Abstract
Maintaining the physiological acid–base balance of the skin is critical to preserving the integrity of the epidermal barrier and preventing irritation. This study investigates the short-term effects of natural soaps, prepared using cold and hot processes, on skin surface pH. A double-blind, controlled design was applied to assess changes in pH following application of soap formulations. pH levels were measured in vivo using non-invasive instrumentation at baseline and 2, 15 and 30 min post-application in 41 adult volunteers. The results demonstrated a significant increase in skin pH immediately after exposure to both types of natural soap, with elevated values persisting for up to 30 min. These changes were associated with potential disruption of the skin’s acid mantle and reduced buffering capacity. The findings highlight the importance of pH considerations in the formulation and routine use of natural cleansers. Although natural soaps are often perceived as gentle alternatives, their alkalinity may transiently disturb the skin’s acid–base homeostasis, potentially leading to increased transepidermal water loss and barrier impairment. This study supports the need for reformulation strategies and consumer awareness regarding the physicochemical impact of cleansing agents on skin health.
1. Introduction
1.1. Stratum Corneum Structure
The stratum corneum (SC) is the outermost part of the epidermis, acting as a biochemically complex but highly organized structure between the organism and the external environment [1]. The stratum corneum consists of corneocytes, which are terminally differentiated, without a nucleus, and lipids secreted by lamellar bodies. The barrier protecting against damaging factors, i.e., mechanical, chemical and physical, is formed in the normal process of keratinization of epidermal cells. The structure of SC is often compared to “bricks and mortar”, where flattened, protein-rich corneocytes (dead keratinocytes devoid of cell organelles) constitute the “bricks” and the lipid matrix surrounding them acts as the “mortar”. In the deeper layers of SC, lipids form a densely packed, orthorhombic crystalline structure that effectively limits fluid transport. On the other hand, closer to the surface of SC, they are arranged in a less ordered, hexagonal network, allowing for easier fluid flow [2,3,4].
SC lipid precursors are formed during keratinocyte differentiation and then packed into lamellar bodies in an orderly manner. After undergoing enzymatic modifications, they are secreted into the SC intercellular space. Stratum corneum lipids spontaneously assemble into multilayered structures that interact with lipids associated with the corneocyte envelope [5]. The main components of the lipid matrix of SC are ceramides (40–50%), cholesterol (25%) and free fatty acids (10–15%). These three classes of lipids differ in their biophysical and biochemical properties compared to typical components of eukaryotic membranes such as glycerolipids or sterols, which are responsible for the structure and function of the lipid bilayer of cell membranes [6].
The pH of the epidermis is influenced by endogenous factors such as age, anatomical location, genetic predisposition, sweat secretion or ethnic differences, and exogenous factors such as cleaning agents—soaps, detergents and cosmetic products—and local antibacterial agents [2,3,7].
The proper pH of the epidermis is crucial for the physiological functions of the skin, including the integrity of the stratum corneum and antimicrobial protection. Altered pH is likely to be associated with clinical symptoms: rough and dry skin, which causes itching, increased susceptibility to skin infections and contact allergies [8].
The surface of the stratum corneum is acidic and approaches more neutral values (pH 7–7.4) in the living layers of the epidermis [9]. In preliminary studies in mice, the upper surface of the stratum corneum was found to have a neutral pH, probably due to contact with a neutral environment. The upper surface of the stratum corneum has been reported to exhibit a nearly neutral pH, which contrasts with the generally acidic pH observed in human skin [10,11]. This discrepancy likely results from differences in species-specific skin physiology and experimental design. Mice have thinner epidermal layers, distinct lipid profiles and higher skin permeability compared to humans, all of which may influence surface pH readings. Therefore, caution must be exercised when extrapolating pH-related data from animal models to human skin, as these physiological differences can significantly affect the acid–base characteristics of the epidermis. When the skin was exposed to topical buffers of different pH, the external environment of the upper stratum corneum changed dynamically, whereas the pH of the deeper layers of the epidermis remained acidic [10,11].
An increase in the pH of the epidermis poses a particular risk. The correct, slightly acidic pH of the epidermis is one of the mechanisms of protection against bacterial and fungal infections [12]. The pH of the human epidermis generally ranges from 4.5 to 5.5, with 5.5 often cited as a reference value for healthy skin. However, this value may vary depending on anatomical location, age, sex, ethnicity and individual skin characteristics. The commonly accepted reference value of pH 5.5 is based primarily on measurements performed on the volar forearm or facial skin of healthy adults.
A human skin pH of around 5.5 can partially inhibit the growth of pathogenic bacteria such as Staphylococcus aureus, which proliferates more efficiently at neutral to slightly alkaline pH. Cutibacterium acnes grows at pH 6.3 [13]. Pseudomonas aeruginosa, whose optimal growth environment is in a pH between 7 and 7.4 [14], is the same as Escherichia spp. [15] (Table 1).

Table 1.
The optimal growth pH ranges of key skin microorganisms.
In relation to Candida albicans, fungal dimorphism has also been shown to be pH dependent. This morphological switch is regulated in part by the Rim101 signaling pathway, which becomes activated in alkaline conditions and promotes hyphal formation—associated with increased pathogenicity. The blastospore form is more common at slightly acidic pH, while the filamentous form, which may be pathogenic, occurs at neutral or alkaline pH [15,16]. A model of experimentally induced infection with C. albicans at different pH in healthy humans showed that the resulting changes were more pronounced in skin buffered at pH 6.0 compared to pH 4.5 [16]. Skin with a pH less than 5.0 has a better barrier function, i.e., a higher resistance to induced dermatitis, less flaking and a higher level of hydration, than skin with a pH higher than 5.0.
1.2. The Effect of Soaps on the Stratum Corneum
The daily skin hygiene, especially with soaps and detergents, can significantly affect the integrity of the stratum corneum. Soaps, due to their amphiphilic properties, can dissolve and wash away SC structural lipids, which leads to a weakening of the skin barrier. The solution of soap is characterized by a high pH, which disrupts the physiological pH of the skin (approx. 4.5–5.5), promoting the degradation of lipids and structural proteins. Sodium soaps, which are a mixture of sodium salts of higher fatty acids, undergo anionic hydrolysis in water, causing the solution to become alkaline [15,17].
Long-term use of strong detergents results in increased transepidermal water loss (TEWL), which can lead to roughness, irritation and a weakening of the protective function of the hydrolipid barrier. In addition, the removal of natural lipids from the SC can lead to increased susceptibility to irritants, allergens and pathogenic microorganisms. Therefore, it is recommended to use milder cleansing agents, such as syndets (synthetic detergents), which are characterized by a lower irritation potential and less impact on the lipid structure of the SC [18,19].
In recent years, natural soaps based on vegetable fats subjected to the cold saponification process have gained great popularity as an alternative to drugstore cleaning agents. Consumers are increasingly reaching for products with a short, natural composition, believing that they are gentler on the skin, moisturize better and do not damage its protective barrier [20]. But are natural soaps really better than conventional ones?
1.3. Composition and Production Process of Natural Soaps
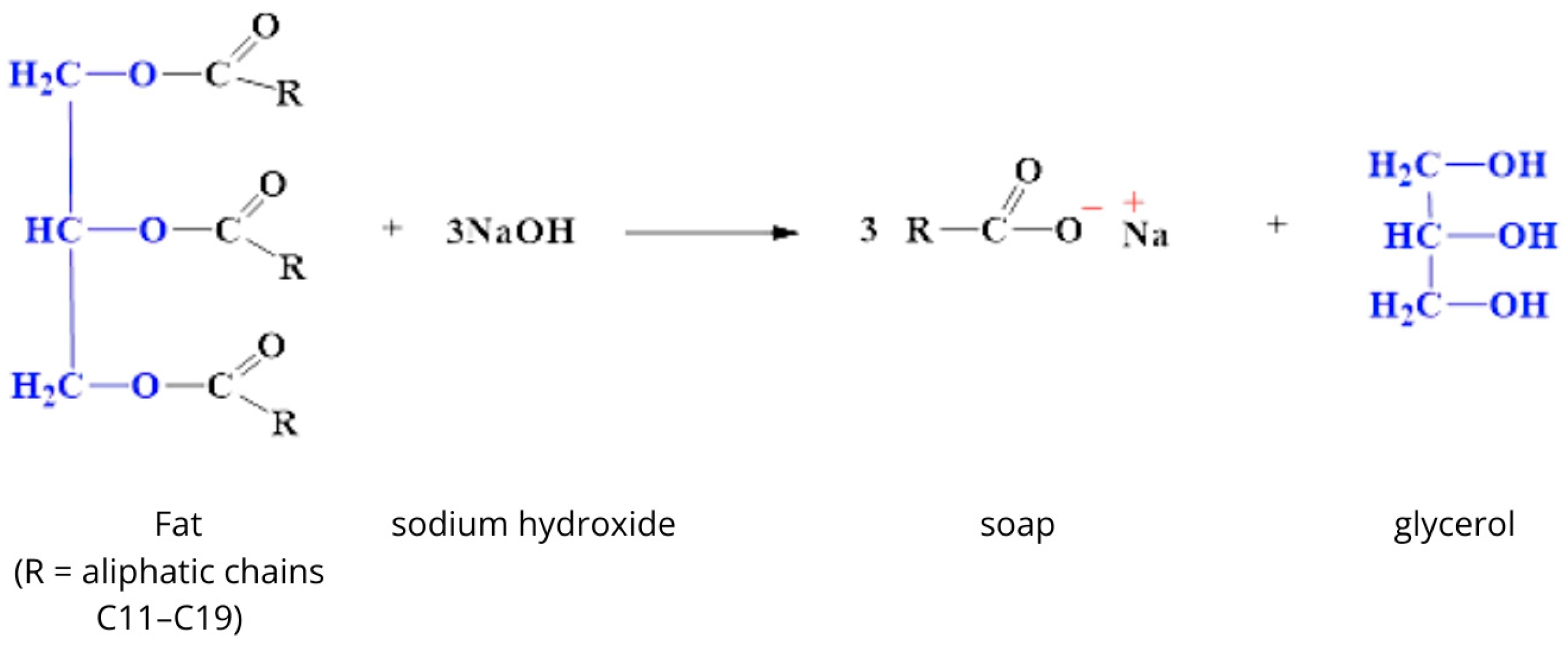
Natural soaps are produced by traditional saponification of vegetable or animal fats using sodium hydroxide (NaOH). This process leads to the formation of sodium salts of fatty acids and natural glycerin, which is hygroscopic (Figure 1) [21,22].
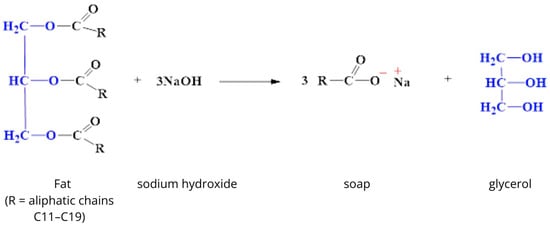
Figure 1.
Saponification reaction [own study].
Unlike drugstore soaps, which often contain synthetic detergents (e.g., SLS—Sodium Lauryl Sulfate), natural soaps are usually based on vegetable oils, such as olive oil, sunflower oil, shea butter and others, which contain fatty acids that support the hydrolipid barrier. This is often a marketing argument of manufacturers, indicating the good care properties of natural soap. Additionally, manufacturers often enrich these products with plant extracts, essential oils and cosmetic clays, which are supposed to support skin care. Popular additions include, for example, activated carbon with cleansing properties, honey with a soothing effect or aloe, which helps maintain skin moisture [23].
Despite the increasing popularity of so-called “natural soaps”, there is a notable lack of scientific literature examining the dermatological effects of soaps produced using the traditional cold process method. Most commercially available soap bars marketed as natural are actually manufactured using hot process or industrial saponification techniques, which aim to accelerate production by completing the reaction between fats and alkali under elevated temperatures. This results in products that differ significantly from cold-processed soaps in terms of composition, residual glycerin content and skin interaction potential.
Cold-processed soaps, in contrast, are typically handcrafted and cured over several weeks. They retain glycerol, avoid synthetic surfactants and often include unreacted oils (superfatting), which may influence their effect on the skin’s acid–base balance. However, their physiological impact, particularly on skin surface pH, remains underexplored.
The aim of the study was to produce soap using the cold and hot methods and then assess their effect on the pH of the epidermis. The study attempted to compare both production methods in terms of their effect on the skin’s pH balance after using soap, which is a key aspect for skin safety and health.
The main objectives of the study were as follows:
- Cold and hot process soap production—In the first stage of the study, soap samples were prepared using two different production methods to obtain representative samples for further analysis.
- The assessment of the effect of soaps on epidermal pH—The effect of cold and hot process soaps on skin pH after application was investigated. The epidermal pH measurements were taken before and after soap application to assess changes in skin pH in response to soap contact.
- The comparison of the effects of both production methods on skin pH—The aim of this stage of the study was to compare which method (cold or hot) causes the soap to have a relative pH change on the skin, which is important for maintaining the proper pH balance of the epidermis and preventing skin irritation or dryness.
The aim of the study was not only to obtain soaps using two different manufacturing methods, but also to evaluate their effect on the pH of the epidermis, which is crucial to determining their safety and suitability for everyday use.
This study addresses a clear research gap by evaluating and comparing the short-term effects of cold- and hot-processed natural soaps on skin surface pH using a double-blind, in vivo approach.
2. Materials and Methods
2.1. The Soap Production
Four soaps were made, three of which were manufactured using the cold method and one using the hot method in accordance with good laboratory practice and basic principles of soap production (Table 2). The soaps were produced under standardized conditions. For the hot process, the saponification reaction was carried out at approximately 70 °C for 2 h, ensuring complete reaction between the oils and sodium hydroxide. For the cold process, the ingredients were mixed at ambient temperature (approx. 22–24 °C), then poured into molds and left to cure for a minimum of 6 weeks in a controlled environment (humidity ~50%, temperature ~20 °C) to allow for full saponification and pH stabilization. To isolate the influence of the production method itself, we did not use commercial soap bars in this study. Instead, we created both cold- and hot-processed soaps using controlled, replicable laboratory conditions with similar base ingredients. This approach enabled a direct comparison of how saponification temperature and process influence the soap’s interaction with the epidermal acid mantle. After the curing period, the surface pH of each soap was measured using a calibrated laboratory pH meter to ensure that the products had reached chemical stability and were safe for skin application. Only formulations that demonstrated stable pH values within the expected alkaline range for traditional soaps (pH 8.5–10.0) were used in the study. This procedure ensured the comparability of samples and the reliability of the results in terms of their effect on the skin’s acid–base balance.

Table 2.
Recipe for manufacturing soaps 1, 2 and 3 (cold method) and soap 4 (hot method). * Essential oils and tocopherol amounts were originally given in drops (15 drops ≈ 0.75 mL; 5 drops ≈ 0.25 mL), converted here for reproducibility.
2.2. Instrumental Tests
A dermatological silver chloride electrode Skin-pH-Meter PH 905 (Courage & Khazaka electronic GmbH, Cologne, Germany) with a measurement accuracy of 0.01 pH units was used to measure the soap surface and the skin pH. The measurements were taken in a room with strictly defined conditions: temperature 20–23 °C, humidity 50–60%.
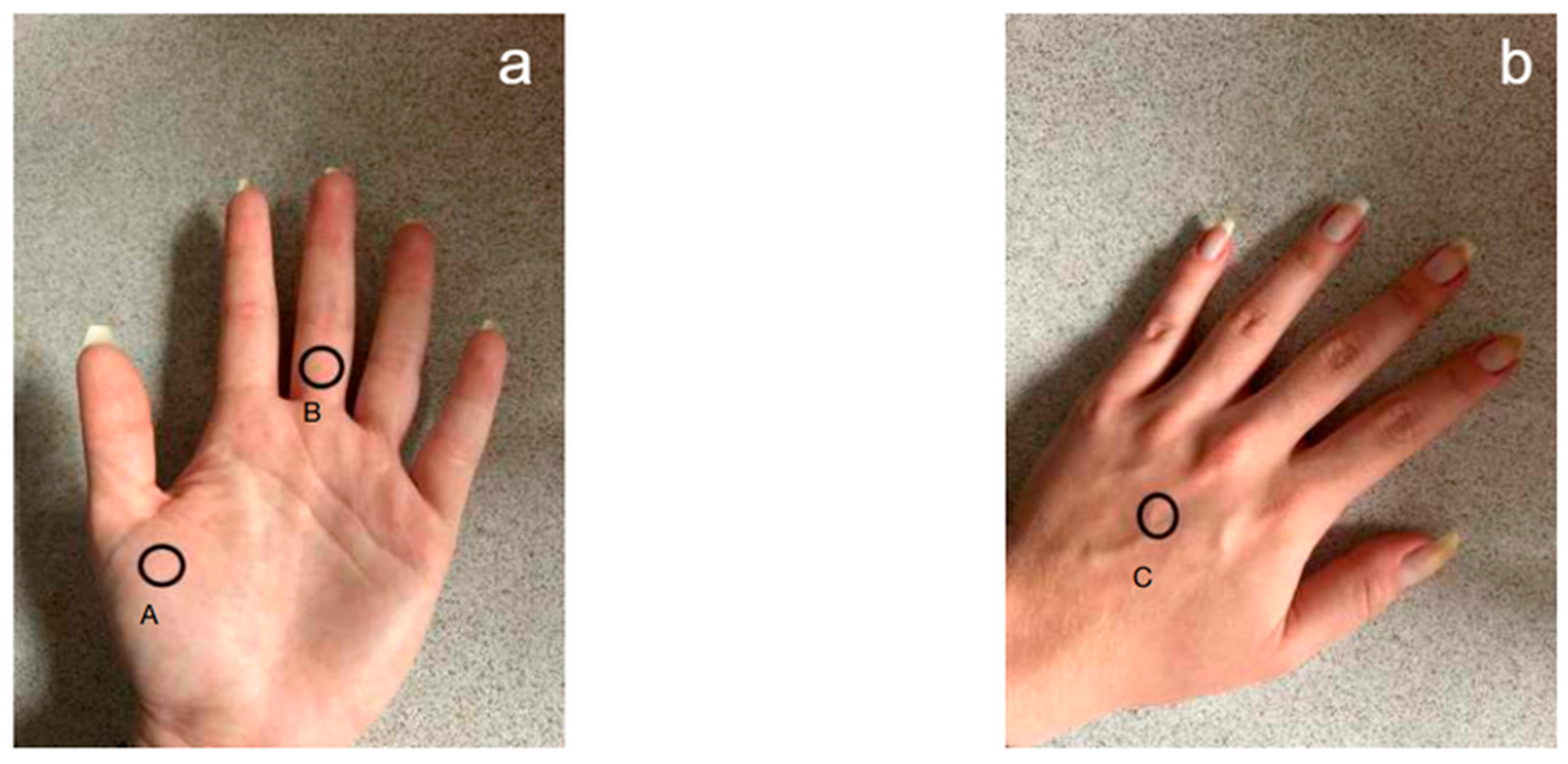
The measurement was performed in three locations on the right and left hand: the back and palm of the hand and the middle finger (Figure 2). Before using the test soaps, the volunteers did not wash their hands for at least 3 h before the test. Two time points were selected: before the hygienic washing procedure and 2, 15 and 30 min after application of the preparation.
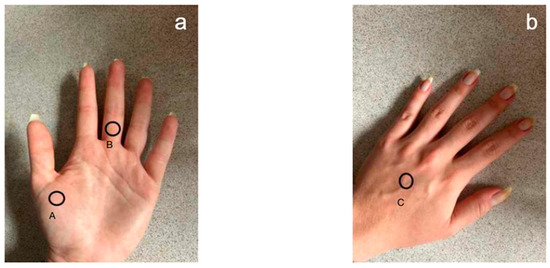
Figure 2.
Location of the electrode for measuring the pH of the epidermis of the hands. (a) Palmar side: A—first metacarpal bone; B—middle part of the proximal phalanx. (b) Dorsal side of the hand: C—middle part of the third metacarpal bone.
2.3. Study Group
The skin examination before using soaps was performed on a group of 41 healthy volunteers, divided into 23 women and 18 men (average 21 ± 2). Hands were washed according to the procedure of hygienic hand washing. The examination was repeated within 2 weeks. This study was approved by the Ethics Committee of Jan Dlugosz University in Czestochowa number KE-U/8/2024 from 22 May 2024. All participants provided written informed consent before participation. All participants were required to be clinically healthy and free from any dermatological conditions that could influence skin pH or barrier function. Exclusion criteria included current or past diagnosis of atopic dermatitis, seborrheic dermatitis, eczema, psoriasis, chronic hand dermatitis, hypersensitivity reactions, and visible skin irritation or inflammation at the measurement sites. These criteria were established to ensure the integrity of the data by eliminating potential confounding factors related to existing disruptions in the acid–base balance of the skin.
2.4. Double-Blind Study
The study was conducted in double-blind conditions in order to minimize the influence of subjective factors on the results. The process included two independent stages: preparation of soaps and taking measurements, and then analysis of the obtained data. The person responsible for preparing and carrying out the hand washing procedure did not participate in the analysis of the results, which allowed us to avoid unconscious influence on the interpretation of the data. In addition, the person performing the statistical analysis did not have information about the type of soaps used, thanks to which the objectivity of the assessment of changes in skin pH after the use of individual preparations was ensured.
2.5. Statistical Analysis
Excel 2016 and Statistica 13.3 software were used for statistical analysis of results. In order to assess the normality of the distribution, the Shapiro–Wilk test and quantile–quantile plot were performed. Comparisons of group results before application in particular anatomical points were made using the one-way ANOVA. The significance of the pH differences in measurements over time was assessed using the Friedman ANOVA, followed by the post hoc test. Differences at p < 0.05 were considered statistically significant.
3. Results
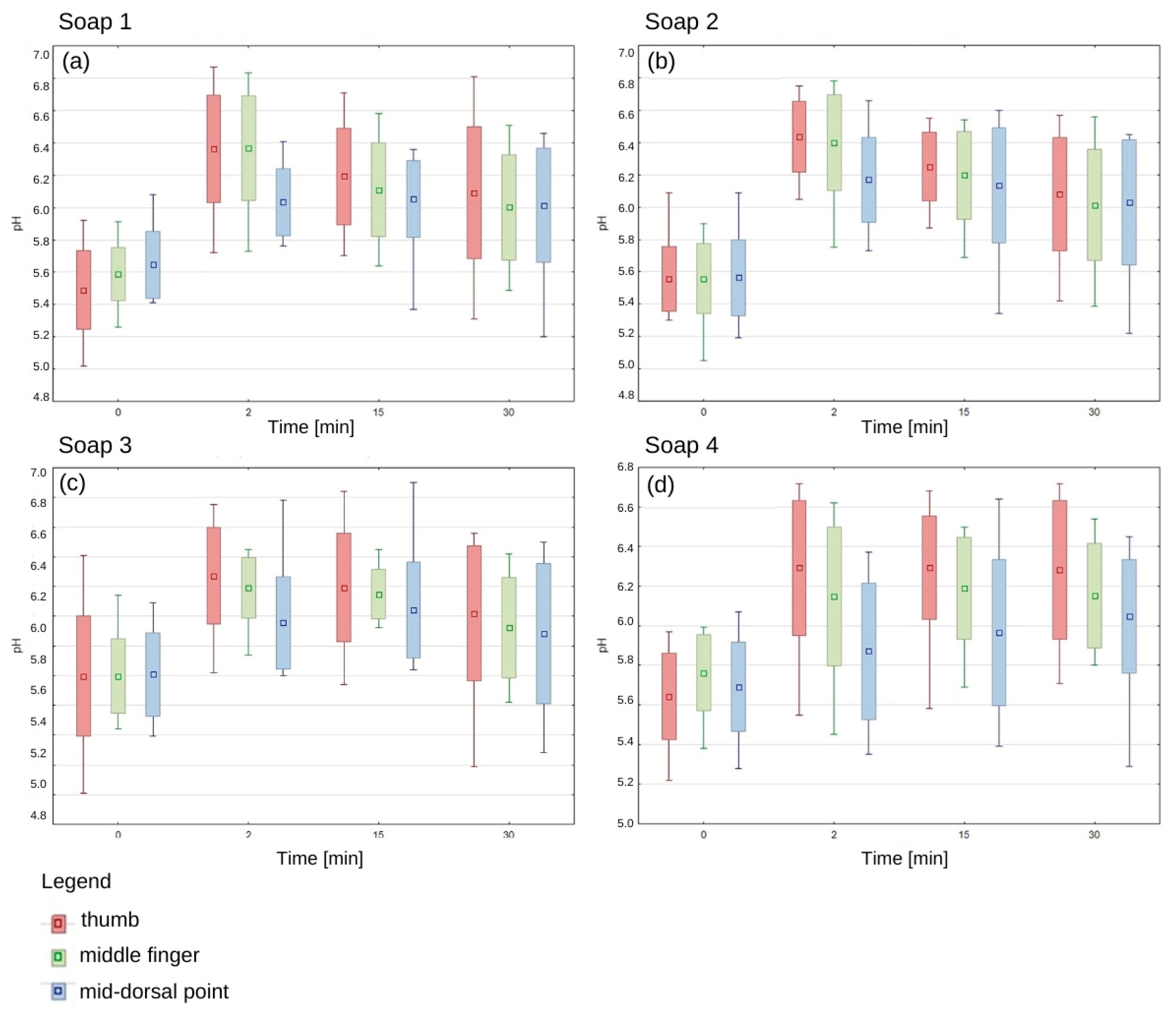
The pH values of the epidermis before application of each of the tested soaps oscillated in the range of 5.5–5.8, which confirms the physiological, slightly acidic skin environment. There was no statistically significant difference between the pH of the right and left hand and between women and men. Immediately after application of each soap, there was an increase in pH, with the highest values recorded in the thumb area (Figure 3).
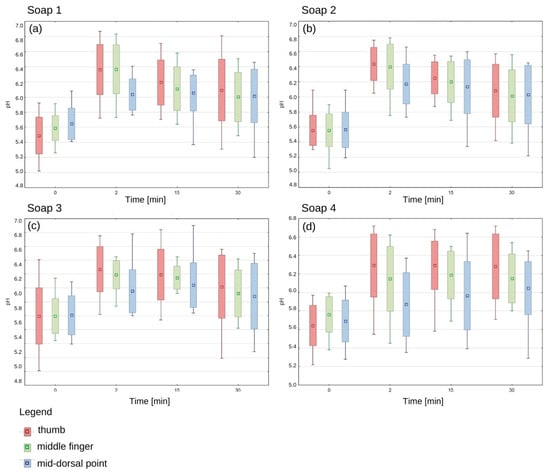
Figure 3.
The change in epidermis pH over time after using the manufactured soaps. Average; standard deviation; Min-Max. (a) The epidermis pH after soap 1 application, (b) The epidermis pH after soap 2 application, (c) The epidermis pH after soap 3 application, (d) The epidermis pH after soap 4 application.
After 15 min of hand washing, a gradual decrease in pH was observed for each soap, but it was still higher than the baseline values. For the first three soaps, the decreasing trend was clearly marked, while soap 4 showed a different pattern of action, as pH values remained elevated even 30 min after application. The pH of the thumb epidermis was higher with the first soap than in the other areas, and this was also the case with the second and third soaps (Figure 3). These results suggest that different parts of the hand may show different susceptibility to pH changes depending on the type of soap used.
The increase in pH of the epidermis in the skin of the hand—on the thumb, middle finger and dorsal part of the hand—after the application of soap is statistically significant compared to the initial value (p < 0.001). The p value, defining the level of statistical significance, allows for an unequivocal statement that the observed changes are not accidental and have a significant impact on the physiological properties of the skin.
In summary, all tested soaps increased the pH of the hand epidermis, with the degree and dynamics of return to the initial value differing depending on the tested soap and the measurement area. The long-term effect of soap 4 seems to be particularly important, as it maintained an increased pH even after 30 min, which may suggest its different composition or mechanism of action compared to the other preparations.
4. Discussion
The study of the effect of soap on skin pH is one of many aspects of dermatological and cosmetological analysis. The literature also discusses issues related to the chemical composition of care products [24], their effect on skin hydration [25], the integrity of the hydrolipid barrier [26] and the potential risk of irritation and allergic reactions [27]. In the context of existing studies on skin pH, it is important to determine the differences between the experiment conducted and previous scientific works.
Analyzing the results of the study by Zgoda et al. [28], it can be seen that the pH values of the skin surface after regular use of drugstore cosmetics show an effect on the activity of hydrogen ions (aH+), and thus on the process of amino acid conversion in the stratum corneum of the epidermis. The authors of this work showed that cosmetics with a pH range higher than 4.5–5.5 can lead to the degradation of the skin’s protective barrier, which weakens its defense capabilities and increases the risk of allergic reactions. The study also observed that both artisanal and drugstore soaps can violate the hydrolipid barrier, but differences in their composition influenced the degree of this phenomenon. Soaps, the solutions of which are alkaline, lead to the ionization of the α-carboxyl groups of amino acids present in the stratum corneum (arginine, ornithine, citrulline, glutamic and aspartic acids). This ionization has an impact on the following:
- Reducing the skin’s buffering capacity;
- Disturbing the water–lipid balance;
- Slowing down the amino acid conversion cycle, which leads to a weakening of the protective barrier and an increase in TEWL.
Soaps—regardless of the production method—usually have a pH in the range of 8–10, and their application can definitely exceed the limit of physiological skin tolerance (pH 4.5–5.5).
Schmid-Wendtner et al. [4] conducted a study comparing the effects of soaps and syndets on skin pH, showing that alkaline soaps increased pH, while acidic syndets helped maintain epidermal homeostasis. Furthermore, it was shown that higher pH values can promote the growth of pathogenic microorganisms such as Staphylococcus aureus, which can have significant clinical consequences. The study also found that artisanal soaps increased skin pH, supporting the use of acidic products in dermatological care.
The results of this study indicate that soaps can disrupt the acid–base balance of the skin and slow down its return to physiological values. Analysis of the results in the context of available scientific research confirms that the pH of cosmetics has a significant impact on the skin barrier function, and products with an alkaline pH can lead to a weakening of its protective properties.
The results of instrument tests showing that the pH remains elevated for 30 min after applying soap indicate that the skin is unable to quickly buffer this change, which in turn can lead to the following:
- Destabilization of the skin microbiome;
- Dryness (due to a disrupted amino acid cycle);
- Greater susceptibility to inflammation and allergic reactions, and reduced effectiveness of subsequent applications of active substances.
In the optimal pH range (4.5–5.5), amino acids undergo almost complete dissociation (αa ≥ 0.99), which increases their solubility, facilitates diffusion and supports the regeneration of the stratum corneum because they have a good ability to form complexes with lipids. Meanwhile, alkaline soaps shift the pH beyond this range, which can limit this diffusion, leading to local disturbances of water balance and the formation of micro-damages to the skin [29].
The application of cleansing agents with alkaline pH, including cold- and hot-processed soaps, has notable thermodynamic implications for the skin’s protective functions. These effects are particularly evident in the disruption of the lipid barrier of the stratum corneum and the modulation of acid–base equilibrium at the skin surface.
An increase in skin surface pH enhances the ionization of lipids, amino acids and proteins. This biochemical shift leads to the destabilization of their native structures, increased entropy (i.e., molecular disorder) and a greater thermodynamic cost required to restore homeostasis—such as the reconstruction of intercellular lipids and re-acidification of the skin environment.
The epidermal lipid barrier, composed primarily of ceramides, cholesterol and free fatty acids, forms a highly ordered lamellar system essential for maintaining skin hydration and defense. Exposure to alkaline substances induces the partial dissolution or saponification of these lipids, thereby disturbing the molecular order. The resulting disorganization introduces intercellular gaps, which facilitate increased skin permeability and accelerate transepidermal water loss (TEWL) [30,31].
Under physiological pH conditions (4.5–5.5), skin lipids and proteins are stabilized by ionic, hydrogen and hydrophobic interactions. Alkaline pH disrupts these non-covalent bonds, impairs the structural organization of lipid lamellae and reduces the overall cohesion of the stratum corneum. Consequently, the barrier becomes more vulnerable to external aggressors [32].
Additionally, the skin’s buffering capacity—conferred by amino acids (natural moisturizing factors, NMFs) and weak organic acids—is compromised following exposure to soaps with elevated pH values. Such conditions promote excessive dissociation and dispersion of amino acids, interrupt their regenerative turnover, and impair the skin’s ability to resist pH fluctuations. This shift increases the risk of local irritation and inflammation [33].
Moreover, surface-active agents present in soaps can self-assemble into micellar structures that effectively solubilize lipids from the intercellular matrix. These surfactants act as lipophilic solvents, extracting key lipid components such as ceramides and cholesterol. This process further compromises the structural integrity of the lipid barrier, potentiating TEWL and impairing the skin’s capacity to maintain hydration and selective permeability [34,35].
Collectively, these thermodynamic disturbances illustrate the necessity of selecting skin cleansers that preserve physiological pH and minimize interference with the native architecture of the stratum corneum.
Although the soap formulations used in this study were based on vegetable oils such as olive oil, grape seed oil, sunflower oil, or shea butter—known to contain beneficial phytochemical compounds including tocopherols, unsaturated fatty acids and polyphenols. The primary focus was to assess the impact of soaps produced using different saponification methods on the skin surface pH. The antioxidant or emollient properties of the oils were not within the scope of this investigation. Moreover, the actual concentration and activity of such compounds in the final soap product may be altered by the saponification process. While the positive influence of fatty acids [36,37] and antioxidants [38,39,40] on the skin barrier is well documented, we did not assess parameters such as transepidermal water loss (TEWL), lipid structure, or occlusive effect, which would be necessary to establish any relationship between soap composition and barrier function. Future research could explore this dimension in combination with pH monitoring.
Although participants were instructed to use a standardized amount of soap (approximately 1 g) and rub their hands gently and evenly for 30 s in accordance with WHO guidelines, it is possible that certain areas, such as the thumbs, experienced greater mechanical friction due to their dominant role in gripping and movement. This may have led to increased accumulation or retention of soap in these regions and contributed to the higher local pH values observed immediately after washing.
The results obtained clearly indicate that the selection of a cleansing agent plays a key role in maintaining the health and condition of the skin. Comparative analysis showed that soap produced using the “hot” method causes the greatest disturbances in the process of restoring the physiological pH of the skin. Other soaps produced using the “cold” method also contributed to extending the time to return to the natural pH, but their effect was less intense. Human skin is characterized by a slightly acidic pH, which plays a fundamental role in protection against microorganisms, maintaining the hydrolipid barrier and regulating the processes of epidermal exfoliation. We chose not to include commercial soaps to reduce formulation variability and to maintain control over ingredient composition. Future studies should include commercial products as controls to improve generalizability. Therefore, the optimal solution seems to be cleansing preparations with a pH close to the physiological reaction of the skin, as they minimize the risk of disorders of the epidermal barrier. This is particularly important for people with sensitive skin or prone to dermatoses, such as atopic dermatitis [41], eczema [42] or seborrheic dermatitis [43].
5. Conclusions
From the point of view of dermatology and cosmetology, soap manufacturers should strive to develop formulas that support the maintenance of the skin’s natural pH, while ensuring effective cleansing and conditioning. Dry skin is more susceptible to irritation, micro-damage and transepidermal water loss (TEWL), which can lead to increased inflammation and a deterioration of its overall condition.
In summary, the research results indicate the need for conscious selection of cleansing agents, especially in the context of protecting the physiological mechanisms of the skin. Soaps with a high pH can significantly disrupt the barrier functions of the epidermis, leading to excessive drying and irritation. Introducing cleansing products with a pH close to physiological and enriched with moisturizing substances into daily care can effectively support skin homeostasis and reduce the risk of dermatological complications.
Author Contributions
Conceptualization, M.D.; methodology, M.D. and J.Z.-N.; formal analysis, J.Z.-N.; investigation, S.A.; resources, S.A.; data curation, S.A.; writing—original draft preparation, S.A. and J.Z.-N.; writing—review and editing, M.D.; supervision, M.D.; project administration, J.Z.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Jan Dlugosz University (date of approval 22 January 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Téot, L. Structure de la peau et cicatrisation cutanée [Skin structure and cutaneous scarring]. Rev Infirm. 2002, 80, 20–23. [Google Scholar]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and Function of the Epidermis Related to Barrier Properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Darlenski, R.; Fluhr, J.W. Influence of Skin Type, Race, Sex, and Anatomic Location on Epidermal Barrier Function. Clin. Dermatol. 2012, 30, 269–273. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.-H.; Korting, H.C. The PH of the Skin Surface and Its Impact on the Barrier Function. Ski. Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Masur, C.; Reich, H.; Knie, U.; Dähnhardt, D.; Dähnhardt-Pfeiffer, S.; Abels, C. Skin Acidification with a Water-in-Oil Emulsion (PH 4) Restores Disrupted Epidermal Barrier and Improves Structure of Lipid Lamellae in the Elderly. J. Dermatol. 2019, 46, 457–465. [Google Scholar] [CrossRef]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A Simple Sphingolipid with Unique Biophysical Properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef]
- Bornkessel, A.; Flach, M.; Arens-Corell, M.; Elsner, P.; Fluhr, J.W. Functional Assessment of a Washing Emulsion for Sensitive Skin: Mild Impairment of Stratum Corneum Hydration, PH, Barrier Function, Lipid Content, Integrity and Cohesion in a Controlled Washing Test. Ski. Res. Technol. 2005, 11, 53–60. [Google Scholar] [CrossRef]
- Behm, B.; Kemper, M.; Babilas, P.; Abels, C.; Schreml, S. Impact of a Glycolic Acid-Containing PH 4 Water-in-Oil Emulsion on Skin PH. Ski. Pharmacol. Physiol. 2015, 28, 290–295. [Google Scholar] [CrossRef]
- Schreml, S.; Meier, R.J.; Wolfbeis, O.S.; Landthaler, M.; Szeimies, R.-M.; Babilas, P. 2D Luminescence Imaging of PH in Vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 2432–2437. [Google Scholar] [CrossRef]
- Turner, N.G.; Cullander, C.; Guy, R.H. Determination of the PH Gradient across the Stratum Corneum. J. Investig. Dermatol. Symp. Proc. 1998, 3, 110–113. [Google Scholar] [CrossRef]
- Furuichi, Y.; Matsui, T.; Amagai, M. Real Time 3D in Vivo PH Imaging of Stratum Corneum Revealed Complex Morphology-Based Regulation in Mice. J. Dermatol. Sci. 2017, 86, e40. [Google Scholar] [CrossRef]
- Walsh, T.R.; Efthimiou, J.; Dréno, B. Systematic Review of Antibiotic Resistance in Acne: An Increasing Topical and Oral Threat. Lancet Infect. Dis. 2016, 16, e23–e33. [Google Scholar] [CrossRef]
- Proksch, E. PH in Nature, Humans and Skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Andonova, M.; Urumova, V. Immune Surveillance Mechanisms of the Skin against the Stealth Infection Strategy of Pseudomonas Aeruginosa—Review. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 433–448. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural Skin Surface PH Is on Average below 5, Which Is Beneficial for Its Resident Flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Runeman, B.; Faergemann, J.; Larkö, O. Experimental Candida Albicans Lesions in Healthy Humans: Dependence on Skin PH. Acta Derm.-Venereol. 2000, 80, 421–424. [Google Scholar] [CrossRef]
- Gfatter, R.; Hackl, P.; Braun, F. Effects of Soap and Detergents on Skin Surface PH, Stratum Corneum Hydration and Fat Content in Infants. Dermatology 1997, 195, 258–262. [Google Scholar] [CrossRef]
- Hachem, J.-P.; Crumrine, D.; Fluhr, J.; Brown, B.E.; Feingold, K.R.; Elias, P.M. PH Directly Regulates Epidermal Permeability Barrier Homeostasis, and Stratum Corneum Integrity/Cohesion. J. Investig. Dermatol. 2003, 121, 345–353. [Google Scholar] [CrossRef]
- Hachem, J.-P.; Man, M.-Q.; Crumrine, D.; Uchida, Y.; Brown, B.E.; Rogiers, V.; Roseeuw, D.; Feingold, K.R.; Elias, P.M. Sustained Serine Proteases Activity by Prolonged Increase in PH Leads to Degradation of Lipid Processing Enzymes and Profound Alterations of Barrier Function and Stratum Corneum Integrity. J. Investig. Dermatol. 2005, 125, 510–520. [Google Scholar] [CrossRef]
- Mijaljica, D.; Spada, F.; Harrison, I.P. Skin Cleansing without or with Compromise: Soaps and Syndets. Molecules 2022, 27, 2010. [Google Scholar] [CrossRef]
- Kawano, T.; Andou, Y. Synthesis and Mechanical Performance of Thermoformable Cellulose Fatty Acid Esters Using Natural Soap. RSC Adv. 2023, 13, 24286–24290. [Google Scholar] [CrossRef]
- Borhan, F.P.; Abd Gani, S.S.; Shamsuddin, R. The Use of D-Optimal Mixture Design in Optimising Okara Soap Formulation for Stratum Corneum Application. Sci. World J. 2014, 2014, 173979. [Google Scholar] [CrossRef]
- Blaak, J.; Staib, P. The Relation of PH and Skin Cleansing. Curr. Probl. Dermatol. 2018, 54, 132–142. [Google Scholar] [CrossRef]
- Plum, F.; Yüksel, Y.T.; Agner, T.; Nørreslet, L.B. Skin Barrier Function after Repeated Short-Term Application of Alcohol-Based Hand Rub Following Intervention with Water Immersion or Occlusion. Contact Dermat. 2020, 83, 215–219. [Google Scholar] [CrossRef]
- Houben, E.; De Paepe, K.; Rogiers, V. Skin Condition Associated with Intensive Use of Alcoholic Gels for Hand Disinfection: A Combination of Biophysical and Sensorial Data. Contact Dermat. 2006, 54, 261–267. [Google Scholar] [CrossRef]
- Törmä, H.; Lindberg, M.; Berne, B. Skin Barrier Disruption by Sodium Lauryl Sulfate-Exposure Alters the Expressions of Involucrin, Transglutaminase 1, Profilaggrin, and Kallikreins during the Repair Phase in Human Skin in Vivo. J. Investig. Dermatol. 2008, 128, 1212–1219. [Google Scholar] [CrossRef]
- di Nardo, A.; Sugino, K.; Wertz, P.; Ademola, J.; Maibach, H.I. Sodium Lauryl Sulfate (SLS) Induced Irritant Contact Dermatitis: A Correlation Study between Ceramides and in Vivo Parameters of Irritation. Contact Dermat. 1996, 35, 86–91. [Google Scholar] [CrossRef]
- Zgoda, M.M.; Piechota-Urbańska, M.; Kołodziejska, J. Wpływ wybranych klas kosmetyków na cykl przemian aminokwasów w warstwie rogowej naskórka i poziom równowagi kwasowo-zasadowej powierzchni skóry. Pol. J. Cosmetol. 2001, 1, 38–53. [Google Scholar]
- Visscher, M.; Robinson, M.; Wickett, R. Stratum Corneum Free Amino Acids Following Barrier Perturbation and Repair. Int. J. Cosmet. Sci. 2011, 33, 80–89. [Google Scholar] [CrossRef]
- Hama, T.; Kouchi, A.; Watanabe, N.; Shioya, N.; Shimoaka, T.; Hasegawa, T. In Vivo Characterization of the Structures of Films of a Fatty Acid and an Alcohol Adsorbed on the Skin Surface. Biophys. Chem. 2020, 266, 106459. [Google Scholar] [CrossRef]
- Tapfumaneyi, P.; Imran, M.; Alavi, S.E.; Mohammed, Y. Science of, and Insights into, Thermodynamic Principles for Dermal Formulations. Drug Discov. Today 2023, 28, 103521. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.T.; Barua, S.; Yoo, S.-Y.; Hong, S.-C.; Lee, K.B.; Lee, J. Seeking Better Topical Delivery Technologies of Moisturizing Agents for Enhanced Skin Moisturization. Expert Opin. Drug Deliv. 2018, 15, 17–31. [Google Scholar] [CrossRef]
- Baldwin, H.E.; Arrowitz, C.; Del Rosso, J. Natural Moisturizing Factor-Enriched Formulations Compared to a Ceramide-Based Cream. J. Drugs Dermatol. 2024, 23, 141–145. [Google Scholar]
- Yadav, N.P.; Meher, J.G.; Pandey, N.; Luqman, S.; Yadav, K.S.; Chanda, D. Enrichment, Development, and Assessment of Indian Basil Oil Based Antiseptic Cream Formulation Utilizing Hydrophilic-Lipophilic Balance Approach. BioMed Res. Int. 2013, 2013, 410686. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Q.; Chen, Z.; Wu, W.; Zhu, Q.; Lu, Y. In Vitro and in Vivo Correlation for Lipid-Based Formulations: Current Status and Future Perspectives. Acta Pharm. Sin. B 2021, 11, 2469–2487. [Google Scholar] [CrossRef]
- Huang, T.-H.; Wang, P.-W.; Yang, S.-C.; Chou, W.-L.; Fang, J.-Y. Cosmetic and Therapeutic Applications of Fish Oil’s Fatty Acids on the Skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef]
- Lin, M.-H.; Khnykin, D. Fatty acid transporters in skin development, function and disease. Biochim. Biophys Acta 2014, 1841, 362–368. [Google Scholar] [CrossRef]
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Tamagawa-Mineoka, R.; Katoh, N. Atopic Dermatitis: Identification and Management of Complicating Factors. Int. J. Mol. Sci. 2020, 21, 2671. [Google Scholar] [CrossRef] [PubMed]
- Inuzuka, Y.; Natsume, O.; Matsunaga, M.; Monna, Y.; Okada, E.; Kato, Y.; Taguchi, T. Washing with Water Alone versus Soap in Maintaining Remission of Eczema. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2020, 62, 663–668. [Google Scholar] [CrossRef]
- Waldroup, W.; Scheinfeld, N. Medicated Shampoos for the Treatment of Seborrheic Dermatitis. J. Drugs Dermatol. 2008, 7, 699–703. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).