Multi-Target Anti-Aging Mechanisms of Multani Mitti (Fuller’s Earth): Integrating Enzyme Inhibition and Molecular Docking for Cosmeceuticals
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. INAA Methodology
2.3. GCMS Analysis
2.4. Antioxidant Assay
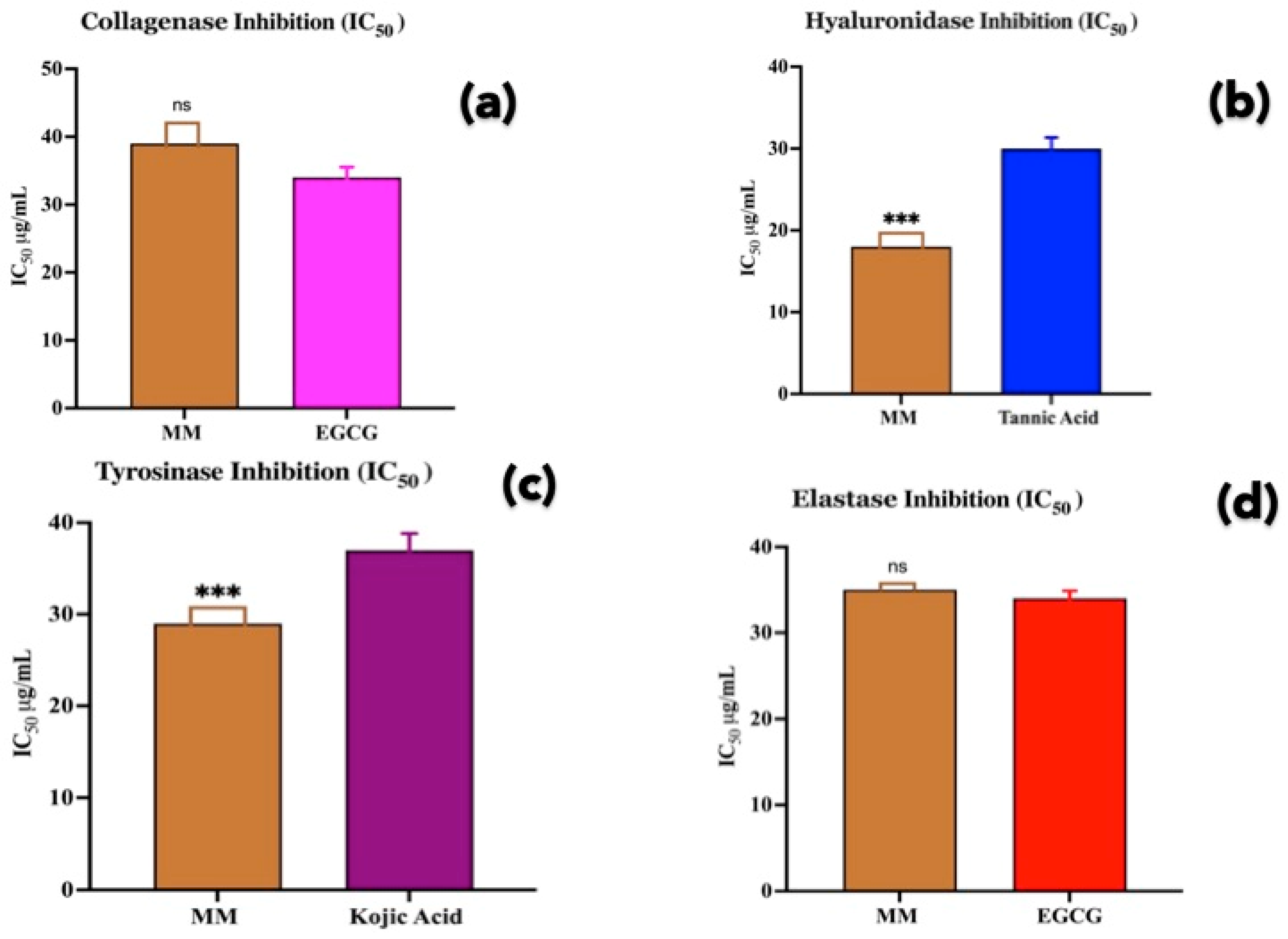
2.5. Collagenase Enzyme Inhibitory Assay
2.6. Elastase Enzyme Inhibitory Assay
2.7. Hyaluronidase Enzyme Inhibitory Assay
2.8. Tyrosinase Enzyme Inhibitory Assay
2.9. Statistical Analysis
2.10. Molecular Docking Studies
- Collection and Optimization of Active Compounds
- Retrieval and Preparation of Receptor Proteins
- Molecular Docking
- Drug Scanning through Pharmacokinetics Parameters
3. Results
3.1. INAA Analysis
| N° | Element | Symbol | PubChem ID/CID | Concentration (mg/g) | Uncertainty (±) | Detection Limit | Biological Role |
|---|---|---|---|---|---|---|---|
| 01 | Silicon | Si | 5461123 | 169.3742 | 20.5 | 10.0 | Connective tissue health [42] |
| 02 | Aluminum | Al | 5359268 | 118.2931 | 18.0 | 8.5 | Cellular transport [46] |
| 03 | Manganese | Mn | 23930 | 123.6701 | 13.0 | 3.05 | Antioxidant [43] |
| 04 | Zinc | Zn | 23994 | 130.1224 | 22.5 | 19.0 | Immune function [44] |
| 05 | Calcium | Ca | 5460341 | 96.7853 | 15.0 | 7.0 | Bone structure, signaling [47] |
| 06 | Vanadium | V | 23990 | 91.4083 | 22 | 33.5 | Insulin mimetic [48] |
| 07 | Rubidium | Rb | 5357696 | 79.5790 | 6.50 | 15.5 | Potassium substitute [49] |
| 08 | Chromium | Cr | 23976 | 75.2774 | 9.8 | 5.85 | Glucose metabolism [50] |
| 09 | Barium | Ba | 5355457 | 72.5890 | 17 | 85 | Bone density [51] |
| 10 | Cobalt | Co | 23974 | 14.6253 | 1.5 | 1.12 | Vitamin B12 component [52] |
| 11 | Strontium | Sr | 5359327 | 14.6253 | 2.3 | 29.5 | Bone formation [51] |
| 12 | Cesium | Cs | 5354618 | 4.8661 | 0.85 | 0.91 | Cellular fluid balance [53] |
| 13 | Tin | Sn | 5352426 | 2.9842 | 0.66 | 0.72 | Metabolic activity [54] |
| 14 | Iron | Fe | 23925 | 1.9465 | 0.15 | 0.098 | Oxygen transport [45] |
| 15 | Molybdenum | Mo | 23932 | 1.5324 | 0.22 | 2.00 | Enzyme cofactor [55] |
| 16 | Potassium | K | 5462222 | 1.2958 | 0.22 | 0.19 | Electrolyte balance [56] |
| 17 | Magnesium | Mg | 5462224 | 0.3414 | 0.045 | 0.22 | Enzyme cofactor [57] |
| 18 | Sodium | Na | 5360545 | 0.2839 | 0.025 | 110 | Nerve function [58] |
| 19 | Titanium | Ti | 23963 | 0.2645 | 0.042 | 0.27 | Biocompatible metal [59] |
| 20 | Selenium | Se | 6326970 | 0.1355 | 0.031 | 0.27 | Antioxidant [60] |
3.2. GCMS Analysis
3.3. Antioxidant Assay
3.4. Enzyme Inhibition Activity
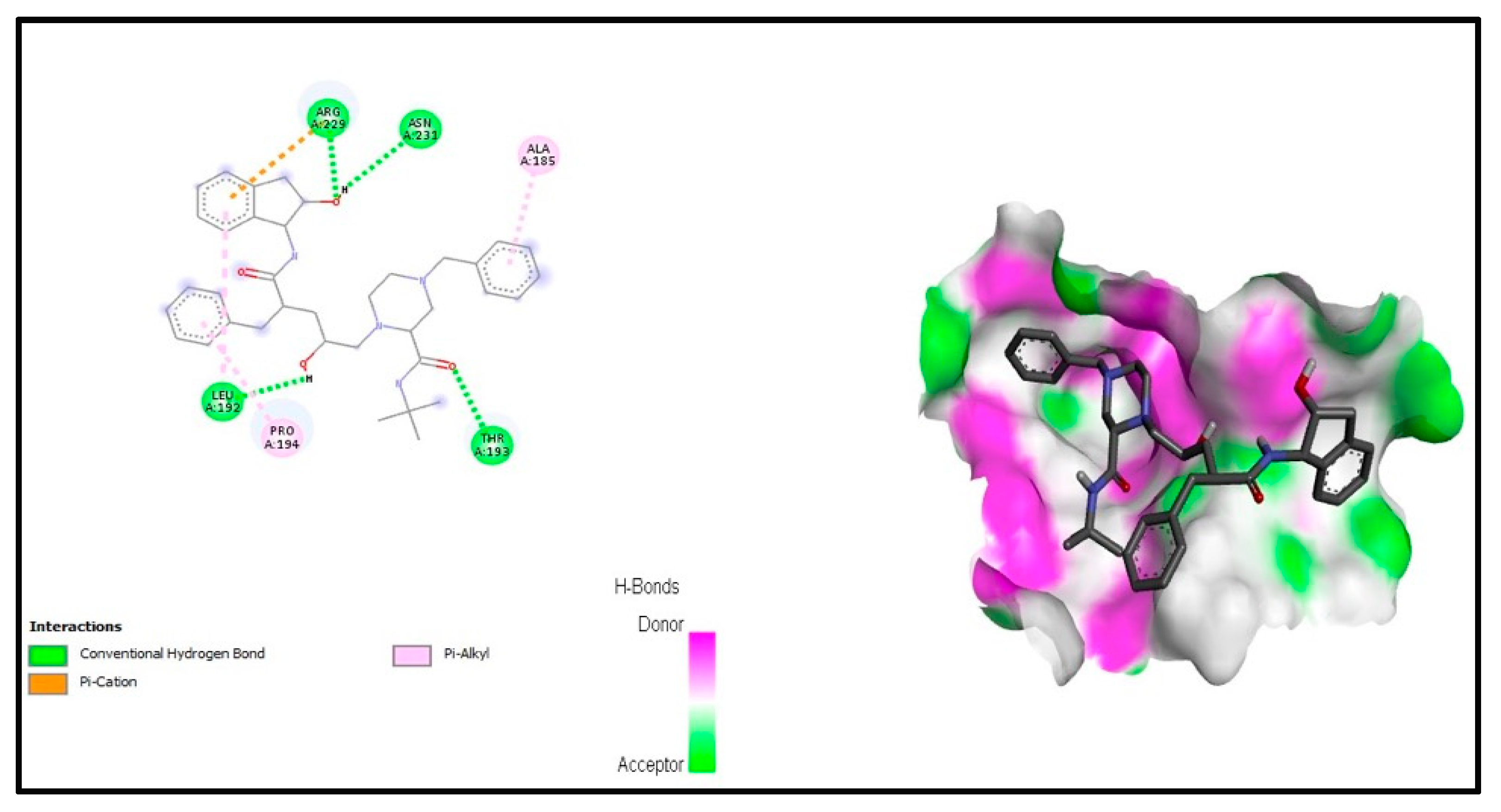
3.5. Molecular Docking Studies
3.5.1. Interaction Analysis
3.5.2. Anti-Tyrosinase Activity
3.5.3. Anti-Hyaluronidase Activity
3.5.4. Druggability Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, I.A.; Mikail, M.A. Diet and skin health: The good and the bad. Nutrition 2024, 119, 112350. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.S.; Pal, Y.; Wal, P. In-House Preparation and Standardization of Herbal Face Pack. Open Dermatol. J. 2017, 11, 72–80. [Google Scholar] [CrossRef]
- Kumar, P. Multani mitti—Is it more than a placebo? J. Pak. Assoc. Dermatol. 2019, 29, 345–348. [Google Scholar]
- Waheed, S.; Faiz, Y.; Rahman, S.; Siddique, N. Toxic element composition of multani mitti clay for nutritional safety. J. Radioanal. Nucl. Chem. 2012, 295, 143–150. [Google Scholar] [CrossRef]
- Pal, R.S.; Pal, Y.; Wal, P.; Wal, A.; Saraswat, N. Composition and Quality Standards of Naturally Derived Anti Tan Medicine. Open Biol. J. 2021, 9, 40–46. [Google Scholar] [CrossRef]
- Waheed, S.; Siddique, N.; Faiz, Y. Rare Earth and High Field-Strength Elements in the Multani Mitti Clay: A Study Using INAA. Geostand. Geoanalytical Res. 2013, 37, 197–205. [Google Scholar] [CrossRef]
- Hosterman, J.W.; Patterson, S.H. Bentonite and Fuller’s Earth Resources of the United States; U.S. Government Publishing Office: Washington, DC, USA, 1992. [CrossRef]
- Patterson, S.H. Fuller’s Earth and Other Industrial Mineral Resources of the Meigs-Attapulgus-Quincy District, Georgia and Florida; U.S. Government Publishing Office: Washington, DC, USA, 1974; p. 828.
- Idoine, N.E.; Raycraft, E.R.; Shaw, R.A.; Hobbs, S.F.; Deady, E.A.; Everett, P.; Evans, E.J.; Mills, A.J. World Mineral Production; British Geological Survey: Nottingham, UK, 2016. [Google Scholar]
- Thakur, P.; Garg, R.K. New developing reagent for latent fingermark visualization: Fuller’s earth (Multani Mitti). Egypt. J. Forensic Sci. 2016, 6, 449–458. [Google Scholar] [CrossRef]
- Bhola, K.L. Fuller’s Earth in India. Trans. Indian Ceram. Soc. 2014, 5, 104–124. [Google Scholar] [CrossRef]
- Klein, C.; Dutrow, B. Manual of Mineral Science; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kora, A.J. Cosmetic and religious applications of natural nanoclay: An Indian scenario. In Nanoclay-Based Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2024; pp. 429–447. [Google Scholar]
- Tiwari, D.; Prakash, K.; Gupta, P.; Shukla, T.; Yadav, P. Formulation And Evaluation of Face Scrub In Modern Pharmaceutics: A Herbal Formulation. Int. J. Pharm. Sci. 2024, 2, 579–588. [Google Scholar] [CrossRef]
- Bhavani, M.S.; Naveena, C.H.; Nagamani, P.; Sowmya, B. Formulation and Evaluation of Herbal Face Cream. Int. J. Pharm. Sci. Rev. Res. 2023, 83, 74–78. [Google Scholar] [CrossRef]
- Kumar, R. Komal Formulation and evaluation of herbal face pack. Asian J. Pharm. Res. 2021, 11, 9–12. [Google Scholar] [CrossRef]
- Gomes, C.; Silva, J. Minerals and clay minerals in medical geology. Appl. Clay Sci. 2007, 36, 4–21. [Google Scholar] [CrossRef]
- Kamble, Y.; Choudhary, G.; Kishore, K.; Sonawale, N.; Choudhary, A.; Baviskar, R. Formulation and evaluation of multi herbal face pack for glowing skin. World J. Pharm. Res. 2023, 12, 1393–1401. Available online: https://www.wjpr.net/abstract_file/21843 (accessed on 8 March 2024).
- Tiwari, D.S.; Singh, S.; Kumari, A.; Yadav, S.; Bind, D. The role of ayurvedic herbs in skin rejuvenation by face mask: Ficus religiosa and beyond. Int. J. Pharm. Res. Dev. 2024, 6, 143–150. [Google Scholar] [CrossRef]
- Dalavi, P.R.K.; Ziya, K. Formulation and Evaluation of Herbal Face Pack. Int. J. Sci. Res. Technol. 2025, 2, 236–243. [Google Scholar] [CrossRef]
- Eximpedia. Find the Multani Mitti Export Opportunity: Foreign Market Demand Statistics. Available online: https://www.eximpedia.app/blog/multani-mitti-export-from-india (accessed on 8 March 2024).
- Journal, D. Fuller’s Earth Market Share, Revenue, and Forecast 2023–2030. Available online: https://www.digitaljournal.com/pr/news/fuller-s-earth-market-share-nbsp-revenue-nbsp-and-forecast-2023-2030 (accessed on 8 March 2024).
- Muralidharan, N.P.; Dhanasekaran, L. Antimicrobial Activity of Fuller’s Earth, Turmeric, and Sandalwood Against Streptococcus Mutans, Micrococci and Coagulase Negative Staphylococci—An In Vitro Study. J. Pharm. Res. Int. 2021, 33, 65–73. [Google Scholar] [CrossRef]
- Park, D.-B.; Lee, Y.-J.; Kim, W.-S.; Kim, Y.-T. Anti-skin Aging and Antihypertensive Activities of Different Solvent Extracts from Sargassum thunbergii. Korean J. Fish. Aquat. Sci. 2025, 58, 140–145. [Google Scholar] [CrossRef]
- Hussein, R.S.; Bin Dayel, S.; Abahussein, O.; El-Sherbiny, A.A. Influences on Skin and Intrinsic Aging: Biological, Environmental, and Therapeutic Insights. J. Cosmet. Dermatol. 2025, 24, e16688. [Google Scholar] [CrossRef]
- Jung, H. Hyaluronidase: An overview of its properties, applications, and side effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef]
- Gendreau, I.; Angers, L.; Jean, J.; Pouliot, R. Pigmented Skin Models: Understand the Mechanisms of Melanocytes. In Regenerative Medicine and Tissue Engineering; Andrades, J.A., Ed.; Intech: Houston, TX, USA, 2013. [Google Scholar]
- He, X.; Gao, X.; Guo, Y.; Xie, W. Research Progress on Bioactive Factors against Skin Aging. Int. J. Mol. Sci. 2024, 25, 3797. [Google Scholar] [CrossRef]
- Hong, Y.H.; Jung, E.Y.; Noh, D.O.; Suh, H.J. Physiological effects of formulation containing tannase-converted green tea extract on skin care: Physical stability, collagenase, elastase, and tyrosinase activities. Integr. Med. Res. 2014, 3, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.-T.; Jang, Y.; Myung, S.-W.; Lee, J.M.; Kim, H.-S.; Ko, S.-C.; Lee, S.-H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 62–72. [Google Scholar] [CrossRef]
- Waheed, S.; Rahman, S.; Faiz, Y.; Siddique, N. Neutron activation analysis of essential elements in Multani mitti clay using miniature neutron source reactor. Appl. Radiat. Isot. 2012, 70, 2362–2369. [Google Scholar] [CrossRef]
- Profumo, A.; Gorroni, A.; Guarnieri, S.A.; Mellerio, G.G.; Cucca, L.; Merli, D. GC-MS qualitative analysis of the volatile, semivolatile and volatilizable fractions of soil evidence for forensic application: A chemical fingerprinting. Talanta 2020, 219, 121304. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Elgamal, A.M.; El Raey, M.A.; Gaara, A.; Abdelfattah, M.A.O.; Sobeh, M. Phytochemical profiling and anti-aging activities of Euphorbia retusa extract: In silico and in vitro studies. Arab. J. Chem. 2021, 14, 103159. [Google Scholar] [CrossRef]
- Sklirou, A.D.; Angelopoulou, M.T.; Argyropoulou, A.; Chaita, E.; Boka, V.I.; Cheimonidi, C.; Niforou, K.; Mavrogonatou, E.; Pratsinis, H.; Kalpoutzakis, E.; et al. Phytochemical Study and In Vitro Screening Focusing on the Anti-Aging Features of Various Plants of the Greek Flora. Antioxidants 2021, 10, 1206. [Google Scholar] [CrossRef]
- Fikry, E.; Mahdi, I.; Bugra Ortaakarsu, A.; Tawfeek, N.; Adhiambo Ochieng, M.; Ben Bakrim, W.; Ao Abdelfattah, M.; Omari, K.W.; Mahmoud, M.F.; Sobeh, M. Dermato-cosmeceutical properties of Pseudobombax ellipticum (Kunth) Dugand: Chemical profiling, in vitro and in silico studies. Saudi Pharm. J. 2023, 31, 101778. [Google Scholar] [CrossRef] [PubMed]
- PubChem, National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 8 March 2024).
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- Shaweta, S.; Akhil, S.; Utsav, G. Molecular Docking studies on the Anti-fungal activity of Allium sativum (Garlic) against Mucormycosis (black fungus) by BIOVIA discovery studio visualizer 21.1.0.0. Ann. Antivir. Antiretrovir. 2021, 5, 028–032. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Majid, M.; Ghaffar, A.; Yameen, M.; Samad, H.A.; Mahrosh, H.S. Screening and molecular docking of selected phytochemicals against NS5B polymerase of hepatitis c virus. Pak. J. Pharm. Sci. 2020, 33, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Gotz, W.; Tobiasch, E.; Witzleben, S.; Schulze, M. Effects of Silicon Compounds on Biomineralization, Osteogenesis, and Hard Tissue Formation. Pharmaceutics 2019, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Carver, P.L. Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; De Gruyter: Berlin, Germany, 2019. [Google Scholar]
- Bonaventura, P.; Benedetti, G.; Albarede, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Duck, K.A.; Connor, J.R. Iron uptake and transport across physiological barriers. Biometals 2016, 29, 573–591. [Google Scholar] [CrossRef]
- Exley, C.; Mold, M.J. The binding, transport and fate of aluminium in biological cells. J. Trace Elem. Med. Biol. 2015, 30, 90–95. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Jurowska, A.; Hodorowicz, M.; Kazek, G.; Głuch-Lutwin, M.; Sapa, J. Ligand role on insulin-mimetic properties of vanadium complexes. Structural and biological studies. Inorganica Chim. Acta 2021, 516, 120135. [Google Scholar] [CrossRef]
- Zheng, S.; Cheng, S.; Xiao, S.; Hu, L.; Chen, Z.; Huang, B.; Liu, Q.; Yang, J.; Chen, Q. Partial replacement of K by Rb to improve electrochemical performance of K3V2(PO4)3 cathode material for potassium-ion batteries. J. Alloys Compd. 2020, 815, 152379. [Google Scholar] [CrossRef]
- Vincent, J.B. New Evidence against Chromium as an Essential Trace Element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef]
- Panahifar, A.; Chapman, L.D.; Weber, L.; Samadi, N.; Cooper, D.M.L. Biodistribution of strontium and barium in the developing and mature skeleton of rats. J. Bone Miner. Metab. 2019, 37, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Osman, D.; Cooke, A.; Young, T.R.; Deery, E.; Robinson, N.J.; Warren, M.J. The requirement for cobalt in vitamin B(12): A paradigm for protein metalation. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118896. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of cesium ions from aqueous solutions using various separation technologies. Rev. Environ. Sci. Bio/Technol. 2019, 18, 231–269. [Google Scholar] [CrossRef]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Biological activity of soil contaminated with cobalt, tin, and molybdenum. Environ. Monit. Assess. 2016, 188, 398. [Google Scholar] [CrossRef]
- Mayr, S.J.; Mendel, R.R.; Schwarz, G. Molybdenum cofactor biology, evolution and deficiency. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118883. [Google Scholar] [CrossRef]
- Shrimanker, I.; Bhattarai, S. Electrolytes. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541123/ (accessed on 8 March 2024).
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Osteopath. Med. 2018, 118, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ou, S.W.; Wang, Y.J. Distribution and function of voltage-gated sodium channels in the nervous system. Channels 2017, 11, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Grover, T.; Pandey, A.; Kumari, S.T.; Awasthi, A.; Singh, B.; Dixit, P.; Singhal, P.; Saxena, K.K. Role of titanium in bio implants and additive manufacturing: An overview. Mater. Today Proc. 2020, 26, 3071–3080. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Ge, X.; Cao, Z.; Chu, L. The Antioxidant Effect of the Metal and Metal-Oxide Nanoparticles. Antioxidants 2022, 11, 791. [Google Scholar] [CrossRef]
- Saeed, M.; Shoaib, A.; Tasleem, M.; Alabdallah, N.M.; Alam, M.J.; Asmar, Z.E.; Jamal, Q.M.S.; Bardakci, F.; Alqahtani, S.S.; Ansari, I.A.; et al. Assessment of Antidiabetic Activity of the Shikonin by Allosteric Inhibition of Protein-Tyrosine Phosphatase 1B (PTP1B) Using State of Art: An In Silico and In Vitro Tactics. Molecules 2021, 26, 2996. [Google Scholar] [CrossRef] [PubMed]
- Ribas, J.; Cubero, E.; Luque, F.J.; Orozco, M. Theoretical study of alkyl-pi and aryl-pi interactions. Reconciling theory and experiment. J. Org. Chem. 2002, 67, 7057–7065. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T.I. BDDCS, the Rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Chao, J. Skincare for Your Soul: Achieving Outer Beauty and Inner Peace with Korean Skincare; Mango Media Inc.: Balmain, Australia, 2021. [Google Scholar]
- Gupta, M.A.; Gilchrest, B.A. Psychosocial aspects of aging skin. Dermatol. Clin. 2005, 23, 643–648. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. GC/MS Profiling, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase Activities of a Stenocarpus sinuatus Leaves Extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef]
- Mishra, B.B.; Choudhary, S.K.; Roy, R. Soil and Industry. In The Soils of India; World Soils Book Series; Springer: Cham, Switzerland, 2020; pp. 243–259. [Google Scholar]
- Pohl, P.; Stelmach, E.; Welna, M.; Szymczycha-Madeja, A. Determination of the Elemental Composition of Coffee Using Instrumental Methods. Food Anal. Methods 2012, 6, 598–613. [Google Scholar] [CrossRef]
- Racles, C.; Dascalu, M.; Bele, A.; Cazacu, M. Reactive and Functional Silicones for Special Applications. In Reactive and Functional Polymers Volume One; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–291. [Google Scholar]
- Chaves, J.O.; de Souza Mesquita, L.M.; Strieder, M.M.; Contieri, L.S.; Pizani, R.S.; Sanches, V.L.; Viganó, J.; Neves Bezerra, R.M.; Rostagno, M.A. Eco-friendly and high-performance extraction of flavonoids from lemon peel wastes by applying ultrasound-assisted extraction and eutectic solvents. Sustain. Chem. Pharm. 2024, 39, 101558. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Gao, X.; Shi, X.; Chen, S.; Xu, Y.; Tang, K. Comparison of the Aroma-Active Compounds and Sensory Characteristics of Different Grades of Light-Flavor Baijiu. Foods 2023, 12, 1238. [Google Scholar] [CrossRef]
- Baser, B.; Yousaf, B.; Yetis, U.; Abbas, Q.; Kwon, E.E.; Wang, S.; Bolan, N.S.; Rinklebe, J. Formation of nitrogen functionalities in biochar materials and their role in the mitigation of hazardous emerging organic pollutants from wastewater. J. Hazard. Mater. 2021, 416, 126131. [Google Scholar] [CrossRef]
- Billings, A.; Jones, K.C.; Pereira, M.G.; Spurgeon, D.J. Plasticisers in the terrestrial environment: Sources, occurrence and fate. Environ. Chem. 2021, 18, 111–130. [Google Scholar] [CrossRef]
- Chandra, S.; Roychoudhury, A. Role of Selenium and Manganese in Mitigating Oxidative Damages. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 597–621. [Google Scholar]
- Bizerea, T.O.; Dezsi, S.G.; Marginean, O.; Stroescu, R.; Rogobete, A.; Bizerea-Spiridon, O.; Ilie, C. The Link Between Selenium, Oxidative Stress and Pregnancy Induced Hypertensive Disorders. Clin. Lab. 2018, 64, 1593–1610. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N.; Verma, M.; Kamal, R.; Tiwari, R.; Sanjay Chivate, M.; Rai, S.N.; Kumar, A.; Singh, A.; Singh, M.P.; et al. Hexavalent-Chromium-Induced Oxidative Stress and the Protective Role of Antioxidants against Cellular Toxicity. Antioxidants 2022, 11, 2375. [Google Scholar] [CrossRef]
- Dwivedi, J.; Sachan, P.; Wal, P. A Mechanistic Approach on Structural, Analytical and Pharmacological Potential of Beta-sitosterol: A Promising Nutraceutical. Curr. Nutr. Food Sci. 2024, 20, 932–951. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Habtemariam, S.; Adejare, A. Chemistry and Pharmacology of Alkylamides from Natural Origin. Rev. Bras. Farmacogn. 2020, 30, 622–640. [Google Scholar] [CrossRef] [PubMed]
- Mortada, S.; Karrouchi, K.; Hamza, E.H.; Oulmidi, A.; Bhat, M.A.; Mamad, H.; Aalilou, Y.; Radi, S.; Ansar, M.; Masrar, A.; et al. Synthesis, structural characterizations, in vitro biological evaluation and computational investigations of pyrazole derivatives as potential antidiabetic and antioxidant agents. Sci. Rep. 2024, 14, 1312. [Google Scholar] [CrossRef]
- Inglis, K.; Tettoni, L.I. Ayurveda: Asian Secrets of Wellness, Beauty and Balance; Tuttle Publishing: North Clarendon, VT, USA, 2012. [Google Scholar]
- Nandi, S.; Nag, A.; Khatua, S.; Sen, S.; Chakraborty, N.; Naskar, A.; Acharya, K.; Calina, D.; Sharifi-Rad, J. Anticancer activity and other biomedical properties of beta-sitosterol: Bridging phytochemistry and current pharmacological evidence for future translational approaches. Phytother. Res. 2024, 38, 592–619. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, P.; Li, J.; Wang, S. Preparation of Biocompatible Manganese Selenium-Based Nanoparticles with Antioxidant and Catalytic Functions. Molecules 2023, 28, 4498. [Google Scholar] [CrossRef]
- Yasin, M.; Younis, A.; Javed, T.; Akram, A.; Ahsan, M.; Shabbir, R.; Ali, M.M.; Tahir, A.; El-Ballat, E.M.; Sheteiwy, M.S.; et al. River Tea Tree Oil: Composition, Antimicrobial and Antioxidant Activities, and Potential Applications in Agriculture. Plants 2021, 10, 2105. [Google Scholar] [CrossRef]
- Yadav, E.; Kumar, S.; Mahant, S.; Khatkar, S.; Rao, R. Tea tree oil: A promising essential oil. J. Essent. Oil Res. 2016, 29, 201–213. [Google Scholar] [CrossRef]
- Kumar, P.; Karam, N.; Choudhury, D. Assessment of the Phytochemical Constituents and Metabolites in the Medicinal Plants and Herbal Medicine Used in the Treatment and Management of Skin Diseases. In Herbal Medicine Phytochemistry; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–41. [Google Scholar]








| Peak | RT (Min) | SI (%) | Compound Name | PubChem ID | Molecular Formula | Molecular Weight (g/mol) |
|---|---|---|---|---|---|---|
| 1 | 08.120 | 83 | 3-Heptanol, 1-Octanol | 957 | C8H18O | 130 |
| 2 | 10.060 | 83 | Cyclohexasiloxane | 10911 | C12H36O6Si6 | 444.92 |
| 3 | 15.929 | 93 | Nonadecane | 15979 | C19H40 | 268.5 |
| 4 | 15.995 | 90 | Pentacosane | 12406 | C25H52 | 352.7 |
| 5 | 16.060 | 91 | Heptacosane | 11636 | C27H56 | 380.7 |
| 6 | 17.020 | 92 | Nonacosane | 12409 | C29H60 | 408.8 |
| 7 | 17.130 | 92 | Tetrapentacontane | 545963 | C54H108Br2 | 917.2 |
| 8 | 23.975 | 82 | Oleth-2 | 13387455 | C22H44O3 | 356.6 |
| 9 | 24.170 | 94 | Docosanamide | 76468 | C22H45NO | 339.6 |
| 10 | 25.705 | 94 | Benzyl-piperazine-carboxamide | 455963 | C37H48N4O4 | 612.8 |
| 11 | 26.580 | 75 | Bis(2-ethylhexyl) phthalate | 8343 | C24H38O4 | 390.6 |
| 12 | 28.650 | 83 | Ganaxolone | 6918305 | C22H36O2 | 332.5 |
| 13 | 36.110 | 77 | Beta-sitosterol | 222284 | C29H50O | 414.7 |
| Sample | IC50 |
|---|---|
| MM Powder (Fuller’s Earth) | 31.938 μg/mL |
| Ascorbic Acid | 8.5 μg/mL |
| Name | PubChem ID | Binding Affinity (kcal/mol) with 3NQ1 | Interacting Amino Acids with 3NQ1 | Binding Affinity (kcal/mol) with 1FCV | Interacting Amino Acids with 1FCV |
|---|---|---|---|---|---|
| Benzyl-piperazine-carboxamide | 455963 | −8.6 | PheB:258, AspA:36, ProB:51, LysA:30, ProA:145, ThrA:137, GluA:31, ProB:52 and LeuA:27 | −8.1 | ArgA:229, AsnA:231, AlaA:185, LeuA:192, ProA:194 and ThrA:193 |
| Bis(2-ethylhexyl) phthalate | 8343 | −8.0 | AsnB:249, GlnB:242, HisB:279, IleB:243 and LysB:281 | −5.9 | TyrA:168, AlaA:185, ArgA:244, SerA:225, TyrA:190 and ArgA:116 |
| Heptacosane | 11636 | −8.0 | PheB:124, AspB:123 and LysB:150 | −4.6 | TyrA:227, TrpA:301, TrpA:267, TrA:184, TyrA:55 and AspA:111 |
| Ganaxolone | 6918305 | −7.2 | PheA:262, ProA:273, ValA:276, MetA:266, ProA:67, TrpA:68, GluA:71 and ValA:276 | −7.4 | SerA:304, AspA:111, AspA:305, Tyra:55, AspA:56, PheA:112, GluA:113, SerA:303 and AspA:305 |
| Beta-sitosterol | 222284 | −7.1 | AsnA:249, PheA:262, MetA:277, tyrA:250, GluA:274, ProA:273, ValA:276, ProA:67, GluA:71, TyrA:72, trpA:269, TrpA:68, ThrA:272, ArgA:70, AspA:275 and MetA:266 | −8.5 | LysA:45, PheA:20, ProA:100, IleA:99, IleA:99, ProA:105 and AspA:101 |
| Molecular Properties | |||||||
|---|---|---|---|---|---|---|---|
| Ligand | Molecular Mass (≤500 Dalton) | Hydrogen Bond Donor (≤5) | Hydrogen Bond Acceptor (≤10) | No. of Rotatable Bonds (≤10) | LogP (≤5) | Refractivity (40–130) | Violations |
| Benzyl-piperazine-carboxamide | 612.80 | 4 | 6 | 14 | 3.65 | 184.83 | 0 |
| Bis(2-ethylhexyl) phthalate | 390.6 | 0 | 4 | 16 | 4.77 | 116.30 | 1 |
| Heptacosane | 380.73 | 0 | 0 | 24 | 7.32 | 130.1 | 1 |
| Ganaxolone | 332.52 | 1 | 2 | 1 | 3.29 | 100.29 | 0 |
| Beta-sitosterol | 414.71 | 1 | 1 | 6 | 5.05 | 133.23 | 0 |
| Compounds | |||||
|---|---|---|---|---|---|
| Benzyl-piperazine-carboxamide | Bis(2-ethylhexyl) phthalate | Heptacosane | Ganaxolone | Beta-sitosterol | |
| Absorption and Distribution | |||||
| BBB | −−− | +++ | −−− | +++ | −−− |
| HIA | −−− | −−− | −−− | −−− | −−− |
| Caco-2 Permeability | −5.246 | −4.918 | −5.074 | −4.806 | −5.122 |
| Volume of Distribution (Vd) | 0.001 | 0.94 | 3.009 | 0.205 | −0.244 |
| Plasma Protein Binding (PPB) | 95.1% | 98.7% | 105.5% | 67.4% | 86.9% |
| Excretion | |||||
| CLplasma | 4.928 | 5.79 | 4.545 | 16.978 | 13.205 |
| T1/2 | 0.865 | 0.324 | 3.031 | 0.97 | 0.541 |
| Toxicity | |||||
| Skin Sensitization | 0.807 | 0.938 | 0.998 | 0.992 | 0.99 |
| Carcinogenicity | 0.13 | 0.328 | 0.164 | 0.983 | 0.688 |
| Human Hepatotoxicity | 0.916 | 0.086 | 0.473 | 0.854 | 0.573 |
| AMES Toxicity | 0.129 | 0.018 | 0.012 | 0.183 | 0.139 |
| Drug-Likeness Rules | |||||
| Acute Aquatic Toxicity Rule | 0 | 0 | 1 | 2 | 1 |
| Skin Sensitization Rule | 1 | 0 | 0 | 2 | 0 |
| Lipinski Rule | Yes | Yes | Yes | Yes | Yes |
| Pfizer Rule | Yes | No | No | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, M.J.; Loren, P.; Burgos, V.; Salazar, L.A. Multi-Target Anti-Aging Mechanisms of Multani Mitti (Fuller’s Earth): Integrating Enzyme Inhibition and Molecular Docking for Cosmeceuticals. Cosmetics 2025, 12, 124. https://doi.org/10.3390/cosmetics12030124
Iqbal MJ, Loren P, Burgos V, Salazar LA. Multi-Target Anti-Aging Mechanisms of Multani Mitti (Fuller’s Earth): Integrating Enzyme Inhibition and Molecular Docking for Cosmeceuticals. Cosmetics. 2025; 12(3):124. https://doi.org/10.3390/cosmetics12030124
Chicago/Turabian StyleIqbal, Muhammad Javid, Pía Loren, Viviana Burgos, and Luis A. Salazar. 2025. "Multi-Target Anti-Aging Mechanisms of Multani Mitti (Fuller’s Earth): Integrating Enzyme Inhibition and Molecular Docking for Cosmeceuticals" Cosmetics 12, no. 3: 124. https://doi.org/10.3390/cosmetics12030124
APA StyleIqbal, M. J., Loren, P., Burgos, V., & Salazar, L. A. (2025). Multi-Target Anti-Aging Mechanisms of Multani Mitti (Fuller’s Earth): Integrating Enzyme Inhibition and Molecular Docking for Cosmeceuticals. Cosmetics, 12(3), 124. https://doi.org/10.3390/cosmetics12030124








