Ash Characteristics and Selected Critical Elements (Ga, Sc, V) in Coal and Ash in Polish Deposits
Abstract
1. Introduction
2. Materials and Methods
- (0.00–0.20)—Weak correlation, the relationship is almost insignificant;
- (<0.20–0.40)—Low correlation, the relationship is clearly visible;
- (<0.40–0.70)—Strong correlation, the relationship is clearly visible and small;
- (<0.70–0.90)—Strong correlation, significant relationship;
- (<0.90–1.00)—Strong correlation and relationship.
3. Results and Discussion
3.1. Proximate and Ultimate Analysis
3.2. Petrographic Analysis of the Examined Coal
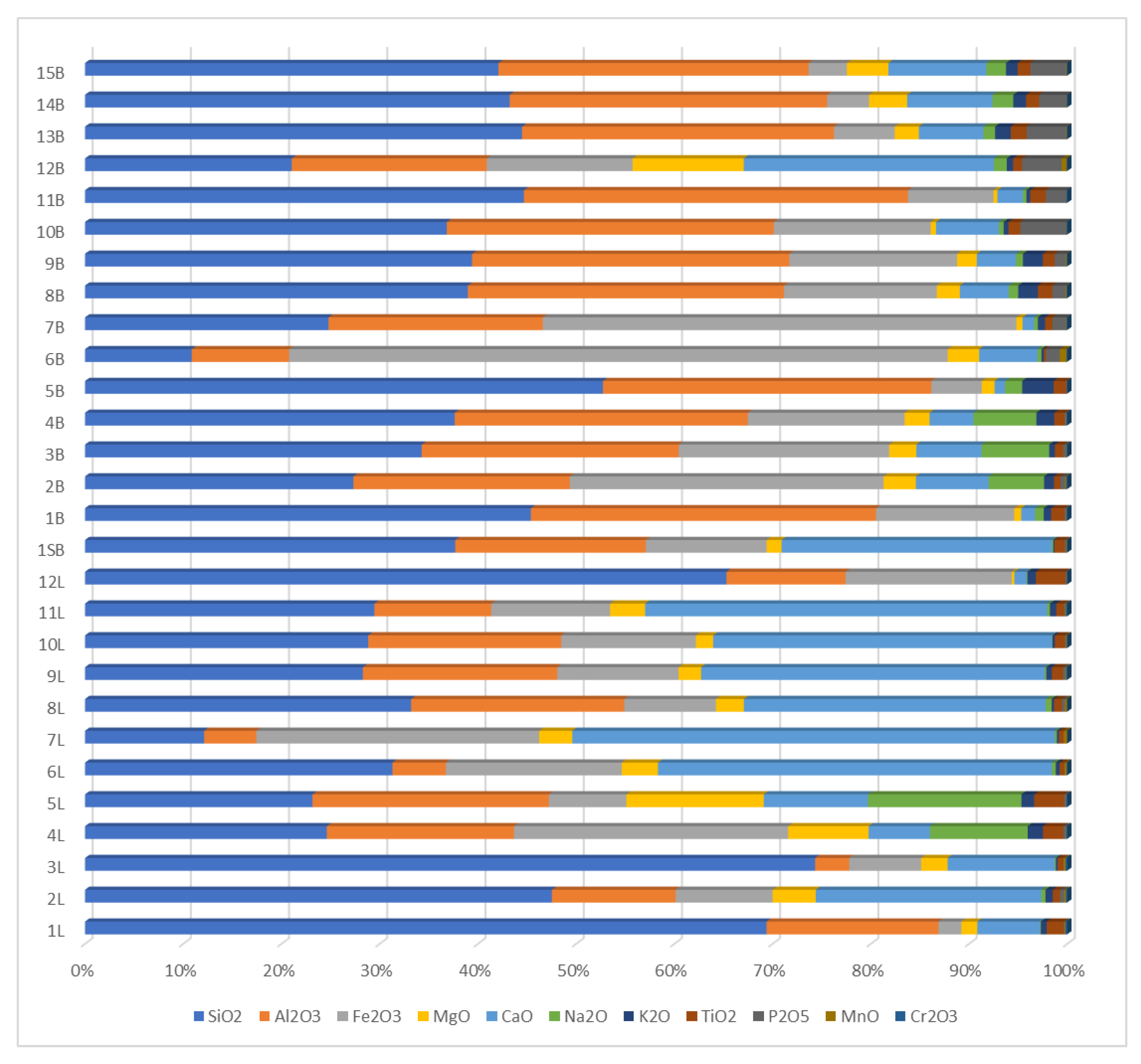
3.3. Oxide Composition of Ash and Its Properties
- -
- Carbonates decompose with the release of carbon dioxide: CaCO3 → CaO + CO2;
- -
- Silicates and aluminosilicates lose crystallization water;
- -
- Pyrite and marcasite oxidize to Fe2O3 and SO2: 4FeS2 + 11O2 → 2Fe2O3 + 8SO2;
- -
- Calcium carbonate, when broken down into calcium oxide, can bind SO2 resulting from the oxidation of pyrites and organic sulfur to calcium sulfate (IV): CaO + SO2 → CaSO3, which then further oxidizes to calcium (VI) sulfate: 2CaSO3 + O2 → 2CaSO4;
- -
- Alkali metal chlorides sublime (volatilize, e.g., NaCl);
- -
- Oxidation of organometallic compounds.
- (1)
- alkalinity/acidity (base/acid (B/A) ratio)where SiO2, Al2O3,TiO3, Fe2O3, CaO, MgO, Na2O, and K2O represent the percentages of individual oxides in the ashes.B/A = %(Fe2O3 + CaO + MgO + Na2O + K2O)/[%(SiO2 + Al2O3 + TiO2)]
- (2)
- Slagging (slagging index) (Rs)where Std is the total sulfur content in coal, dry basis.Rs = B/A×StdClassification of slagging potential using Slagging Index Rs
- <0.6 low
- ≤0.6–2.0 medium
- 2.0–2.6 high
- >2.6 severe
- (3)
- SiO2 ratio (silica value, SV)Depending on the silica value SV, the coal will showSV = (SiO2 × 100)/(SiO2 + Fe2O3 + CaO + MgO)
- ≥72 low tendency to slagging
- 65–72 medium tendency to slagging
- ≤65 high tendency to slagging
- (4)
- The tendency to heating surfaces slagging (Fouling Index Rf)Classification of fouling potential using Fouling Index RfRf = B/A×(Na2O + K2O)
- <0.6 low
- 0.6–40 high
- >40.0 severe
- (5)
- Alkalinity AKwhere Ad is the percentage of ash content in coal (dry basis).AK = Na2O + 0.96559K2O × (Ad/100)Alkali indexes AK:
- <0.3 low tendency to pollute the alkaline components
- 0.3–0.45 medium tendency to pollute the alkaline components
- 0.45–0.6 high tendency to pollute the alkaline components
- >0.6 severe tendency to pollute the alkaline components
3.4. Critical Elements in Coal and Ash
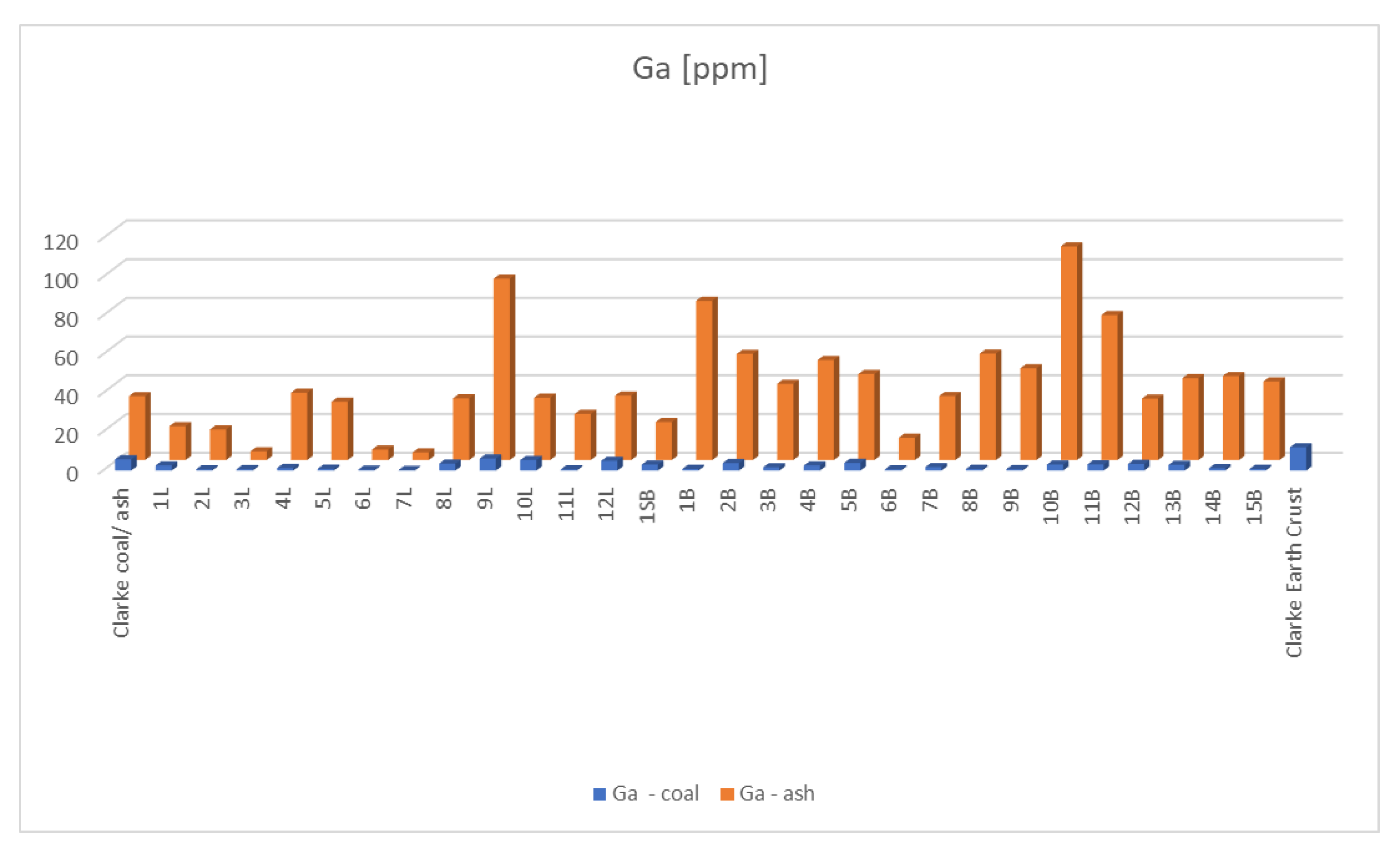
3.4.1. Ga—Gallium
- -
- As an admixture in the production of semiconductors and transistors;
- -
- In the production of mirrors (to moisten the glass);
- -
- In the production of low-melting alloys;
- -
- To improve the properties of solders;
- -
- For the production of high temperature thermometers;
- -
- As a catalyst in the production of hydrogen from water by aluminum oxidation;
- -
3.4.2. V—Vanadium
3.4.3. Sc—Scandium
4. Conclusions
Funding
Conflicts of Interest
Appendix A
| SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | MgO (%) | CaO (%) | Na2O (%) | ||
| Mad (%) | −0.3 | −0.7 | −0.3 | 0.1 | 0.7 | 0.1 | |
| Adb (%) | 0.6 | −0.1 | −0.3 | −0.3 | −0.1 | −0.3 | |
| V daf (%) | −0.2 | −0.8 | −0.2 | 0.1 | 0.7 | 0 | |
| Stdb (%) | 0 | −0.3 | 0.5 | −0.4 | 0 | −0.2 | |
| GCV (MJ/kg) | 0.1 | 0.8 | 0.3 | 0 | −0.7 | 0.1 | |
| Ash Sintering Temperature (°C) | −0.1 | −0.2 | 0.1 | 0.1 | 0 | 0.3 | |
| Ash Softening Temperature (°C) | 0.5 | 0.4 | 0 | −0.5 | −0.5 | −0.3 | |
| Ash Melting Temperature (°C) | 0.5 | 0.4 | 0.2 | −0.4 | −0.6 | −0.3 | |
| Ash Fluid Temperature (°C) | 0.5 | 0.4 | 0.2 | −0.4 | −0.7 | −0.2 | |
| C daf (%) | 0.1 | 0.8 | 0.3 | −0.1 | −0.7 | 0.1 | |
| H daf (%) | −0.3 | −0.2 | 0.1 | 0.3 | 0 | 0.4 | |
| N daf (%) | 0 | 0.6 | 0.5 | −0.2 | −0.6 | −0.1 | |
| O daf (%) | −0.1 | −0.8 | −0.4 | 0.1 | 0.7 | −0.1 | |
| Total porosity (%) | 0.1 | −0.7 | −0.3 | 0.1 | 0.5 | 0 | |
| Huminite/Vitrinite (%) | −0.3 | −0.7 | −0.1 | 0.2 | 0.5 | 0 | |
| Inertinite (%) | 0.3 | 0.7 | −0.1 | −0.2 | −0.5 | 0 | |
| Liptinite (%) | 0.1 | 0.6 | 0.2 | −0.2 | −0.5 | 0 | |
| Mineral Matter (%) | 0.2 | −0.4 | 0 | −0.2 | 0.1 | −0.3 | |
| Random Reflectance (Ro) (%) | 0.1 | 0.8 | 0.2 | −0.1 | −0.6 | 0 | |
| Aromaticity Factor (fa) | 0.3 | 0.7 | 0.2 | −0.2 | −0.6 | −0.1 | |
| SiO2 (%) | 1 | 0.3 | −0.4 | −0.4 | −0.6 | −0.2 | |
| Al2O3 | 0.3 | 1 | −0.1 | −0.2 | −0.7 | 0.1 | |
| Fe2O3 (%) | −0.4 | −0.1 | 1 | −0.1 | −0.3 | 0 | |
| MgO (%) | −0.4 | −0.2 | −0.1 | 1 | 0.1 | 0.7 | |
| CaO (%) | −0.6 | −0.7 | −0.3 | 0.1 | 1 | −0.3 | |
| Na2O (%) | −0.2 | 0.1 | 0 | 0.7 | −0.3 | 1 | |
| K2O (%) | 0.3 | 0.6 | −0.1 | 0.1 | −0.6 | 0.3 | |
| TiO2 (%) | 0.5 | 0.4 | −0.3 | 0.2 | −0.6 | 0.3 | |
| P2O5 (%) | 0 | 0.5 | 0 | 0.1 | −0.2 | −0.1 | |
| MnO (%) | −0.4 | −0.4 | 0.5 | 0.2 | 0.2 | −0.2 | |
| Cr2O3 (%) | 0.5 | −0.2 | −0.1 | 0 | −0.2 | 0 | |
| LOI and Others (%) | −0.7 | −0.8 | −0.2 | 0.3 | 0.9 | 0 | |
| C in ash (%) | −0.5 | −0.4 | −0.1 | 0.2 | 0.6 | −0.1 | |
| S in ash (%) | −0.5 | −0.7 | −0.2 | 0.2 | 0.9 | −0.1 | |
| Base/Acid (B/A) ratio | −0.7 | −0.6 | 0.5 | 0.1 | 0.5 | −0.1 | |
| Slagging Index (Rs) | −0.5 | −0.2 | 0.5 | 0.3 | 0.1 | −0.2 | |
| Silica Value (SV) | 0.9 | 0.6 | −0.5 | −0.3 | −0.6 | −0.1 | |
| Fouling Index (Rf) | −0.4 | 0 | 0.2 | 0.8 | −0.2 | 0.9 | |
| Alkalinity (AK) | −0.2 | 0.1 | 0 | 0.7 | −0.3 | 1 | |
| Ga (ppm)—coal | 0.1 | 0.1 | −0.2 | −0.3 | 0.1 | −0.1 | |
| Ga (ppm)—ash | 0.1 | 0.7 | −0.1 | −0.2 | −0.3 | 0 | |
| Sc (ppm)—coal | 0.2 | 0 | −0.1 | −0.1 | −0.1 | 0 | |
| Sc (ppm)—ash | 0.1 | 0.7 | 0.1 | 0.1 | −0.6 | 0.4 | |
| V (ppm)—coal | −0.1 | 0.2 | 0.1 | −0.1 | −0.1 | 0.1 | |
| V (ppm)—ash | −0.1 | 0.4 | 0.1 | 0 | −0.3 | 0.2 | |
| K2O (%) | TiO2 (%) | P2O5 (%) | MnO (%) | Cr2O3 (%) | LOI and Others (%) | ||
| Mad (%) | −0.4 | −0.2 | −0.7 | −0.1 | 0.2 | 0.8 | |
| Adb (%) | −0.1 | 0.2 | −0.2 | −0.1 | 0.4 | −0.2 | |
| Vdaf (%) | −0.5 | −0.2 | −0.5 | 0 | 0.2 | 0.8 | |
| Stdb (%) | −0.3 | −0.1 | −0.3 | 0.1 | 0.1 | 0 | |
| GCV (MJ/kg) | 0.4 | 0.2 | 0.5 | 0 | −0.2 | −0.7 | |
| Ash Sintering Temperature (°C) | 0 | 0 | −0.4 | −0.1 | 0 | 0.1 | |
| Ash Softening Temperature (°C) | 0.2 | 0.2 | 0 | −0.1 | 0.1 | −0.5 | |
| Ash Melting Temperature (°C) | 0.2 | 0.2 | 0.1 | 0 | 0.1 | −0.6 | |
| Ash Fluid Temperature (°C) | 0.3 | 0.2 | 0.2 | 0.1 | 0.2 | −0.7 | |
| Cdaf (%) | 0.4 | 0.1 | 0.5 | 0 | −0.2 | −0.7 | |
| Hdaf (%) | 0 | 0.2 | −0.2 | −0.2 | 0 | 0.2 | |
| Ndaf (%) | 0.4 | 0.1 | 0.6 | 0.2 | −0.3 | −0.7 | |
| Odaf (%) | −0.4 | −0.2 | −0.5 | 0 | 0.2 | 0.8 | |
| Total porosity (%) | −0.4 | 0 | −0.6 | −0.1 | 0.4 | 0.5 | |
| Huminite/Vitrinite (%) | −0.4 | −0.2 | −0.5 | 0.2 | 0.3 | 0.6 | |
| Inertinite (%) | 0.4 | 0.1 | 0.4 | −0.3 | −0.2 | −0.6 | |
| Liptinite (%) | 0.3 | 0.1 | 0.5 | −0.2 | −0.3 | −0.5 | |
| Mineral Matter (%) | −0.1 | 0.1 | −0.3 | 0.1 | −0.1 | 0.1 | |
| Random Reflectance (Ro) (%) | 0.3 | 0.1 | 0.5 | 0 | −0.2 | −0.6 | |
| Aromaticity Factor (fa) | 0.5 | 0.2 | 0.5 | 0 | −0.2 | −0.8 | |
| SiO2 (%) | 0.3 | 0.5 | 0 | −0.4 | 0.5 | −0.7 | |
| Al2O3 | 0.6 | 0.4 | 0.5 | −0.4 | −0.2 | −0.8 | |
| Fe2O3 (%) | −0.1 | −0.3 | 0 | 0.5 | −0.1 | −0.2 | |
| MgO (%) | 0.1 | 0.2 | 0.1 | 0.2 | 0 | 0.3 | |
| CaO (%) | −0.6 | −0.6 | −0.2 | 0.2 | −0.2 | 0.9 | |
| Na2O (%) | 0.3 | 0.3 | −0.1 | −0.2 | 0 | 0 | |
| K2O (%) | 1 | 0.4 | 0.1 | −0.3 | −0.1 | −0.5 | |
| TiO2 (%) | 0.4 | 1 | 0.1 | −0.5 | 0 | −0.5 | |
| P2O5 (%) | 0.1 | 0.1 | 1 | 0.1 | −0.2 | −0.4 | |
| MnO (%) | −0.3 | −0.5 | 0.1 | 1 | 0 | 0.2 | |
| Cr2O3 (%) | −0.1 | 0 | −0.2 | 0 | 1 | −0.2 | |
| LOI and Others (%) | −0.5 | −0.5 | −0.4 | 0.2 | −0.2 | 1 | |
| C in ash (%) | −0.3 | −0.3 | −0.1 | 0.3 | −0.4 | 0.6 | |
| S in ash (%) | −0.5 | −0.4 | −0.5 | 0 | −0.1 | 0.9 | |
| Base/Acid (B/A) ratio | −0.4 | −0.5 | −0.2 | 0.6 | −0.3 | 0.6 | |
| Slagging Index (Rs) | −0.2 | −0.3 | 0.4 | 0.7 | −0.2 | 0.1 | |
| Silica Value (SV) | 0.5 | 0.6 | 0.2 | −0.5 | 0.3 | −0.7 | |
| Fouling Index (Rf) | 0.2 | 0.3 | −0.2 | −0.1 | 0 | 0.1 | |
| Alkalinity (AK) | 0.3 | 0.4 | −0.1 | −0.2 | 0 | −0.1 | |
| Ga (ppm)—coal | 0 | 0.2 | 0 | −0.2 | −0.2 | 0 | |
| Ga (ppm)—ash | 0.2 | 0.3 | 0.4 | −0.4 | −0.1 | −0.4 | |
| Sc (ppm)—coal | 0.1 | 0.5 | −0.1 | −0.3 | −0.3 | −0.1 | |
| Sc (ppm)—ash | 0.4 | 0.6 | 0.3 | −0.5 | −0.2 | −0.5 | |
| V (ppm)—coal | 0.2 | 0.2 | 0.1 | −0.3 | −0.1 | −0.1 | |
| V (ppm)—ash | 0.3 | 0.3 | 0.2 | −0.3 | 0 | −0.2 | |
| C in ash (%) | S in ash (%) | Base/Acid (B/A) Ratio | Slagging Index (Rs) | Silica Value (SV) | Fouling Index (Rf) | Alkalinity (AK) | |
| Mad (%) | 0.3 | 0.9 | 0.3 | −0.2 | −0.4 | 0.2 | 0.1 |
| Adb (%) | −0.3 | −0.1 | −0.3 | −0.2 | 0.5 | −0.3 | −0.3 |
| Vdaf (%) | 0.3 | 0.8 | 0.3 | −0.1 | −0.4 | 0.1 | 0 |
| Stdb (%) | −0.4 | 0.1 | 0 | 0.2 | −0.2 | −0.1 | −0.2 |
| GCV (MJ/kg) | −0.2 | −0.7 | −0.2 | 0.1 | 0.2 | 0 | 0.1 |
| Ash Sintering Temperature (°C) | 0.1 | 0.1 | 0.2 | −0.3 | −0.1 | 0.4 | 0.3 |
| Ash Softening Temperature (°C) | −0.4 | −0.5 | −0.3 | −0.1 | 0.6 | −0.4 | −0.3 |
| Ash Melting Temperature (°C) | −0.4 | −0.6 | −0.2 | 0.1 | 0.5 | −0.3 | −0.3 |
| Ash Fluid Temperature (°C) | −0.5 | −0.7 | −0.3 | 0.1 | 0.5 | −0.2 | −0.2 |
| Cdaf (%) | −0.2 | −0.8 | −0.2 | 0.1 | 0.3 | 0 | 0.1 |
| Hdaf (%) | 0.1 | 0.2 | 0.2 | −0.2 | −0.2 | 0.5 | 0.3 |
| Ndaf (%) | −0.2 | −0.7 | 0 | 0.4 | 0.1 | −0.2 | −0.1 |
| Odaf (%) | 0.3 | 0.8 | 0.2 | −0.2 | −0.3 | 0 | −0.1 |
| Total porosity (%) | 0.3 | 0.6 | 0.2 | −0.3 | −0.1 | 0.1 | 0 |
| Huminite/Vitrinite (%) | 0.3 | 0.6 | 0.4 | 0.1 | −0.4 | 0.2 | 0 |
| Inertinite (%) | −0.3 | −0.6 | −0.4 | −0.2 | 0.5 | −0.2 | 0 |
| Liptinite (%) | −0.2 | −0.6 | −0.2 | 0.1 | 0.2 | −0.1 | 0 |
| Mineral Matter (%) | 0.3 | 0 | 0.3 | −0.1 | 0 | −0.2 | −0.3 |
| Random Reflectance (Ro) (%) | −0.2 | −0.7 | −0.2 | 0.2 | 0.3 | −0.2 | 0 |
| Aromaticity Factor (fa) | −0.3 | −0.8 | −0.3 | 0.2 | 0.4 | −0.2 | −0.1 |
| SiO2 (%) | −0.5 | −0.5 | −0.7 | −0.5 | 0.9 | −0.4 | −0.2 |
| Al2O3 | −0.4 | −0.7 | −0.6 | −0.2 | 0.6 | 0 | 0.1 |
| Fe2O3 (%) | −0.1 | −0.2 | 0.5 | 0.5 | −0.5 | 0.2 | 0 |
| MgO (%) | 0.2 | 0.2 | 0.1 | 0.3 | −0.3 | 0.8 | 0.7 |
| CaO (%) | 0.6 | 0.9 | 0.5 | 0.1 | −0.6 | −0.2 | −0.3 |
| Na2O (%) | −0.1 | −0.1 | −0.1 | −0.2 | −0.1 | 0.9 | 1 |
| K2O (%) | −0.3 | −0.5 | −0.4 | −0.2 | 0.5 | 0.2 | 0.3 |
| TiO2 (%) | −0.3 | −0.4 | −0.5 | −0.3 | 0.6 | 0.3 | 0.4 |
| P2O5 (%) | −0.1 | −0.5 | −0.2 | 0.4 | 0.2 | −0.2 | −0.1 |
| MnO (%) | 0.3 | 0 | 0.6 | 0.7 | −0.5 | −0.1 | −0.2 |
| Cr2O3 (%) | −0.4 | −0.1 | −0.3 | −0.2 | 0.3 | 0 | 0 |
| LOI and Others (%) | 0.6 | 0.9 | 0.6 | 0.1 | −0.7 | 0.1 | −0.1 |
| C in ash (%) | 1 | 0.3 | 0.7 | 0.2 | −0.5 | 0 | −0.1 |
| S in ash (%) | 0.3 | 1 | 0.3 | 0 | −0.6 | 0.1 | −0.1 |
| Base/Acid (B/A) ratio | 0.7 | 0.3 | 1 | 0.4 | −0.8 | 0.1 | −0.1 |
| Slagging Index (Rs) | 0.2 | 0 | 0.4 | 1 | −0.5 | −0.1 | −0.2 |
| Silica Value (SV) | −0.5 | −0.6 | −0.8 | −0.5 | 1 | −0.3 | −0.1 |
| Fouling Index (Rf) | 0 | 0.1 | 0.1 | −0.1 | −0.3 | 1 | 0.9 |
| Alkalinity (AK) | −0.1 | −0.1 | −0.1 | −0.2 | −0.1 | 0.9 | 1 |
| Ga (ppm)—coal | −0.3 | 0.1 | −0.3 | 0.1 | 0.1 | −0.2 | −0.1 |
| Ga (ppm)—ash | −0.4 | −0.3 | −0.4 | 0 | 0.3 | −0.1 | 0 |
| Sc (ppm)—coal | −0.3 | 0 | −0.3 | −0.1 | 0.2 | 0 | 0 |
| Sc (ppm)—ash | −0.4 | −0.4 | −0.4 | −0.1 | 0.2 | 0.4 | 0.4 |
| V (ppm)—coal | −0.4 | 0.1 | −0.2 | 0.1 | 0 | 0.1 | 0.1 |
| V (ppm)—ash | −0.3 | −0.1 | −0.2 | 0.1 | 0 | 0.3 | 0.2 |
| Ga (ppm)—coal | Ga (ppm)—ash | Sc (ppm)—coal | Sc (ppm)—ash | V (ppm)—coal | V (ppm)—ash | ||
| Mad (%) | 0.1 | −0.5 | 0.1 | −0.5 | 0 | −0.3 | |
| Adb (%) | 0.4 | −0.3 | 0.4 | −0.4 | 0 | −0.4 | |
| Vdaf (%) | 0.1 | −0.5 | 0.1 | −0.5 | 0 | −0.2 | |
| Stdb (%) | 0.4 | −0.1 | 0.4 | 0 | 0.4 | 0.1 | |
| GCV (MJ/kg) | −0.3 | 0.5 | −0.2 | 0.6 | 0 | 0.4 | |
| Ash Sintering Temperature (°C) | 0 | −0.2 | 0.2 | 0.1 | 0 | 0 | |
| Ash Softening Temperature (°C) | 0.1 | 0.3 | 0.1 | 0.2 | 0.1 | 0.1 | |
| Ash Melting Temperature (°C) | 0.1 | 0.4 | 0 | 0.2 | 0.2 | 0.1 | |
| Ash Fluid Temperature (°C) | 0.1 | 0.4 | 0 | 0.2 | 0.1 | 0.1 | |
| Cdaf (%) | −0.3 | 0.5 | −0.2 | 0.6 | 0 | 0.3 | |
| Hdaf (%) | −0.4 | −0.1 | −0.2 | 0.2 | 0.1 | 0.3 | |
| Ndaf (%) | −0.1 | 0.3 | 0 | 0.4 | 0.1 | 0.2 | |
| Odaf (%) | 0.2 | −0.5 | 0.1 | −0.6 | 0 | −0.3 | |
| Total porosity (%) | −0.1 | −0.5 | 0 | −0.5 | −0.3 | −0.3 | |
| Huminite/Vitrinite (%) | 0 | −0.2 | −0.1 | −0.2 | 0 | 0 | |
| Inertinite (%) | −0.1 | 0.3 | 0 | 0.3 | −0.1 | 0 | |
| Liptinite (%) | 0 | 0.2 | 0.1 | 0.3 | 0.1 | 0.1 | |
| Mineral Matter (%) | 0.2 | −0.4 | 0.5 | −0.3 | −0.1 | −0.4 | |
| Random Reflectance (Ro) (%) | −0.1 | 0.5 | −0.2 | 0.5 | 0 | 0.2 | |
| Aromaticity Factor (fa) | 0.2 | 0.5 | 0.1 | 0.4 | 0 | 0.1 | |
| SiO2 (%) | 0.1 | 0.1 | 0.2 | 0.1 | −0.1 | −0.1 | |
| Al2O3 | 0.1 | 0.7 | 0 | 0.7 | 0.2 | 0.4 | |
| Fe2O3 (%) | −0.2 | −0.1 | −0.1 | 0.1 | 0.1 | 0.1 | |
| MgO (%) | −0.3 | −0.2 | −0.1 | 0.1 | −0.1 | 0 | |
| CaO (%) | 0.1 | −0.3 | −0.1 | −0.6 | −0.1 | −0.3 | |
| Na2O (%) | −0.1 | 0 | 0 | 0.4 | 0.1 | 0.2 | |
| K2O (%) | 0 | 0.2 | 0.1 | 0.4 | 0.2 | 0.3 | |
| TiO2 (%) | 0.2 | 0.3 | 0.5 | 0.6 | 0.2 | 0.3 | |
| P2O5 (%) | 0 | 0.4 | −0.1 | 0.3 | 0.1 | 0.2 | |
| MnO (%) | −0.2 | −0.4 | −0.3 | −0.5 | −0.3 | −0.3 | |
| Cr2O3 (%) | −0.2 | −0.1 | −0.3 | −0.2 | −0.1 | 0 | |
| LOI and Others (%) | 0 | −0.4 | −0.1 | −0.5 | −0.1 | −0.2 | |
| C in ash (%) | −0.3 | −0.4 | −0.3 | −0.4 | −0.4 | −0.3 | |
| S in ash (%) | 0.1 | −0.3 | 0 | −0.4 | 0.1 | −0.1 | |
| Base/Acid (B/A) ratio | −0.3 | −0.4 | −0.3 | −0.4 | −0.2 | −0.2 | |
| Slagging Index (Rs) | 0.1 | 0 | −0.1 | −0.1 | 0.1 | 0.1 | |
| Silica Value (SV) | 0.1 | 0.3 | 0.2 | 0.2 | 0 | 0 | |
| Fouling Index (Rf) | −0.2 | −0.1 | 0 | 0.4 | 0.1 | 0.3 | |
| Alkalinity (AK) | −0.1 | 0 | 0 | 0.4 | 0.1 | 0.2 | |
| Ga (ppm)—coal | 1 | 0.4 | 0.7 | 0.1 | 0.6 | 0.1 | |
| Ga (ppm)—ash | 0.4 | 1 | 0.1 | 0.7 | 0.6 | 0.7 | |
| Sc (ppm)—coal | 0.7 | 0.1 | 1 | 0.3 | 0.4 | 0 | |
| Sc (ppm)—ash | 0.1 | 0.7 | 0.3 | 1 | 0.5 | 0.7 | |
| V (ppm)—coal | 0.6 | 0.6 | 0.4 | 0.5 | 1 | 0.7 | |
| V (ppm)—ash | 0.1 | 0.7 | 0 | 0.7 | 0.7 | 1 | |
References
- Kurama, H.; Kaya, M. Usage of coal combustion bottom ash in concrete mixture. Constr. Build. Mater. 2008, 22, 1922–1928. [Google Scholar] [CrossRef]
- Torrey, S. Coal ash utilization. Fly ash, bottom ash and slag. In Coal Ash Utilization. Fly Ash, Bottom Ash and Slag; Noyes Data Corporation: Park Ridge, NJ, USA, 1978. [Google Scholar]
- Mondragon, F.; Rincon, F.; Sierra, L.; Escobar, J.; Ramirez, J.; Fernandez, J. New perspectives for coal ash utilization: Synthesis of zeolitic materials. Fuel 1990, 69, 263–266. [Google Scholar] [CrossRef]
- Manz, O.E. Worldwide production of coal ash and utilization in concrete and other products. Fuel 1997, 76, 691–696. [Google Scholar] [CrossRef]
- EUR-Lex−52011DC0025-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52011DC0025 (accessed on 25 May 2020).
- EUR-Lex−52017DC0490-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52017DC0490 (accessed on 25 May 2020).
- Hagelüken, C.; Caffrey, M. Raw materials: Sustainable exploration, extraction, processing, recycling and substitution. In Proceedings of the MIT No. EU-US Workshop, Boston, MA, USA, 3 December 2010. [Google Scholar]
- Dai, S.; Zhao, L.; Peng, S.; Chou, C.-L.L.; Wang, X.; Zhang, Y.; Li, D.; Sun, Y. Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant, Inner Mongolia, China. Int. J. Coal Geol. 2010, 81, 320–332. [Google Scholar] [CrossRef]
- Fernandez-Turiel, J.L.; Georgakopoulos, A.; Gimeno, D.; Papastergios, G.; Kolovos, N. Ash deposition in a pulverized coal-fired power plant after high-calcium lignite combustion. Energy Fuels 2004. [Google Scholar] [CrossRef]
- Filippidis, A.; Georgakopoulos, A.; Kassoli-Fournaraki, A. Mineralogical components of some thermally decomposed lignite and lignite ash from the Ptolemais basin, Greece. Int. J. Coal Geol. 1996, 30, 303–314. [Google Scholar] [CrossRef]
- Huffman, G.P.; Huggins, F.E.; Shah, N.; Shah, A. Behavior of basic elements during coal combustion. Progress Energy Combust. Sci. 1990, 16, 243–251. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Karayigit, A.I.; Bulut, Y.; Alastuey, A.; Querol, X. Phase-mineral and chemical composition of composite samples from feed coals, bottom ashes and fly ashes at the Soma power station, Turkey. Int. J. Coal Geol. 2005, 61, 35–63. [Google Scholar] [CrossRef]
- Kostova, I.; Vassileva, C.; Dai, S.; Hower, J.C.; Apostolova, D. Influence of surface area properties on mercury capture behaviour of coal fly ashes from some Bulgarian power plants. Int. J. Coal Geol. 2013, 116–117, 227–235. [Google Scholar] [CrossRef]
- Seredin, V.V.; Shpirt, M.Y. Metalliferous coals: A new potential source of valuable trace elements as by-products. Coal Sci. Technol. 1995, 24, 1649–1652. [Google Scholar] [CrossRef]
- Seredin, V.V. Rare earth element-bearing coals from the Russian Far East deposits. Int. J. Coal Geol. 1996, 30, 101–129. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Dai, S.; Li, D.; Chou, C.L.; Zhao, L.; Zhang, Y.; Ren, D.; Ma, Y.; Sun, Y. Mineralogy and geochemistry of boehmite-rich coals: New insights from the Haerwusu Surface Mine, Jungar Coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2008, 74, 185–202. [Google Scholar] [CrossRef]
- Dai, S.; Wang, X.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; O’Keefe, J.M.K.; Huang, W.; Li, T.; Li, X.; Liu, H.; et al. Petrology, mineralogy, and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit, Inner Mongolia, China: New data and genetic implications. Int. J. Coal Geol. 2012, 72–99. [Google Scholar] [CrossRef]
- Lefticariu, L.; Klitzing, K.L.; Kolker, A. Rare earth elements and yttrium (REY) in coal mine drainage from the Illinois Basin, USA. Int. J. Coal Geol. 2020, 217. [Google Scholar] [CrossRef]
- Lin, R.; Soong, Y.; Granite, E.J. Evaluation of trace elements in U.S. coals using the USGS COALQUAL database version 3.0. Part I: Rare earth elements and yttrium (REY). Int. J. Coal Geol. 2018, 192, 1–13. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, X.; Xie, Q.; Finkelman, R.B.; Han, S.; Huan, B.; Pan, X. Petrological characteristics and trace element partitioning of gasification residues from slagging entrained-flow gasifiers in Ningdong, China. Energy Fuels 2018. [Google Scholar] [CrossRef]
- Bielowicz, B.; Botor, D.; Misiak, J.; Wagner, M. Critical elements in fly ash from the combustion of bituminous coal in major polish power plants. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 35. [Google Scholar]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Xing, Y.; Zhang, W.; Song, W.; Wang, P. Enrichment of U–Se–Mo–Re–V in coals preserved within marine carbonate successions: Geochemical and mineralogical data from the Late Permian Guiding Coalfield, Guizhou, China. Miner. Depos. 2015, 50, 159–186. [Google Scholar] [CrossRef]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal fly ash as a resource for rare earth elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef]
- Kataka, M.O.; Matiane, A.R.; Odhiambo, B.D.O. Chemical and mineralogical characterization of highly and less reactive coal from Northern Natal and Venda-Pafuri coalfields in South Africa. J. Afr. Earth Sci. 2018, 137, 278–285. [Google Scholar] [CrossRef]
- Kolker, A.; Hower, J.C.; Karamalidis, A.K. Introduction to critical elements in coal and coal ash and their recovery, a virtual special issue. Int. J. Coal Geol. 2019, 206, 19–20. [Google Scholar] [CrossRef]
- Valentim, B.; Abagiu, A.T.; Anghelescu, L.; Flores, D.; French, D.; Gonçalves, P.; Guedes, A.; Popescu, L.G.; Predeanu, G.; Ribeiro, J.; et al. Assessment of bottom ash landfilled at Ceplea Valley (Romania) as a source of rare earth elements. Int. J. Coal Geol. 2019, 201, 109–126. [Google Scholar] [CrossRef]
- ISO-ISO 579:2013-Coke—Determination of Total Moisture. Available online: https://www.iso.org/standard/62608.html (accessed on 17 June 2020).
- ISO-ISO 1171:2010-Solid Mineral Fuels—Determination of Ash. Available online: https://www.iso.org/standard/55944.html (accessed on 17 June 2020).
- ISO-ISO 562:2010-Hard Coal and Coke—Determination of Volatile Matter. Available online: https://www.iso.org/standard/55943.html (accessed on 17 June 2020).
- ISO-ISO 351:1996-Solid Mineral Fuels—Determination of Total Sulfur—High Temperature Combustion Method. Available online: https://www.iso.org/standard/4304.html (accessed on 17 June 2020).
- ISO-ISO 1928:2009-Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value. Available online: https://www.iso.org/standard/41592.html (accessed on 17 June 2020).
- ISO-ISO 540:2008-Hard Coal and Coke—Determination of Ash Fusibility. Available online: https://www.iso.org/standard/41484.html (accessed on 17 June 2020).
- ISO-ISO 609:1996-Solid Mineral Fuels—Determination of Carbon and Hydrogen—High Temperature Combustion Method. Available online: https://www.iso.org/standard/4724.html (accessed on 17 June 2020).
- ISO. Methods for the Petrographic Analysis of Coals-Part 3: Method of Determining Maceral Group Composition; 7404−3:2009; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ISO 7404−5:2009-Methods for the Petrographic Analysis of Coals-Part 5: Method of Determining Microscopically the Reflectance of Vitrinite. Available online: https://www.iso.org/standard/42832.html (accessed on 30 January 2018).
- ISO 7404−4:2017-Methods for the Petrographic Analysis of Coals-Part 4: Method of Determining Microlithotype, Carbominerite and Minerite Composition. Available online: https://www.iso.org/standard/69224.html (accessed on 30 January 2018).
- Guilford, J. Fundamental Statistics in Psychology and Education, 4th ed.; McGraw-Hill: New York, NY, USA, 1965. [Google Scholar]
- Kwiecińska, B.; Wagner, M. Classification of Qualitative Features of Brown Coal From Polish Deposits According to Petrographical, Chemical and Technological Criteria; Wydawnictwo Centrum PPGSMiE PAN: Kraków, Poland, 1997. [Google Scholar]
- Wagner, M.; Lipiarski, I.; Misiak, J. A Petrographic Atlas of Subbituminous and Bituminous Coal from the Polish Deposits and From Uneconomic Occurrences; AGH Uczelniane Wydawnictwa Naukowo-Dydaktyczne: Kralow, Poland, 2008. [Google Scholar]
- Kwiecińska, B.; Wagner, M. A Petrographic Atlas of Brown Coals (Lignites) from the Polish Deposits; Wydawnictwo JAK Andrzej Choczewski: Kraków, Poland, 2001. [Google Scholar]
- Suárez-Ruiz, I.; Crelling, J. Applied Coal Petrology; Elsevier Ltd: Amsterdam, The Netherlands, 2008; ISBN 9780080450513. [Google Scholar]
- Rajan, S.; Raghavan, J.K. Coal mineral matter transformation during combustion and its effects on gas turbine blade deposition and erosion. J. Eng. Gas Turbines Power 1993, 115, 634–640. [Google Scholar] [CrossRef]
- Qiu, J.R.; Li, F.; Zheng, C.G. Mineral transformation during combustion of coal blends. Int. J. Energy Res. 1999, 23, 453–463. [Google Scholar] [CrossRef]
- Karayigit, A.I.; Ozlem, Y.; Iserli, S.; Querol, X.; Mastalerz, M.; Oska, R.; Hower, J.C. Mineralogy and geochemistry of feed coals and combustion residues from tunçbilek and eyitömer coal-fired power plants in western Turkey. Coal Combust. Gasif. Prod. 2019. [Google Scholar] [CrossRef]
- Shirazi, A.R.; Börtin, O.; Eklund, L.; Lindqvist, O. The impact of mineral matter in coal on its combustion, and a new approach to the determination of the calorific value of coal. Fuel 1995, 74, 247–251. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 4. Characterization of heavy concentrates and improved fly ash residues. Fuel 2005, 84, 973–991. [Google Scholar] [CrossRef]
- Burchill, P.; Richards, D.G.G.; Warrington, S.B. A study of the reactions of coals and coal minerals under combustion-related conditions by thermal analysis-mass spectrometry and other techniques. Fuel 1990, 69, 950–956. [Google Scholar] [CrossRef]
- Reifenstein, A.P.; Kahraman, H.; Coin, C.D.A.; Calos, N.J.; Miller, G.; Uwins, P. Behaviour of selected minerals in an improved ash fusion test: Quartz, potassium feldspar, sodium feldspar, kaolinite, illite, calcite, dolomite, siderite, pyrite and apatite. Fuel 1999, 78, 1449–1461. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S. Coal-derived unburned carbons in fly ash: A review. Int. J. Coal Geol. 2017, 179, 11–27. [Google Scholar] [CrossRef]
- Wagner, M. Zmienność Petrologiczno-Sedymentologiczna i Własności Technologiczne Kredy Jeziornej w Osadach Neogenu Typu Wapiennego Zapadliska Tektonicznego na Przykładzie Zloża Węgla Brunatnego “Szczerców”; AGH Uczelniane Wydawn. Naukowo-Dydaktyczne: Krakow, Poland, 2007; ISBN 9788374641289. [Google Scholar]
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: An updated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Wagner, M. Doppleritization of xylitic coal in the light of petrographic and chemical investigations. Int. J. Coal Geol. 1982, 2, 181–194. [Google Scholar] [CrossRef]
- Slomka, T.; Wagner, M. Polska Akademia Nauk; Komisja Nauk Geologicznych. Charakter Petrograficzny i Warunki Sedymentacji Wybranych Kompleksów Litologicznych z Profilu Miocenu w Złożu Węgla Brunatnego Bełchatów; IGSMiE PAN: Krakow, Poland, 2000; ISBN 9788387854768. [Google Scholar]
- Christanis, K.; Georgakopoulos, A.; Fernández-Turiel, J.L.; Bouzinos, A. Geological factors influencing the concentration of trace elements in the Philippi peatland, eastern Macedonia, Greece. Int. J. Coal Geol. 1998, 36, 295–313. [Google Scholar] [CrossRef]
- Kalaitzidis, S.; Christanis, K.; Georgakopoulos, A.; Fernández-Turiel, J.L.; Papazisimou, S. Influence of geological conditions during peat accumulation on trace element affinities and their behavior during peat combustion. Energy Fuels 2002, 16, 1476–1482. [Google Scholar] [CrossRef]
- van Dyk, J.C.; Melzer, S.; Sobiecki, A. Mineral matter transformation during Sasol-Lurgi fixed bed dry bottom gasification-utilization of HT-XRD and FactSage modelling. Miner. Eng. 2006. [Google Scholar] [CrossRef]
- Collot, A.-G.G. Matching gasification technologies to coal properties. Int. J. Coal Geol. 2006, 65, 191–212. [Google Scholar] [CrossRef]
- Skodras, G.; Sakellaropoulos, G.P. Mineral matter effects in lignite gasification. Fuel Process. Technol. 2002, 77–78, 151–158. [Google Scholar] [CrossRef]
- Kong, L.; Bai, J.; Bai, Z.; Guo, Z.; Li, W. Effects of CaCO3 on slag flow properties at high temperatures. Fuel 2013, 109, 76–85. [Google Scholar]
- Su, S.; Pohl, J.H.; Holcombe, D. Fouling propensities of blended coals in pulverized coal-fired power station boilers. Fuel 2003, 82, 1653–1667. [Google Scholar] [CrossRef]
- Hamala, K.; Róg, L. Influence of chemical composition and coal physic-chemical specificity and its ashes on indicators of slagging and pollution of surface of heating energy boiler. Pr. Nauk. GIG. Gór. Śr./Gł. Inst. Gór. 2004, 3, 81–109. [Google Scholar]
- Bielowicz, B. A new technological classification of low-rank coal on the basis of Polish deposits. Fuel 2012, 96, 497–510. [Google Scholar] [CrossRef]
- Atakül, H.; Hilmioǧlu, B.; Ekinci, E. The relationship between the tendency of lignites to agglomerate and their fusion characteristics in a fluidized bed combustor. Fuel Process. Technol. 2005, 86, 1369–1383. [Google Scholar] [CrossRef]
- The Composition of the Earth’s Crust-Frank Wigglesworth Clarke, Henry Stephens Washington-Google Książki. Available online: https://books.google.pl/books/about/The_Composition_of_the_Earth_s_Crust.html?id=Ht9WAAAAMAAJ&redir_esc=y (accessed on 8 May 2020).
- Ronov, A.B.; Yaroshevsky, A.A.; Migdisov, A.A. Chemical composition of the earth’s crust and geochemical balance of main elements. Sci. Pub. House Mosc. 1990, 33, 192. [Google Scholar]
- Ketris, M.P.; Yudovich, Y.E. Estimations of clarkes for carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Phipps, G.; Mikolajczak, C.; Guckes, T. Indium and Gallium: Long-term supply. Renew. Energy Focus 2008, 9. [Google Scholar] [CrossRef]
- Moskalyk, R.R. Gallium: The backbone of the electronics industry. Miner. Eng. 2003, 16, 921–929. [Google Scholar] [CrossRef]
- Fahlquist, H.; Noréus, D.; Callear, S.; David, W.I.F.; Hauback, B.C. Two new cluster ions, Ga[GaH3]45- with a neopentane structure in Rb8Ga5H15 and [GaH 2]nn- with a polyethylene structure in Rb n(GaH2)n, represent a new class of compounds with direct Ga-Ga bonds mimicking common hydrocarbons. J. Am. Chem. Soc. 2011, 133, 14574–14577. [Google Scholar] [CrossRef]
- Foley, N.; Jaskula, B. Gallium—A Smart Metal; U.S. Geological Survey Fact Sheet 2013–3006; USGS: Reston, VA, USA, 2013.
- Gallium Statistics and Information. Available online: https://www.usgs.gov/centers/nmic/gallium-statistics-and-information (accessed on 9 May 2020).
- Qin, S.; Sun, Y.; Li, Y.; Wang, J.; Zhao, C.; Gao, K. Coal deposits as promising alternative sources for gallium. Earth-Sci. Rev. 2015, 150, 95–101. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.L.; Li, S.; Jiang, Y. Mineralogy and geochemistry of the No. 6 Coal (Pennsylvanian) in the Junger Coalfield, Ordos Basin, China. Int. J. Coal Geol. 2006, 66, 253–270. [Google Scholar] [CrossRef]
- Zou, J.; Tian, H.; Wang, Z. Leaching process of rare earth elements, gallium and niobium in a coal-bearing strata-hosted rare metal deposit—A case study from the late permian tuff in the zhongliangshan mine, chongqing. Metals 2017, 7, 174. [Google Scholar] [CrossRef]
- Wang, J.; Yamada, O.; Nakazato, T.; Zhang, Z.G.; Suzuki, Y.; Sakanishi, K. Statistical analysis of the concentrations of trace elements in a wide diversity of coals and its implications for understanding elemental modes of occurrence. Fuel 2008, 87, 2211–2222. [Google Scholar] [CrossRef]
- Wang, W.; Sang, S.; Hao, W.; Wang, R.; Zhang, J.; Duan, P.; Qin, Y.; Xu, S. A cut-off grade for gold and gallium in coal. Fuel 2015, 147, 62–66. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Li, S. Discovery of the superlarge gallium ore deposit in Jungar, Inner Mongolia, North China. Chin. Sci. Bull. 2006, 51, 2243–2252. [Google Scholar] [CrossRef]
- Seredin, V.V. From Coal Science to Metal Production and Environmental Protection: A New Story of Success; Geoinformmark: Moscow, Russia, 2012; Volume 90–91, pp. 1–3. [Google Scholar]
- Arroyo, F.; Font, O.; Chimenos, J.M.; Fernández-Pereira, C.; Querol, X.; Coca, P. IGCC fly ash valorisation. Optimisation of Ge and Ga recovery for an industrial application. Fuel Process. Technol. 2014, 124, 222–227. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, Q.; Jia, S.; Zou, J.; Nechaev, V.P.; Dai, S. Origin of minerals and critical metals in an argillized tuff from the Huayingshan Coalfield, Southwestern China. Minerals 2017, 7, 92. [Google Scholar] [CrossRef]
- Dill, H.G.; Wehner, H. The depositional environment and mineralogical and chemical compositions of high ash brown coal resting on early Tertiary saprock (Schirnding Coal Basin, SE Germany). Int. J. Coal Geol. 1999, 39, 301–328. [Google Scholar] [CrossRef]
- Zhou, J.; Zhuang, X.; Alastuey, A.; Querol, X.; Li, J. Geochemistry and mineralogy of coal in the recently explored Zhundong large coal field in the Junggar basin, Xinjiang province, China. Int. J. Coal Geol. 2010, 82, 51–67. [Google Scholar] [CrossRef]
- Diakonov, I.I.; Pokrovski, G.S.; Bénézeth, P.; Schott, J.; Dandurand, J.L.; Escalier, J. Gallium speciation in aqueous solution. Experimental study and modelling: Part 1. Thermodynamic properties of Ga(OH)4− to 300 °C. Geochim. Cosmochim. Acta 1997, 61, 1333–1343. [Google Scholar] [CrossRef]
- Seredin, V.V.; Finkelman, R.B. Metalliferous coals: A review of the main genetic and geochemical types. Int. J. Coal Geol. 2008, 76, 253–289. [Google Scholar] [CrossRef]
- Bielowicz, B.; Misiak, J. The forms of occurrence and chemical composition of sulfides in the LW Bogdanka bituminous coal depositof the Lublin coal basin. Gospod. Surow. Miner./Miner. Res. Manag. 2018, 34, 37–52. [Google Scholar] [CrossRef]
- Chinach, W. Powstaje Rekordowy Magazyn Energii Oparty o Nowe Ogniwa Wanadowe-ŚwiatOZE.pl. Available online: https://swiatoze.pl/w-chinach-powstaje-rekordowy-magazyn-energii-oparty-o-nowe-ogniwa-wanadowe/ (accessed on 13 May 2020).
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Dai, S.; Ren, D.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Dai, S.; Zheng, X.; Wang, X.; Finkelman, R.B.; Jiang, Y.; Ren, D.; Yan, X.; Zhou, Y. Stone coal in China: A review. Int. Geol. Rev. 2018, 60, 736–753. [Google Scholar] [CrossRef]
- Premović, P.I.; Nikolić, N.D.; Pavlović, M.S.; Jovanović, L.S.; Premović, M.P. Origin of vanadium in coals: Parts of the Western Kentucky (USA) No. 9 coal rich in vanadium. Geol. Soc. Lond. Spec. Publ. 1997, 125, 273–286. [Google Scholar] [CrossRef]
- Tonstein from the coal seam No. 385 in the Lublin Formation (Lower Westphalian) from the Lublin Coal Basin Lipiarski. Geological Quarterly. Available online: https://gq.pgi.gov.pl/article/view/8485 (accessed on 15 May 2020).
- Gola, M.R.; Karger, M.; Gazda, L. Dystrybucja biomarkerów i dojrzałość termiczna materii organicznej w tonsteinie i węglu kamiennym z pokładu 385/2 z kopalni Bogdanka (Lubelskie Zagłębie Węglowe). Prz. Geol. 2011, 59, 777–784. [Google Scholar]
- Ilaste skały kaolinitowe (paratonsteiny) ze złoża węgla brunatnego Bełchatów Wagner. Geological Quarterly. Available online: https://gq.pgi.gov.pl/article/view/8634 (accessed on 15 May 2020).
- Handbook of Non-Ferrous Metal Powders; Elsevier: Amsterdam, The Netherlands, 2019.
- Scandium in Russia and World: Production, Market and Forecast (4th Edition)-GII. Available online: https://www.giiresearch.com/report/info578149-scandium-russia-world-production-market-forecast−3.html (accessed on 17 May 2020).
- Recovering and Producing Scandium and Rare Earths from Coal Deposits-International Mining. Available online: https://im-mining.com/2016/12/23/recovering-producing-scandium-rare-earths-coal-deposits/ (accessed on 17 May 2020).
- Arbuzov, S.I.; Maslov, S.G.; Il’enok, S.S. Modes of occurrence of scandium in coals and peats (A review). Solid Fuel Chem. 2015, 49, 167–182. [Google Scholar] [CrossRef]
- Ren, D.; Zhao, F.; Wang, Y.; Yang, S. Distributions of minor and trace elements in Chinese coals. Int. J. Coal Geol. 1999, 40, 109–118. [Google Scholar] [CrossRef]
- Finkelman, R.B. Trace and Minor Elements in Coal; Springer: Berlin/Heidelberg, Germany, 1993; pp. 593–607. [Google Scholar]
- Arbuzov, S.I.; Volostnov, A.V.; Mezhibor, A.M.; Rybalko, V.I.; Ilenok, S.S. Scandium (Sc) geochemistry in coals (Siberia, Russian Far East, Mongolia, Kazakhstan, and Iran). Int. J. Coal Geol. 2014, 125, 22–35. [Google Scholar] [CrossRef]











| Rank | Sample No. | Deposit |
|---|---|---|
| Lignite | 1L | Pątnów (Jóźwin opencast), Middle Miocene (1st seam), detritic |
| 2L | Pątnów (Jóźwin opencast), Middle Miocene (1st seam), xylitic | |
| 3L | Pątnów (Jóźwin opencast), Middle Miocene (1st seam), detro-xylitic | |
| 4L | Turów, Early Miocene (3rd seam), xylitic | |
| 5L | Turów, Early Miocene (3rd seam), xylo-detritic | |
| 6L | Sieniawa, Early-Middle Miocene (2nd seam), xylo-detritic | |
| 7L | Sieniawa, Early-Middle Miocene (2nd seam), detritic | |
| 8L | Bełchatów, Early Miocene (3rd seam), xylo-detritic | |
| 9L | Bełchatów, Lower Miocene, xylitic | |
| 10L | Bełchatów, Early Miocene (3rd seam), detritic | |
| 11L | Szczerców, Early-Middle Miocene (2rd seam), xylitic | |
| 12L | Szczerców, Early-Middle Miocene (2rd seam), xylo-detritic | |
| Subbituminous coal | 1SB | Poręba, Early Jurassic, detritic |
| Bituminous coal | 1B | Jas-Mos, Westphalian A (seam 510), coking coal |
| 2B | Janina, Westphalian D (seam 119/2), bright coal | |
| 3B | Janina, Westphalian D (seam 119/2), dull coal | |
| 4B | Janina, Westphalian C (seam 203/2), bright coal | |
| 5B | Janina, Westphalian C (seam 203/2), dull coal | |
| 6B | Bogdanka, Westphalian B (seam 391), bright coal | |
| 7B | Bogdanka, Westphalian B (seam 391), dull coal | |
| 8B | Bogdanka, Westphalian B (seam 381), bright coal | |
| 9B | Bogdanka, Westphalian B (seam 381), dull coal | |
| 10B | Bogdanka, Westphalian B (seam 385/2), bright coal | |
| 11B | Bogdanka, Westphalian B (seam 385/2), dull coal | |
| 12B | Wesoła, Westphalian B (seam 308), bright coal | |
| 13B | Wesoła, Westphalian B (seam 308), dull coal | |
| 14B | Bielszowice, Upper Namurian (seam 405/2), bright coal | |
| 15B | Bielszowice Upper Namurian (seam 405/2), dull coal |
| Proximate Analysis | Ultimate Analysis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Mad (%) | Adb (%) | Vdaf (%) | Stdb (%) | GCV (MJ/kg) | Ash Sintering Temperature (tS) (°C) | Ash Softening Temperature (tA) (°C) | Ash Melting Temperature (tB)(°C) | Ash Fluid Temperature (tC) (°C) | Total Porosity (%) | C daf (%) | H daf (%) | N daf (%) | O daf (%) | Aromaticity Factor (fa) |
| 1L | 7.6 | 46.2 | 56.2 | 1.6 | 23.9 | 890 | 1310 | 1400 | 1470 | 33.4 | 64.0 | 4.7 | 0.6 | 27.7 | 0.7 |
| 2L | 8.2 | 6.2 | 58.8 | 1.6 | 25.9 | 920 | 1220 | 1230 | 1240 | 25.0 | 64.9 | 5.3 | 0.2 | 27.8 | 0.6 |
| 3L | 9.6 | 24.6 | 56.9 | 2.8 | 26.1 | 990 | 1480 | 1510 | 1550 | 31.5 | 66.3 | 5.2 | 0.4 | 24.5 | 0.6 |
| 4L | 8.4 | 5.0 | 57.4 | 1.9 | 28.9 | 1110 | 1270 | 1310 | 1350 | 11.5 | 69.2 | 5.8 | 0.4 | 22.5 | 0.6 |
| 5L | 10.0 | 3.8 | 56.8 | 0.8 | 29.2 | 900 | 1210 | 1280 | 1320 | 26.5 | 70.7 | 6.0 | 0.5 | 22.0 | 0.6 |
| 6L | 10.5 | 6.9 | 55.8 | 1.0 | 26.2 | 920 | 1190 | 1290 | 1300 | 35.9 | 66.0 | 5.2 | 0.5 | 27.2 | 0.7 |
| 7L | 9.3 | 6.5 | 58.0 | 0.5 | 26.3 | 1020 | 1250 | 1280 | 1290 | 22.9 | 66.5 | 5.3 | 0.8 | 26.9 | 0.6 |
| 8L | 9.8 | 16.2 | 55.8 | 1.7 | 25.6 | 980 | 1230 | 1250 | 1260 | 8.4 | 65.7 | 5.0 | 0.7 | 26.6 | 0.7 |
| 9L | 8.7 | 7.2 | 56.2 | 3.6 | 26.9 | nd | nd | nd | nd | 19.9 | 63.4 | 5.2 | 0.2 | 27.3 | 0.7 |
| 10L | 12.0 | 19.8 | 55.4 | 3.6 | 25.5 | 910 | 1260 | 1280 | 1300 | 18.3 | 65.4 | 4.8 | 1.0 | 24.4 | 0.7 |
| 11L | 11.6 | 2.7 | 64.2 | 1.0 | 26.7 | 910 | 1300 | 1310 | 1320 | 28.6 | 65.0 | 5.8 | 0.1 | 28.1 | 0.5 |
| 12L | 4.6 | 23.2 | 49.4 | 3.9 | 27.6 | 980 | 1390 | 1430 | 1450 | 25.9 | 67.4 | 5.2 | 0.9 | 21.5 | 0.7 |
| 1SB | 9.7 | 22.4 | 58.3 | 3.6 | 25.2 | nd | nd | nd | nd | 11.1 | 65.1 | 5.0 | 0.8 | 24.6 | 0.6 |
| 1B | 0.6 | 6.5 | 22.0 | 1.1 | 36.4 | 960 | 1540 | 1550 | 1550 | 3.6 | 89.7 | 4.8 | 1.1 | 3.2 | 0.8 |
| 2B | 5.1 | 8.6 | 39.3 | 2.6 | 31.2 | 1090 | 1260 | 1340 | 1430 | 10.5 | 76.7 | 4.8 | 1.2 | 14.5 | 0.8 |
| 3B | 4.6 | 6.2 | 40.5 | 1.6 | 31.8 | 990 | 1240 | 1280 | 1380 | 9.2 | 77.9 | 5.1 | 1.3 | 14.0 | 0.7 |
| 4B | 5.1 | 7.8 | 35.4 | 1.4 | 31.2 | 900 | 1230 | 1270 | 1360 | 7.1 | 77.6 | 4.7 | 1.2 | 15.1 | 0.8 |
| 5B | 3.8 | 24.1 | 38.8 | 1.0 | 30.5 | 950 | 1550 | 1550 | 1550 | 6.1 | 76.0 | 5.1 | 1.2 | 16.4 | 0.8 |
| 6B | 1.5 | 8.3 | 39.5 | 3.0 | 33.4 | 900 | 1350 | 1510 | 1550 | 2.9 | 80.4 | 5.3 | 2.0 | 9.0 | 0.7 |
| 7B | 1.2 | 11.3 | 39.8 | 5.3 | 33.6 | 920 | 1330 | 1400 | 1410 | 2.6 | 79.4 | 5.4 | 1.8 | 7.5 | 0.7 |
| 8B | 1.1 | 3.3 | 35.1 | 1.2 | 33.9 | 940 | 1330 | 1380 | 1430 | 3.3 | 81.9 | 5.2 | 2.0 | 9.6 | 0.8 |
| 9B | 1.2 | 3.7 | 35.2 | 1.5 | 33.4 | 910 | 1340 | 1420 | 1440 | 3.5 | 81.1 | 5.2 | 2.0 | 10.1 | 0.8 |
| 10B | 1.1 | 6.0 | 38.0 | 1.3 | 33.9 | 830 | 1320 | 1450 | 1470 | 3.3 | 81.2 | 5.4 | 1.7 | 10.3 | 0.7 |
| 11B | 1.0 | 10.6 | 37.8 | 1.2 | 33.6 | 930 | 1540 | 1550 | 1550 | 3.8 | 81.7 | 5.3 | 1.5 | 10.1 | 0.7 |
| 12B | 2.6 | 10.4 | 40.0 | 0.9 | 29.0 | 890 | 1250 | 1300 | 1370 | 3.1 | 74.1 | 4.4 | 1.3 | 19.2 | 0.8 |
| 13B | 2.5 | 15.1 | 38.2 | 0.7 | 29.7 | 940 | 1270 | 1380 | 1420 | 4.0 | 74.1 | 4.5 | 1.3 | 19.3 | 0.8 |
| 14B | 0.6 | 10.5 | 32.1 | 0.4 | 35.0 | 930 | 1310 | 1340 | 1420 | 2.3 | 85.2 | 5.1 | 1.4 | 7.9 | 0.8 |
| 15B | 0.6 | 10.1 | 33.7 | 0.3 | 35.3 | 910 | 1260 | 1310 | 1380 | 2.3 | 85.5 | 5.3 | 1.4 | 7.4 | 0.8 |
| No. | Huminite Vitrinite (%) | Inertinite (%) | Liptinite (%) | Mineral Matter (%) | Sulfides | Carbonates | Quartz + Clays | Random Reflectance (Ro) (%) |
|---|---|---|---|---|---|---|---|---|
| 1L | 90.6 | 2.5 | 2.3 | 4.6 | 1.0 | 0.0 | 3.6 | 0.24 |
| 2L | 74.8 | 19.9 | 1.7 | 3.6 | 0.0 | 0.0 | 3.6 | 0.24 |
| 3L | 89.6 | 3.8 | 2.0 | 4.6 | 0.0 | 0.0 | 4.6 | 0.24 |
| 4L | 94.0 | 0.3 | 2.7 | 3.0 | 0.0 | 0.0 | 3.0 | 0.28 |
| 5L | 94.5 | 0.8 | 3.4 | 1.3 | 0.0 | 0.0 | 1.3 | 0.28 |
| 6L | 93.7 | 0.6 | 2.9 | 2.7 | 0.0 | 0.0 | 2.7 | 0.25 |
| 7L | 84.8 | 1.6 | 4.0 | 9.6 | 0.8 | 0.0 | 8.8 | 0.25 |
| 8L | 89.9 | 1.9 | 2.7 | 5.6 | 0.4 | 0.4 | 4.8 | 0.28 |
| 9L | 95.8 | 1.5 | 1.8 | 1.0 | 0.0 | 0.0 | 1.0 | 0.28 |
| 10L | 92.7 | 1.6 | 2.6 | 3.1 | 1.6 | 0.0 | 1.6 | 0.28 |
| 11L | 97.1 | 0.8 | 1.7 | 0.4 | 0.0 | 0.0 | 0.4 | 0.26 |
| 12L | 73.6 | 6.8 | 8.5 | 11.1 | 0.8 | 0.0 | 10.4 | 0.26 |
| 1SB | 83.0 | 3.2 | 8.4 | 5.2 | 0.1 | 0.0 | 0.3 | 0.36 |
| 1B | 76.5 | 23.0 | 0.0 | 0.5 | 0.5 | 0.0 | 0.0 | 1.07 |
| 2B | 77.8 | 13.2 | 7.8 | 1.2 | 0.6 | 0.0 | 0.6 | 0.51 |
| 3B | 40.4 | 30.4 | 28.2 | 1.0 | 0.8 | 0.0 | 0.2 | 0.51 |
| 4B | 68.8 | 24.4 | 5.2 | 1.6 | 0.6 | 0.0 | 1.0 | 0.54 |
| 5B | 55.4 | 23.2 | 13.8 | 7.6 | 1.4 | 0.0 | 6.2 | 0.54 |
| 6B | 85.0 | 4.8 | 7.6 | 2.6 | 2.0 | 0.0 | 0.6 | 0.62 |
| 7B | 47.6 | 20.8 | 27.4 | 4.2 | 3.8 | 0.0 | 0.4 | 0.62 |
| 8B | 80.8 | 9.0 | 9.6 | 0.6 | 0.0 | 0.0 | 0.6 | 0.59 |
| 9B | 54.0 | 19.4 | 26.0 | 0.6 | 0.4 | 0.0 | 0.2 | 0.59 |
| 10B | 75.2 | 10.2 | 13.4 | 1.2 | 0.8 | 0.0 | 0.4 | 0.61 |
| 11B | 46.8 | 24.6 | 27.0 | 1.6 | 0.2 | 0.0 | 1.4 | 0.61 |
| 12B | 78.8 | 8.4 | 9.6 | 3.2 | 0.0 | 0.4 | 2.8 | 0.63 |
| 13B | 40.0 | 31.2 | 26.8 | 2.0 | 0.4 | 0.0 | 1.6 | 0.63 |
| 14B | 69.2 | 22.6 | 8.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.70 |
| 15B | 42.0 | 38.2 | 18.6 | 1.2 | 0.0 | 0.0 | 1.2 | 0.70 |
| No. | SiO2 (%) | Al2O3 | Fe2O3 (%) | MgO (%) | CaO (%) | Na2O (%) | K2O (%) | TiO2 (%) | P2O5 (%) | MnO (%) | Cr2O3 (%) | LOI and Others (%) | C in Ash (%) | S in Ash (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1L | 66.8 | 16.9 | 2.2 | 1.6 | 6.1 | 0.1 | 0.6 | 1.7 | 0.0 | 0.1 | 0.2 | 3.8 | 0.1 | 1.4 |
| 2L | 37.4 | 9.9 | 7.8 | 3.5 | 18.1 | 0.3 | 0.6 | 0.6 | 0.3 | 0.1 | 0.1 | 21.2 | 0.2 | 8.7 |
| 3L | 64.3 | 3.0 | 6.3 | 2.3 | 9.4 | 0.1 | 0.2 | 0.5 | 0.0 | 0.2 | 0.2 | 13.6 | 0.1 | 5.7 |
| 4L | 19.3 | 15.0 | 21.9 | 6.4 | 4.9 | 7.8 | 1.2 | 1.6 | 0.2 | 0.0 | 0.1 | 21.5 | 0.3 | 6.5 |
| 5L | 18.2 | 19.0 | 6.2 | 11.0 | 8.4 | 12.3 | 1.0 | 2.4 | 0.2 | 0.0 | 0.1 | 21.2 | 0.8 | 6.7 |
| 6L | 20.4 | 3.5 | 11.7 | 2.4 | 26.1 | 0.3 | 0.3 | 0.3 | 0.0 | 0.1 | 0.0 | 34.9 | 2.3 | 9.6 |
| 7L | 7.4 | 3.2 | 17.6 | 2.1 | 30.0 | 0.1 | 0.1 | 0.3 | 0.0 | 0.2 | 0.0 | 38.9 | 3.4 | 8.3 |
| 8L | 25.5 | 16.7 | 7.2 | 2.2 | 23.6 | 0.5 | 0.2 | 0.6 | 0.2 | 0.2 | 0.0 | 23.1 | 0.8 | 7.8 |
| 9L | 19.7 | 13.8 | 8.6 | 1.6 | 24.4 | 0.1 | 0.4 | 0.8 | 0.2 | 0.1 | 0.1 | 30.3 | 0.2 | 12.5 |
| 10L | 20.4 | 13.9 | 9.7 | 1.3 | 24.5 | 0.0 | 0.2 | 0.7 | 0.1 | 0.1 | 0.0 | 29.1 | 0.2 | 11.7 |
| 11L | 18.4 | 7.4 | 7.6 | 2.2 | 25.6 | 0.2 | 0.4 | 0.5 | 0.1 | 0.1 | 0.1 | 37.6 | 0.6 | 13.2 |
| 12L | 63.4 | 11.8 | 16.4 | 0.3 | 1.2 | 0.1 | 0.8 | 2.9 | 0.1 | 0.0 | 0.0 | 2.9 | 0.1 | 0.5 |
| 1SB | 28.2 | 14.5 | 9.2 | 1.1 | 20.6 | 0.1 | 0.1 | 0.8 | 0.1 | 0.1 | 0.0 | 25.1 | 0.2 | 10.2 |
| 1B | 45.1 | 35.0 | 14.0 | 0.7 | 1.4 | 0.9 | 0.8 | 1.4 | 0.2 | 0.0 | 0.1 | 0.6 | 0.2 | 3.3 |
| 2B | 25.6 | 20.7 | 29.9 | 3.1 | 7.0 | 5.3 | 1.0 | 0.6 | 0.5 | 0.1 | 0.1 | 6.2 | 0.1 | 1.8 |
| 3B | 31.2 | 23.8 | 19.5 | 2.5 | 6.0 | 6.3 | 0.5 | 0.8 | 0.3 | 0.0 | 0.0 | 9.1 | 0.1 | 0.5 |
| 4B | 35.1 | 27.9 | 14.9 | 2.4 | 4.2 | 6.0 | 1.7 | 1.0 | 0.2 | 0.0 | 0.1 | 6.7 | 0.6 | 8.6 |
| 5B | 50.7 | 32.1 | 4.9 | 1.3 | 1.0 | 1.7 | 3.1 | 1.2 | 0.1 | 0.0 | 0.0 | 3.9 | 0.6 | 7.6 |
| 6B | 9.6 | 8.7 | 58.9 | 2.8 | 5.2 | 0.4 | 0.2 | 0.2 | 1.2 | 0.6 | 0.1 | 12.1 | 0.2 | 1.9 |
| 7B | 24.0 | 21.1 | 46.6 | 0.6 | 1.1 | 0.4 | 0.7 | 0.7 | 1.3 | 0.1 | 0.1 | 3.3 | 0.2 | 0.3 |
| 8B | 38.4 | 31.7 | 15.3 | 2.3 | 4.9 | 1.0 | 2.0 | 1.5 | 1.3 | 0.1 | 0.1 | 1.4 | 0.4 | 1.6 |
| 9B | 38.9 | 31.9 | 16.8 | 2.0 | 3.9 | 0.7 | 2.0 | 1.2 | 1.1 | 0.1 | 0.1 | 1.3 | 0.5 | 1.1 |
| 10B | 36.3 | 32.8 | 15.8 | 0.5 | 6.3 | 0.5 | 0.5 | 1.2 | 4.6 | 0.0 | 0.1 | 1.4 | 0.2 | 0.4 |
| 11B | 43.3 | 37.9 | 8.4 | 0.4 | 2.5 | 0.4 | 0.4 | 1.5 | 2.1 | 0.0 | 0.0 | 3.0 | 0.2 | 0.3 |
| 12B | 16.3 | 15.3 | 11.5 | 8.8 | 19.7 | 1.0 | 0.5 | 0.7 | 3.1 | 0.4 | 0.0 | 22.8 | 1.5 | 4.5 |
| 13B | 41.9 | 29.9 | 5.8 | 2.3 | 6.2 | 1.1 | 1.5 | 1.5 | 3.8 | 0.1 | 0.0 | 5.8 | 0.3 | 1.3 |
| 14B | 39.8 | 29.8 | 3.9 | 3.6 | 8.0 | 2.0 | 1.2 | 1.2 | 2.6 | 0.0 | 0.0 | 8.0 | 0.3 | 0.4 |
| 15B | 39.3 | 29.5 | 3.6 | 4.0 | 9.3 | 1.9 | 1.1 | 1.2 | 3.4 | 0.1 | 0.0 | 6.6 | 0.7 | 1.9 |
| No. | Ga (ppm) Coal | EF Ga Coal | Ga (ppm) Ash | EF Ga Ash | Sc (ppm) Coal | EF Sc Coal | Sc (ppm) Ash | EF Sc Ash | V (ppm) Coal | EF V Coal | V (ppm) Ash | EF V Ash |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Clarke [49] | 5.8 | 33.0 | 3.9 | 23.0 | 25.0 | 155.0 | ||||||
| 1L | 2.6 | 0.2 | 17.5 | 1.5 | 1.3 | 0.1 | 6.0 | 0.3 | 12.0 | 0.1 | 70.0 | 0.6 |
| 2L | 0.6 | 0.1 | 15.8 | 1.3 | 0.6 | 0.0 | 10.0 | 0.5 | 11.0 | 0.1 | 231.0 | 1.9 |
| 3L | 0.7 | 0.1 | 4.5 | 0.4 | 0.7 | 0.0 | 3.0 | 0.1 | 6.0 | 0.1 | 27.0 | 0.2 |
| 4L | 1.3 | 0.1 | 34.8 | 2.9 | 3.4 | 0.2 | 62.0 | 2.8 | 69.0 | 0.6 | 1426.0 | 11.9 |
| 5L | 0.9 | 0.1 | 30.1 | 2.5 | 1.9 | 0.1 | 51.0 | 2.3 | 20.0 | 0.2 | 559.0 | 4.7 |
| 6L | 0.4 | 0.0 | 5.4 | 0.5 | 0.4 | 0.0 | 5.0 | 0.2 | 3.0 | 0.0 | 69.0 | 0.6 |
| 7L | 0.3 | 0.0 | 3.9 | 0.3 | 0.5 | 0.0 | 6.0 | 0.3 | 2.0 | 0.0 | 31.0 | 0.3 |
| 8L | 3.6 | 0.3 | 31.9 | 2.7 | 2.6 | 0.1 | 17.0 | 0.8 | 18.0 | 0.2 | 162.0 | 1.4 |
| 9L | 6.2 | 0.5 | 93.7 | 7.8 | 2.0 | 0.1 | 30.0 | 1.4 | 90.0 | 0.8 | 1404.0 | 11.7 |
| 10L | 5.4 | 0.5 | 32.2 | 2.7 | 6.4 | 0.3 | 35.0 | 1.6 | 58.0 | 0.5 | 363.0 | 3.0 |
| 11L | 0.6 | 0.1 | 23.8 | 2.0 | 0.4 | 0.0 | 12.0 | 0.5 | 4.0 | 0.0 | 178.0 | 1.5 |
| 12L | 5.0 | 0.4 | 33.3 | 2.8 | 8.0 | 0.4 | 43.0 | 2.0 | 25.0 | 0.2 | 176.0 | 1.5 |
| Average Lignite | 2.3 | 0.2 | 27.2 | 2.3 | 2.4 | 0.1 | 23.3 | 1.1 | 26.5 | 0.2 | 391.3 | 3.3 |
| 1SB | 3.1 | 0.3 | 19.6 | 1.6 | 3.7 | 0.2 | 18.0 | 0.8 | 37.0 | 0.3 | 226.0 | 1.9 |
| 1B | 0.8 | 0.1 | 82.3 | 6.9 | 0.9 | 0.0 | 64.0 | 2.9 | 11.0 | 0.1 | 626.0 | 5.2 |
| 2B | 3.9 | 0.3 | 54.8 | 4.6 | 2.2 | 0.1 | 32.0 | 1.5 | 17.0 | 0.1 | 277.0 | 2.3 |
| 3B | 1.7 | 0.1 | 39.4 | 3.3 | 1.8 | 0.1 | 32.0 | 1.5 | 12.0 | 0.1 | 227.0 | 1.9 |
| 4B | 2.6 | 0.2 | 51.7 | 4.3 | 2.2 | 0.1 | 33.0 | 1.5 | 37.0 | 0.3 | 554.0 | 4.6 |
| 5B | 4.0 | 0.3 | 44.4 | 3.7 | 4.1 | 0.2 | 29.0 | 1.3 | 44.0 | 0.4 | 285.0 | 2.4 |
| 6B | 0.6 | 0.1 | 11.5 | 1.0 | 0.6 | 0.0 | 10.0 | 0.5 | 15.0 | 0.1 | 210.0 | 1.8 |
| 7B | 1.8 | 0.2 | 33.1 | 2.8 | 2.0 | 0.1 | 38.0 | 1.7 | 45.0 | 0.4 | 539.0 | 4.5 |
| 8B | 0.8 | 0.1 | 55.0 | 4.6 | 0.7 | 0.0 | 45.0 | 2.0 | 26.0 | 0.2 | 1112.0 | 9.3 |
| 9B | 0.7 | 0.1 | 47.4 | 4.0 | 0.8 | 0.0 | 41.0 | 1.9 | 35.0 | 0.3 | 1277.0 | 10.6 |
| 10B | 3.1 | 0.3 | 110.4 | 9.2 | 2.0 | 0.1 | 61.0 | 2.8 | 56.0 | 0.5 | 1234.0 | 10.3 |
| 11B | 3.2 | 0.3 | 74.9 | 6.2 | 2.2 | 0.1 | 40.0 | 1.8 | 39.0 | 0.3 | 545.0 | 4.5 |
| 12B | 3.4 | 0.3 | 31.7 | 2.6 | 2.3 | 0.1 | 24.0 | 1.1 | 17.0 | 0.1 | 193.0 | 1.6 |
| 13B | 2.9 | 0.2 | 42.3 | 3.5 | 3.1 | 0.1 | 32.0 | 1.5 | 23.0 | 0.2 | 261.0 | 2.2 |
| 14B | 1.2 | 0.1 | 43.4 | 3.6 | 1.6 | 0.1 | 32.0 | 1.5 | 19.0 | 0.2 | 287.0 | 2.4 |
| 15B | 0.8 | 0.1 | 40.6 | 3.4 | 1.5 | 0.1 | 35.0 | 1.6 | 15.0 | 0.1 | 280.0 | 2.3 |
| Average bituminous coal | 2.2 | 0.2 | 48.9 | 4.1 | 2.0 | 0.1 | 35.4 | 1.6 | 28.0 | 0.2 | 508.3 | 4.2 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielowicz, B. Ash Characteristics and Selected Critical Elements (Ga, Sc, V) in Coal and Ash in Polish Deposits. Resources 2020, 9, 115. https://doi.org/10.3390/resources9090115
Bielowicz B. Ash Characteristics and Selected Critical Elements (Ga, Sc, V) in Coal and Ash in Polish Deposits. Resources. 2020; 9(9):115. https://doi.org/10.3390/resources9090115
Chicago/Turabian StyleBielowicz, Barbara. 2020. "Ash Characteristics and Selected Critical Elements (Ga, Sc, V) in Coal and Ash in Polish Deposits" Resources 9, no. 9: 115. https://doi.org/10.3390/resources9090115
APA StyleBielowicz, B. (2020). Ash Characteristics and Selected Critical Elements (Ga, Sc, V) in Coal and Ash in Polish Deposits. Resources, 9(9), 115. https://doi.org/10.3390/resources9090115





