Evolution of Thread Lifting: Advancing Toward Bioactive Polymers and Sustained Hyaluronic Acid Delivery
Abstract
1. Introduction
2. Materials and Methods
3. Results
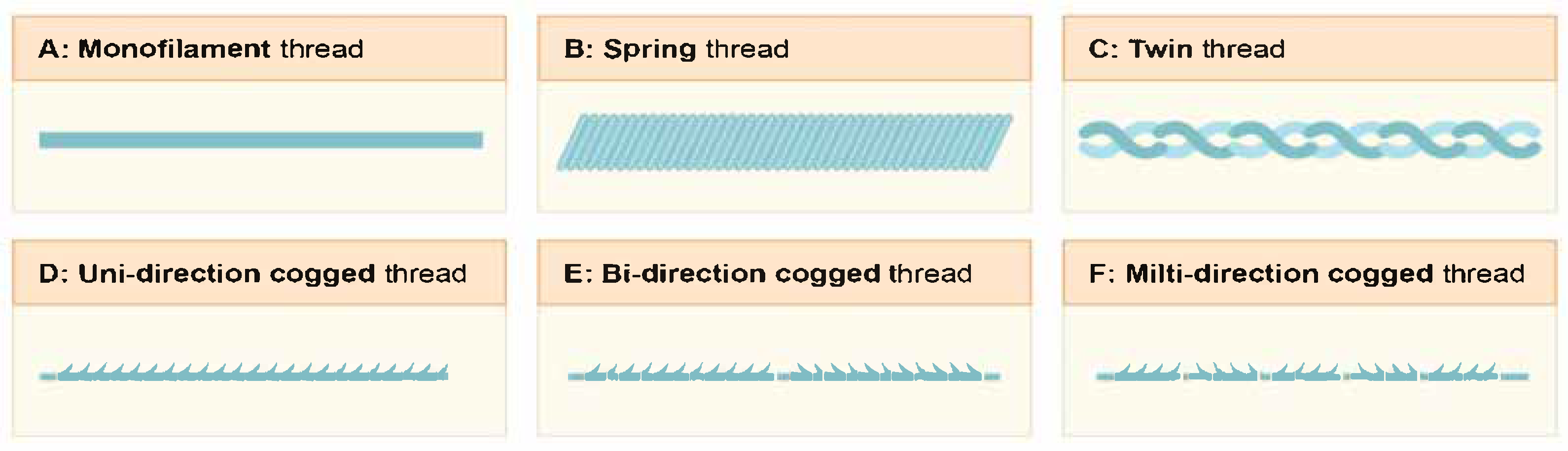
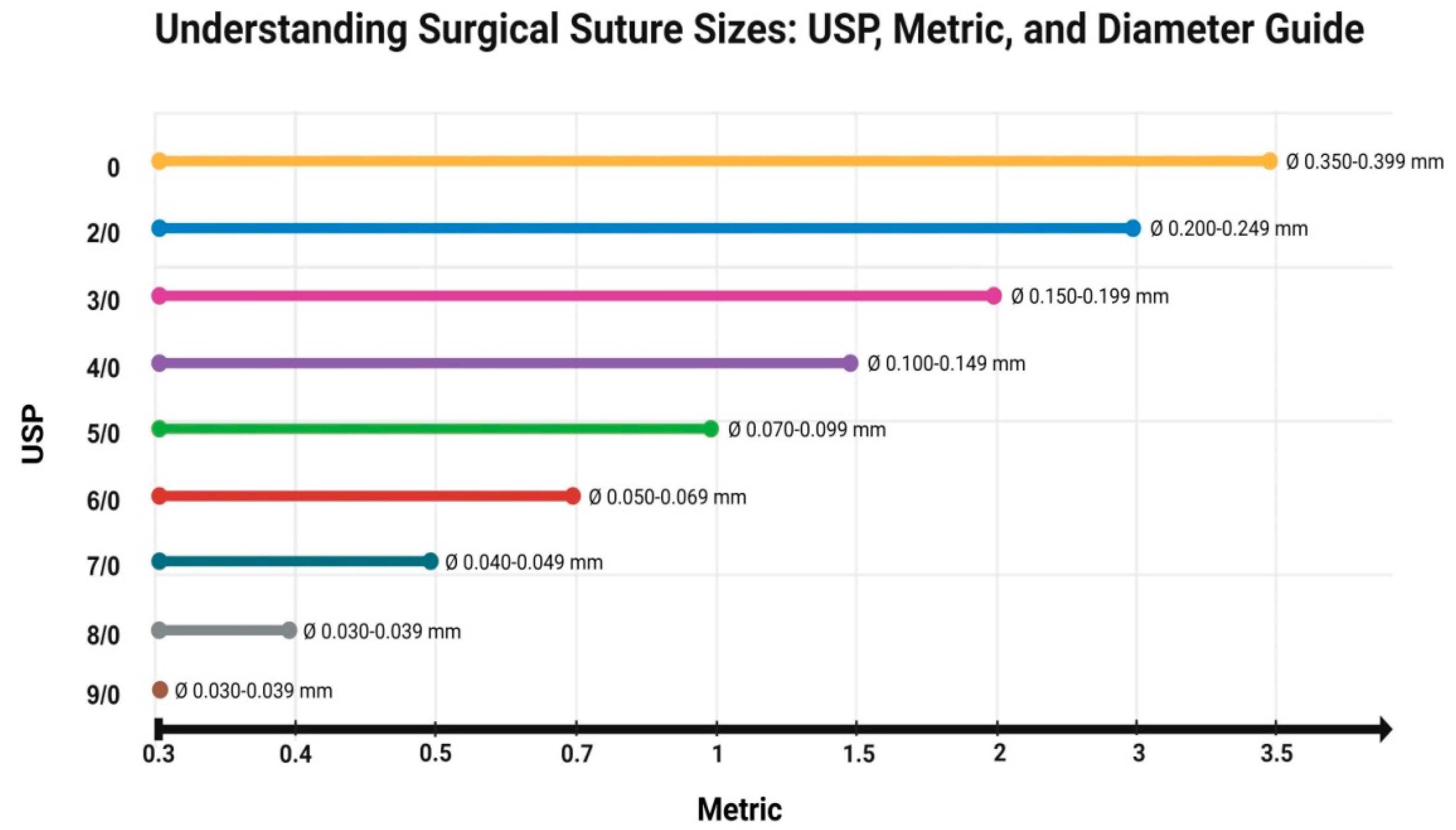
3.1. Types of Threads and the Materials They Are Made of
- −
- Monofilament (Mono) sutures are smooth-surfaced threads designed to minimize tissue trauma during insertion. They are most often used for reinforcement. These threads are quite thin and are therefore ideal for working with delicate areas, such as thin skin and regions with minimal subcutaneous fat. Their primary function is to stimulate the formation of a collagen framework in the dermis and to improve skin quality by inducing microtrauma in the tissues.
- −
- Spring/Twin threads are a type of thread implant characterized by pronounced elastomeric properties, including “shape memory” capabilities. After implantation into tissues, they tend to return to their original helical configuration, providing a pronounced mechanical lifting effect through the distribution of tension vectors within the three-dimensional structure of the dermis and hypodermis. This design feature helps not only in repositioning soft tissues but also in effectively correcting local asymmetry, shaping desired contours, and restoring lost volume in areas affected by age-related involution.
- −
- Barbed sutures, featuring barbed structures along the entire length of the thread, provide mechanical fixation within the SMAS layer, contributing to the formation of a stable tension vector and a prolonged lifting effect. The morphology of the barbs varies depending on the clinical task and the intended area of correction. Currently, designs with unidirectional, bidirectional, or multidirectional barb orientation are used, depending on the clinical task and the intended area of correction. These modifications optimize the tension distribution, achieve uniform tissue traction along the implantation line, and minimize the risk of displacement. Thus, barbed sutures combine a mechanical lifting effect with the biostimulating potential associated with the tissue response to microtrauma, making them a highly effective modality for the aesthetic correction of age-related changes.
3.2. Function of Hyaluronan in Cutaneous Wound Repair
3.3. Hyaluronic Acid: Clinical Application in Aesthetic Medicine and Synergy with Thread Technologies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borzykh, O.B. Predictors of Skin Aging and the System of Comprehensive Correction of Involutive Skin Changes. D.Sc. Thesis, Peoples’ Friendship University of Russia named after Patrice Lumumba, Moscow, Russia, 27 February 2024. (In Russian). [Google Scholar]
- Goodman, G.J. Lasers and Lights. In Facial Rejuvenation: A Total Approach; Goldberg, D.J., Ed.; Springer International Publishing: Berlin, Germany, 2007; pp. 1–48. [Google Scholar]
- Gold, M. Photodynamic Photorejuvenation. In Facial Rejuvenation: A Total Approach; Goldberg, D.J., Ed.; Springer International Publishing: Berlin, Germany, 2007; pp. 49–70. [Google Scholar]
- Weiss, R.A. LED Low-Level Light Therapy. In Facial Rejuvenation: A Total Approach; Goldberg, D.J., Ed.; Springer International Publishing: Berlin, Germany, 2007; pp. 71–78. [Google Scholar]
- Jones, D. Dermal Fillers. In Facial Rejuvenation: A Total Approach; Goldberg, D.J., Ed.; Springer International Publishing: Berlin, Germany, 2007; pp. 105–123. [Google Scholar]
- Campbell, R.M.; Monheit, G.D. Chemical Peeling. In Facial Rejuvenation: A Total Approach; Goldberg, D.J., Ed.; Springer International Publishing: Berlin, Germany, 2007; pp. 125–146. [Google Scholar]
- Rotunda, A.M. Mesotherapy and Injectable Lipolysis. In Facial Rejuvenation: A Total Approach; Goldberg, D.J., Ed.; Springer International Publishing: Berlin, Germany, 2007; pp. 147–165. [Google Scholar]
- Samizadeh, S. (Ed.) Thread Types and Materials. In Thread Lifting Techniques for Facial Rejuvenation and Recontouring; Springer International Publishing: Cham, Germany, 2024; pp. 179–198. [Google Scholar]
- Chu, C.C. 10—Types and Properties of Surgical Sutures. In Biotextiles as Medical Implants; King, M.W., Gupta, B.S., Guidoin, R., Eds.; Woodhead Publishing: Philadelphia, PA, USA, 2013; pp. 231–273. [Google Scholar]
- Kubaeva, A.S.; Batkaev, E.A.; Astashev, V.V. Polydioxanone Filaments in Combination with Preparations Based on Polynucleotides for the Prevention and Correction of Involutive Changes in Facial Tissues. Literature Review. Post-Qualif. Med. Educ. Her. 2022, 2, 39–44. (In Russian) [Google Scholar]
- Gruzdev, D.A.; Kodyakov, A.A.; Fedorov, P.G. A New Approach to the Classification of Threads for Facial and Neck Skin Rejuvenation. J. New Med. Technol. 2014, 21, 104–109. (In Russian) [Google Scholar]
- Borzykh, O.B.; Karpova, E.I.; Shnayder, N.A.; Demina, O.M. Contemporary View on Thread Lifting: Histological and Anatomical Approaches. Russ. Open Med. J. 2022, 11, e0107. [Google Scholar] [CrossRef]
- Fukaya, M. Two Mechanisms of Rejuvenation Using Thread Lifting. Plast. Reconstr. Surg. Glob. Open 2018, 6, e2068. [Google Scholar] [CrossRef]
- Fukaya, M. Long-Term Effect of the Insoluble Thread-Lifting Technique. Clin. Cosmet. Investig. Dermatol. 2017, 10, 483–491. [Google Scholar] [CrossRef]
- Al-Mubarak, L.; Al-Haddab, M. Cutaneous Wound Closure Materials: An Overview and Update. J. Cutan. Aesthet. Surg. 2013, 6, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Razumov, M.; Gornukhina, O.; Golubchikov, O.; Vershinina, I.; Vashurin, A. Polypropylene Suture Material with Anti-Inflammatory Action. Iran. Polym. J. 2018, 27, 629–634. [Google Scholar] [CrossRef]
- Samizadeh, S.; Sulamanidze, G.; Albina, K.; Sulamanidze, K.; Sulamanidze, M. P(LA/CL)-l-Polylactide-Σ-Caprolactone Threads—APTOS Threads: APTOS Solution-APTOS Methods and Threads. In Thread Lifting Techniques for Facial Rejuvenation and Recontouring; Samizadeh, S., Ed.; Springer International Publishing: Cham, Germany, 2024; pp. 223–235. [Google Scholar]
- Sapountzis, S.; Nikkhah, D.; Kim, J.H.; Seo, J.D. Novel Polypropylene Barbed Threads for Midface Lift-“REEBORN Lifting”. Plast. Reconstr. Surg. Glob. Open 2014, 2, e250. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Yan, C.; Ng, J.N.C.; Fundarò, S. Absorbable Barbed Threads for Lower Facial Soft-Tissue Repositioning in Asians. Dermatol. Ther. 2021, 11, 1395–1408. [Google Scholar] [CrossRef]
- Park, T.H.; Park, H.J.; Whang, K.W. Functional Vaginal Rejuvenation with Elastic Silicone Threads: A 4-Year Experience with 180 Patients. J. Plast. Surg. Hand Surg. 2015, 49, 36–39. [Google Scholar] [CrossRef]
- Cao, L.; Qiu, H.; Yu, D.; Shuo, L.; Wang, H. Comparison of Different Thread Products for Facial Rejuvenation: Materials and Barb Designs. J. Cosmet. Dermatol. 2023, 22, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.-W.; Kim, S.-B.; Park, S.Y.; Wan, J.; Yi, K.-H. Thread Lifting Materials: A Review of Its Difference in Terms of Technical and Mechanical Perspective. Clin. Cosmet. Investig. Dermatol. 2024, 17, 999–1006. [Google Scholar] [CrossRef]
- Cobo, R. Use of Polydioxanone Threads as an Alternative in Nonsurgical Procedures in Facial Rejuvenation. Facial Plast. Surg. 2020, 36, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Jang, H.W.; Lee, S.J.; Lee, W.S.; Ryu, H.J. Outcomes of Polydioxanone Knotless Thread Lifting for Facial Rejuvenation. Dermatol. Surg. 2015, 41, 720–725. [Google Scholar] [CrossRef]
- Samizadeh, S. (Ed.) Polydioxanone (PDO) Threads. In Thread Lifting Techniques for Facial Rejuvenation and Recontouring; Springer International Publishing: Cham, Germany, 2024; pp. 199–209. [Google Scholar]
- Suárez-Vega, D.V.; de Maldonado, G.J.V.; Ortíz, R.L.; García-Guevara, V.J.; Miller-Kobisher, B. In Vitro Degradation of Polydioxanone Lifting Threads in Hyaluronic Acid. J. Cutan. Aesthet. Surg. 2019, 12, 145–148. [Google Scholar] [CrossRef]
- Tavares, J.P.; Oliveira, C.A.C.P.; Torres, R.P.; Bahmad, F., Jr. Facial Thread Lifting with Suture Suspension. Braz. J. Otorhinolaryngol. 2017, 83, 712–719. [Google Scholar] [CrossRef]
- Fundaro, S.P.; Goh, C.L.; Hau, K.C.; Moon, H.; Lao, P.P.; Salti, G. Expert Consensus on Soft-Tissue Repositioning Using Absorbable Barbed Suspension Double-Needle Threads in Asian and Caucasian Patients. J. Cutan. Aesthet. Surg. 2021, 14, 1–13. [Google Scholar] [CrossRef]
- Amuso, D.; Amore, R.; Iorio, E.L.; Dolcemascolo, R.; Reggiani, L.B.; Leonardi, V. Histological Evaluation of a Biorevitalisation Treatment with PDO Wires. Aesthetic Med. 2015, 1, 111–117. [Google Scholar]
- Karimi, K.; Reivitis, A. Lifting the Lower Face With an Absorbable Polydioxanone (PDO) Thread. J. Drugs Dermatol. 2017, 16, 932–934. [Google Scholar]
- Lewandowska, K.; Staniszewska, I.; Baran, M.; Turczynowicz, M.; Retman, P. Complications after Polydioxanone Threads (PDO) for Facial Lifting—A Literature Review. J. Educ. Heal. Sport. 2024, 66, 50072. [Google Scholar] [CrossRef]
- Samizadeh, S.; Hong, K. Poly-l-Lactic Acid Cone Threads: Silhouette Soft Threads. In Thread Lifting Techniques for Facial Rejuvenation and Recontouring; Samizadeh, S., Ed.; Springer International Publishing: Cham, Germany, 2024; pp. 315–330. [Google Scholar]
- Bobel, A.C.; Lohfeld, S.; Shirazi, R.N.; McHugh, P.E. Experimental Mechanical Testing of Poly (l-Lactide) (PLLA) to Facilitate Pre-Degradation Characteristics for Application in Cardiovascular Stenting. Polym. Test. 2016, 54, 150–158. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Lowe, N.J. Optimizing Poly-L-Lactic Acid Use. J. Cosmet. Laser Ther. 2008, 10, 43–46. [Google Scholar] [CrossRef]
- Bohnert, K.; Dorizas, A.; Lorenc, P.; Sadick, N.S. Randomized, Controlled, Multicentered, Double-Blind Investigation of Injectable Poly-L-Lactic Acid for Improving Skin Quality. Dermatol. Surg. 2019, 45, 718–724. [Google Scholar] [CrossRef]
- Nikishin, D.V.; Sulamanidze, G.M.; Kadzhaya, A.A. Histological Examination of the Implantation Area of Lifting Threads Based on Poly-L-Lactic Acid (PLA) Coated with Hyaluronic Acid (HA) in a Long-Term Experiment (1 Year). Univ. Proc. Volga Reg. Med. Sci. 2020, 54, 87–98. (In Russian) [Google Scholar] [CrossRef]
- Wong, V.; Rafiq, N.; Kalyan, R.; Hsenriksen, A.; Funner, R. Hanging by a Thread: Choosing the Right Thread for the Right Patient. J. Dermat. Cosmetol. 2017, 1, 86–88. [Google Scholar] [CrossRef]
- Cho, S.W.; Shin, B.H.; Heo, C.Y.; Shim, J.H. Efficacy Study of the New Polycaprolactone Thread Compared with Other Commercialized Threads in a Murine Model. J. Cosmet. Dermatol. 2021, 20, 2743–2749. [Google Scholar] [CrossRef]
- Ha, Y.I.; Kim, J.H.; Park, E.S. Histological and Molecular Biological Analysis on the Reaction of Absorbable Thread; Polydioxanone and Polycaprolactone in Rat Model. J. Cosmet. Dermatol. 2022, 21, 2774–2782. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Mishra, P. Thread Lift in Aesthetic and Regenerative Gynecology. In Aesthetic and Regenerative Gynecology; Jindal, P., Malhotra, N., Joshi, S., Eds.; Springer: Singapore, 2022; pp. 153–163. [Google Scholar]
- Soen, M.; Hidayat, M.; Widowati, W. Enhancing Dermal Collagen Density towards Youthfulness: A Comparative Study of PCL, PLLA, and PDO Thread Implantation in Aging Rats Model. Iran. J. Basic. Med. Sci. 2025, 28, 151–157. [Google Scholar]
- Wong, V. The Science of Absorbable Poly(L-Lactide-Co-ε-Caprolactone) Threads for Soft Tissue Repositioning of the Face: An Evidence-Based Evaluation of Their Physical Properties and Clinical Application. Clin. Cosmet. Investig. Dermatol. 2021, 14, 45–54. [Google Scholar] [CrossRef]
- Ramot, Y.; Nyska, A.; Markovitz, E.; Dekel, A.; Klaiman, G.; Zada, M.H.; Domb, A.J.; Maronpot, R.R. Long-Term Local and Systemic Safety of Poly(L-Lactide-Co-Epsilon-Caprolactone) after Subcutaneous and Intra-Articular Implantation in Rats. Toxicol. Pathol. 2015, 43, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Kasperczyk, J.; Li, S.; Dobrzynski, P.; Janeczek, H.; Jarzabek, B. Novel Poly(L-Lactide-Co-ε-Caprolactone) Matrices Obtained with the Use of Zr[Acac]4 as Nontoxic Initiator for Long-Term Release of Immunosuppressive Drugs. Biomed. Res. Int. 2013, 2013, 607351. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Cirillo, P.; Fundaro, S.P.; Hau, K.C.; Moon, H.J.; Salti, G.; Goh, C.L.; Lao, P.P.; Savoia, A.; Coleman, S.R.; et al. Reshaping with Barbed Threads; Minerva Medica: Torino, Italy, 2020. [Google Scholar]
- Burko, P.; Sulamanidze, G.; Nikishin, D. Efficacy of Lifting Threads Composed of Poly(L-Lactide-Co-ε-Caprolactone) Copolymers Coated With Hyaluronic Acid: A Long-Term Study on Biorevitalizing Properties in Skin Remodeling. J. Cosmet. Dermatol. 2025, 24, e70077. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Piperigkou, Z.; Theocharis, A.D.; Watanabe, H.; Franchi, M.; Baud, S.; Brézillon, S.; Götte, M.; Passi, A.; Vigetti, D.; et al. Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics. Chem. Rev. 2018, 118, 9152–9232. [Google Scholar] [CrossRef]
- Manou, D.; Caon, I.; Bouris, P.; Triantaphyllidou, I.E.; Giaroni, C.; Passi, A.; Karamanos, N.K.; Vigetti, D.; Theocharis, A.D. The Complex Interplay Between Extracellular Matrix and Cells in Tissues. Methods Mol. Biol. 2019, 1952, 1–20. [Google Scholar]
- Hascall, V.; Esko, J. Hyaluronan. In Essentials of Glycobiology; Varki, A., Cummings, R., Esko, J., Al, E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009. [Google Scholar]
- Cowman, M.K.; Lee, H.-G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front. Immunol. 2015, 6, 261. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a Crucial Regulator of Inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular Size-Dependent Signaling and Biological Functions in Inflammation and Cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Sigaeva, N.N.; Kolesov, S.V.; Nazarov, P.V.; Vildanova, R.R. Chemical Modification of Hyaluronic Acid and Its Application in Medicine. Vestn. Bashkir Univ. 2012, 17, 1220–1241. (In Russian) [Google Scholar]
- Mikhaylova, N.P.; Bazarny, V.V.; Kochurova, I.V. Immunotropic Effects of Hyaluronic Acid in Dermatology. Mesotherapy 2012, 17, 30–36. (In Russian) [Google Scholar]
- Wang, Y.; Maytin, E.V. The Role of Hyaluronan in Skin Wound Healing. In Hyaluronan: Structure, Biology and Biotechnology; Passi, A., Ed.; Springer International Publishing: Cham, Germany, 2023; pp. 189–204. [Google Scholar]
- Dunphy, J.E. Wound Healing. Surg. Clin. N. Am. 1978, 58, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Shirali, A.C.; Goldstein, D.R. Activation of the Innate Immune System by the Endogenous Ligand Hyaluronan. Curr. Opin. Organ. Transpl. 2008, 13, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Tammi, R.; Pasonen-Seppänen, S.; Kolehmainen, E.; Tammi, M. Hyaluronan Synthase Induction and Hyaluronan Accumulation in Mouse Epidermis Following Skin Injury. J. Investig. Dermatol. 2005, 124, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Monslow, J.; Sato, N.; Mack, J.A.; Maytin, E.V. Wounding-Induced Synthesis of Hyaluronic Acid in Organotypic Epidermal Cultures Requires the Release of Heparin-Binding EGF and Activation of the EGFR. J. Investig. Dermatol. 2009, 129, 2046–2058. [Google Scholar] [CrossRef]
- Singleton, P.A.; Mirzapoiazova, T.; Guo, Y.; Sammani, S.; Mambetsariev, N.; Lennon, F.E.; Moreno-Vinasco, L.; Garcia, J.G. High-Molecular-Weight Hyaluronan Is a Novel Inhibitor of Pulmonary Vascular Leakiness. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L639–L651. [Google Scholar] [CrossRef]
- Aya, K.L.; Stern, R. Hyaluronan in Wound Healing: Rediscovering a Major Player. Wound Repair. Regen. 2014, 22, 579–593. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Fan, J.; Yu, S.; Chen, S.; Luo, Y.; Prestwich, G.D.; Mascarenhas, M.M.; Garg, H.G.; Quinn, D.A.; et al. Regulation of Lung Injury and Repair by Toll-Like Receptors and Hyaluronan. Nat. Med. 2005, 11, 1173–1179. [Google Scholar] [CrossRef]
- Bollyky, P.L.; Bogdani, M.; Bollyky, J.B.; Hull, R.L.; Wight, T.N. The Role of Hyaluronan and the Extracellular Matrix in Islet Inflammation and Immune Regulation. Curr. Diab Rep. 2012, 12, 471–480. [Google Scholar] [CrossRef]
- Pardue, E.L.; Ibrahim, S.; Ramamurthi, A. Role of Hyaluronan in Angiogenesis and Its Utility to Angiogenic Tissue Engineering. Organogenesis 2008, 4, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Enoch, S.; Leaper, D.J. Basic Science of Wound Healing. Surgery 2005, 23, 37–42. [Google Scholar]
- Meyer, L.J.; Stern, R. Age-Dependent Changes of Hyaluronan in Human Skin. J. Investig. Dermatol. 1994, 102, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; King, A.; Marsh, E.; LeSaint, M.; Bhattacharya, S.S.; Han, N.; Dhamija, Y.; Ranjan, R.; Le, L.D.; Bollyky, P.L.; et al. The Role of Interleukin-10 and Hyaluronan in Murine Fetal Fibroblast Function In Vitro: Implications for Recapitulating Fetal Regenerative Wound Healing. PLoS ONE 2015, 10, e0124302. [Google Scholar] [CrossRef]
- Shang, L.; Li, M.; Xu, A.; Zhuo, F. Recent Applications and Molecular Mechanisms of Hyaluronic Acid in Skin Aging and Wound Healing. Med. Nov. Technol. Devices 2024, 23, 100320. [Google Scholar] [CrossRef]
- Walker, K.; Basehore, B.M.; Goyal, A.; Zito, P.M. Hyaluronic Acid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Raab, S.; Yatskayer, M.; Lynch, S.; Manco, M.; Oresajo, C. Clinical Evaluation of a Multi-Modal Facial Serum That Addresses Hyaluronic Acid Levels in Skin. J. Drugs Dermatol. 2017, 16, 884–890. [Google Scholar]
- Lee, D.H.; Oh, I.Y.; Koo, K.T.; Suk, J.M.; Jung, S.W.; Park, J.O.; Kim, B.J.; Choi, Y.M. Improvement in Skin Wrinkles Using a Preparation Containing Human Growth Factors and Hyaluronic Acid Serum. J. Cosmet. Laser Ther. 2015, 17, 20–23. [Google Scholar] [CrossRef]
- Pavicic, T.; Gauglitz, G.G.; Lersch, P.; Schwach-Abdellaoui, K.; Malle, B.; Korting, H.C.; Farwick, M. Efficacy of Cream-Based Novel Formulations of Hyaluronic Acid of Different Molecular Weights in Anti-Wrinkle Treatment. J. Drugs Dermatol. 2011, 10, 990–1000. [Google Scholar] [PubMed]
- Fanian, F.; Deutsch, J.-J.; Bousquet, M.T.; Boisnic, S.; Andre, P.; Catoni, I.; Beilin, G.; Lemmel, C.; Taieb, M.; Gomel-Toledano, M.; et al. A Hyaluronic Acid-Based Micro-Filler Improves Superficial Wrinkles and Skin Quality: A Randomized Prospective Controlled Multicenter Study. J. Dermatol. Treat. 2023, 34, 2216323. [Google Scholar] [CrossRef]
- Fundarò, S.P.; Salti, G.; Malgapo, D.M.H.; Innocenti, S. The Rheology and Physicochemical Characteristics of Hyaluronic Acid Fillers: Their Clinical Implications. Int. J. Mol. Sci. 2022, 23, 10518. [Google Scholar] [CrossRef]
- Raspaldo, H. Volumizing Effect of a New Hyaluronic Acid Sub-Dermal Facial Filler: A Retrospective Analysis Based on 102 Cases. J. Cosmet. Laser Ther. 2008, 10, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Morley, A.M.S.; Malhotra, R. Use of Hyaluronic Acid Filler for Tear-Trough Rejuvenation as an Alternative to Lower Eyelid Surgery. Ophthal Plast. Reconstr. Surg. 2011, 27, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Berguiga, M.; Galatoire, O. Tear Trough Rejuvenation: A Safety Evaluation of the Treatment by a Semi-Cross-Linked Hyaluronic Acid Filler. Orbit 2017, 36, 22–26. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Horne, K.N.; Rajadas, J. Threads of Hyaluronic Acid and/or Derivatives Thereof, Methods of Making Thereof and Uses Thereof. U.S. Patent № 9,861,570, 9 January 2018. [Google Scholar]
- Kim, J.-H.; Han, M.W.; Lee, M.-H.; Kweon, D.-K.; Park, Y.J.; Heo, C.Y. Comparative In Vivo Study of Solid-Type Pure Hyaluronic Acid in Thread Form: Safety and Efficacy Compared to Hyaluronic Acid Filler and Polydioxanone Threads. Aesthetic Plast. Surg. 2024, 48, 221–227. [Google Scholar] [CrossRef]
- Casabona, G. Dermatologist Have Now Developed a 3 Dimensional View of Facial Changes While Aging. Dermatol. Surg. 2015, 41, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Salti, G.; Rauso, R. Facial Rejuvenation with Fillers: The Dual Plane Technique. J. Cutan. Aesthet. Surg. 2015, 8, 127–133. [Google Scholar] [CrossRef]
- Moon, H.; Fundaro, S.P.; Goh, C.L.; Hau, K.C.; Paz-Lao, P.; Salti, G. A Review on the Combined Use of Soft Tissue Filler, Suspension Threads, and Botulinum Toxin for Facial Rejuvenation. J. Cutan. Aesthet. Surg. 2021, 14, 147–155. [Google Scholar] [CrossRef]
- Liao, K.L.; Liao, K.H. Study and Analysis of the Clinical Effects and Maintenance Duration of Facial Rejuvenation Treatment in Middle-Aged and Elderly Individuals through the Combined Use of Facial Hyaluronic Acid Fillers and PPDO Thread Lift. Altern. Ther. Health Med. 2024, 19, AT10596. [Google Scholar]
- Urdiales-Gálvez, F.; Martín-Sánchez, S.; Maíz-Jiménez, M.; Castellano-Miralla, A.; Lionetti-Leone, L. Concomitant Use of Hyaluronic Acid and Laser in Facial Rejuvenation. Aesthetic Plast. Surg. 2019, 43, 1061–1070. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Rai, R.; Kumar, S.; Mitra, B.; Chopra, A.; Singh, G.K.; Mitra, D.; Patil, C.; Sandhu, S. Safety and Efficacy of Restoring Facial Symmetry Using Polydioxanone Thread Face Lift Technique in Patients with Facial Palsy. J. Clin. Aesthet. Dermatol. 2022, 15, 26–29. [Google Scholar]
- Goel, A.; Rai, K. Non-Surgical Facelift-by PDO Threads and Dermal Filler: A Case Report. J. Cosmet. Dermatol. 2022, 21, 4241–4244. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.-H.; Kim, S.-B.; Hu, H.; Bae, H.; Park, H.-J.; Yoon, J.-H.; Kim, H.-J. Self-Crossing Hyaluronic Acid Filler with Combination Use of Polydioxanone Thread in Minipig Model. J. Cosmet. Dermatol. 2024, 23, 2821–2828. [Google Scholar] [CrossRef]
- Wan, J.; Chan, L.K.W.; Lee, K.W.A.; Cartier, H.; Yi, K.H. Volumizing Threads and Hyaluronic Acid Filler for Lip Augmentation. Skin. Res. Technol. 2024, 30, e13797. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.S. Minimally Invasive Rhinoplasty Technique Using a Hyaluronic Acid Filler and Polydioxanone Threads: An Effective Combination. Facial Plast. Surg. 2019, 35, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.W.; Yi, K.H. Integration of Liposuction, Fat Transplantation, and Filler Treatments with Thread Lifting in Managing Facial Aesthetics. Skin. Res. Technol. 2024, 30, e13767. [Google Scholar] [CrossRef]
- Kang, S.H.; Moon, S.H.; Kim, H.S. Nonsurgical Rhinoplasty with Polydioxanone Threads and Fillers. Dermatol. Surg. 2020, 46, 664–670. [Google Scholar] [CrossRef]
- Li, K.; Meng, F.; Li, Y.R.; Tian, Y.; Chen, H.; Jia, Q.; Cai, H.; Jiang, H.B. Application of Nonsurgical Modalities in Improving Facial Aging. Int. J. Dent. 2022, 2022, 8332631. [Google Scholar] [CrossRef]
- Girgin, A. The Effectiveness of PLLA/PCL Aptos Thread on Skin Quality. Aesthet. Med. 2019, 5, 25. [Google Scholar]
- Avelar, L.E.; Cazerta, C.E. The Improvement of the Skin Quality with the Use of PLLA. J. Dermat. Cosmetol. 2018, 2, 101–102. [Google Scholar] [CrossRef]
- Irina, P.; Albina, K. Single-Blind Comparative Study of the Aesthetic Outcome of Armouring Procedures with PLLA/PCL-and HA-Enriched Absorbable Threads. Trichology Cosmetol. Open J. 2019, 3, 15–20. [Google Scholar] [CrossRef]
- Nikishin, D.V.; Sulamanidze, G.M.; Kajaia, A.A. Effectiveness of Using Poly Lactide and Caprolactone Acid with Hyaluronic Acid Material. Adv. Plast. Reconstr. Surg. 2019, 3, 274–284. [Google Scholar]
- Tezel, A.; Fredrickson, G.H. The Science of Hyaluronic Acid Dermal Fillers. J. Cosmet. Laser Ther. 2008, 10, 35–42. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking Method of Hyaluronic-Based Hydrogel for Biomedical Applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef]
- Wang, F.; Garza, L.A.; Kang, S.; Varani, J.; Orringer, J.S.; Fisher, G.J.; Voorhees, J.J. In Vivo Stimulation of de Novo Collagen Production Caused by Cross-Linked Hyaluronic Acid Dermal Filler Injections in Photodamaged Human Skin. Arch. Dermatol. 2007, 143, 155–163. [Google Scholar] [CrossRef]
- Khabarov, V.N. Hyaluronic Acid in Injection Cosmetology; GEOTAR-Media: Moscow, Russia, 2017. (In Russian) [Google Scholar]
- Jeong, C.H.; Kim, D.H.; Yune, J.H.; Kwon, H.C.; Shin, D.-M.; Sohn, H.; Lee, K.H.; Choi, B.; Kim, E.S.; Kang, J.H.; et al. In Vitro Toxicity Assessment of Crosslinking Agents Used in Hyaluronic Acid Dermal Filler. Toxicol. Vitr. 2021, 70, 105034. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jeong, C.H.; Han, J.H.; Lim, S.J.; Kwon, H.C.; Kim, Y.J.; Keum, D.H.; Lee, K.H.; Han, S.G. Comparative Toxicity Study of Hyaluronic Acid Fillers Crosslinked with 1,4-Butanediol Diglycidyl Ether or Poly (Ethylene Glycol) Diglycidyl Ether. Int. J. Biol. Macromol. 2025, 296, 139620. [Google Scholar] [CrossRef] [PubMed]
- Wojtkiewicz, M.; Stachura, A.; Roszkowski, B.; Winiarska, N.; Kazimierska, K.; Stachura, K. Are We Overlooking Harms of BDDE-Cross-Linked Dermal Fillers? A Scoping Review. Aesthetic Plast. Surg. 2024, 48, 5147–5154. [Google Scholar] [CrossRef]
- Sulamanidze, M.A.; Nikishin, D.V.; Sulamanidze, G.M.; Sulamanidze, K.M.; Kadzhaya, G.N. Method for Production of Medical Implant Shell, Medical Implant Shell. Russ. Fed. Patent № 2782112, 21 October 2022. (In Russian). [Google Scholar]
- Burko, P.; Sulamanidze, G.; Nikishin, D. Long-Term Efficacy of Poly(L-Lactide-Co-ε-Caprolactone) Copolymer Lifting Threads with Encapsulated MICROscale Hyaluronic Acid Particles Using NAMICA Technology: Investigating Biorevitalizing Effects in Skin Remodeling (Part 1). Cosmetics 2025, 12, 20. [Google Scholar] [CrossRef]
- Burko, P.; Sulamanidze, G.; Nikishin, D. NAMICA Encapsulation Technology in an Animal Model: MICROscale vs. NANOscale Hyaluronic Acid Particles in Skin Remodeling (Part 2). Cosmetics 2025, 12, 55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burko, P.; Miltiadis, I. Evolution of Thread Lifting: Advancing Toward Bioactive Polymers and Sustained Hyaluronic Acid Delivery. Cosmetics 2025, 12, 127. https://doi.org/10.3390/cosmetics12030127
Burko P, Miltiadis I. Evolution of Thread Lifting: Advancing Toward Bioactive Polymers and Sustained Hyaluronic Acid Delivery. Cosmetics. 2025; 12(3):127. https://doi.org/10.3390/cosmetics12030127
Chicago/Turabian StyleBurko, Pavel, and Ilias Miltiadis. 2025. "Evolution of Thread Lifting: Advancing Toward Bioactive Polymers and Sustained Hyaluronic Acid Delivery" Cosmetics 12, no. 3: 127. https://doi.org/10.3390/cosmetics12030127
APA StyleBurko, P., & Miltiadis, I. (2025). Evolution of Thread Lifting: Advancing Toward Bioactive Polymers and Sustained Hyaluronic Acid Delivery. Cosmetics, 12(3), 127. https://doi.org/10.3390/cosmetics12030127






