Abstract
Compounding is currently an integral part of pharmacy practice, and it is essential to the provision of healthcare. Compounding is an important therapeutic option in all areas of medicine, with relevance to dermatological treatments. Compounding topical medicines can be time-consuming and requires specialized equipment. In this regard, the use of proprietary topical liquid and semisolid vehicles/bases can be a good alternative and a more sustainable approach. This review provides an overview of existing proprietary vehicles/bases, summarizing their properties and applications and identifying existing commercial and information gaps. Creams emerge as the foremost topical base, followed by gels and ointments. Besides acting locally on the skin, almost a third of these proprietary bases/vehicles are also suitable for the transdermal application of APIs. Information regarding composition and compatibilities/incompatibilities with APIs is not always provided by the manufacturer, constraining a complete analysis of all proprietary topical bases/vehicles considered. The collection and organization of this information are important not only for pharmacy practice and physician prescription, helping to select the best vehicles/bases, but also for the industry to identify opportunities for innovation.
1. Introduction
Topical medicines are one of the most popular therapeutic approaches for several diseases. The vehicle is designated as the carrier of the active pharmaceutical ingredients (APIs) in liquid preparations, being composed of one or more excipients. They are denominated bases when the formulations are solid and semisolid [1].
Topical drugs in general are made up of active ingredients incorporated in a vehicle/base that facilitates application to the skin. With the help of the vehicle/base, the API acts only at the application site and target organs, thus achieving the desired effect. When selecting a vehicle or a base, it is pivotal to consider existing skin diseases and associated cutaneous manifestations, as well as the skin type and skin barrier needs. For example, oil-free emulsions are targeted for oily skin and associated conditions, such as acne and seborrheic dermatitis, while W/O cream, ointments, and oleogels are adequate for dry skin or diseases like atopic dermatitis, psoriasis, and ichthyosis [2,3].
When choosing the vehicle, the solubility of the API in the vehicle; the release rate of the ingredient from the vehicle; the ability of the vehicle to hydrate the stratum corneum, thus increasing penetration; the stability of the therapeutic agent in the vehicle; and the chemical and physical interactions between the vehicle, stratum corneum, and API should be taken into consideration. Depending on the vehicle/base, dermatological formulations can be classified into solutions, suspensions, lotions, gels, aerosols, powders, pastes, creams, foams, and ointments [4].
2. Vehicles/Bases for Topical Medicines
Topical drug formulations are complex and use many different ingredients because each product must have properties tailored to the desired application. In addition to the skin surface, topical medicines can be applied to other body areas such as the scalp, eyelids, lips, genital external skin, or nails [2].
The formulation of a topical product involves three fundamental elements: vehicle/base, active ingredients, and additives. Topical vehicles are frequently composed of one or more substances whose purpose is to provide structure to the product as well as to promote or modulate the effects of the drugs. Topical bases/vehicles include solutions; suspensions and shampoos; lotions and creams; gels (aqueous or oily); sprays and foams; and micro and nanosystems (micelles, liposomes, micro- and nanoemulsions, micro- and nanoparticles) [4]. Several authors have developed reviews about the definition and characterization of topical vehicles [5], the advantages and disadvantages of semisolid vehicles [6], and nanosystems for topical and transdermal drug delivery applications [7,8].
The selection of the most suitable vehicle depends on technological issues for drug stability and administration, the type of skin and skin disorder specifications, and patient preferences and needs. These attributes contribute to medication adherence and therapeutic success and comprise a Patient-Centric Drug Product Design approach to achieve a target product specific to a patient [2].
2.1. Topical Liquids: Solutions, Suspensions, Shampoos, Sprays, Foams, and Lotions
Solutions are essential vehicles for pharmaceutical products. They are homogeneous systems composed of a single liquid phase (aqueous or oily) in which there is a complete dissolution of one or more compounds [9].
Although apparently homogeneous, there are pharmaceutical forms in which the particles are not completely dissolved in the solvent. Therefore, they are not true solutions, but heterogeneous systems. These systems are classified according to the size of the particles (smaller or larger than a few micrometers) and their consistency (liquid or solid).
A suspension is a pharmaceutical form that has two phases: a liquid phase, also called external, dispersing, or continuous phase, and a solid phase called internal, dispersed, or discontinuous. The solid particles are insoluble in the liquid phase; they remain suspended for some time and tend to sediment due to their size (>1000 nm). The sediment formed, however, should be easily dispersed by stirring or shaking [4]. The liquid phase should have a certain viscosity (by using suspending agents) and may be of an aqueous or oily nature. Suspensions may be used in preparations for oral, parenteral, dermatological, ophthalmic, nasal, otological, and rectal use among others [10].
However, there are also other dispersed systems in which the suspended particles, due to their very small size (1 to 1000 nm), remain uniformly dispersed in the medium due to repulsion phenomena, in which the effect of gravity is felt less. These are called colloidal dispersions [4]. These systems are widely used today either in the form of shampoos, aerosols, and foams.
Shampoos consist of liquid or, on occasion, semisolid formulations designed for application to the scalp and subsequent rinsing with water. They are composed of a surfactant dispersion suitable for scalp application [1].
Sprays are aerosol solutions packed under pressure with a propellent solvent. This provides a continuous and homogeneous flow and, when applied to the skin, produces a thin film [4,11].
Foams are usually three-phase products of water, oil, and a volatile solvent [10]. They are packaged in a special highly resistant container, composed of a bottle and a valve that regulates the passage of the product with the gas [4]. The formulation of foams is performed with ingredients that increase the skin penetration and absorption of the active ingredients and are designed for the patients’ needs, thus optimizing the therapeutic results, which are achieved in less time [12]. Both foams and sprays prevent oxidation and contamination as they do not allow the product to make contact with the environment’s oxygen. The formulas are stable and safe, both for those who produce it and those who use it [13]. The major attributes of sprays and foams are their ease of use, low contamination, and variety of application features, such as seborrheic dermatitis [14], psoriasis [15], and eczema [16].
Emulsions are the most common dermatological and cosmetic vehicle. An emulsion is a heterogeneous system consisting of two immiscible liquid or semisolid phases in which one of the phases is dispersed, internal, or discontinuous in another dispersant, external, or continuous phase [4]. Depending on the composition of the internal and external phases, they are classified as W/O emulsions (with oil as the outer and continuous phase) or O/W emulsions (with water as the outer and continuous phase). The water- and oil-phase components and the emulsifying system determine the type of emulsion and its occlusive properties. Their consistency can be very diverse from liquid or slightly viscous (lotions) to semisolid (creams). Lotions are fluid emulsions generally applied to intertriginous areas, due to is lubricant effect [10]
Topical liquids are adequate to be applied on large skin surfaces or when rapid vehicle vanishment by evaporation is needed, which is achieved with the use of volatile solvents. Due to their consistency, they are prone to be applied in scalp or hairy skin areas. Aqueous liquids generally have no emollient or moisturizing effect. The common presence of alcohol is not suitable for sensitive or injured skin. Scalp psoriasis [17,18], atopic dermatitis [19], and seborrheic dermatitis [20,21] are examples of skin disorders where liquid vehicles are applied successfully.
2.2. Topical Semisolids: Creams, Ointments, and Gels
Creams are opaque, soft solids or thick liquids that are intended for external application [10]. Creams are classified according to the type of dispersant or continuous phase; thus, the emulsions are water/oil (W/O) when the external phase is oily and oil/water (O/W) when the external phase is aqueous [10]. There are also silicone/water (S/W) emulsions, where the oily phase is replaced by a silicone phase, and these are the basis of oil-free emulsions, highly targeted for oily skin diseases such as acne and seborrheic dermatitis. Since there are two immiscible phases in an emulsion, they can only be kept dispersed by the use of emulsifiers or surfactants. Depending on the chemical nature of these compounds, emulsions are classified as amphoteric, anionic, cationic, or non-ionic [22]. While anionic and cationic surfactants are considered less innocuous to the skin, leading to some irritation skin reactions, the non-ionic surfactants, with special emphasis on glucoside-based emulsions, are preferable for sensitive skin [23]. Emulgels or cream gels are low-fat emulsions composed of a gelled aqueous phase and a small amount of oily phase, leading to a pleasant, less greasy, and easy-to-spread topical form [24].
The amount of oily phase determines its application in relation to the type of skin and the desired occlusion degree. Emulsions have a wide range of applications. W/O emulsions provide more occlusive and greasy properties for drier skin areas with sparse hair, and O/W emulsions are easy to spread, moisturizing, and suitable for all body areas, especially flexural or exudative zones [5,6]. The fat content can be controlled to adapt to the patients’ needs in order to become more or less occlusive, moisturizing, or greasy. Thus, emulsions can be used to treat a massive variety of skin diseases such as atopic dermatitis [25], seborrheic dermatitis [26], acne [27], or rosacea [28].
Ointments consist of a pharmaceutical form of one single phase, generally made of oily and absorption excipients, and can include lipophilic substances or small amounts of hydrophilic components dissolved in a co-solvent [4]. They are preferred for dry skin lesions such as seborrheic dermatitis [26] or psoriasis [29].
Gels are a type of semisolid preparation that corresponds to a particular state of colloidal dispersion [10]. Gels are formed by a three-dimensional network made up of macromolecules (gelling agents), between which the liquid is distributed [1]. According to the nature of the solvent, gels can be classified as hydrogels—aqueous gels, whose excipients are usually water, glycerin, and propylene glycol gelled with appropriate gelling agents, such as starch, cellulose derivatives, carbomers, or magnesium aluminum silicates—and oleogels—lipophilic gels, the excipient of which is generally liquid paraffin with the addition of polyethylene compounds or fatty oils gelled with colloidal silica or aluminum or zinc soap [4,30,31]. Organogels are typically composed of an organic liquid phase, exhibiting a broad functionality range that results from the combination of the organic liquid with gelator components [32]. They are applied for transdermal drug delivery, such as pluronic lecithin organogel (PLO gel) [33]. However, hydrogels continue to be the pharmaceutical forms of choice when ease of spreading, non-greasy, and non-occlusive properties are required for application to hairy or injured skin, or in acne [27], atopic dermatitis [34], or seborrheic dermatitis [35].
Semisolid bases allow the retention of drugs on the skin surface and have good spreadability and desired degrees of lubricating features. It is also known that bases can have more effects than just drug carriers. The correct choice of a base can, in itself, contribute to a clinical improvement in the disease [25,36].
3. Micro- and Nanosystems
Nanotechnology has developed new systems that can be applied at a topical level, in particular for the optimization of skin permeation. Microsystems are structures at the micrometer scale, while nanosystems’ structures are typically within the range of 1 to 100 nanometers. Through the appropriate selection of the micro- or nanosystem to be used, it is possible to produce controlled-release systems, achieving constant concentration profiles, maximizing the effect, and reducing adverse reactions related to high-dose administration, besides simplifying the administration for the patient [37].
Micelles are structures formed by amphiphilic molecules (surfactants or detergents) characterized by the coexistence of a hydrophobic and a hydrophilic portion. Micellar cleansers, which consist of dispersions of micellar nanoparticles, can be considered a practical application of nanotechnology in skincare for their ability to efficiently remove makeup and skin impurities [38,39]. Polymeric micelles, a specific class of micelles, are colloidal systems formed of copolymers consisting of both hydrophilic and hydrophobic monomer units. Polymeric micelles demonstrate advantageous attributes as cutaneous nanocarriers both for cosmetic applications and as a topical medicine, encompassing enhanced drug solubilization within the skin, heightened partitioning of hydrophilic drugs into the stratum corneum (SC), targeted drug localization within hair follicles and keratinocytes across distinct epidermal layers, and the establishment of controlled and sustained release through the skin [40,41].
Liposomes are spherical vesicles composed of one or more lipid bilayers separated by internal aqueous compartments, thus being composed of amphiphilic molecules, containing a hydrophobic and a hydrophilic portion, and being able to transport large quantities of both lipophilic and hydrophilic drugs. These systems have been widely studied as possible agents of drug vectorization through the skin, presenting some benefits such as the stabilization of drugs, decreased systemic absorption, and the ability to increase the permeability of a molecule at the topical level due to their ability to increase the fluidity of the lipids that constitute the stratum corneum; in addition to that, conjugated with other molecules that aid permeation, they can guarantee a sufficient amount of the drug in the target site. However, they still present problems of stability and toxicity [42]. When phospholipids of herbal origin and phytochemicals are used, the vesicles are called phytosomes, with improved bioavailability and stability [43]. Non-ionic surfactants and cholesterol-like lipids originate self-assembly nanovesicles, called niosomes, that can also trap several molecules including phytochemicals, increasing their stability and encapsulation efficacy. This type of vesicle presents a better stability and sustainability than classical liposomes and is used as commercial ingredients [44].
Micro- and nanoemulsions are, concisely, emulsions in which the droplets of the dispersed or discontinuous phase are reduced to micro- or nanometric sizes, respectively; thus, the difference between macro-, micro-, and nanoemulsions lies in the size of the particles that reflects the physical stability of the dispersed system [45]. Impaired stability in these emulsions may compromise their use for commercial purposes.
Micro- and nanoparticles are solid systems generally composed of polymers (natural, synthetic, or semi-synthetic) capable of organizing themselves into vesicles, thus functioning as possible drug vehicles. As regards their structure, the terms microparticle and nanoparticle cover micro- and nanospheres and micro- and nanocapsules. Micro- and nanospheres refer to vesicles in which the drug is confined to the core (which may be liquid or solid), surrounded by the shell. Micro- and nanocapsules are vesicles in which the drug is homogeneously distributed throughout the matrix. These systems have wide applicability, with ocular, intramuscular, oral, and dermal uses [46].
Scale-up problems, a lack of industrial batch uniformity, physical instability, clinical performance, and a deficient regulatory framework are the main obstacles to the marketing of nanosystems, which are more widespread in cosmetics than in pharmaceutical products [46,47].
4. Compounded Medicines
Compounded medicines are unique, tailor-made products for a patient that take into account not only their therapeutic needs but also their individual characteristics, enhancing the success of the treatment. They are prepared based on a physicians’ prescription, or a formulary or pharmacopoeia, and formulated and dispensed under the responsibility of a pharmacist, either in community or hospital pharmacies [48,49].
Compounded medicines in general can overcome the standardization of pharmaceutical industrial products and may solve several patient problems in obtaining their medication [48,50]:
- The absence of marketed medicines. The pharmaceutical industry often discontinues drug commercialization due to technical or commercial reasons. Also, in the case of rare diseases, there are only a few insufficient options [51];
- Unsuitable pharmaceutical forms for patients with routes of administration unavailable or unconscious patients, or in the case of routes of administration that are difficult to use, such as solid oral forms for children or the elderly;
- Inadequate dosages for the pathophysiological needs of patients such as pediatric, geriatric, and renal or hepatic impairment, or for dosage titration needs;
- Patients intolerant to some substances (e.g., lactose), diabetics (e.g., sucrose), allergic patients (e.g., parabens), or where one needs to avoid drug interactions [52];
- The association of active substances to improve and facilitate the dosage regimen in the case of comorbidities;
- The adequacy of the amount of product to be dispensed, allowing adjustment to the patient’s regimen needs;
- The use of unstable active substances with a short shelf life and complex stability, which may be an obstacle to their marketing;
- Improving therapeutic adherence by optimizing vehicles according to patient preferences (organoleptic characteristics) and disease characteristics;
- Small-scale formulations for clinical trial application [53].
Compounded medicines have their drawbacks. Since they are produced on a small scale and outside an industrial setting, there may be a less uniform presentation. Chemical interactions between ingredients may occur since the formulations are not as standardized as an industrialized product. Therefore, quality issues should be taken into account in a risk–benefit analysis, considering the patient context. Additionally, the definition of a shelf life is often not defined based on stability studies of the final product, but rather indirectly based on information of the active substances, the type of vehicle, and the packaging. Compounded medicines can be subject to formulation errors with very serious consequences. Most errors are due to contamination (particularly critical in parenteral formulations) or dosage (causing under- or overdosage) [54,55]. Finally, there are economic issues to consider, as compounded medicines are often more expensive than industrialized ones and may vary among dispensing pharmacies.
Dermatology and aesthetic medicine are medical specialties that are increasingly resorting to the prescription of compounded medicines as a form of therapeutic customization and increased therapeutic effectiveness [56]. Manipulation allows the choice of the excipients best suited to the characteristics of the lesions and the patient’s needs, as well as the best combination of active substances in a single formulation that is most suitable individually [57]. Much of the therapeutic success is centered on the appropriateness of individualized therapeutic doses and the choice of the most suitable vehicles/bases [2].
5. Proprietary Vehicles/Bases
Therapy focused on the patient and their individual needs is a desirable approach when the goal is to obtain the best results with the highest effectiveness. A tailored therapy through compounded medicines requires skills from the prescribing physician and also from the formulating pharmacist to solve scenarios that can sometimes be complex. Furthermore, the product choice and the concern over sensory aspects become fundamental for user adherence [50].
It is expected that topical compounding medicines present pleasant sensorial characteristics, easy application and spreadability, and physicochemical and microbiological stability, among others defined by the intended purpose. It is the pharmacist’s responsibility to ensure the quality and safety of the compounded product delivered [2]. Since the development and formulation of suitable pharmaceutical forms can be a very complex task, the use of proprietary bases/vehicles can be of great importance.
Proprietary bases and vehicles are semi-finished products that are ready to be used in the preparation of compounding products. They present advantages such as the following: they are optimized in terms of stability and organoleptic characteristics so the safety and quality are assured because they are often prepared in industrialized units; they simplify medical prescriptions; they are easy to prepare without requiring in-depth knowledge of the formulation and reduce the manufacturing time and need for specialized equipment; they guarantee product uniformity between different batches and manufacturers; and there may be economic advantages by reducing the number of raw materials to be purchased. Proprietary bases/vehicles have been extensively studied regarding their microbiological, chemical, and physical stability and their compatibility/incompatibility with several APIs. Overall, a major reason to use semi-finished products as proprietary bases/vehicles is to obtain a higher-quality final product with a more reliable safety and stability profile when compared to non-standardized preparations. Proprietary vehicles/bases can be intended for local action, which means that the APIs only minimally penetrate the stratum corneum or can also be appropriate for transdermal action, including permeation enhancers to potentiate the delivery of adequate drug quantities into the systemic circulation or deeper subcutaneous tissues [6]. Moreover, it is always necessary to verify possible incompatibilities between the active substances and the excipients that constitute the vehicles/bases, as well as to follow good manufacturing practices for compounding medicines.
This study aims to comprehensively analyze the properties and applications of existing proprietary bases/vehicles and assess their coverage of current cosmetic formulation and compounding-medicine needs.
6. Search Method
The websites and catalogues of known suppliers of proprietary compounding formulations distributed in the EU and USA were searched to compile the bases/vehicles commercially available for topical application. The commercial designation and composition of each base/vehicle (when available) are described in Table S1 (Supplementary Material). The frequency of bases/vehicles corresponding to the properties, namely application area, physical form, typical application, type of dosage form, and claims made in each proprietary base/vehicle analyzed, were calculated. The data collection was performed until 20 October 2023.
7. Results
The main characteristics and composition, among other information regarding proprietary bases and vehicles currently commercially available, was thoroughly analyzed. Nine suppliers and 138 proprietary topical bases/vehicles were identified (Table S1) [58,59,60,61,62,63,64,65,66].
Semisolid products (84.1%) were more common than liquid formulations (15.9%). Cream was the most popular topical base, whilst foam was the most used vehicle. Among creams and lotions, O/W systems were by far the preferred ones (Figure 1).
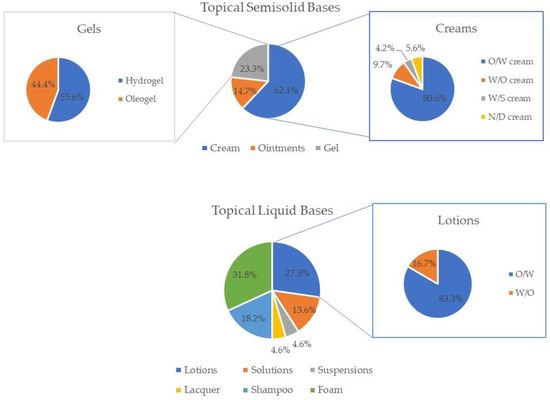
Figure 1.
Frequency of each type of dosage form of the topical semisolid bases (creams, ointments, and gels) and topical vehicles (lotions, solutions, suspensions, lacquer, shampoos, and foams) analyzed.
Several anhydrous formulations are also available (12.3%), probably to address the instability in water of some APIs (Table 1). Most topical products (68.8%) do not specify the application area. Some free-from claims, such as “free from parabens” (40.6%), are mentioned. Few nanodelivery systems (5.8%), such as liposomes, are used in the commercial formulations analyzed. The majority of the vehicles/bases mention either their compatibilities or incompatibilities with some APIs (58.0%). The use of natural excipients is pursued by some suppliers (21.7%).

Table 1.
Categorization of proprietary topical vehicles/bases according to information indicated by the manufacturer (body area and therapeutic application, physical form and type of dosage form, reported claims, compatibilities/incompatibilities, and composition).
8. Discussion
Proprietary bases/vehicles offer a convenient solution for personalized dermatological treatment and skincare. The choice of the vehicle or base must consider the type of skin, the pathological condition, and the application area [2,67]. It is also known that vehicles/bases play an important role in the effectiveness of topical therapy, not only because they influence drug stability and permeation, but they also influence patient satisfaction with treatment. Thus, a product that meets every patient’s preference is unrealistic. The wide array of textures, forms, and sensory attributes presented by the different commercial proprietary bases/vehicles analyzed is highly useful to allow selecting a product best suited to the disease, affected area, and patients’ preferences. The improvement in patient satisfaction with treatment is also a key factor to promote adherence to medication, which is known to be particularly low for topical treatments [18]. According to the analyzed products, 84.1% are bases, whereas 15.9% are vehicles.
8.1. Topical Liquids: Solutions, Micellar Solutions, Suspensions, Shampoos, Sprays, Foams, and Lotions
Considering the topical vehicles found, the majority are foams (5.1%), followed by lotions (4.3%) and shampoos (2.9%). The greater availability of foams, to the detriment of other types of topical vehicles, may be due to the innovation in their technology, which has moved from hydroethanolic-based formulations to aqueous or emulsion-based foam [68]. Foams are a great contributor to improving patient compliance since they are generally well tolerated, minimally irritating (as they contain fewer preservatives and alcohol), and do not cause a greasy feeling on the skin [68]. Capillary foam bases or hydroalcoholic solutions are examples of suitable vehicles for this purpose that are also commercially available. Hair products are intended for contact with the hair and scalp, so they should be light in texture, not cause discomfort upon application, and provide maximum invisibility. From the shampoos analyzed, it was clear that half made claims of “sulfate-free” and “SLS-free”, respectively, while the other half presented, as the anionic detergent, sodium laureth sulfate. Sodium laureth sulphate is considered less irritating than sodium lauryl sulphate, which justifies its increasing use [69]. The majority of lotions analyzed are classified as O/W emulsions (83.3%), whereas only 16.7% corresponds to W/O lotions. O/W lotions can be especially useful in the treatment of exudative dermatoses, as they can provide a cooling effect to the skin as the aqueous phase rapidly evaporates, and when the application area is the scalp or another site with hair as they are easy to spread, mainly due to their low viscosity [6]. On the other hand, since W/O lotions deposit as a film on the skin, they are stickier and more difficult to spread than O/W lotions, constraining patient compliance. Skin folds or pilous areas, special body areas that have unique characteristics, require formulations adapted to the application site, like solutions, which could be found in 2.2% of the analyzed products, or suspensions (0.7%), when the API is not soluble in the solvent.
8.2. Topical Semisolids: Creams, Ointments, and Gels
Conventional (ointments and creams) and more recent formulations (cream gels, oily gels, W/S emulsions, foams) were found in the analyzed commercial products. In fact, cream gels and oily gels demonstrated unique rheological properties, allowing for easy spreadability and enhanced skin penetration [70]. W/S emulsions exhibited stability and versatility, accommodating both hydrophilic and lipophilic active ingredients [71]. This is not only a demonstration of the advances in formulation technology but also that manufacturers rely on formulations that have been extensively studied and have proved their quality and effectiveness, thus avoiding unnecessary duplication and costs. Conventional formulations were the most common among the bases analyzed, including creams (52.2%), ointments (12.3%), and gels (19.6%). Specifically, bases for O/W creams were the most predominant of all (Figure 1), which might be due to the fact that they are significantly less greasy, less viscous, and more spreadable than W/O creams and ointments, therefore being more appealing to some patients [6]. The commercial application of O/W emulsion technology is limited by the higher formulation cost, the need for special emulsifiers, and the generally unappealing esthetics due to an oily, heavy sensation when applied to the skin [3,72].
Formulations for dry skin contain an emollient, humectant, and moisturizing properties. Emulsified systems that contain more oil and less water while maintaining the hydration of the stratum corneum, such as W/O cream, ointments, and oleogels, and while maintaining the hydration of the stratum corneum are adequate for dry skin [2,3]. This is the case of W/O systems that were also found from many suppliers. On the other hand, formulations to be applied on oily skin or acne-prone skin are based on oil-free vehicles, with more water and less or no oil, such as hydrogel, cream gel, or solutions. Attributes such as non-greasy (20.3%) and suitability for oily skin (13.0%) were claimed for several products. Normal or combination skins should use moderate-fat-content formulas so they do not increase oiliness in the most prone areas, namely O/W creams. Emulsified O/W systems comply with this requirement and were widely represented in the proprietary products analyzed. Sensitive skin is especially prone to irritation and sensitization. Formulations which are hypoallergenic (13.0%), fragrance-free (15.2%), non-irritating (8.7%) or deemed suitable for sensitive skin (12.3%) were also reasonably represented in the pool of topical products analyzed.
8.2.1. Nanosystems
Nanosystems (5.8%) are seldom used and include liposomes, niosomes, and phytosomes. These innovative drug delivery systems may have improved drug stability and skin penetration [73]. However, since these formulations can be expensive, scale-up process can be troublesome, and the regulatory framework is more demanding [74].
8.2.2. Therapeutic Application
The different proprietary bases analyzed can also be distinguished by their therapeutic application. Of the bases/vehicles analyzed for topical application, 39.9% were conceived specifically for transdermal application, namely for pain treatment (17.4%), immunotherapy (2.9%), and hormone replacement therapy (19.6%). Even though several bases revealed more than one typical application, the majority were revealed to be adequate for dermatology applications (84.1%), namely with the incorporation of corticosteroids, antifungals, and antibacterial active substances. Many of the scrutinized bases/vehicles claim to facilitate the transdermal administration of diverse APIs, including analgesics for pain management and hormones like testosterone and estriol for hormone replacement therapy. In fact, several bases can be used for both local and transdermal applications, depending on the APIs that are incorporated. Bases for transdermal use have been studied regarding API systemic distribution and rate of absorption. A wide range of specialized excipients improve the performance and customization of formulations. Some examples are the use of poloxamer 407/pluronic F127 for the enhancement of transdermal delivery, non-ionic glucoside-based emulsifiers (e.g., cetearyl glucoside) for sensitive skin, and silicones to modulate texture and elaborate oil-free products.
8.2.3. Claims
Some free-from claims, such as “free from parabens” (40.6%) and “free from colorings” (13.8%), are relevant in addressing formulations for patients with allergies and hypersensitivity to topical excipients. The claim “free from preservatives” (4,4%) is mainly associated with anhydrous proprietary bases/vehicles. Other free-from claims are probably more related to sustainability issues such as the absence of petrolatum. It is crucial to characterize the API compatibilities/incompatibilities of each proprietary base. More than a third of the vehicles/bases (42.0%) did not mention incompatibilities with common dermatological drugs, which constrains their application and raises stability issues. Anhydrous formulations were also found (12.3%). In some cases, it was possible to avoid the use of preservatives. These products are also helpful to improve the stability of APIs. Good sensory attributes were claimed for several topical products (24.6%), whilst others emphasized performance attributes like moisturizing properties (27.5%). These properties are relevant from a patient perspective, especially for long-term treatments.
Sustainability plays a central role in today’s societal development. The pharmaceutical industry is also addressing the goals of sustainable development, having adopted sustainable management practices. Some of the pharmaceutical excipients are classified as persistent and can accumulate in the environment. For a more sustainable approach that preserves resource availability and decreases the impact on the environment, it is important to decrease the use of excipients like oils, whose refinement generates significant waste, water, and petroleum derivatives that are not biodegradable and use minimalistic compositions. These concerns are depicted in some of the formulations which are free from petrolatum or surfactants, or even water-free. Saving manufacturing time, energy, and resources can also be achieved by using these ready-to-use products.
A wide array of products and applications were covered by the commercial formulations. Even so, some unmet needs could be identified like vehicles/bases for otological application. Sticks or related pre-prepared bases, which can be useful for application in small areas and are practical to carry, were also missing. The possibility of using a base/vehicle to obtain sprays was not mentioned in the characteristics provided by the manufacturers, although several liquid formulations can be dispensed as sprays. Few products reported the application area, which is useful information for base/vehicle selection for the intended application. In our examination of proprietary dermatological and cosmetic bases/vehicles, it was observed that nearly half of the suppliers either did not disclose the complete composition or did not provide any information. Constituents like ceramides and cholesterol, major components of the stratum corneum, would be anticipated in proprietary bases/vehicles for application on compromised, dehydrated, or dry skin [75]. However, our analysis revealed the absence of these ingredients in the bases/vehicles considered in this study. It is essential to acknowledge, however, that their absence in our findings does not definitively imply their non-existence, given that certain compositions remain undisclosed.
Only 2.2% reported high usage flexibility (face, body, and hair), which can be an interesting approach to reduce costs for compounding pharmacies. The information provided by suppliers, like composition or incompatibility with common dermatological APIs, is often incomplete, which constrains the decision making when selecting the formulation. Furthermore, it is essential to acknowledge the absence of a comprehensive database encompassing all suppliers and their respective proprietary topical bases/vehicles, implying that our study might not include every available option. Additionally, the variability in the information provided for each base/vehicle poses a limitation to our research analysis.
9. Conclusions
The application of topical formulations is a resource widely used for the maintenance of skin health and treatment of numerous skin disorders. Proprietary topical bases/vehicles play an increasingly important role in the formulation of products for topical application, with some aiming to treat superficial skin disorders or maintain/restore skin integrity, and others aiming to reach the systemic circulation that are useful in pain management or hormone replacement therapy. The constant development of new excipients and formulation technology has provided advancements in products with light and pleasant textures, better performance, and stability, enhancing patient satisfaction with treatments.
The availability of proprietary bases/vehicles offers the potential to decrease the manufacturing expenses associated with compounded medications, as a singular base can encompass various APIs catering to diverse therapeutic applications. The proprietary bases/vehicles analyzed exhibit a broad range of dosage forms, textures, and sensory attributes that cover a wide array of applications, namely dermatological and cosmetic, from conventional to more modern dosage forms. The majority of the products included in this study belong to the category of semisolids. Among these, creams emerge as the foremost topical base, followed by gels and ointments. Besides acting locally on the skin, almost a third of these proprietary bases/vehicles have also been studied in terms of the transdermal application of APIs.
Although some information is missing (e.g., composition and compatibilities/incompatibilities with APIs) for a complete assessment of each proprietary topical base/vehicle, the compilation of this information is not described elsewhere and is an unvaluable tool for pharmacists and prescribing physicians.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics11010016/s1, Table S1: List of the analyzed proprietary bases/vehicles organized by supplier, and respective composition.
Author Contributions
Conceptualization, I.F.A. and R.O.; methodology, A.T.; data curation, A.T.; R.O. writing—original draft preparation, A.T.; writing—review and editing, I.F.A. and R.O.; supervision, I.F.A. and R.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the projects UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences—UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB. Ana Torres acknowledges her Ph.D. grant (2023.03685.BD).
Acknowledgments
The authors are thankful to Ana Catarina Guedes for her assistance in data collection.
Conflicts of Interest
A. Torres and R. Oliveira declare no conflicts of interest. I. F. Almeida has coordinated contract services (stability studies) with PCCA at the Faculty of Pharmacy.
References
- Council of Europe. European Pharmacopoeia, 11th ed.; Directorate for the Quality of Medicines and HealthCare of the Council of Europe (EDQM): Strasbourg, France, 2022. [Google Scholar]
- Oliveira, R.; Almeida, I.F. Patient-Centric Design of Topical Dermatological Medicines. Pharmaceuticals 2023, 16, 617. [Google Scholar] [CrossRef] [PubMed]
- Maxon, B.; Starch, M. Formulating Skin Care Products with Silicones: Approaches and Strategies. In Handbook of Formulating Dermal Applications; Wiley: Weinheim, Germany, 2016; pp. 59–114. [Google Scholar]
- Aulton, M.E.; Taylor, K. (Eds.) Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2021; p. 968. [Google Scholar]
- Mayba, J.N.; Gooderham, M.J. A Guide to Topical Vehicle Formulations. J. Cutan. Med. Surg. 2018, 22, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.M.; Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. Vehicles for Drug Delivery and Cosmetic Moisturizers: Review and Comparison. Pharmaceutics 2021, 13, 2012. [Google Scholar] [CrossRef] [PubMed]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- Lein, A.; Ng, S.W. Dermal. In Practical Pharmaceutics: An International Guideline for the Preparation, Care and Use of Medicinal Products, 2nd ed.; Le Brun, P., Crauste-Manciet, S., Krämer, I., Smith, J., Woerdenbag, H., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 439–471. [Google Scholar]
- Swarbrick, J. Encyclopedia of Pharmaceutical Technology, 3th ed.; Swarbrick, J., Ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Allen, L.V. The Art, Science, and Technology of Pharmaceutical Compounding, 5th ed.; American Pharmacists Association: Washington, DC, USA, 2016. [Google Scholar]
- Umar, A.K.; Butarbutar, M.; Sriwidodo, S.; Wathoni, N. Film-Forming Sprays for Topical Drug Delivery. Drug Des. Devel Ther. 2020, 14, 2909–2925. [Google Scholar] [CrossRef]
- Schramm, L.L. Personal Care Product Applications. In Emulsions, Foams, and Suspensions; Schramm, L.L., Ed.; Wiley: Weinheim, Germany, 2005; pp. 337–346. [Google Scholar]
- Schramm, L.L. Dispersion and Dispersed Species Characterization. In Emulsions, Foams, and Suspensions; Schramm, L.L., Ed.; Wiley: Weinheim, Germany, 2005; pp. 13–51. [Google Scholar]
- Elewski, B.E.; Abramovits, W.; Kempers, S.; Schlessinger, J.; Rosen, T.; Gupta, A.K.; Abraham, S.; Rowell, R. A novel foam formulation of ketoconazole 2% for the treatment of seborrheic dermatitis on multiple body regions. J. Drugs Dermatol. 2007, 6, 1001–1008. [Google Scholar]
- Leonardi, C.; Bagel, J.; Yamauchi, P.; Pariser, D.; Xu, Z.; Olesen, M.; Østerdal, M.L.; Stein Gold, L. Efficacy and Safety of Calcipotriene Plus Betamethasone Dipropionate Aerosol Foam in Patients with Psoriasis Vulgaris—A Randomized Phase III Study (PSO-FAST). J. Drugs Dermatol. 2015, 14, 1468–1477. [Google Scholar]
- Fowler, J.; Fowler, L. Physician and Patient Assessment of Triamcinolone Acetonide Spray for Steroid-responsive Dermatoses. J. Clin. Aesthet. Dermatol. 2010, 3, 27–31. [Google Scholar]
- Schlager, J.G.; Rosumeck, S.; Werner, R.N.; Jacobs, A.; Schmitt, J.; Schlager, C.; Nast, A. Topical treatments for scalp psoriasis. Cochrane Database Syst. Rev. 2016, 2, Cd009687. [Google Scholar] [CrossRef]
- Teixeira, A.; Teixeira, M.; Almeida, V.; Gaio, R.; Torres, T.; Magina, S.; Cunha, C.; Sousa Lobo, J.M.; Almeida, I.F. Does the Vehicle Matter? Real-World Evidence on Adherence to Topical Treatment in Psoriasis. Pharmaceutics 2021, 13, 1539. [Google Scholar] [CrossRef]
- Kvenshagen, B.K.; Carlsen, K.H.; Mowinckel, P.; Berents, T.L.; Carlsen, K.C. Can early skin care normalise dry skin and possibly prevent atopic eczema? A pilot study in young infants. Allergol. Immunopathol. 2014, 42, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Emtestam, L.; Svensson, Å.; Rensfeldt, K. Treatment of seborrhoeic dermatitis of the scalp with a topical solution of urea, lactic acid, and propylene glycol (K301): Results of two double-blind, randomised, placebo-controlled studies. Mycoses 2012, 55, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, J.P.; Nikkels, A.F.; Reich, K.; Ponce Olivera, R.M.; Lee, J.H.; Kerrouche, N.; Sidou, F.; Faergemann, J. Efficacious and safe management of moderate to severe scalp seborrhoeic dermatitis using clobetasol propionate shampoo 0.05% combined with ketoconazole shampoo 2%: A randomized, controlled study. Br. J. Dermatol. 2011, 165, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Schramm, L.L. Interfacial Energetics. In Emulsions, Foams, and Suspensions; Schramm, L.L., Ed.; Wiley: Weinheim, Germany, 2005; pp. 53–100. [Google Scholar]
- Lukic, M.; Pantelic, I.; Savic, S. An Overview of Novel Surfactants for Formulation of Cosmetics with Certain Emphasis on Acidic Active Substances. Tenside Surfactants Deterg. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Ajazuddin; Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Danby, S.G.; Draelos, Z.D.; Gold, L.F.S.; Cha, A.; Vlahos, B.; Aikman, L.; Sanders, P.; Wu-Linhares, D.; Cork, M.J. Vehicles for atopic dermatitis therapies: More than just a placebo. J. Dermatol. Treat. 2022, 33, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A.A.; Yenilmez, E. Pharmacological and Pharmaceutical Technological Overview for Seborrheic Dermatitis: A Review About Topical Application and New Approaches. Acta Pharm. Sci. 2018, 56, 57–80. [Google Scholar] [CrossRef]
- Hoffman, L.K.; Bhatia, N.; Zeichner, J.; Kircik, L.H. Topical Vehicle Formulations in the Treatment of Acne. J. Drugs Dermatol. 2018, 17, S6–S10. [Google Scholar]
- Jackson, J.M.; Pelle, M. Topical rosacea therapy: The importance of vehicles for efficacy, tolerability and compliance. J. Drugs Dermatol. 2011, 10, 627–633. [Google Scholar]
- Mason, A.R.; Mason, J.; Cork, M.; Dooley, G.; Hancock, H. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst. Rev. 2013, 3, Cd005028. [Google Scholar] [CrossRef]
- Rajkapoor, B.; Sughir, A.; Damodar, G. Oleogel: A promising base for transdermal formulations. Asian J. Pharm. 2012, 6, 1–9. [Google Scholar] [CrossRef]
- Almeida, I.F.; Gaio, A.R.; Bahia, M.F. Hedonic and Descriptive Skinfeel Analysis of Two Oleogels: Comparison with Other Topical Formulations. J. Sens. Stud. 2008, 23, 92–113. [Google Scholar] [CrossRef]
- Kuzina, M.A.; Kartsev, D.D.; Stratonovich, A.V.; Levkin, P.A. Organogels versus Hydrogels: Advantages, Challenges, and Applications. Adv. Funct. Mater. 2023, 33, 2301421. [Google Scholar] [CrossRef]
- Jun Yang, S.; Yoon, K.S. Preparation and Evaluation of Pluronic Lecithin Organogels in Cosmetics. J. Cosmet. Sci. 2021, 72, 325–346. [Google Scholar]
- Barbosa, A.I.; Torres, T.; Lima, S.A.C.; Reis, S. Hydrogels: A Promising Vehicle for the Topical Management of Atopic Dermatitis. Adv. Ther. 2021, 4, 2100028. [Google Scholar] [CrossRef]
- Schlesinger, T.; Rowland Powell, C. Efficacy and safety of a low molecular weight hyaluronic Acid topical gel in the treatment of facial seborrheic dermatitis final report. J. Clin. Aesthet. Dermatol. 2014, 7, 15–18. [Google Scholar]
- Van Zuuren, E.J.; Fedorowicz, Z.; Christensen, R.; Lavrijsen, A.; Arents, B.W.M. Emollients and moisturisers for eczema. Cochrane Database Syst. Rev. 2017, 2, Cd012119. [Google Scholar] [CrossRef]
- Antunes, A.F.; Pereira, P.; Reis, C.; Rijo, P.; Reis, C. Nanosystems for Skin Delivery: From Drugs to Cosmetics. Curr. Drug Metab. 2017, 18, 412–425. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Mohd-Nasir, H.; Ahmad, A.; Mohd, S.H.; Peng, W.L.; Chuo, S.C.; Khatoon, A.; Umar, K.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Role of Nanotechnology for Design and Development of Cosmeceutical: Application in Makeup and Skin Care. Front. Chem. 2019, 7, 739. [Google Scholar] [CrossRef]
- Makhmalzade, B.S.; Chavoshy, F. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J. Adv. Pharm. Technol. Res. 2018, 9, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Jarak, I.; Santos, A.; Veiga, F.; Figueiras, A. Polymeric Micelles: A Promising Pathway for Dermal Drug Delivery. Materials 2021, 14, 7278. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Alam, K.; Beg, S.; Anwar, F.; Kumar, V. Chapter 6—Liposomes as topical drug delivery systems: State of the arts. In Biomedical Applications of Nanoparticles; Rahman, M.; Alam, K.; Beg, S.; Anwar, F.; Kumar, V. William Andrew Publishing: Amsterdam, The Netherlands, 2019; pp. 149–161. [Google Scholar]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.J.; Tharmavaram, M.; Rawtani, D.; Prajapati, P.; Pandya, H.; Dey, A. Niosomes as cutting edge nanocarrier for controlled and targeted delivery of essential oils and biomolecules. J. Drug Deliv. Sci. Technol. 2022, 73, 103438. [Google Scholar] [CrossRef]
- Nastiti, C.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Osorio-Blanco, E.R.; Sonzogni, A.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for Skin Applications: Where Do We Stand? Angew. Chem. Int. Ed. 2022, 61, e202107960. [Google Scholar] [CrossRef]
- Hofmann-Amtenbrink, M.; Hofmann, H.; Hool, A.; Roubert, F. Nanotechnology in medicine: European research and its implications. Swiss Med. Wkly. 2014, 144, w14044. [Google Scholar] [CrossRef]
- Shrewsbury, R.P. Applied Pharmaceutics in Contemporary Compounding; Morton Publishing Company: Englewood, CO, USA, 2015. [Google Scholar]
- Elder, D.L. A Practical Guide to Contemporary Pharmacy Practice and Compounding; Wolters Kluwer: Philadelphia, PA, USA, 2017. [Google Scholar]
- Carvalho, M.; Almeida, I.F. The Role of Pharmaceutical Compounding in Promoting Medication Adherence. Pharmaceuticals 2022, 15, 1091. [Google Scholar] [CrossRef]
- Dooms, M.; Carvalho, M. Compounded medication for patients with rare diseases. Orphanet J. Rare Dis. 2018, 13, 1. [Google Scholar] [CrossRef]
- Nagel-Edwards, K.M.; Ko, J.Y. Excipient choices for special populations. Int. J. Pharm. Compd. 2008, 12, 426–430. [Google Scholar]
- Allen, L.V., Jr. Compounding for Investigational and Clinical Studies. Int. J. Pharm. Compd. 2022, 26, 201–209. [Google Scholar] [PubMed]
- Watson, C.J.; Whitledge, J.D.; Siani, A.M.; Burns, M.M. Pharmaceutical Compounding: A History, Regulatory Overview, and Systematic Review of Compounding Errors. J. Med. Toxicol. 2021, 17, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Sacks, G.S. Compounding parenteral nutrition: Reducing the risks. Nutr. Clin. Pract. 2009, 24, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Regaña, M.; Llambí-Mateos, F.; Salleras-Redonnet, M.; Iglesias Sancho, M.; Collgros Totosaus, H.; Umbert-Millet, P. Compounding as a Current Therapeutic Option in Dermatology. Actas Dermo-Sifiliográficas 2013, 104, 738–756. [Google Scholar] [CrossRef] [PubMed]
- Abarca Lachén, E.; Hernando Martínez, P.; Gilaberte Calzada, Y. The Most Useful Pharmaceutical Formulations (Individualized Medications) in Pediatric Dermatology: A Review. Actas Dermo-Sifiliográficas 2021, 112, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Acofarma. Acofarma Formulas Magistrales. Available online: https://formulasmagistrales.acofarma.com (accessed on 20 October 2023).
- Fragron. Fagron Advanced Derma. Available online: https://d2p6xt2uil81ec.cloudfront.net/fagron/uploads/2021/11/25100332/FAD-productflyer.pdf (accessed on 20 October 2023).
- Guinama. Bases. Available online: https://www.guinama.com/bases-jarabes-y-emulsionantes/bases.html (accessed on 20 October 2023).
- Medisca. Cream Base Reference Chart. Available online: https://www.medisca.com/Files/ReferenceCharts/Cream%20&%20Gel%20Bases%20Reference%20Chart%20-%20MUS.pdf (accessed on 20 October 2023).
- PCCA. PCCA’s Dermatology Bases. Available online: https://www.pccarx.com/BuyProducts/CompoundingBaseTechnology/DermatologyBases (accessed on 20 October 2023).
- RX, S. Technical Data Sheets. Available online: https://specializedrx.com/pages/tech-sheets (accessed on 20 October 2023).
- Medical, L. Creams & Bases. Available online: https://www.letcomedical.com/creams-bases (accessed on 20 October 2023).
- Issuu. Letco Catalog 2021. Available online: https://issuu.com/letcomed/docs/let_2021_catalog (accessed on 20 October 2023).
- Compounding Chemicals.com.au. Spectrum Chemical MFG Corp. Available online: https://compoundingchemicals.com.au/pages/bases (accessed on 20 October 2023).
- Iversen, L.; Jakobsen, H.B. Patient Preferences for Topical Psoriasis Treatments are Diverse and Difficult to Predict. Dermatol. Ther. 2016, 6, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Hoc, D.; Haznar-Garbacz, D. Foams as unique drug delivery systems. Eur. J. Pharm. Biopharm. 2021, 167, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, V.; Morrison, B.M.; Paye, M.; Maibach, H.I. Subclinical, non-erythematous irritation with an open assay model (washing): Sodium lauryl sulfate (SLS) versus sodium laureth sulfate (SLES). Food Chem. Toxicol. 2001, 39, 279–286. [Google Scholar] [CrossRef]
- Liao, Y.; Sun, Y.; Wang, Z.; Zhong, M.; Li, R.; Yan, S.; Qi, B.; Li, Y. Structure, rheology, and functionality of emulsion-filled gels: Effect of various oil body concentrations and interfacial compositions. Food Chem. X 2022, 16, 100509. [Google Scholar] [CrossRef]
- Baki, G.; Alexander, K.S. Introduction to Cosmetic Formulation and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Epstein, H. Pre-formulation Design and Considerations. In Handbook of Formulating Dermal Applications; Wiley: Weinheim, Germany, 2016; pp. 1–27. [Google Scholar]
- Choi, M.J.; Maibach, H.I. Liposomes and niosomes as topical drug delivery systems. Skin Pharmacol. Physiol. 2005, 18, 209–219. [Google Scholar] [CrossRef]
- Okoro, U.; John, D.N.O.; Anthony, A.A. Nanoparticles for Dermal and Transdermal Drug Delivery. In Application of Nanotechnology in Drug Delivery; Ali Demir, S., Ed.; IntechOpen: Rijeka, Croatia, 2014; p. 6. [Google Scholar]
- Torres, A.; Rego, L.; Martins, M.S.; Ferreira, M.S.; Cruz, M.T.; Sousa, E.; Almeida, I.F. How to Promote Skin Repair? In-Depth Look at Pharmaceutical and Cosmetic Strategies. Pharmaceuticals 2023, 16, 573. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).