 10, 396. [Google Scholar] [CrossRef]
- Shakir, N.; Anwaar, S.; Jabeen, N.; Anwar, T.; Qureshi, H.; Munazir, M.; Zaman, W.; Soufan, W. Impact of NaCl Stress on Phytoconstituents and Bioactivity of Matricaria chamomilla: A Multi-Analytical Approach. Sci. Rep. 2024, 14, 19717. [Google Scholar] [CrossRef]
- Hendawy, S.F.; Omer, E.A.; El-Gohary, A.E.; El-Gendy, A.G.; Hussein, M.S.; Salaheldin, S.; Soliman, W.S. Effect of Soil and Irrigation Water Salinity on the Productivity and Essential Oil Constituents of Chamomile (Chamomilla recutita L.). J. Essent. Oil Bear. Plants 2019, 22, 962–971. [Google Scholar] [CrossRef]
- Jeshni, M.G.; Mousavinik, M.; Khammari, I.; Rahimi, M. The Changes of Yield and Essential Oil Components of German Chamomile (Matricaria recutita L.) under Application of Phosphorus and Zinc Fertilizers and Drought Stress Conditions. J. Saudi Soc. Agric. Sci. 2017, 16, 60–65. [Google Scholar] [CrossRef]
- Zarezadeh, S.; Riahi, H.; Shariatmadari, Z.; Sonboli, A. Effects of Cyanobacterial Suspensions as Bio-Fertilizers on Growth Factors and the Essential Oil Composition of Chamomile (Matricaria chamomilla L.). J. Appl. Phycol. 2020, 32, 1231–1241. [Google Scholar] [CrossRef]
- Rathore, S.; Kumar, R. Agronomic Interventions Affect the Growth, Yield, and Essential Oil Composition of German Chamomile (Matricaria chamomilla L.) in the Western Himalaya. Ind. Crops Prod. 2021, 171, 113873. [Google Scholar] [CrossRef]
- Ghasemi, M.; Babaeian Jelodar, N.; Modarresi, M.; Bagheri, N.; Jamali, A. Increase of Chamazulene and α-Bisabolol Contents in the Essential Oil of German Chamomile (Matricaria chamomilla L.) Using Salicylic Acid Treatments under Normal and Heat Stress Conditions. Foods 2016, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Subhdara, K.; Dhyani Jakhmola, K.; Khanduri, S.; Chandra, G. Heat Stress-Induced Changes in Growth and Essential Oil Profile of German Chamomile (Matricaria chamomilla). Asian J. Biol. Life Sci. 2024, 13, 432–438. [Google Scholar] [CrossRef]
- Galea, C.; Cocos, D.; Feier, R.; Moales, D. The Use of Essential Oils in the Development of Dermato-Cosmetic Products. Med. Mater. 2023, 3, 31–36. [Google Scholar] [CrossRef]
- Pastare, L.; Berga, M.; Kienkas, L.; Boroduskis, M.; Ramata-Stunda, A.; Reihmane, D.; Senkovs, M.; Skudrins, G.; Nakurte, I. Exploring the Potential of Supercritical Fluid Extraction of Matricaria chamomilla White Ray Florets as a Source of Bioactive (Cosmetic) Ingredients. Antioxidants 2023, 12, 1092. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, Y.E.; Soliman, S.S.; Ismail, T.A.; Hassan, M.A.; Abdelkader, M.A.; Abdel Latef, A.A.H.; Al-Khayri, J.M.; Alshamrani, S.M.; Safhi, F.A.; Awad, M.F.; et al. Improvement of German Chamomile (Matricaria recutita L.) for Mechanical Harvesting, High Flower Yield and Essential Oil Content Using Physical and Chemical Mutagenesis. Plants 2022, 11, 2940. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Lončar, B.; Kiprovski, B.; Stanković-Jeremić, J.; Todosijević, M.; Pezo, L.L.; Jeremić, J. Chamomile Essential Oil Quality after Postharvest Separation Treatments. Ratar. Povrt. 2021, 58, 72–78. [Google Scholar] [CrossRef]
- Abbas, A.M.; Seddik, M.A.; Gahory, A.-A.; Salaheldin, S.; Soliman, W.S. Differences in the Aroma Profile of Chamomile (Matricaria chamomilla L.) after Different Drying Conditions. Sustainability 2021, 13, 5083. [Google Scholar] [CrossRef]
- Karaca, N.; Demirci, B.; Gavahian, M.; Demirci, F. Enhanced Bioactivity of Rosemary, Sage, Lavender, and Chamomile Essential Oils by Fractionation, Combination, and Emulsification. ACS Omega 2023, 8, 10941–10953. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, Z.; Dang, J.; Li, D.; Yu, D.; Qu, C.; Wu, Q. GC–MS Combined with Fast GC E-Nose for the Analysis of Volatile Components of Chamomile (Matricaria chamomilla L.). Foods 2024, 13, 1865. [Google Scholar] [CrossRef]
- European Pharmacopoeia (Ph. Eur.), 10th ed.; EDQM Council of Europe: Strasbourg, France, 2022; p. 1531.
- ISO 19332:2020; Oil of Blue Chamomile [Chamomilla recutita (L.) Rauschert syn. Matricaria chamomilla auct.]. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO/FDIS 24600:2025; Essential oil of roman chamomile (Chamaemelum nobile (L.) All. syn. Anthemis nobilis L.). International Organization for Standardization: Geneva, Switzerland, 2025. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:24600:dis:ed-1:v1:en (accessed on 3 April 2025).
- Final Report of the Safety Assessment of Chamomilla recutita (Matricaria)-Derived Ingredients as Used in Cosmetics; Cosmetic Ingredient Review Expert Panel: Washington, DC, USA, 2013.
- Johnson, W.; Boyer, I.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Amended Safety Assessment of Chamomilla recutita-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2018, 37 (Suppl. S2), 51S–79S. [Google Scholar] [CrossRef]
- Avonto, C.; Wang, Z.; Ahn, J.; Verma, R.P.; Sadrieh, N.; Dale, O.; Khan, S.I.; Chittiboyina, A.G.; Khan, I.A. Integrated Testing Strategy for the Safety of Botanical Ingredients: A Case Study with German Chamomile Constituents. Appl. Vitr. Toxicol. 2021, 7, 126–136. [Google Scholar] [CrossRef]
- Vella, F.M.; Cautela, D.; Laratta, B. Determination of Antioxidant Activity and Sun Protection Factor of Commercial Essential Oils. Biol. Life Sci. Forum 2021, 6, 96. [Google Scholar] [CrossRef]
- Zhou, Y.; He, L.; Wang, W.; Wei, G.; Ma, L.; Liu, H.; Yao, L. Artemisia sieversiana Ehrhart ex Willd. Essential Oil and Its Main Component, Chamazulene: Their Photo-protective Effect against UVB-Induced Cellular Damage and Potential as Novel Natural Sunscreen Additives. ACS Sustain. Chem. Eng. 2023, 11, 17675–17686. [Google Scholar] [CrossRef]
- Humaira, N.; Aisyah, Y.; Muzaifa, M.; Irfan, I.; Erika, C. Evaluation of Sun Protection Factor (SPF) Value of Essential Oils and Its Application in Sunscreen Cream. IOP Conf. Ser. Earth Environ. Sci. 2025, 1476, 012050. [Google Scholar] [CrossRef]
- Ivanov, I.; Petkova, N.; Tumbarski, J.; Dincheva, I.; Badjakov, I.; Denev, P.; Pavlov, A. GC-MS Characterization of N-Hexane Soluble Fraction from Dandelion (Taraxacum officinale Weber ex F.H. Wigg.) Aerial Parts and Its Antioxidant and Antimicrobial Properties. Z. Naturforsch. C 2017, 73, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Vrancheva, R.; Marchev, A.; Petkova, N.; Aneva, I.; Denev, P.; Georgiev, V.; Pavlov, A. Antioxidant Activities and Phenolic Compounds in Bulgarian Fumaria Species. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 296–306. [Google Scholar]
- Kaur, C.D.; Saraf, S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacogn. Res. 2010, 2, 22–25. [Google Scholar] [CrossRef]
- Malsawmtluangi, C.; Nath, D.K.; Jamatia, I.; Lianhimgthangi; Zarzoliana, E.; Pachuau, L. Determination of Sun Protection Factor (SPF) number of some aqueous herbal extracts. J. App. Pharm. Sci. 2013, 3, 150–151. [Google Scholar] [CrossRef]
- Diffey, B.L.; Tanner, P.R.; Matts, P.J.; Nash, J.F. In vitro assessment of the broad-spectrum ultraviolet protection of sunscreen products. J. Am. Acad. Dermatol. 2000, 43, 1024–1035. [Google Scholar] [CrossRef]
- Springsteen, A.; Yurek, R.; Frazier, M.; Carr, K.F. In vitro measurement of sun protection factor of sunscreens by diffuse transmittance. Anal. Chim. Acta 1999, 380, 155–164. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Ivanov, I.; Todorova, M.; Gerasimova, A.; Dincheva, I.; Makedonski, L.; Nikolova, K. Chemical Composition and Biological Activities of St John’s Wort (Hypericum perforatum L.) Essential Oil from Bulgaria. Appl. Sci. 2024, 14, 11754. [Google Scholar] [CrossRef]
- Satyal, P.; Shrestha, S. Composition and Bioactivities of an (E)-β-Farnesene Chemotype of Chamomile (Matricaria chamomilla) Essential Oil from Nepal. Nat. Prod. Commun. 2015, 10, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Acimovic, M.; Stankovic, J.; Cvetkovic, M.; Kiprovski, B.; Todosijevic, M. Essential Oil Quality of Tetraploid Chamomile Cultivars Grown in Serbia. J. Essent. Oil Bear. Plants 2018, 21, 15–22. [Google Scholar] [CrossRef]
- Acimovic, M.; Jeremić, J.; Simić, K.; Ivanović, S.; Ljujić, J.; Cabarkapa, I.; Radojcin, M.; Todosijević, M.; Cvetkovic, M. Essential Oil Quality of Chamomile Grown in Province of Vojvodina. Letop. Naucn. Rad. Poljopr. Fak. 2021, 45, 63–70. [Google Scholar]
- Rather, M.A.; Dar, B.A.; Dar, M.Y.; Wani, B.A.; Shah, W.A.; Bhat, B.A.; Ganai, B.A.; Bhat, K.A.; Anand, R.; Qurishi, M.A. Chemical Composition, Antioxidant and Antibacterial Activities of the Leaf Essential Oil of Juglans regia L. and Its Constituents. Phytomedicine 2012, 19, 1185–1190. [Google Scholar] [CrossRef]
- Alimi, D.; Hraoui, M.; Hajri, A.; Taamalli, W.; Selmi, S.; Sebai, H. Bioactivity and Molecular Docking Studies of Selected Plant Compounds. J. Sci. Food Agric. 2024, 104, 4391–4399. [Google Scholar] [CrossRef]
- Wu, H.; Yang, K.; Dong, L.; Ye, J.; Xu, F. Classification, Distribution, Biosynthesis, and Regulation of Secondary Metabolites in Matricaria chamomilla. Horticulturae 2022, 8, 1135. [Google Scholar] [CrossRef]
- Noori, K.; Omidi, H.; Pirahmadi, L. Morphological Characteristics, Essential Oil, Chamazulene Percentage, and Anti-Oxidation Enzymes Activity Changes of Chamomile (Matricaria recutita) Under the Soil and Water Salinity. J. Fundam. Appl. Sci. 2016, 8, 2293–2310. [Google Scholar]
- Mollova, S.; Stanev, S.; Bozhilov, D.; Manolov, S.; Mazova, N.; Koleva, Y.; Stoyanova, A. Chemical Composition and Antioxidant Activity of Roman Chamomile (Anthemis nobilis L.) Essential Oil. Nat. Prod. Commun. 2024, 264, 264–271. [Google Scholar]
- Johnson, W., Jr.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Anthemis nobilis-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2017, 36 (Suppl. S1), 57S–66S. [Google Scholar] [CrossRef]
- Fırat, Z.; Demirci, F.; Demirci, B. Antioxidant Activity of Chamomile Essential Oil and Main Components. Nat. Volatiles Essent. Oils 2018, 5, 11–16. [Google Scholar]
- Couteau, C.A.C.; Paparis, E.; Coiffard, L.J.M. An in vitro study of fixed and essential oils claimed to have photoprotective properties. J. Photochem. Photobiol. A Chem. 2022, 426, 113743. [Google Scholar] [CrossRef]
- Shahrivari, S.; Alizadeh, S.; Ghassemi-Golezani, K.; Aryakia, E. A comprehensive study on essential oil compositions, antioxidant, anticholinesterase and antityrosinase activities of three Iranian Artemisia species. Sci. Rep. 2022, 12, 7234. [Google Scholar] [CrossRef] [PubMed]
- Gabbanini, S.; Neba, J.N.; Matera, R.; Valgimigli, L. Photochemical and Oxidative Degradation of Chamazulene Contained in Artemisia, Matricaria and Achillea Essential Oils and Setup of Protection Strategies. Molecules 2024, 29, 2604. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).