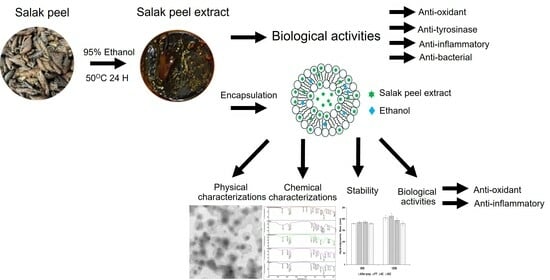
Exploration of Salak Peel Extract Activities for Cosmeceutical Applications and Its Encapsulation in Ethosomes Using Green Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Salak Peel Extraction Process and Characterizations
2.3. Chemical Analysis of Salak Peel Extract
2.3.1. Antiradical Capacity
2.3.2. Anti-Tyrosinase Activity Assay
2.4. Biological Activities of Salak Peel Extract
2.4.1. Cytotoxicity Assay
2.4.2. Anti-Inflammatory Assay
2.4.3. Anti-Bacterial Activity Assay
2.4.4. Biocompatibility Assay
2.5. Encapsulation of Salak Peel Extract
2.6. Characterizations of Salak Peel Ethosomes
2.7. Encapsulation Efficiency of Salak Peel Ethosomes
2.8. Stability Study
2.9. Statistical Analysis
3. Results and Discussion
3.1. Salak Peel Extraction and Characterizations
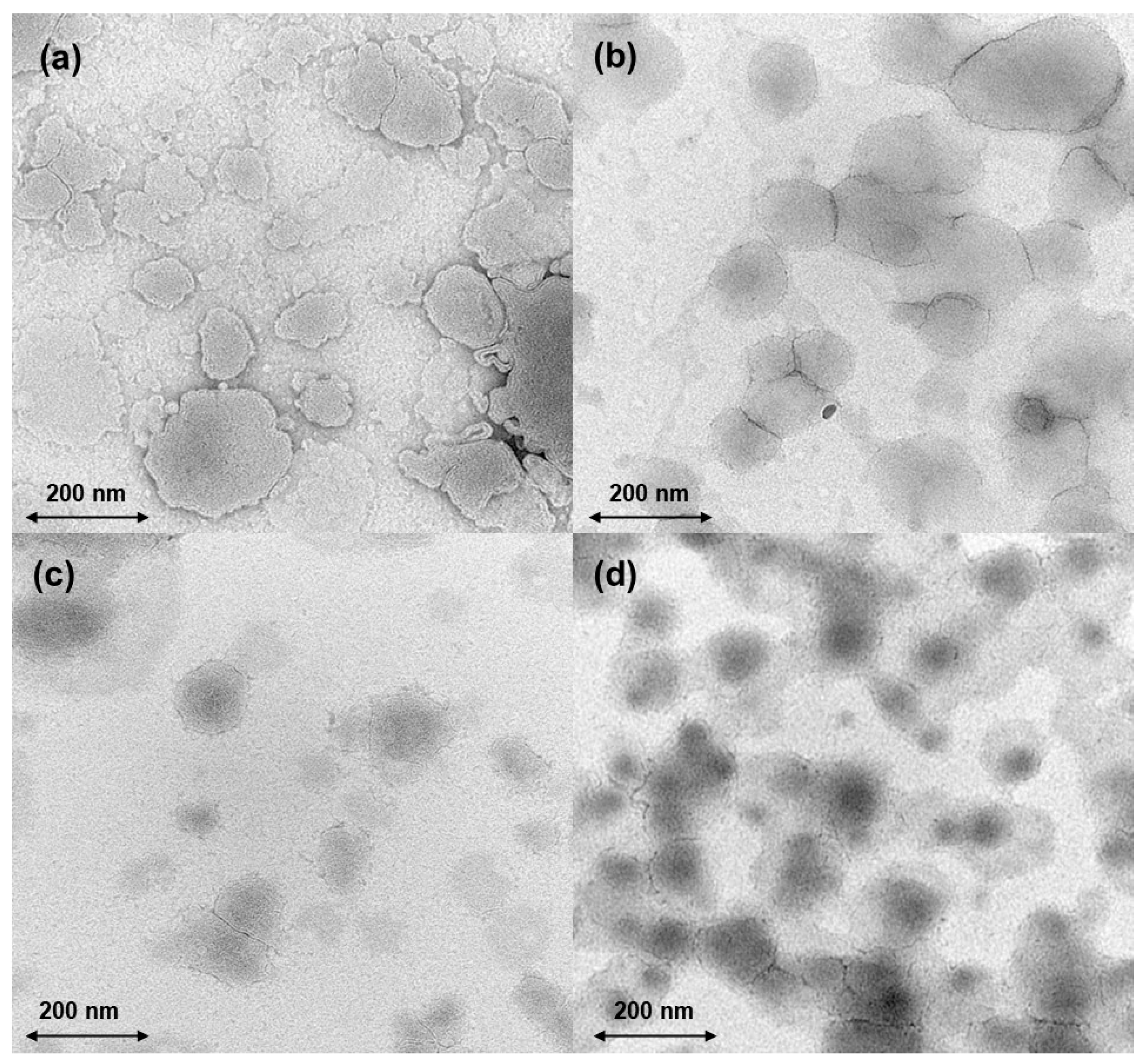
3.2. Salak Peel Ethosome Preparation and Characterizations
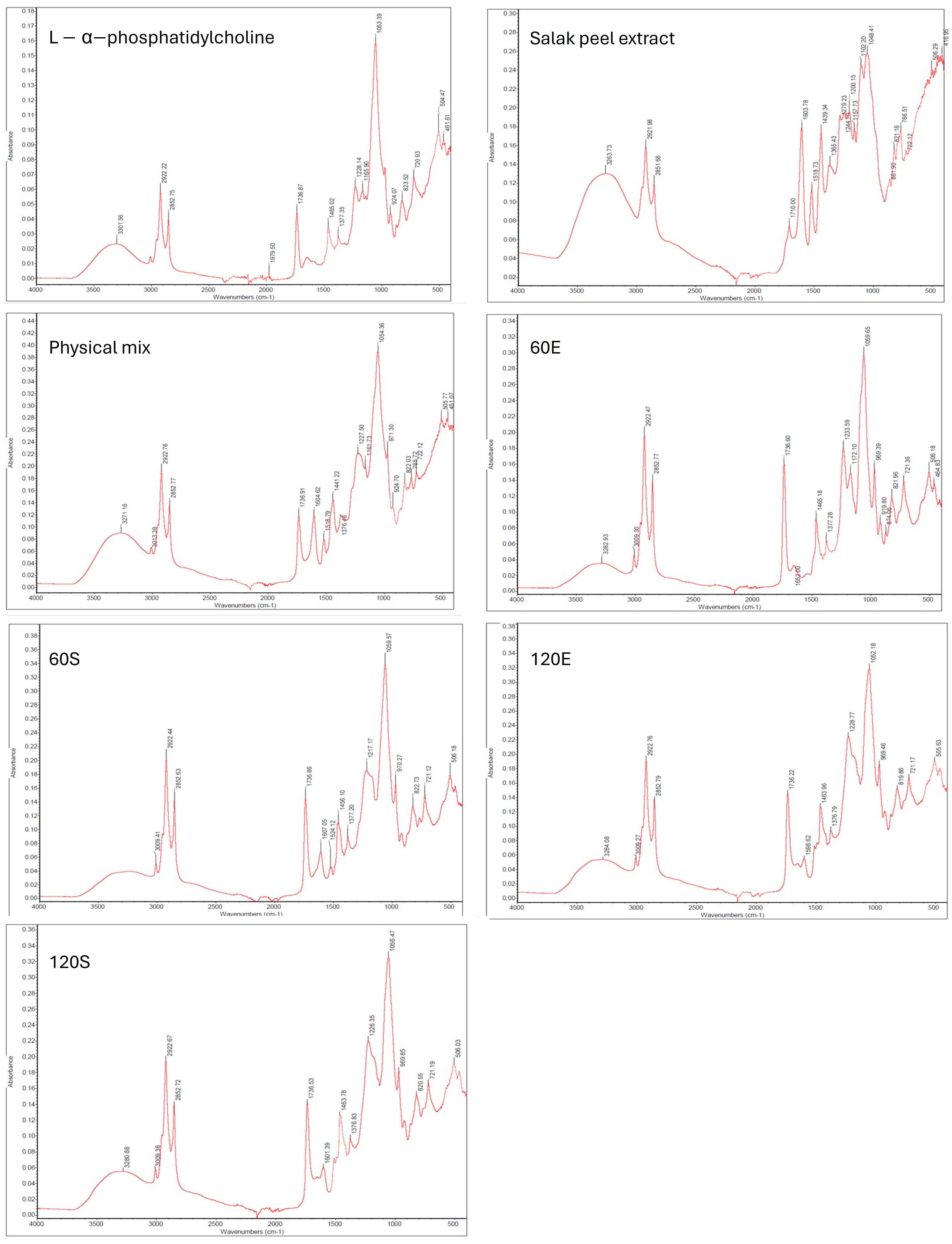
3.3. Other Characterizations of Ethosome-Encapsulated Salak Peel Extract
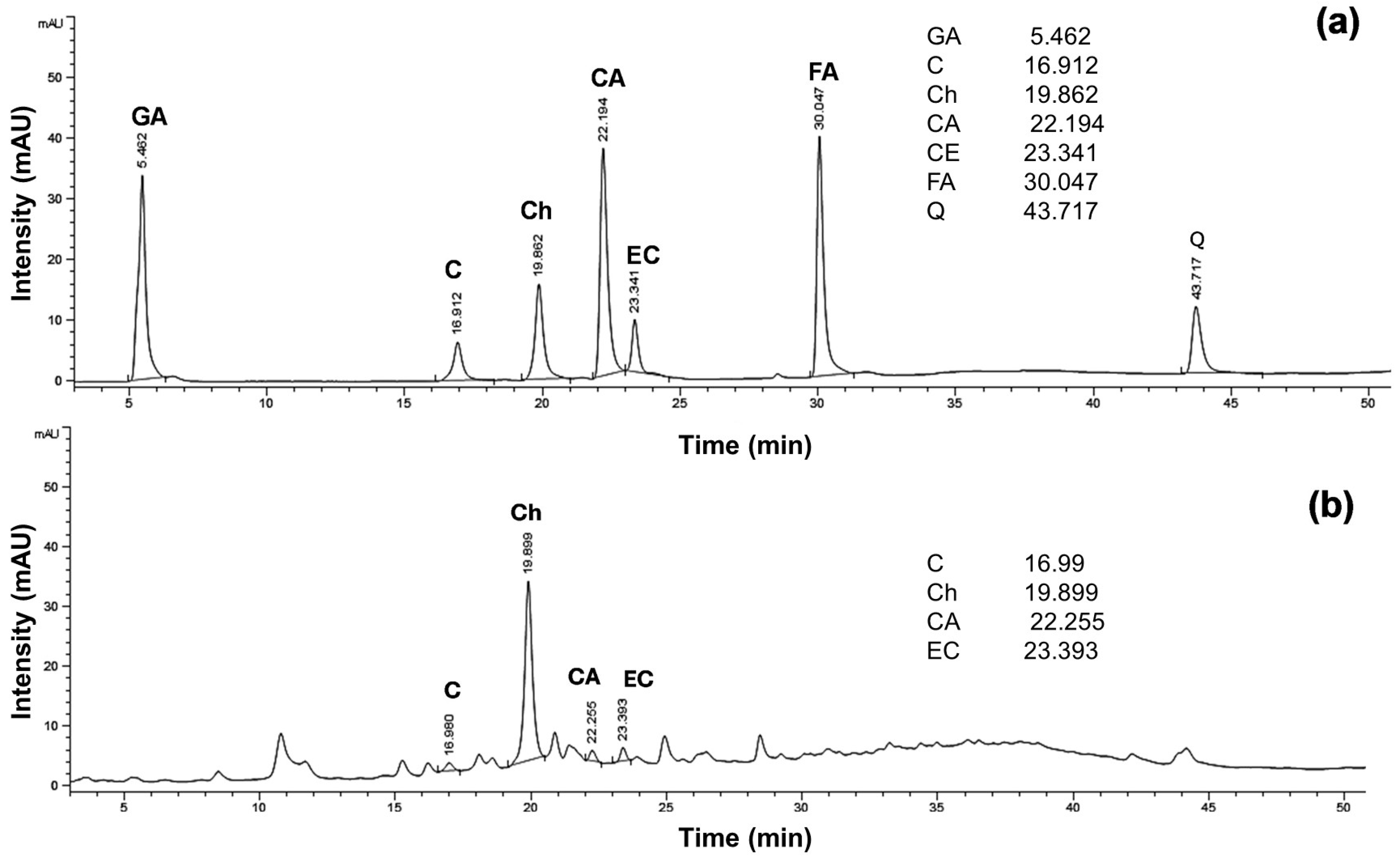
3.4. Chemical Analysis and Biological Activities of Salak Peel Extract and Salak Peel Ethosomes
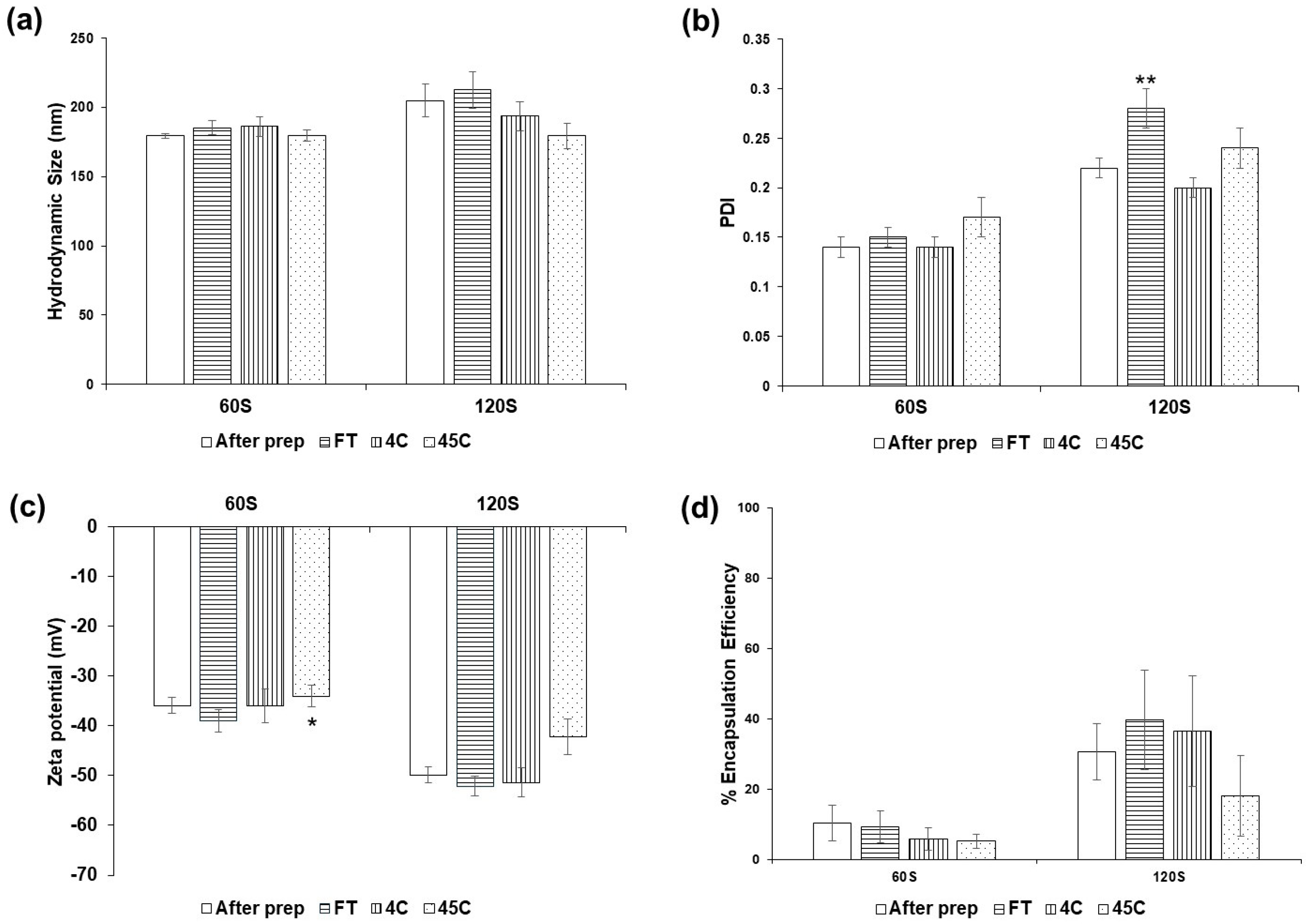
3.5. Stability of Ethosomes and Salak Peel Ethosomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleh, M.S.; Siddiqui, M.J.; Mediani, A.; Ismail, N.H.; Ahmed, Q.U.; So’ad, S.Z.M.; Saidi-Besbes, S. Salacca zalacca: A short review of the palm botany, pharmacological uses and phytochemistry. Asian Pac. J. Trop. Med. 2018, 11, 645–652. [Google Scholar]
- Kanlayavattanakul, M.; Lourith, N.; Ospondpant, D.; Ruktanonchai, U.; Pongpunyayuen, S.; Chansriniyom, C. Salak plum peel extract as a safe and efficient antioxidant appraisal for cosmetics. Biosci. Biotechnol. Biochem. 2013, 77, 1068–1074. [Google Scholar] [CrossRef]
- Tan, S.S.; Tan, S.T.; Tan, C.X. Antioxidant, hypoglycemic and anti-hypertensive properties of extracts derived from peel, fruit and kernel of Salak. Br. Food J. 2020, 122, 3029–3038. [Google Scholar] [CrossRef]
- Sunandar, A.P.; Aris, A.; Fitri, R.L. Evaluation of inhibitory activity of salak peel extract and lotion formula on Trichophyton mentagrophytes. AIP Conf. Proc. 2023, 2556, 030001. [Google Scholar]
- Saleh, M.S.; Siddiqui, M.J.; So’ad, S.Z.M.; Murugesu, S.; Khatib, A.; Rahman, M.M. Antioxidant and α-Glucosidase Inhibitory Activities and Gas Chromatography–Mass Spectrometry Profile of Salak (Salacca zalacca) Fruit Peel Extracts. Pharmacogn. Res. 2018, 10, 385–390. [Google Scholar]
- Hcini, K.; Lozano-Pérez, A.A.; Luis Cenis, J.; Quílez, M.; José Jordán, M. Extraction and encapsulation of phenolic compounds of tunisian rosemary (Rosmarinus officinalis L.) extracts in silk fibroin nanoparticles. Plants 2021, 10, 2312. [Google Scholar] [CrossRef] [PubMed]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate (Punica granatum L.) peel extract as a natural source of antioxidants. Nanomaterials 2021, 11, 1439. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Paul, S.; Khuda-Bukhsh, A.R. Encapsulated plant extract (Gelsemium sempervirens) poly (lactide-co-glycolide) nanoparticles enhance cellular uptake and increase bioactivity in vitro. Exp. Biol. Med. 2010, 235, 678–688. [Google Scholar] [CrossRef]
- Gortzi, O.; Lalas, S.; Chinou, I.; Tsaknis, J. Reevaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes. Eur. Food Res. Technol. 2008, 226, 583–590. [Google Scholar] [CrossRef]
- Miere, F.; Vicas, S.I.; Timar, A.V.; Ganea, M.; Zdrinca, M.; Cavalu, S.; Fritea, L.; Vicas, L.; Muresan, M.; Pallag, A. Preparation and characterization of two different liposomal formulations with bioactive natural extract for multiple applications. Processes 2021, 9, 432. [Google Scholar] [CrossRef]
- Madhunithya, E.; Venkatesh, G.; Shyamala, G.; Manjari, V.; Ramesh, S.; Karuppaiah, A.; Sankar, V. Development of ethosome comprising combined herbal extracts and its effect on hair growth. Adv. Tradit. Med. 2021, 21, 131–141. [Google Scholar] [CrossRef]
- Somwanshi, S.B.; Hiremath, S.N. In-vivo evaluation of the wound healing activity of the Sesamum indicum L. seed extract in novel ethosomal vesicular system. J. Drug Deliv. Ther. 2018, 8, 411–420. [Google Scholar] [CrossRef]
- Yücel, Ç.; Şeker Karatoprak, G.; Degim, I.T. Anti-aging formulation of rosmarinic acid-loaded ethosomes and liposomes. J. Microencapsul. 2019, 36, 180–191. [Google Scholar] [CrossRef]
- Kusumawati, I.; Kurniawan, K.O.; Rohmania, R.; Pratama, B.A.; Pratama, Y.A.; Rullyansyah, S.; Warsito, M.F.; Widyowati, R.; Hestianah, E.P.; Matsunami, K. Comparative study of liposomal and ethosomal formulations of Curcuma heyneana rhizome extract in a transdermal delivery system. Pharm. Nanotechnol. 2025, 13, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Anwar, E.; Farhana, N. Formulation and evaluation of phytosome-loaded maltodextrin-gum Arabic microsphere system for delivery of Camellia sinensis extract. J. Young Pharm. 2018, 10, S56. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef]
- Kidd, P.; Head, K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (Siliphos). Altern. Med. Rev. 2005, 10, 193–203. [Google Scholar]
- Bombardelli, E.; Spelta, M.; Loggia, R.; Sosa, S.; Tubaro, A. Aging skin: Protective effect of silymarin-PHYTOSOME. Fitoterapia 1991, 62, 115–122. [Google Scholar]
- Sunagar, R.R.; Sreerama, Y.N. Implication of solvent polarities on browntop millet (Urochloa ramosa) phenolic antioxidants and their ability to protect oxidative DNA damage and inhibit α-amylase and α-glucosidase enzymes. Food Chem. 2023, 411, 135474. [Google Scholar] [CrossRef]
- AAT Bioquest, Inc. 4 October 2024. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 13 December 2024).
- Hsu, C.-K.; Chou, S.-T.; Huang, P.-J.; Mong, M.-C.; Wang, C.-K.; Hsueh, Y.-P.; Jhan, J.-K. Crude ethanol extracts from grape seeds and peels exhibit anti-tyrosinase activity. J. Cosmet. Sci. 2012, 63, 225–232. [Google Scholar]
- Onyebuchi, C.; Kavaz, D. Effect of extraction temperature and solvent type on the bioactive potential of Ocimum gratissimum L. extracts. Sci. Rep. 2020, 10, 21760. [Google Scholar] [CrossRef] [PubMed]
- Spigno, G.; De Faveri, D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Nasri, S.; Ebrahimi-Hosseinzadeh, B.; Rahaie, M.; Hatamian-Zarmi, A.; Sahraeian, R. Thymoquinone-loaded ethosome with breast cancer potential: Optimization, in vitro and biological assessment. J. Nanostructure Chem. 2020, 10, 19–31. [Google Scholar] [CrossRef]
- Bodade, S.S.; Kamble, M.S.; Shaikh, K.S.; Chaudhari, P.D. A study on ethosomes as mode for transdermal delivery of an antidiabetic drug. Drug Deliv. 2013, 20, 40–46. [Google Scholar] [CrossRef]
- Bellefroid, C.; Lechanteur, A.; Evrard, B.; Mottet, D.; Debacq-Chainiaux, F.; Pie, G. In vitro skin penetration enhancement techniques: A combined approach of ethosomes and microneedles. Int. J. Pharm. 2019, 572, 118793. [Google Scholar] [CrossRef]
- Sallustio, V.; Farruggia, G.; di Cagno, M.P.; Tzanova, M.M.; Marto, J.; Ribeiro, H.; Goncalves, L.M.; Mandrone, M.; Chiocchio, I.; Cerchiara, T.; et al. Design and characterization of an ethosomal gel encapsulating rosehip extract. Gels 2023, 9, 362. [Google Scholar] [CrossRef]
- Arjmandi, N.; Van Roy, W.; Lagae, L.; Borghs, G. Measuring the Electric Charge and Zeta Potential of Nanometer-Sized Objects Using Pyramidal-Shaped Nanopores. Anal. Chem. 2012, 84, 8490–8496. [Google Scholar] [CrossRef]
- Limsuwan, T.; Boonme, P.; Khongkow, P.; Amnuaikit, T. Ethosomes of phenylethyl resorcinol as vesicular delivery system for skin lightening applications. Biomed Res. Int. 2017, 2017, 8310979. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal ph of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Saleh, M.S.; Siddiqui, M.J.; Mat So’ad, S.Z.; Roheem, F.O.; Saidi-Besbes, S.; Khatib, A. Correlation of FT-IR fingerprint and α-glucosidase inhibitory activity of salak (Salacca zalacca) fruit extracts utilizing orthogonal partial least square. Molecules 2018, 23, 1434. [Google Scholar] [CrossRef]
- Pereira, A.; Mallya, R. Formulation and evaluation of a photoprotectant cream containing Phyllanthus emblica extract-phospholipid complex. J. Pharmacogn. Phytochem. 2015, 4, 232–240. [Google Scholar]
- Ong, S.P.; Law, C.L. Drying kinetics and antioxidant phytochemicals retention of salak fruit under different drying and pretreatment conditions. Dry. Technol. 2011, 29, 429–441. [Google Scholar] [CrossRef]
- Maryana, W.; Rachmawati, H.; Mudhakir, D. Formation of phytosome containing silymarin using thin layer-hydration technique aimed for oral delivery. Mater. Today Proc. 2016, 3, 855–866. [Google Scholar] [CrossRef]
- Xiao, Y.; Song, Y.; Chen, Z.; Ping, Q. The preparation of silybin–phospholipid complex and the study on its pharmacokinetics in rats. Int. J. Pharm. 2006, 307, 77–82. [Google Scholar]
- Predescu, N.C.; Papuc, C.; Nicorescu, V.; Gajaila, I.; Goran, G.V.; Petcu, C.D.; Stefan, G. The influence of solid-to-solvent ratio and extraction method on total phenolic content, flavonoid content and antioxidant properties of some ethanolic plant extracts. Rev. Chim 2016, 67, 1922–1927. [Google Scholar]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Adjeng, A.N.T.; Akib, N.I.; Hairah, S.; Herman, S. Formulation and antioxidants evaluation of liquid soap of Salacca zalacca (Gaertn.) Voss. peels ethanol extract 96%. J. Cakrawala Ilm. 2022, 1, 1913–1920. [Google Scholar] [CrossRef]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Khu, A.; Samin, B.; Widowati, W.; Wibowo, S.; Rizal, R. Chemical constituents of snake fruit (Salacca zalacca (Gaert.) Voss) peel and in silico anti-aging analysis. Mol. Cell. Biomed. Sci. 2019, 3, 122–128. [Google Scholar] [CrossRef]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Nasution, S.L.; Suhartina, S.; Munshy, U.Z.; Rizal, R.; Widowati, W. Antioxidant and Anti-inflammatory Activity of Salacca zalacca (Gaertn.) Voss Peel Ethanolic Extract on Lead Induced Fibroblast Cells. In Proceedings of the International Conference on Advance Molecular Bioscience Biomedical Engineering, Newport Beach, CA, USA, 6–9 January 2019; pp. 68–73. [Google Scholar]
- Girsang, E.; Ginting, C.N.; Lister, I.N.E.; yashfa Gunawan, K.; Widowati, W. Anti-inflammatory and antiaging properties of chlorogenic acid on UV-induced fibroblast cell. PeerJ 2021, 9, e11419. [Google Scholar] [CrossRef]
- Wahyuni, U.N.; Sihotang, Y.M.; Marpaung, M.P.; Prasetyo, D. Perbandingan Pelarut Etanol Dengan Etil Asetat Terhadap Aktivitas Antibakteri Ekstrak Kulit Buah Salak (Salacca zalacca Reinw) Pada Pertumbuhan Bakteri Staphylococcus Aureus ATCC 25923. Madani J. Ilm. Multidisiplin 2024, 2, 637–643. [Google Scholar]
- Li, Y.; Xu, F.; Li, X.; Chen, S.Y.; Huang, L.Y.; Bian, Y.Y.; Wang, J.; Shu, Y.T.; Yan, G.J.; Dong, J.; et al. Development of curcumin-loaded composite phospholipid ethosomes for enhanced skin permeability and vesicle stability. Int. J. Pharm. 2021, 592, 119936. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; Grande, F.; De Luca, M.; Occhiuzzi, M.A.; Garofalo, A.; Ragno, G. Photodegradation of anti-inflammatory drugs: Stability tests and lipid nanocarriers for their photoprotection. Molecules 2021, 26, 5989. [Google Scholar] [CrossRef] [PubMed]
- Sydykov, B.; Oldenhof, H.; Sieme, H.; Wolkers, W.F. Storage stability of liposomes stored at elevated subzero temperatures in DMSO/sucrose mixtures. PLoS ONE 2018, 13, e0199867. [Google Scholar] [CrossRef] [PubMed]
- Karpuz, M.; Gunay, M.S.; Ozer, A.Y. Chapter 18—Liposomes and phytosomes for phytoconstituents. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Singh, M.R., Singh, D., Kanwar, J.R., Chauhan, N.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 525–553. [Google Scholar]
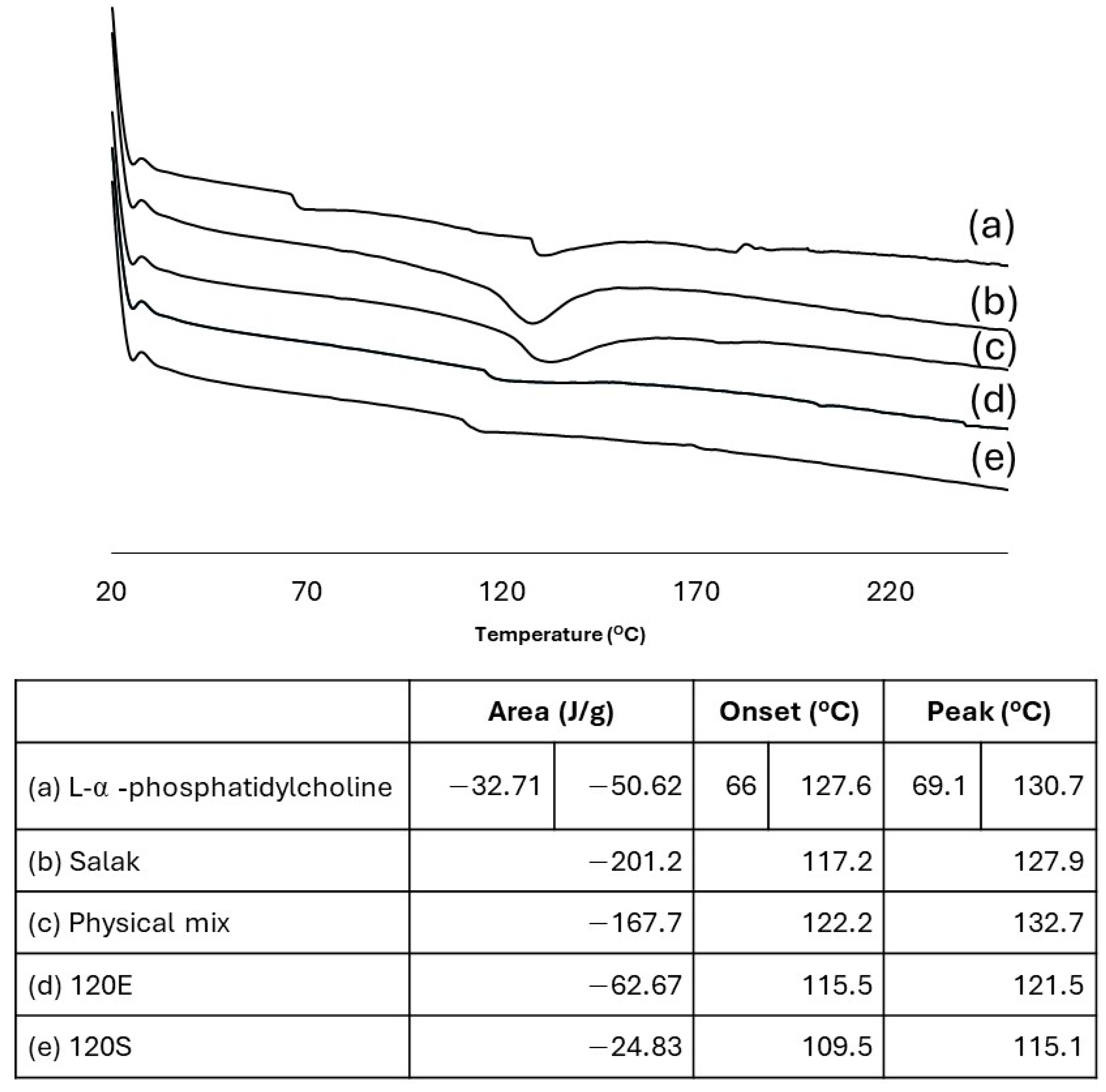
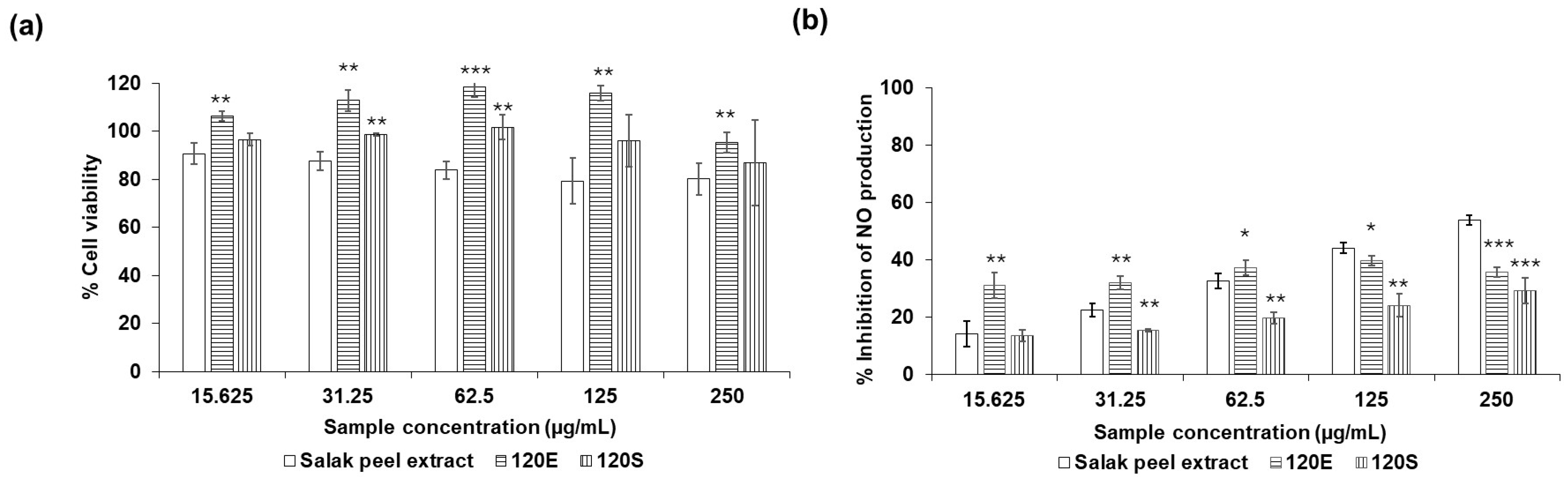
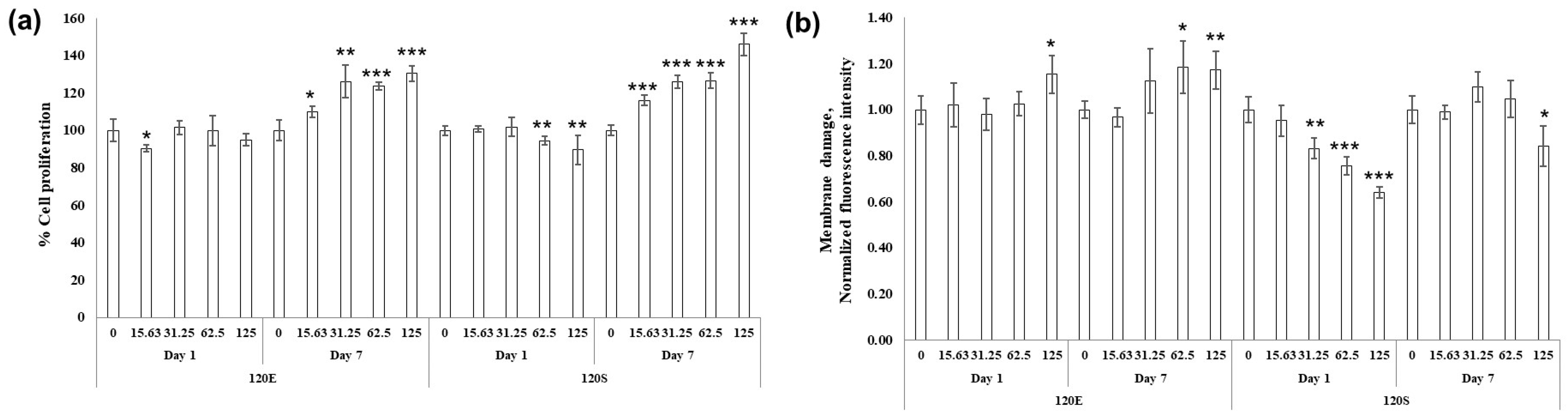
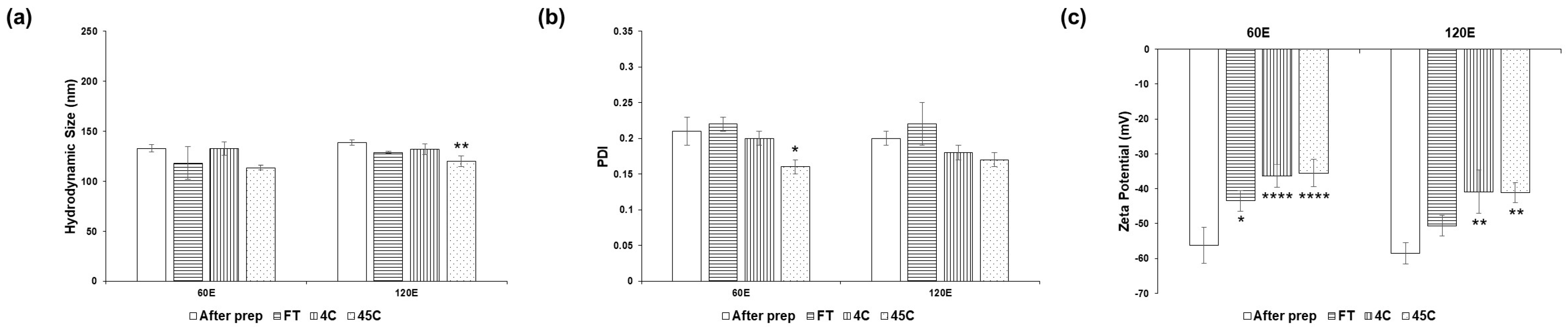
| Escherichia coli | Staphylococcus aureus | Cutibacterium acnes | |
|---|---|---|---|
| Media Used for MIC | Mueller–Hinton broth | Reinforced Clostridial Broth | |
| Media Used for MBC | Mueller–Hinton Agar | Brain Heart Infusion Agar | |
| Incubation Time (hours) | 24 | 72 | |
| Incubation Condition | Aerobic | Anaerobic | |
| Formulation Codes | Amount of L-α-Phosphatidylcholine (mg) | Amount of Salak Peel Extract (mg) | Total Amount of Ethanol (mL) | Total Amount of Type I Water (mL) |
|---|---|---|---|---|
| Ethosomes | ||||
| 30E | 30 | - | 2.75 | 3.5 |
| 60E | 60 | - | 2.75 | 3.5 |
| 120E | 120 | - | 2.75 | 3.5 |
| Salak Peel Ethosomes | ||||
| 30S | 30 | 30 | 2.75 | 3.5 |
| 60S | 60 | 30 | 2.75 | 3.5 |
| 120S | 120 | 30 | 2.75 | 3.5 |
| Phenolic Content | Concentration (µg/mL) | %Content (g/100 g·Sample) |
|---|---|---|
| Catechin | 0.48 ± 0.05 | 0.05 ± 0.00 |
| Chlorogenic acid | 4.36 ± 0.05 | 0.44 ± 0.00 |
| Caffeic acid | 0.10 ± 0.00 | 0.01 ± 0.00 |
| Epicatechin | 0.48 ± 0.00 | 0.05 ± 0.00 |
| Formulations | pH | Hydrodynamic Size (nm) | PDI | Zeta Potential (mV) | % Encapsulation Efficiency (Total Phenolic Content) |
|---|---|---|---|---|---|
| Ethosomes | |||||
| 30E | 5.2 ± 0.1 | 133.8 ± 2.8 | 0.22 ± 0.00 | −53.5 ± 2.9 | - |
| 60E | 5.5 ± 0.2 | 132.8 ± 3.7 | 0.21 ± 0.02 | −56.2 ± 5.1 | - |
| 120E | 5.1 ± 0.2 | 138.8 ± 2.8 | 0.20 ± 0.01 | −58.5 ± 3.1 | - |
| Salak Peel Ethosomes | |||||
| 30S | 6.0 ± 0.4 | 124.6 ± 11.2 | 0.14 ± 0.02 | −34.5 ± 1.5 | 1.8 ± 0.3 |
| 60S | 5.3 ± 0.1 | 179.4 ± 1.8 | 0.14 ± 0.01 | −35.9 ± 1.6 | 10.5 ± 5.1 |
| 120S | 5.1 ± 0.1 | 204.9 ± 11.8 | 0.22 ± 0.01 | −39.9 ± 1.6 | 30.6 ± 8 |
| Testing Sample | Anti-Oxidant Activity (IC50, mg/mL) | Anti-Tyrosinase Activity (IC50, mg/mL) | |
|---|---|---|---|
| DPPH | ABTS | ||
| L-Ascorbic Acid | 0.038 ± 0.001 | 0.007 ± 0.002 | - |
| Kojic Acid | - | - | 0.078 ± 0.005 |
| Salak Peel Extract | 0.027 ± 0.001 | 0.027 ± 0.004 | 0.193 ± 0.027 |
| Ethosomes (120E) | More than 50 | No activity | No activity |
| Salak Peel Ethosomes (120S) | 0.054 ± 0.008 | 0.448 ± 0.069 | No activity |
| Sample | E. coli ATCC 25922 | S. aureus ATCC 6538 | C. acnes ATCC 6919 | |||
|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| Salak Peel Extract | 100 | 100 | 1.56 | 1.56 | 100 | 100 |
| 20% DMSO | ND | ND | 5% | 10% | ND | ND |
| 70% Ethanol | 17.5% | 35% | 8.75% | 35% | 35% | 35% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tambunlertchai, S.; Thiramanas, R.; Wongngam, Y.; Yodkrahom, P.; Batthong, S.; Suktham, K.; Surassmo, S.; Asawapirom, U.; Polpanich, D. Exploration of Salak Peel Extract Activities for Cosmeceutical Applications and Its Encapsulation in Ethosomes Using Green Method. Cosmetics 2025, 12, 122. https://doi.org/10.3390/cosmetics12030122
Tambunlertchai S, Thiramanas R, Wongngam Y, Yodkrahom P, Batthong S, Suktham K, Surassmo S, Asawapirom U, Polpanich D. Exploration of Salak Peel Extract Activities for Cosmeceutical Applications and Its Encapsulation in Ethosomes Using Green Method. Cosmetics. 2025; 12(3):122. https://doi.org/10.3390/cosmetics12030122
Chicago/Turabian StyleTambunlertchai, Supreeda, Raweewan Thiramanas, Yodsathorn Wongngam, Pimnipa Yodkrahom, Sornsawan Batthong, Kunat Suktham, Suvimol Surassmo, Udom Asawapirom, and Duangporn Polpanich. 2025. "Exploration of Salak Peel Extract Activities for Cosmeceutical Applications and Its Encapsulation in Ethosomes Using Green Method" Cosmetics 12, no. 3: 122. https://doi.org/10.3390/cosmetics12030122
APA StyleTambunlertchai, S., Thiramanas, R., Wongngam, Y., Yodkrahom, P., Batthong, S., Suktham, K., Surassmo, S., Asawapirom, U., & Polpanich, D. (2025). Exploration of Salak Peel Extract Activities for Cosmeceutical Applications and Its Encapsulation in Ethosomes Using Green Method. Cosmetics, 12(3), 122. https://doi.org/10.3390/cosmetics12030122