Abstract
Kaempferia galanga Linn. (KG), a member of the family Zingiberaceae, is native to India, and commonly found in China, Indonesia, and Thailand. It has been used as a food condiment, folk medicine, and to relieve skin diseases due to its biological activities. However, its anti-aging effect has not yet been investigated. In this study, the rhizome of Kaempferia galanga Linn was extracted with solvents of different polarities (deionized water, absolute ethanol, ethyl acetate, and hexane). Phytochemical screening assay, total flavonoid and total phenolic contents, antioxidant activity (DPPH•, FRAP, ABTS +• assay), anti-aging activity (anti-collagenase, anti-elastase), and cell cytotoxicity on human dermal fibroblasts were investigated. The outcomes revealed that the extraction in highly polar solvents resulted in a high extract yield. Flavonoids, phenolic, and terpenoid compounds were detected in KG extracts using all extraction solvents. However, deionized water as a solvent exhibited the lowest level of flavonoids and phenolics, as compared to the other solvents. The highest total flavonoid and phenolic contents were achieved through extraction with absolute ethanol and ethyl acetate, respectively. Interestingly, the extract obtained with absolute ethanol exhibited the most potent antioxidant activities (the IC50 value of DPPH• was 0.612 mg/mL, the FRAP value was 62.79 mmol of Fe2+/g of extract, and TEAC value was 9.21 mg TE/g of extract in ABTS+• assay) and anti-aging properties (the percentages of collagenase inhibitory and elastase were 71.83%, and 66.35%, respectively). Regarding cell cytotoxicity, both KG extracts obtained with deionized water and absolute ethanol showed lower toxicity on human dermal fibroblasts compared to those obtained with ethyl acetate and hexane. Ethanol-based KG extract demonstrated a good antioxidant, anti-aging capacity and is considered safe for cosmeceutical products focused on anti-aging applications.
1. Introduction
Skin aging is a complex biological process influenced by the combination of intrinsic factors (genetics, hormones) and extrinsic factors (radiation, pollution). Both sets of factors can stimulate skin cells to generate reactive oxygen species (ROS), exerting detrimental effects on cell functions such as collagen and elastin synthesis as well as pigmentation [1]. During skin aging, an imbalance occurs between ROS and the antioxidant reserve, referred to as oxidative stress. These unstable ROS readily react with lipids, proteins, and genetic materials within skin cells, triggering the activation of activator protein-1 (AP-1) along with the upregulation of collagen-degrading enzymes like matrix metalloproteinases (MMPs), collagenase, and elastase. With the inhibition of collagen and elastin synthesis, and the simultaneous enhancement of degradation, the depletion of collagen and elastin content becomes evident, leading to wrinkle formation. Antioxidants offer a promising approach to alleviate skin-aging symptoms by neutralizing free radicals, potentially preventing, or slowing, certain types of cellular damage in the human body. Endogenous antioxidants (glutathione and Coenzyme Q) and exogenous antioxidants (vitamins, phenolic acid, polyphenols, flavonoids, and carotenoids) can defend against ROS based on the hydrogen atom transfer, a single electron transfer, and metal chelation [2,3]. Consequently, the prevention of skin aging is most effectively achieved through the inhibition of ROS formation, collagenase, and elastase activation [4,5].
Kaempferia galanga Linn., a valuable medicinal plant from the Zingiberaceae family, is native to tropical Asia including India, China, Indonesia, Malaysia, and Thailand. It has been traditionally used in primary care medicine, particularly for the treatment of hypertension, diabetes, intestinal wounds, and skin diseases. Recent studies have shown that different extracts from Kaemfpferia galangal Linn. present activities such as anti-angiogenic, anti-inflammatory, and antiviral [6,7,8]. Key components found in the rhizome extract of Kaempferia galanga include cinnamic acid derivatives such as ethyl p-methoxycinnamate (EPMC) and ethyl cinnamate, as well as flavonoids such as kaempferol, known for their antioxidant, antimicrobial, and anti-cancer properties [9]. Among the isolated compounds, EPMC is a major compound from the rhizome of KG that has been identified and has a wide range of biological activities, including an anti-inflammatory effect [10,11]. However, the potential on anti-skin aging of Kaempferia galanga rhizome extract specifically through collagenase and elastase inhibition has not yet been investigated.
In this study, our objective is to explore a novel source of anti-aging agents derived from the Kaempferia galanga rhizome. Moreover, we investigated the impact of solvent polarity on extraction yield and on various biological activities, including antioxidant, anti-collagenase, and anti-elastase activities as well as cell cytotoxicity.
2. Materials and Methods
2.1. Chemicals and Reagents
The analytical grade solvents including absolute ethanol, ethyl acetate, hexane, hydrochloric acid, chloroform, sulfuric acid, and dimethyl sulfoxide (DMSO) were purchased from RCI Labscan (Bangkok, Thailand). The standard and chemical compounds including Trolox, gallic acid, catechin, epigallocatechin (EGCG), sodium acetate, calcium chloride, sodium hydroxide, sodium nitrite, sodium carbonate, aluminum chloride, potassium persulphate, Folin-Ciocalteu’s phenol reagent, 2,2-Di(4-tert-octylphenyl)-1-picrylhydrazyl, free radical DPPH, 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, ferrous sulfate, ferric chloride, 2,4,6-tri (2-pyridyl)-1,3,5-triazine, Collagenase from Clostridium histolyticum (EC.3.4.23.3), N-[3-(2-furyl) acryloyl]-Leu-Gly-Pro-Ala (FALGPA), Porcine pancreatic elastase (E.C.3.4.21.36), N-Succinyl-Ala-Ala-Ala-p-nitroanilide, and Tris-HCl were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Dulbecco’s Modified Eagle Medium (DMEM) with high glucose and fetal bovine serum (FBS) were also obtained from Sigma-Aldrich Co. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Bio Basic, Inc. (Markham, ON, Canada).
2.2. Kaempferia galanga Linn. Preparation and Extraction
The rhizome of Kaempferia galanga Linn. (KG) was collected from the north of Thailand and extracted using the maceration method with different solvents (deionized water, absolute ethanol, ethyl acetate, and hexane). The fresh rhizomes of KG were cut into small pieces, washed with clean water, and then dried in a hot-air oven at 50 °C for 3 days. Subsequently, the dried plant material was finely powdered using a hammer mill (Figure 1). Briefly, 100 g of the KG powder was mixed with each solvent (400 mL) and left at room temperature for 48 h in the dark. Afterward, each solvent extract was filtrated using vacuum filtration with Whatman ™ (Maidstone, UK) filter paper No.1. The organic solvent was then removed via a rotary evaporator at 50 °C to obtain crude extracts. The aqueous extract underwent dehydration through lyophilization. Crude extracts were stored at −20 °C for further experiments. The percentage of extraction yield (%) was determined using the following equation:
Extraction yield (%) = (Weight of crude extract/Weight of powdered sample) × 100
Figure 1.
Kaempferia galanga Linn. (KG). (A) Rhizome of KG; (B) small pieces of KG rhizome; (C) finely powdered KG.
2.3. Preliminary Phytochemical Screening of Kaempferia galanga Linn. Extracts
For preliminary screening of the phytochemical compounds (flavonoids, phenolics, and terpenoids), a small portion of the crude extract obtained from each solvent is subjected to phytochemical tests according to the methods reported by Abioye [12] and Harbourne [13] with some modifications. Color formation changes in the phytochemical tests serve as indicators of the presence of the targeted compounds.
2.3.1. Determination of Flavonoid Compounds
Flavonoids are a class of plant secondary metabolites known for their antioxidant properties and various health benefits. Flavonoid compounds were determined using Shinoda’s test. Briefly, 0.2 mg of each crude extract was dissolved in 1 mL of absolute ethanol, and a few fragments of magnesium metal were added. Subsequently, 3 drops of concentrated hydrochloric acid were added to the test solution. The development of orange, red, or magenta coloration indicated the presence of flavonoids (flavone and flavonols).
2.3.2. Determination of Phenolic Compounds
Phenolic compounds are another class of phytochemicals known for their antioxidant properties. The presence of phenolics was determined using ferric chloride (FeCl3) reagent. Briefly, 0.2 mg of each crude extract was dissolved in absolute ethanol 2 mL, and a few drops of 10% w/v ferric chloride solution were added. The formation of violet or deep purple or blue or dark green coloration indicated the presence of phenolics.
2.3.3. Determination of Terpenoid Compounds
The presence of terpenoid compounds was determined by Salkowski’s Test. Briefly, 0.2 mg of each crude extract was dissolved in absolute ethanol (2 mL) and mixed with chloroform (2 mL), followed by slowly adding a few drops of concentrated sulfuric acid to form a layer. The presence of a dish-brown color in the middle layer was indicative of a steroidal ring. The formation of reddish brown indicated the presence of terpenoids.
2.4. Determination of Total Phenolic Contents
Total phenolic contents of all KG extracts were determined using a Folin-Ciocalteu colorimetric method with some modification [14]. The 20 µL of each crude extract solution dissolved in ethanol (1 mg/mL) was mixed with 100 µL of Folin-Ciocalteu’s phenol reagent (10% v/v) in a 96-well microplate. After incubation for 5 min, 20% sodium carbonate solution (80 µL) was added in the well and the reaction mixtures were incubated in the dark for another 30 min at room temperature. The absorbance of the solution was measured at 760 nm using a microplate reader (BioTek Instruments, Winooski, VT, USA). Gallic acid solution at different final concentrations (2–30 µg/mL) was used as a calibration standard (Supplemental Table S1 and Figure S1). The total phenolic content is expressed in terms of milligrams of gallic acid equivalent per gram of the dried crude extracts (mg of GAE/g of dried crude extract).
2.5. Determination of Total Flavonoid Contents
Total flavonoid contents of all KG extracts were determined using an aluminum chloride complexation colorimetric method with some modification [14]. A total of 50 µL of each crude extract dissolved in ethanol (1 mg/mL) was mixed with 30 µL of 5% sodium nitrite solution in a 96-well microplate. After incubating for 5 min, 10% aluminum chloride solution (30 µL) was added to the well, and the reaction mixtures were incubated in the dark for 6 min at room temperature. Then, 90 µL of sodium hydroxide (1 M) was added to the mixtures and incubated for 15 min at room temperature. The absorbance of the solution was measured at 510 nm using a microplate reader (BioTek Instruments). The amount of total flavonoid content in the samples was quantified from the calibration curve of quercetin solution with different final concentrations (0.75–12.5 µg/mL) (Supplemental Table S2 and Figure S2) and expressed in terms of milligrams of catechin equivalent per gram of the dried crude extracts (mg of QE/g of dried crude extract).
2.6. Antioxidant Activities of Kaempferia galanga Linn. Extracts
The in vitro antioxidant activities of four KG extracts (deionized water, absolute ethanol, ethyl acetate, and hexane) were investigated based on DPPH• radical scavenging activity, ABTS+• radical scavenging activity, and ferric reducing antioxidant power, which are the most commonly used methods to determine antioxidant activities of natural products even when present in complex biological mixtures.
2.6.1. DPPH• Radical Scavenging Activity
The DPPH• radical scavenging was developed many years ago and adopted as the standard method to determine antioxidant activity. This assay is based on the transfer of both a hydrogen atom and an electron and the spectrophotometric measurements of the capacity of antioxidants to scavenge DPPH• radicals. The free radical scavenging abilities of all KG extracts were determined using the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) method with some modification [15]. Each KG extract solution (70 µL) at various concentrations (final concentration 0.5–5 mg/mL) (Supplemental Table S3) was mixed with 70 µL of DPPH• solution (0.2 mM ethanolic DPPH• solution). The mixture was incubated for 20 min in the dark at room temperature. The absorbance was determined at 517 nm after incubation using a microplate reader. Trolox was used as the positive control (Supplemental Table S3). All measurements were performed in triplicate. The percentage of inhibition of free radical scavenging ability was calculated by the following equation:
where Ab is the absorbance of the blank solution and As is the absorbance of the sample solution. The antioxidant activities of all extracts were finally expressed as IC50 value, defined as the concentration (mg/mL) of the extract showing 50% inhibition.
% DPPH• radical scavenging activity = [(Ab-As)/Ab] × 100
2.6.2. ABTS+• Radical Scavenging Activity
The ABTS+• radical scavenging is a spectrophotometric technique based on quenching the stability of a colored radical. The antioxidant capacity of the KG extracts was measured using the ABTS+• (2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt free radical assay with some modification [15]. The ABTS+• solution (7 mM) was prepared by dissolving it in deionized water prior to mixing with 140 mM potassium persulphate (ratio 1:1). The mixture was light-protected and incubated at room temperature for 16 h to allow ABTS+• free radicals to be generated. Afterward, it was diluted with distilled water (1:49 v/v) to achieve the optimized absorbance (0.80 ± 0.20) at 734 nm.
Each KG extract solution (70 µL) at a final concentration of 1 mg/mL was mixed with 140 µL of ABTS+• solution. The mixture was incubated for 6 min in the dark at room temperature. The absorbance was determined at 734 nm after incubation using a microplate reader. The results of the ABTS•+ radical assays are presented as Trolox equivalent antioxidant capacity (TEAC) using Trolox (final concentration 0.195–12.5 µg/mL) as the reference standard (Supplemental Table S4 and Figure S3) and expressed in terms of milligrams of Trolox equivalent per gram of the dried crude extracts (mg of TE/g of dried crude extract). All measurements were performed in triplicate.
2.6.3. Ferric Reducing Antioxidant Power (FRAP)
The FRAP test is simple, fast, cost-effective, and does not require specialized equipment. The total reducing power of all KG extracts were measured using a ferric reducing antioxidant power (FRAP) assay, which measures the total capacity of the sample to reduce Fe3+ into Fe2+ with some modification [16]. Briefly, aliquots of 70 µL from each KG extract solution (final concentration 0.5 mg/mL) were mixed with 140 µL of FRAP reagent (300 mM acetate buffer pH 3.6: 10 mM TPTZ solution in 40 mM HCl: 20 mM FeCl3 solution in a ratio of 10:1:1). The absorbance at 593 nm of the mixture solutions was measured after 30-min incubation in the dark at room temperature using a microtiter plate reader. Ferrous sulfate solution (FeSO4) at various concentrations (final concentration 0.008–0.125 mM) served as a calibration standard (Supplemental Table S5 and Figure S4) and Trolox was used as a positive control. The results were expressed as the ferric reducing antioxidant power equivalent concentration (FRAP value). This index is defined as the concentration of the extract having a ferric reducing ability equivalent to that of 1 mM FeSO4. All measurements were conducted in triplicate.
2.7. Anti-Aging Activities
Anti-aging activities were investigated by measuring collagenase and elastase inhibitory activities based on spectrophotometric methods.
2.7.1. Collagenase Inhibitory Activity
The collagenase inhibitory activity of KG extracts was investigated according to the previous method with some modifications [17]. The assay was performed by dissolving collagenase from Clostridium histolyticum (ChC—EC.3.4.23.3) (0.8 U/mL) in 50 mM Tricine buffer (pH 7.5 with 400 mM NaCl and 10 mM CaCl2). The synthetic substrate N-[3-(2-furyl) acryloyl]-Leu-Gly-Pro-Ala (FALGPA) was dissolved in Tricine buffer to obtain a concentration of 2 mM. Briefly, 30 μL of each KG extract solution (0.5 mg/mL in Tricine buffer) was mixed with 60 μL Tricine buffer and 30 μL collagenase. The mixtures were incubated for 10 min at room temperature before adding 60 μL of FALGPA substrate to start the reaction. After that, absorbance at 335 nm was measured immediately and then continuously for 20 min using a microplate reader. Deionized water and epigallocatechin (EGCG) (50 μg/mL) were used as negative and positive controls, respectively. The percentage of inhibition of collagenase activity was calculated by the following equation:
% Collagenase inhibitory activity = [(OD control − OD sample)/OD control] × 100
2.7.2. Elastase Inhibitory Activity
Elastase inhibitory activity of KG extracts were assessed following a modified version of a previously established method [17]. The elastase assay was performed in 0.2 mM Tris-HCl buffer (pH 8.0). Porcine pancreatic elastase (PE—E.C.3.4.21.36) (1 mM) and substrate N-Succinyl-Ala-Ala-Ala-p-nitroanilide (AAAPVN) (2.0 mM) were prepared in the buffer. Briefly, a mixture of 20 μL KG extract (0.5 mg/mL), 90 μL of Tris-HCl buffer, and 10 μL of elastase enzyme was preincubated for 5 min at room temperature. After the preincubation, the reaction was initiated by adding 80 μL of substrate and then incubated for 15 min at room temperature. The absorbance was measured at 410 nm using a microplate reader. Deionized water and epigallocatechin (EGCG) (50 μg/mL) were used as negative and positive controls, respectively. The percentage of inhibition of elastase activity was calculated by the following equation:
% Elastase inhibitory activity = [(OD control − OD sample)/ OD control] × 100
2.8. Cytotoxicity of Kaempferia galanga Linn. Extracts
2.8.1. Cell Culture and Conditions
Human skin fibroblast cells (WS-1) (ATCC CRL-1502) were cultured in high glucose Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS under 5% CO2 humidified atmosphere at 37 °C.
2.8.2. Determination of Cell Viability
The effects of KG extracts prepared by different extraction solvents on fibroblast cell viability were investigated using the MTT assay [18,19]. Briefly, WS-1 cells were seeded at a density of 5.0 × 104 cells/well into 96-well plates and incubated overnight at 37 °C with 5% CO2. Then, cells were treated with different concentrations (1–100 μg/mL) of each KG extract and incubated for 24 h under the same conditions. After incubation, 20 µL of MTT reagent (5 mg/mL) was added into each well and incubated with 5% CO2, at 37 °C for 4 h. Finally, the whole solution was aspirated, and the formazan crystals formed were dissolved with 150 µL of DMSO, and the absorbance was measured at 570 nm using a microplate reader (Enspire, Perkin Elmer, Waltham, MA, USA). Untreated cells cultured in complete medium with 0.1% DMSO were used as a control. The percentage of cell viability was calculated using the following equation:
% Cell viability = (ODtreated cells/ODuntreated cells) × 100
2.9. Statistical Analysis
All data were expressed as mean ± standard deviation (SD) based on independent experiments with triplicate samples (n = 3). Parametric data comparison of more than two groups were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparisons. The significance level was established at p < 0.05. SPSS V.24 for Windows (SPSS, Chicago, IL, USA) was used for statistical analysis.
3. Results and Discussion
3.1. Effect of Solvent Polarity on Kaempferia galanga Linn. Extraction Yield
Rhizomes of Kaempferia galanga Linn (KG) were extracted using the maceration method, a conventional and cost-effective technique. Four solvents with different polarities, namely, deionized water, absolute ethanol, ethyl acetate, and hexane, were employed to extract diverse bioactive compounds from the KG rhizome. Indeed, the selection of the extraction solvent plays a crucial role in separating a desired chemical substance from herbal materials. The impact of solvent polarity on extraction yield is illustrated in Figure 2. The highest extraction yield was observed with deionized water (6.43%), followed by absolute ethanol (5.30%), ethyl acetate (4.62%), and hexane (3.68%), respectively. The results indicated that extraction yield increases as solvent polarity increases. Likewise, previous studies reported that the polarity of the extraction solvent significantly affected the extraction yield [20,21,22].
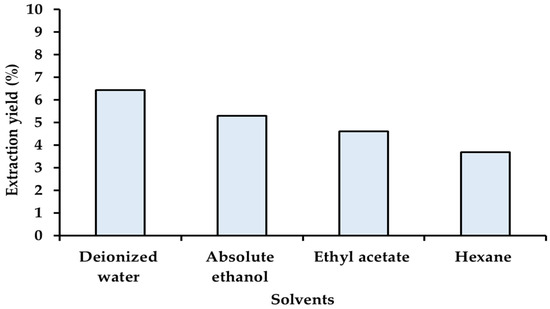
Figure 2.
Percentage of extraction yield. Kaempferia galanga Linn. (KG) extracted by maceration method with different polarity solvents.
3.2. Effect of Solvent Polarity on Phytochemical Screening, Total Phenolic Content, and Total Flavonoids Content
The impact of extraction solvents on phytochemical screening (Table 1) revealed the presence of flavonoid, phenolic, and terpenoid compounds in all extraction solvents. However, deionized water and hexane exhibited a lower intensity of color change compared to other solvents, suggesting that these solvents extract the smallest amounts of flavonoids and phenolic compounds from KG rhizome. The phytochemical composition of the KG rhizome aligned with previous studies, primarily consisting of terpenoids, phenolics, diarylheptanoids, and flavonoids. Notably, flavonoid compounds exhibited a lower presence in aqueous extracts compared to hexane and methanol [23,24]. Additionally, the phytochemical analysis of the KG extract obtained with alcohol showed the presence of saponin, tannin, phenols, and steroids, while extraction with ethyl acetate and hexane identified steroids and tannins [23]. For the extraction of anthocyanins, terpenoids, polypeptide, strarch, phenols, and saponin compounds, water (polarity index = 9.0) was the most effective solvent, whereas polyphenol, flavonols, flavones, and terpenoid compounds were obtained by alcohol extraction (polarity index = 5.2) [25,26]. Furthermore, ethyl acetate (polarity index = 4.4) and hexane (polarity index = 0.0) were used to extract alkaloids, steroids, flavonoids, polyphenols, terpenoids, and anthaquinones [23,25]. These findings suggest that the solvent polarity affects the qualitative characterization of plants. However, the effects of cultivation, harvesting at different ages, climate, and geographic location may also play a role in the phytochemical compounds and biological activities of KG extracts [27,28].

Table 1.
Phytochemical screening of KG extracted with different solvents.
The results of total phenolic and total flavonoid contents aligned with the outcomes of phytochemical screening, as phenolic and flavonoid compounds were found for all solvents (deionized water, absolute ethanol, ethyl acetate, and hexane), independently of polarity (Table 2). The highest flavonoid content was achieved with absolute ethanol, followed by ethyl acetate, hexane, and deionized water, respectively (p < 0.05). The highest phenolic content was obtained by extraction with ethyl acetate, while the lowest was obtained by extraction with deionized water and hexane (p < 0.05). The results show that ethanol and ethyl acetate are the best solvents for extracting flavonoids and phenolic compounds from KG rhizome. Some natural flavonoids (kaempferol, kaempferide, and luteolon) and phenolic compounds (ethyl p-methoxycinnamate, ethyl cinnamate, diarylheptanoid) have been previously identified, as the main phytochemical in KG rhizome [29,30]. These components in KG can be effectively extracted with alcohol and ethyl acetate [31]. However, the total flavonoid and phenolic compounds depend on the extraction solvent, their solvent polarities, the solubility of compounds in the extraction solvents, and the extraction method [20,32,33].

Table 2.
Total flavonoid and total phenolic contents of KG extracts obtained with solvents of different polarities.
3.3. Effect of Solvent Polarity on Antioxidant Activities of Kaempferia galanga Linn. Extracts
The antioxidant activities of KG extracts were evaluated through three assays measuring their abilities to scavenge free radicals using the DPPH• assay and ABTS+• assay as well as the ability to reduce iron (III) ion (Fe3+) using the ferric reducing antioxidant power (FRAP) assay. The results in Table 3 show that ethanolic extract exhibited the greatest antioxidant activity on both the DPPH• assay with an IC50 value of 0.612 mg/mL and the FRAP assay with 62 mmol Fe2+/g extract (p-value < 0.05). Meanwhile, the aqueous extract showed the highest activity in the ABTS +• assay with 11.853 mg TE/g extract (p < 0.05). The ethanolic extract, characterized by its higher flavonoid and phenolic contents (Table 2), showed the greatest antioxidant potential compared to other extracts which exhibited lower antioxidant potency. As has been reported recently, the DPPH• method gives a better response for mostly phenolic compounds [34].

Table 3.
Antioxidant activities of KG extracts prepared with solvents of different polarities.
The major components found in KG rhizome were of the flavonoid group and phenolics, which are well-known for their antioxidant potential [35,36,37]. Other authors observed that the KG methanolic leaf extract exhibited strong antioxidant activity with an IC50 value of 0.611 mg/mL (in the DPPH• assay) [38]. Our findings are similar to the ones observed in a study performed with Kaempferia angustifolia rhizome, a different plant of the same familia. Different solvents were used for extraction, and tested with the DPPH• scavenging assay, showing similar pattern activities compared to our study [39]. The high polarity of methanol allowed for the extraction of more potential antioxidant components from the plant. On the other hand, the antioxidant activity of the extract was decreased by the lower polarity of the solvent used [40], as evidenced by the KG hexane extract showing the lowest antioxidant activity in all experiments due to the low polarity of hexane.
3.4. Effect of Solvent Polarity on Anti-Aging Activities of Kaempferia galanga Linn. Extracts
Skin aging is caused by a natural process involving the alteration of dermal connective tissue and is influenced by intrinsic and extrinsic factors. Both factors can induce skin cells to generate reactive oxygen species (ROS), which have negative effects on skin cell functions such as collagen and elastin synthesis [1]. Collagenase and elastase inhibitory activities are used for assessing anti-aging ability, as collagenase and elastase are responsible for the breakdown of collagen and elastin in the extracellular matrix. The collagenase and elastase inhibitory activity of KG extracts obtained with various solvents of different polarities at a final concentration of 0.5 mg/mL were investigated and presented in Figure 3 and Figure 4. The highest collagenase inhibitory effect was observed with absolute ethanol used as a solvent (71.83 ± 4.65%), followed by hexane (67.43 ± 1.40%), ethyl acetate (56.08 ± 5.48%), and deionized water (37.61 ± 2.97%), respectively. The inhibitory effect of the KG extracts obtained with absolute ethanol on elastase activity exhibited higher anti-elastase activity (66.35 ± 7.21%) than KG extracts obtained with ethyl acetate (43.39 ± 6.75%) and hexane (45.07 ± 6.51%), respectively. The KG extract obtained with deionized water showed the lowest elastase inhibitory effect (22.70 ± 1.63%). The KG extract obtained with absolute ethanol showed similar results as the positive control epigallocatechin (EGCG) with an anti-collagenase activity of 62.38 ± 3.98% and an anti-elastase activity of 71.74 ± 1.73%. The highest collagenase and elastase inhibitory activities, observed with KG extract obtained with absolute ethanol, may be possibly due to its high total phenolic and total flavonoids content (Table 2), along with potent antioxidant activities compared to other solvents (Table 3). Phenolic compounds exhibited significant in vitro antioxidant activity that is effective in inhibiting free radicals and preventing collagen and elastin degradation, resulting in anti-skin aging effects. The results revealed a positive relation between the anti-aging activity and antioxidant activity of KG extracts. Additionally, the effect of different extraction solvents on collagenase and elastase inhibitory activity could be dependent on the part of plant, different stages of harvest, the phytochemical compound in the extract, the concentration of active biological compounds, and their antioxidant activity [15,41,42].
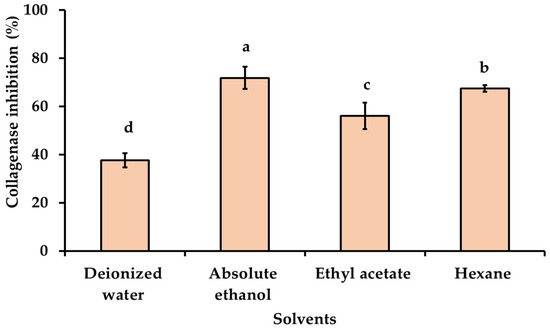
Figure 3.
Collagenase inhibitory activity of KG extracts obtained with solvents of different polarities. The final concentration of the tested samples was 0.5 mg/mL. The values are expressed as the mean ± SD in triplicate experiments. Different alphabet letters indicate a statistically significant difference between solvents (p < 0.05).
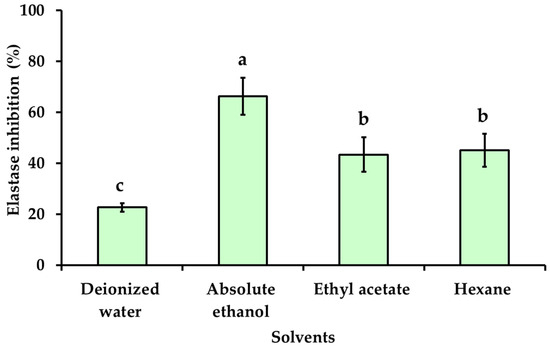
Figure 4.
Elastase inhibitory activity of KG extracts obtained with solvents of different polarities. The final concentration of the tested samples was 0.5 mg/mL. The values are expressed as the mean ± SD in triplicate experiments. Different alphabet letters indicate a statistically significant difference between solvents (p < 0.05).
3.5. Effect of Solvent Polarity on the Cytotoxicity of Kaempferia galanga Linn. Extracts
Dermal fibroblast cells are the main target for anti-aging therapy, playing a crucial role in producing collagen and elastin for skin homeostasis and morphofunctional organization, thereby contributing to anti-aging effects on the skin [43]. The cytotoxicity of all KG rhizome extracts was evaluated on fibroblast cells (WS-1). The WS-1 cell viability after the treatment with KG extracts obtained with different solvents is represented in Figure 5. No cytotoxic effect was observed in fibroblast cells upon exposure to all KG extracts at concentrations ranging from 1 to 25 µg/mL, except for hexane extracts of KG rhizome. However, concentrations higher than 25 µg/mL induced a significant decrease in WS-1 cell viability in a dose-dependent manner (p < 0.05), except for deionized water extracts of KG rhizome which exhibited no cytotoxic effect. Additionally, the KG hexane extract exhibited a higher cytotoxic effect compared to the other extracts. Typically, bioactive phytochemical compounds can be extracted from herbal plants using various solvents of different polarity, especially organic solvents such as hexane, ether, and ethanol [34,44,45]; it is crucial to choose an extraction solvent that is both effective and safe for human use. Thus, concentrations of deionized water and absolute ethanol extracts of KG rhizome less than 50 µg/mL can be considered as permissible limits for applications, especially ethanolic extract due to its higher concentration of polyphenolic and antioxidant and anti-aging activities.
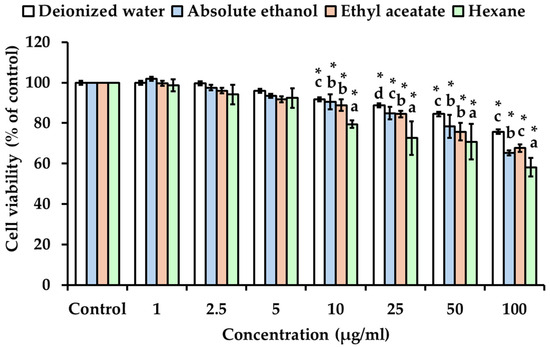
Figure 5.
The cytotoxicity of KG extracts obtained with solvents of different polarity on human fibroblast cells (WS-1). The values are expressed as the mean ± SD in triplicate experiments. The asterisk mark (*) indicates a statistically significant difference at p < 0.05 compared to the control group. Different alphabet letters indicate a statistically significant difference at p < 0.05 among the different solvents.
4. Conclusions
The present study explores the in vitro antioxidant, anti-skin aging, and cytotoxic properties on human fibroblasts of Kaempferia galanga Linn. rhizome (KG). The KG extracted using solvents of different polarity revealed that solvents with higher polarity (water, absolute ethanol) present a higher percentage of extraction yield than low polarity solvents (ethyl acetate, hexane). Interestingly, all extraction solvents effectively dissolve secondary metabolites (flavonoids, phenolics, and terpenoid compounds) from KG. Particularly, absolute ethanol and ethyl acetate extracts exhibited higher phenolic and flavonoid contents. Additionally, the high antioxidant activity and anti-aging properties (anti-collagenase and anti-elastase activity) of the KG extract are potentially attributed to the high content of flavonoids and phenolic compounds. However, different compound polarities in each of the extraction solvents display various antioxidant mechanisms (free radical scavenging ability and ferric reducing antioxidant power). Cytotoxicity assessment of KG in human fibroblasts found that all extraction solvents were noticeable at a concentration of more than 25 µg/mL in a dose-dependent manner. All findings considered, the ethanolic extract of KG revealed the most effective antioxidant activity, collagenase, and elastase inhibitory effects, with none to low cytotoxicity. Therefore, ethanolic KG extract is suitable for the development of cosmeceutical products geared towards anti-aging applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics11030097/s1, Table S1: Raw data of absorbance vs gallic acid concentration, Table S2: Raw data of absorbance vs quercetin concentration, Table S3: Raw data of % DPPH• radical scavenging activity VS sample test (Trolox and KG extract), Table S4: Raw data of absorbance vs FeSO4 concentration, Table S5: Raw data of absorbance vs Trolox concentration, Figure S1: Linearity relationship between absorbance vs concentration of gallic acid, Figure S2: Linearity relationship between absorbance vs concentration of Quercetin, Figure S3: Linearity relationship between absorbance vs concentration of FeSO4 Figure S4. Linearity relationship between absorbance vs concentration of Trolox.
Author Contributions
Conceptualization, P.W.; methodology, P.W. and A.P.; validation, P.W.; formal analysis, P.W. and R.C.; investigation, P.W., R.C., A.P. and W.R.; resources, P.W. and A.P.; data curation, P.W.; writing—original draft preparation, P.W., R.C. and A.P.; writing—review and editing, P.W., M.P.V. and W.R.; visualization, P.W.; supervision, P.W., M.P.V. and W.R.; project administration, P.W.; funding acquisition, P.W. and W.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research (Grant number RGNS 64-133) was supported by the Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innovation (OPS MHESI), Thailand Science Research and Innovation (TSRI) and the University of Phayao.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the finding of this study are available within the article and its Supplementary Materials.
Acknowledgments
The authors are grateful to the Department of Cosmetic Sciences, School of Pharmaceutical Sciences, University of Phayao, and the Natural Products for Neuroprotection and Anti-Ageing Research Unit, Chulalongkorn University for providing instruments and facilities. Acknowledgment is also extended to the support and cooperation of the researchers of Mae Fah Luang University under the Reinventing University Project. The Reinventing University has received funding support from the Office of the Permanent Secretary of the Ministry of Higher Education, Science, Research, and Innovation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohiuddin, A.K. Skin Aging & Modern Age Anti-aging Strategies. Glob. J. Med. Res. B 2019, 19, 209–240. [Google Scholar]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.V.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Akamatsu, H.; Okano, Y.; Matsunaga, K.; Masaki, H. Exogenous nitric oxide enhances the synthesis of type I collagen and heat shock protein 47 by normal human dermal fibroblasts. J. Dermatol. Sci. 2006, 41, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zheng, N.; Niu, N.; Tan, Y.; Li, Y.; Tian, H. Potent anti-angiogenic component in Kaempferia galanga L. and its mechanism of action. J. Ethnopharmacol. 2024, 324, 117811. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, I.S.; Sufiawati, I.; Shafuria, A.; Nittayananta, W.; Levita, J. Formulation and Evaluation of Mucoadhesive Oral Care Gel Containing Kaempferia galanga Extract. Pharmaceutics 2024, 16, 421. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, R.; Hu, H.; Zhao, X.; Yin, Z.; Zou, Y.; Li, L.; Jia, R.; Zhang, Y.; Song, X. Antiviral effect of an extract from Kaempferia galanga L. rhizome in mice infected with pseudorabies virus. J. Virol. Methods. 2022, 307, 114573. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Phytochemistry, pharmacological activities and uses of traditional medicinal plant Kaempferia galanga L.—An overview. J. Ethnopharmacol. 2020, 253, 112667. [Google Scholar] [CrossRef] [PubMed]
- Lallo, S.; Hardianti, B.; Sartini, S.; Ismail, I.; Laela, D.; Hayakawa, Y. Ethyl P-Methoxycinnamate: An Active Anti-Metastasis Agent and Chemosensitizer Targeting NFκB from Kaempferia galanga for Melanoma Cells. Life 2022, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.I.; Asmawi, M.Z.; Sadikun, A.; Atangwho, I.J.; Yam, M.F.; Altaf, R.; Ahmed, A. Bioactivity-guided isolation of ethyl-p-methoxycinnamate, an anti-inflammatory constituent, from Kaempferia galanga L. extracts. Molecules 2012, 17, 8720–8734. [Google Scholar] [CrossRef] [PubMed]
- Abioye, E.O.; Akinpelu, D.A.; Aiyegoro, O.A.; Adegboye, M.F.; Oni, M.O.; Okoh, A.I. Preliminary Phytochemical Screening and Antibacterial Properties of Crude Stem Bark Extracts and Fractions of Parkia biglobosa (Jacq.). Molecules 2013, 18, 8485–8499. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Phytochemical Methods A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Eun, C.-H.; Kang, M.-S.; Kim, I.-J. Elastase/Collagenase Inhibition Compositions of Citrus unshiu and Its Association with Phenolic Content and Anti-Oxidant Activity. Appl. Sci. 2020, 10, 4838. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Vistica, D.T.; Skehan, P.; Scudiero, D.; Monks, A.; Pittman, A.; Boyd, M.R. Tetrazolium-based assays for cellular viability: A critical examination of selected parameters affecting formazan production. Cancer Res. 1991, 51, 2515–2520. [Google Scholar] [PubMed]
- Abarca-Vargas, R.; Pena Malacara, C.F.; Petricevich, V.L. Characterization of Chemical Compounds with Antioxidant and Cytotoxic Activities in Bougainvillea x buttiana Holttum and Standl, (var. Rose) Extracts. Antioxidants 2016, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I.S.M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Sani, S.A.; Faik, A.A.M.; Abdulla, R.; Kunasekaran, S. Phytochemical, antioxidant and antibacterial activities of two kinds of Sabah Zingberaceae. J. Phys. Conf. Ser. 2019, 1358, 012012. [Google Scholar] [CrossRef]
- Uba, G.; Dauda, H.; Aujara, K.M.; Ali, U. Solvent Extraction and its Effects on the Phytochemical Yield and Antioxidant Capacity of Commiphora africana (Burseraceae). Bioremediation Sci. Technol. Res. 2020, 8, 8–11. [Google Scholar] [CrossRef]
- Snyder, L.R. Classification of the solvent properties of common liquids. J. Chromatogr. A 1974, 92, 223–230. [Google Scholar] [CrossRef]
- Rao, A.; Pandey, V.N. Phytochemical Screening of Tubers and Leaf extracts of Sagittaria sagittifolia L.: Newsa (Arrowhead). Int. J. Sci. Res. Publ. 2017, 7, 431–437. [Google Scholar]
- Ncube, B.; Finnie, J.F.; Van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L. Phenological and Geographical Effects on Phenolic and Triterpenoid Content in Vaccinium vitis-idaea L. Leaves. Plants 2021, 10, 1986. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Huang, Y.; Wang, Y.; He, X. Anti-inflammatory diarylheptanoids and phenolics from the rhizomes of kencur (Kaempferia galanga L.). Ind. Crops Prod. 2018, 125, 454–461. [Google Scholar] [CrossRef]
- Swapana, N.; Tominaga, T.; Elshamy, A.I.; Ibrahim, M.A.A.; Hegazy, M.-E.F.; Brajakishor Singh, C.; Suenaga, M.; Imagawa, H.; Noji, M.; Umeyama, A. Kaemgalangol A: Unusual seco-isopimarane diterpenoid from aromatic ginger Kaempferia galanga. Fitoterapia 2018, 129, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, P.C.; Latha, K.P.; Mudgal, J.; Nampurath, G.K. Extraction, characterization and evaluation of Kaempferia galanga L. (Zingiberaceae) rhizome extracts against acute and chronic inflammation in rats. J. Ethnopharmacol. 2016, 194, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Iloki-Assanga, S.B.; Lewis-Lujan, L.M.; Lara-Espinoza, C.L.; Gil-Salido, A.A.; Fernandez-Angulo, D.; Rubio-Pino, J.L.; Haines, D.D. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res. Notes 2015, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Hirano, R.; Sasamoto, W.; Matsumoto, A.; Itakura, H.; Igarashi, O.; Kondo, K. Antioxidant Ability of Various Flavonoids against DPPH Radicals and LDL Oxidation. J. Nutr. Sci. Vitaminol. 2001, 47, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Zdunska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Begum, T.; Gogoi, R.; Sarma, N.; Pandey, S.K.; Lal, M. Novel ethyl p-methoxy cinnamate rich Kaempferia galanga (L.) essential oil and its pharmacological applications: Special emphasis on anticholinesterase, anti-tyrosinase, alpha-amylase inhibitory, and genotoxic efficiencies. PeerJ 2023, 11, e14606. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kabir, M.T.; Islam, M.N.; Muqaddim, M.; Sharmin, S.; Ullah, M.S.; Uddin, M.S. Investigation of Antioxidant and Cytotoxic Activities of Kaempferia galanga L. Res. J. Pharm. Technol. 2019, 12, 6. [Google Scholar] [CrossRef]
- Yeap, Y.S.Y.; Kassim, N.K.; Ng, R.C.; Ee, G.C.L.; Saiful Yazan, L.; Musa, K.H. Antioxidant properties of ginger (Kaempferia angustifolia Rosc.) and its chemical markers. Int. J. Food Prop. 2017, 20, 1158–1172. [Google Scholar] [CrossRef]
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Ćavar Zeljković, S. Influence of Extraction Solvent on the Phenolic Profile and Bioactivity of Two Achillea Species. Molecules 2021, 26, 1601. [Google Scholar] [CrossRef] [PubMed]
- Utami, S.; Sachrowardi, Q.R.; Damayanti, N.A.; Wardhana, A.; Syarif, I.; Nafik, S.; Arrahman, B.C.; Kusuma, H.S.W.; Widowati, W. Antioxidants, anticollagenase and antielastase potentials of ethanolic extract of ripe sesoot (Garcinia picrorrhiza Miq.) fruit as antiaging. J. Herbmed Pharmacol. 2018, 7, 88–93. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. Int. J. Mol. Sci. 2022, 23, 6655. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Santoyo, S.; Jaime, L.; García-Blairsy Reina, G.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Starmans, D.A.J.; Nijhuis, H.H. Extraction of secondary metabolites from plant material: A review. Trends Food Sci. Technol. 1996, 7, 191–197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).