Biofuel, Bioenergy and Feed Valorization of By-Products and Residues from Hevea brasiliensis Cultivation to Enhance Sustainability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Introduction
2.2. Materials
- PB 260
- IRCA 331
- IRCA 109
- GT1
- IRCA 41
2.3. Pre-Treatment
2.4. Chemical Oil Extraction
2.5. Mechanical Oil Extraction
2.6. Chemical-Physical Analysis of Rubber Seed Oil and By-Products/Residues
2.7. Mass and Energy Balances and Possible Production Scenarios
- Mass yield of rubber seed referred to 1 hectare equal to 150 kg ha−1 [2,4,12,13]. Higher yields are reported by other authors [27] but in the present study the lower yield was used to take into account that part of the seeds could be difficult to harvest and should be used to help preserve the ecological balance in the soil.
- According to the authors’ knowledge, there are no data in literature related to capsule yield. The value was estimated from the small quantity obtained and it should be considered as a preliminary indication. This value was also employed to estimate the rubber tree fruit yield.
- Energy content of rubber seed was calculated by energy contents and mass balances of both shell and kernel fractions.
- In the chemical extraction step ethanol was considered as solvent since it represents a more environmentally sustainable scenario.
- Masses were expressed on dry matter basis since moisture content is a parameter strongly dependent by logistic variables.
- Energies were expressed as lower heating value (LHV) to obtain a more realistic estimation of the energetic performances, keeping the dry basis as mentioned above.
- Mass and energy balance were referred to 1 ha of rubber tree cultivation
- Mass percent values were referred on whole seed basis since represents the main residue of the latex chain.
3. Results and Discussion
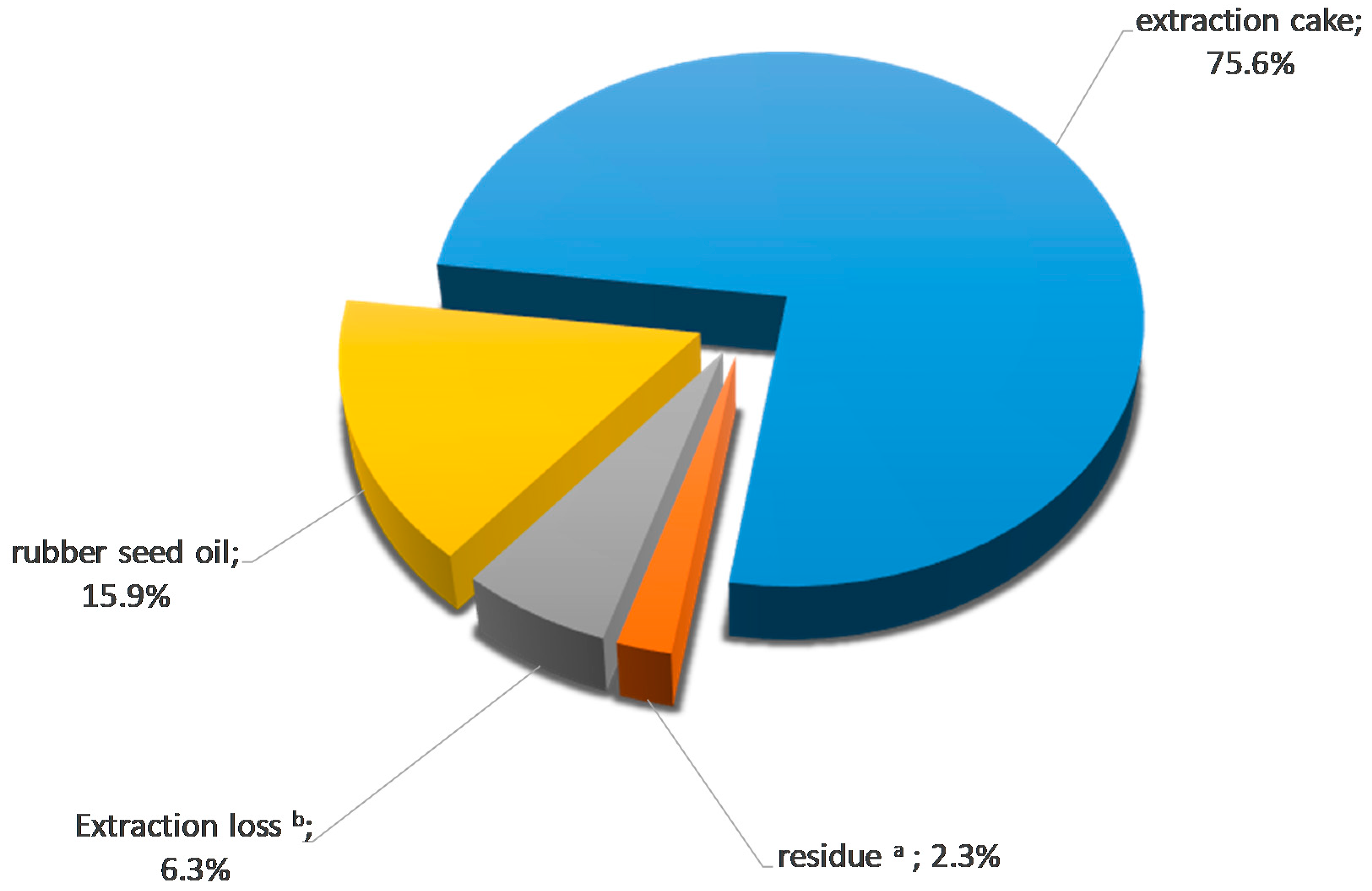
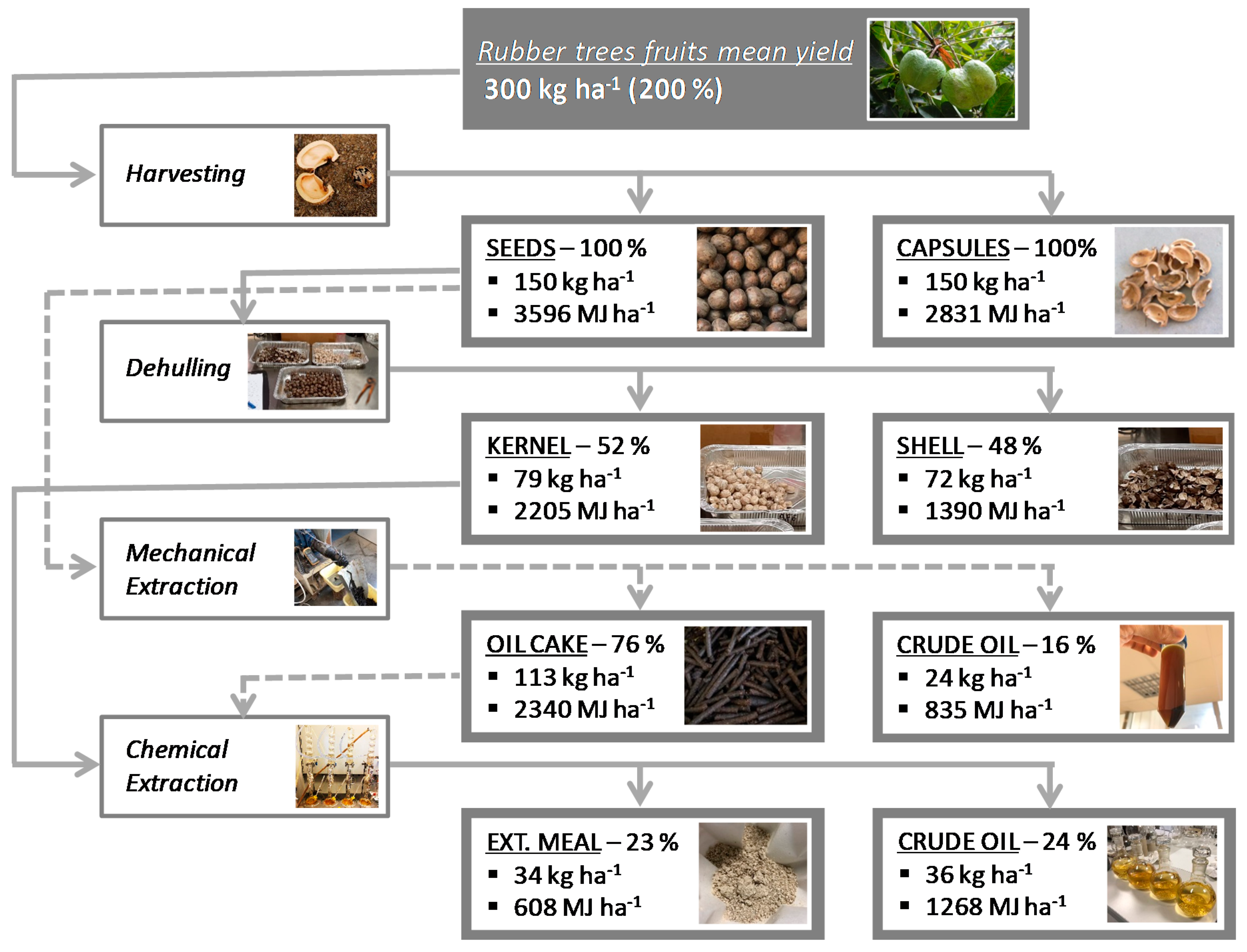
Mass and Energy Balances and Possible Production Scenarios
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ginting, E.M.; Bukit, N.; Frida, E.; Bukit, B.F. The effect of carbon black composition in natural rubber compound. Case Stud. Therm. Eng. 2019, 16, 100566. [Google Scholar]
- Ratnaningsih, E.; Sanders, J.P.M.; Bruins, M.E. Biorefinery methods for separation of protein and oil fractions from rubber seed kernel. Ind. Crops Prod. 2014, 62, 323–332. [Google Scholar]
- Bottier, C. Biochemical composition of Hevea brasiliensis latex: A focus on the protein, lipid, carbohydrate and mineral contents. Adv. Bot. Res. 2019. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Singh, H.K.G.; Yusup, S.; Abdullah, B.; Cheah, K.W.; Azmee, F.N.; Lam, H.L. Refining of crude rubber seed oil as a feedstock for biofuel production. J. Environ. Manag. 2017, 203, 1011–1016. [Google Scholar] [CrossRef]
- Onoji, S.E.; Iyuke, S.E.; Igbafe, A.I.; Daramola, M.O. Transesterification of Rubber Seed Oil to Biodiesel over a Calcined Waste Rubber Seed Shell Catalyst: Modeling and Optimization of Process Variables. Energy Fuels 2017, 31, 6109–6119. [Google Scholar] [CrossRef]
- Abubakar, B. Study on Extraction and Characterization of Rubber Seeds Oil. Aust. J. Basic Appl. Sci. 2014, 8, 7. [Google Scholar]
- Jisieike, C.F.; Betiku, E. Rubber seed oil extraction: Effects of solvent polarity, extraction time and solid-solvent ratio on its yield and quality. Biocatal. Agric. Biotechnol. 2020, 24, 101522. [Google Scholar] [CrossRef]
- Reshad, A.S.; Tiwari, P.; Goud, V.V. Extraction of oil from rubber seeds for biodiesel application: Optimization of parameters. Fuel 2015, 150, 636–644. [Google Scholar] [CrossRef]
- Reshad, A.S.; Tiwari, P.; Goud, V.V. Thermo-chemical conversion of waste rubber seed shell to produce fuel and value-added chemicals. J. Energy Inst. 2018, 91, 940–950. [Google Scholar] [CrossRef]
- Thaiyasuit, P.; Pianthong, K.; Worapun, I. Acid Esterification-Alkaline Transesterification Process for Methyl Ester Production from Crude Rubber Seed Oil. J. Oleo Sci. 2012, 61, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oluodo, L.; Huda, N.; Komilus, C. Potential Utilization of Rubber Seed Meal as Feed and Food. Int. J. Eng. Technol. 2018, 7, 64–71. [Google Scholar]
- Iyayi, A.F.; Akpaka, P.O.; Ukpeoyibo, U. Rubber seed processing for value-added latex production in Nigeria. Afr. J. Agric. Res. 2008, 3, 505–509. [Google Scholar]
- Onoji, S.E.; Iyuke, S.E.; Igbafe, A.I.; Nkazi, D.B. Rubber seed oil: A potential renewable source of biodiesel for sustainable development in sub-Saharan Africa. Energy Convers. Manag. 2016, 110, 125–134. [Google Scholar] [CrossRef]
- Babatunde, G.M.; Pond, W.G.; Peo, E.R. Nutritive value of rubber seed (Hevea brasiliensis) meal: Utilization by growing pigs of semipurified diets in which rubber seed meal partially replaced soybean meal. J. Anim. Sci. 1990, 68, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, B.M.; Salimon, J.; Yousif, E.; Salih, N. Occurrence of cyanogenic glycoside and cyanide in the Malaysian rubber seed oil. J. Assoc. Arab Univ. Basic Appl. Sci. 2013, 14, 83–86. [Google Scholar] [CrossRef]
- Salimon, J.; Abdullah, B.M.; Salih, N. Rubber (Hevea brasiliensis) seed oil toxicity effect and Linamarin compound analysis. Lipids Health Dis. 2012, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Sudyoadsuk, T.; Promarak, V. Rubber seed oil as potential non-edible feedstock for biodiesel production using heterogeneous catalyst in Thailand. Renew. Energy 2017, 101, 937–944. [Google Scholar] [CrossRef]
- Morshed, M.; Ferdous, K.; Khan, M.R.; Mazumder, M.S.I.; Islam, M.A.; Uddin, M.T. Rubber seed oil as a potential source for biodiesel production in Bangladesh. Fuel 2011, 90, 2981–2986. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Aravind, A.; Joy, M.L.; Nair, K.P. Lubricant properties of biodegradable rubber tree seed (Hevea brasiliensis Muell. Arg) oil. Ind. Crops Prod. 2015, 74, 14–19. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Characterization and effect of using rubber seed oil as fuel in the compression ignition engines. Renew. Energy 2005, 30, 795–803. [Google Scholar] [CrossRef]
- Hassan, S.N.A.M.; Ishak, M.A.M.; Ismail, K.; Ali, S.N.; Yusop, M.F. Comparison study of rubber seed shell and kernel (Hevea brasiliensis) as raw material for bio-oil production. Energy Procedia 2014, 52, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Chaireh, S.; Szécsényi, K.M.; Boonsuk, P.; Kaewtatip, K. Preparation of rubber seed shell powder by planetary ball milling and its influence on the properties of starch foam. Ind. Crops Prod. 2019, 135, 130–137. [Google Scholar] [CrossRef]
- Chin, B.L.F.; Yusup, S.; Al Shoaibi, A.; Kannan, P.; Srinivasakannan, C.; Sulaiman, S.A. Comparative studies on catalytic and non-catalytic co-gasification of rubber seed shell and high density polyethylene mixtures. J. Clean. Prod. 2014, 70, 303–314. [Google Scholar] [CrossRef]
- Ishak, M.A.M.; Hassan, S.N.A.M.; Jawad, A.H.; Ismail, K. Characterization of Rubber Seed Shell and Kernel (Hevea brasiliensis) as Raw Materials for Coliquefaction with Low Rank Coal. Ann. Chem. Sci. Res. 2019, 1. [Google Scholar] [CrossRef] [Green Version]
- Wagner, M.; Lippe, M.; Lewandowski, I.; Salzer, M.; Cadisch, G. CO2 Footprint of the Seeds of Rubber (Hevea brasiliensis) as a Biodiesel Feedstock Source. Forests 2018, 9, 548. [Google Scholar] [CrossRef] [Green Version]
- Baümler, E.R.; Carrín, M.E.; Carelli, A.A. Diffusion of tocopherols, phospholipids and sugars during oil extraction from sunflower collets using ethanol as solvent. J. Food Eng. 2017, 194, 1–8. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Garavazo, B.R.; Rodrigues, C.E.C. Liquid-liquid equilibria for systems composed of rice bran oil and alcohol-rich solvents: Application to extraction and deacidification of oil. J. Food Eng. 2012, 110, 418–427. [Google Scholar] [CrossRef]
- Salni, S.; Hariani, P.L.; Hanifa, H.M. Influence the Rubber Seed Type and Altitude on Characteristic of Seed, Oil and Biodiesel. Int. J. Renew. Energy Dev. 2017, 6, 157. [Google Scholar] [CrossRef] [Green Version]
- Abduh, M.Y.; Manurung, R.; Heeres, H.J. The influence of storage time on relevant product properties of rubber seeds, rubber seed oil and rubber seed oil ethyl esters. Sustain. Chem. Process. 2016, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- ISO 17225-2. Solid Biofuels—Fuel Specifications and Classes—Part. 2: Graded Wood Pellets; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- ISO/TS 17225-9. Solid Biofuels—Fuel Specifications and Classes—Part. 9: Graded Hog Fuel and Wood Chips for Industrial Use; Internetional Organization for Standardization: Geneva, Switzerland, 2020. [Google Scholar]
- Mohd-Setapar, S.H.; Nian-Yian, L.; Mohd-Sharif, N.S. Extraction of rubber (hevea brasiliensis) seed oil using soxhlet method. Malays. J. Fundam. Appl. Sci. 2014, 10. [Google Scholar] [CrossRef] [Green Version]
- Attah, J.C.; Ibemesi, J.A. Solvent extraction of the oils of rubber, melon, pumpkin and oilbean seeds. J. Am. Oil Chem. Soc. 1990, 67, 25–27. [Google Scholar] [CrossRef]
- Wildana, A.; Ingrid, A.D.; Hartati, I. Oil Extraction Process from Solid Waste Rubber Seed by Soxhletation and Extraction Solvent by Stirring Methods. In Proceedings of the International Conference on Chemical and Material Engineering, Semarang, Indonesia, 12–13 September 2012. [Google Scholar]
- Ebewele, R.; Iyayi, A.; Hymore, F. Considerations of the extraction process and potential technical applications of Nigerian rubber seed oil. Int. J. Phys. Sci. 2010, 5, 826–831. [Google Scholar]
- Baümler, E.; Carrín, M.; Carelli, A. Extraction of sunflower oil using ethanol as solvent. J. Food Eng. 2016, 178. [Google Scholar] [CrossRef]
- Sabarish, C.S.; Sebastian, J.; Muraleedharan, C. Extraction of Oil from Rubber Seed through Hydraulic Press and Kinetic Study of Acid Esterification of Rubber Seed Oil. Procedia Technol. 2016, 25, 1006–1013. [Google Scholar] [CrossRef] [Green Version]
- Aigbodion, A.I.; Bakare, I. Rubber seed oil quality assessment and authentication. J. Am. Oil Chem. Soc. 2005, 82, 465–469. [Google Scholar] [CrossRef]
- Gensa, U. Review on Cyanide Poisoning in Ruminants. J. Biol. Agric. Healthcare 2019, 9, 12. [Google Scholar]
- Udo, M.D.; Ekpo, U.; Ahamefule, F.O. Effects of processing on the nutrient composition of rubber seed meal. J. Saudi Soc. Agric. Sci. 2018, 17, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Onoji, S.E.; Iyuke, S.E.; Igbafe, A.I. Hevea brasiliensis (Rubber Seed) Oil: Extraction, Characterization, and Kinetics of Thermo-oxidative Degradation Using Classical Chemical Methods. Energy Fuels 2016, 30, 10555–10567. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Mortimer, P.E. The influence of seed and oil storage on the acid levels of rubber seed oil, derived from Hevea brasiliensis grown in Xishuangbanna, China. Energy 2011, 36, 5403–5408. [Google Scholar] [CrossRef]
- Sai, B.A.V.S.L.; Subramaniapillai, N.; Mohamed, K.; Begum, M.S.; Narayanan, A. Optimization of Continuous Biodiesel Production from Rubber Seed Oil (RSO) using Calcined Eggshells as Heterogeneous Catalyst. J. Environ. Chem. Eng. 2019, 103603. [Google Scholar] [CrossRef]
- EN 14214. Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) For Use in Diesel Engines and Heating Applications—Requirements and Test Methods; European Committee for Standardization: Brussels, Belgium, 2012. [Google Scholar]
- Devi, V.N.M.; Prasad, P.N.; Syndia, L.A.M.; Rajakohila, M.; Ariharan, V.N. Physicochemical characterization of rubber seed oil (Hevea brasiliensis)—A promising feedstock for biodiesel production. Int. J. Chem. Anal. Sci. 2012, 3, 1402–1404. [Google Scholar]
| Parameter | Unit of Measure 1 | Reference Method | Principle | Fractions Analyzed 2 |
|---|---|---|---|---|
| Proximate analysis | ||||
| Moisture content | % a.r. | ISO 18134-2 | Drying at 105 ± 2 °C in a forced ventilation oven (mod. M120-VF, MPM Instruments, Bernareggio, Italy) | Cap, Sh, Ker, Flo, Sd |
| Ash content | % d.m. | ISO 18122 | Ignition at 550 ± 10 °C in a thermogravimetric analyzer (mod. TGA 701, LECO, St Joseph, MI, USA) | Cap, Sh, Ker, Flo |
| HHV/LHV | MJ kg−1 | ISO 18125 | Combustion in a bomb calorimeter (mod.C2000 basic, IKA, Staufen im Breisgau, Germany), LHV calculated considering H content | Cap, Sh, Ker, Flo |
| Ultimate analysis | ||||
| Chlorine and Sulfur content | % d.m. | ISO 16994 | Acid combustion gases absorption (calorimetric bomb) and measuring by ion chromatography (mod. 761 IC, Metrohm, Formello, Roma, Italy) | Cap, Sh, Ker, VO |
| Elemental analysis (CHN/O) | % d.m. | ISO 16948 | Analysis with an elemental analyzer (mod. 2400 Series II CHNS/O, Perkin Elmer, Milano, Italy). Oxygen content calculated by difference | Cap, Sh, Ker |
| Feed analysis | ||||
| Crude fiber | % d.m. | ISO 6865 | Gravimetric determination of residue obtained after acid and alkaline digestion | Flo |
| Ether extract | % d.m. | ISO 6492 | Solvent extraction (ether) with a Soxhlet apparatus | Flo |
| Crude protein | % d.m. | ISO 16634-1 | Nitrogen content determination (mod. 2400 Series II CHNS/O, Perkin Elmer, Milano, Italy) and calculation by conversion factor | Flo |
| Nitrogen free extractives | % d.m. | calculated | calculated by difference | Flo |
| Cyanides | ppm | ISO 2164 | Steam distillation and titration with silver nitrate | Flo |
| RSO analysis | ||||
| Kinematic viscosity | cSt | ISO 3104 | Determination with a Cannon-Fenske viscometer tube at 40 °C | VO |
| Density | g cm−3 | ISO 12185 | Determination with oscillating U-tube density meter (mod. Minivis 445, Grabner Instruments Messtechnik, Wien, Austria) | VO |
| Iodine number | gI2 100∙g−1 | ISO 3961 | Iodometric titration (Wijs reagent, potassium iodide and sodium thiosulfate as titrant) | VO |
| Acid number | mg KOH g−1 | ISO 660 | Titration with KOH | VO |
| Saponification number | mg KOH g−1 | ISO 3657 | Saponification (potassium hydroxide) and titration with HCl | VO |
| Fatty acid composition | % | ISO 12966 | FAME production by transmethylation/methylation with boron trifluoride and GC-FID analysis | VO |
| Variety | Seed Moisture | Kernel | Shell |
|---|---|---|---|
| (% w w−1 a.r.) | (% w w−1 d.m.) | (% w w−1 d.m.) | |
| GT1 | 11.9 a | 51.1 a | 48.9 b |
| IRCA 109 | 14.3 a | 50.5 a | 49.5 b |
| IRCA 331 | 19.4 b | 56.8 b | 43.2 a |
| IRCA 41 | 14.6 a | 51.2 a | 48.8 b |
| PB 260 | 20.5 b | 51.8 a | 48.2 b |
| Mean | 16.1 | 52.3 | 47.7 |
| St.Dev. | 3.7 | 2.6 | 2.6 |
| Variety | Fraction | Moisture | Ash | HHV | LHV | C | H | N | O | Cl | S |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (% w w−1a.r.) | (% w w−1d.b.) | (kJ g−1d.m.) | (kJ g−1a.r.) | (% w w−1d.b.) | |||||||
| GT1 | shell | 13.4 b | 0.38 a | 21.07 d | 17.23 b | 51.4 | 5.1 | 0.4 b | 42.7 | <0.01 | 0.01 |
| kernel | 10.5 ab | 3.23 c | 29.22 fg | 25.34 f | 60.7 | 5.7 | 3.3 e | 26.9 | 0.02 | 0.17 | |
| IRCA 109 | shell | 14.8 b | 0.45 a | 20.54 bc | 16.44 a | 50.7 | 5.6 | 0.9 c | 42.3 | 0.01 | 0.01 |
| kernel | 13.7 b | 3.28 c | 28.82 e | 24.14 e | 60.6 | 5.0 | 3.6 fg | 27.4 | 0.02 | 0.11 | |
| IRCA 331 | shell | 16.7 b | 0.41 a | 20.47 bc | 16.12 a | 51.5 | 5.6 | 0.4 b | 42.1 | 0.01 | 0.01 |
| kernel | 21.3 c | 3.40 c | 29.11 ef | 22.87 d | 63.2 | 5.2 | 3.7 g | 24.4 | 0.02 | 0.10 | |
| IRCA 41 | shell | 14.4 b | 0.48 a | 20.64 c | 16.78 ab | 51.2 | 5.6 | 0.4 b | 42.3 | <0.01 | 0.01 |
| kernel | 14.7 b | 3.14 c | 28.91 e | 24.46 e | 52.5 | 4.9 | 2.8 d | 36.6 | 0.01 | 0.10 | |
| PB 260 | shell | 16.8 b | 0.83 a | 20.28 ab | 16.18 a | 50.3 | 5.6 | 0.5 b | 42.8 | 0.01 | 0.02 |
| kernel | 23.6 c | 3.19 c | 29.48 g | 23.81 e | 60.2 | 4.8 | 3.4 ef | 30.3 | 0.02 | 0.11 | |
| Capsule | Capsule | 8.0 a | 1.90 b | 20.04 a | 18.87 c | 50.2 | 5.5 | <0.1 a | 42.3 | 0.01 | 0.03 |
| Mean | 16.0 | 1.88 | 24.86 | 20.34 | 55.2 | 5.3 | 1.9 | 35.8 | 0.02 | 0.06 | |
| St.Dev. | 3.5 | 0.14 | 0.28 | 0.68 | 2.3 | 0.3 | 0.3 | 2.5 | <0.01 | 0.02 | |
| ISO 17225-2 | A1 limit | 10 | 0.7 | 16.5 ≤ LHV ≤ 19.0 | - | - | 0.3 | - | 0.02 | 0.03 | |
| A2 limit | 10 | 1.5 | 16.3 ≤ LHV ≤ 19.0 | - | - | 0.5 | - | 0.02 | 0.03 | ||
| ISO/TS 17225-9 | I1 limit | 45 | 3.0 | - | - | - | 0.5 | - | 0.05 | 0.05 | |
| I4 linit | 60 | 7.0 | - | - | - | 1.5 | - | 0.10 | 0.10 | ||
| Variety | Solvent | Oil 1 | Flour | Residue |
|---|---|---|---|---|
| (% w w−1db) | (% w w−1db) | (% w w−1db) | ||
| GT1 | Ethanol | 43.6 a | 45.0 b | 11.4 |
| Hexane | 48.8 de | 51.2 de | - | |
| IRCA 109 | Ethanol | 47.0 c | 43.4 a | 9.6 |
| Hexane | 47.9 cd | 52.1 e | - | |
| IRCA 331 | Ethanol | 47.6 cd | 42.8 a | 9.6 |
| Hexane | 49.5 ef | 50.5 cd | - | |
| IRCA 41 | Ethanol | 45.1 b | 43.4 a | 11.4 |
| Hexane | 48.6 de | 51.4 de | - | |
| PB 260 | Ethanol | 47.9 cd | 42.5 a | 9.6 |
| Hexane | 50.2 f | 49.8 c | - | |
| Mean | 47.6 | 47.2 | 10.3 | |
| St.Dev. | 1.5 | 1.0 | 1.0 |
| Variety | Solvent | Moisture | Dry Matter | Gross Energy | Ash | Organic Matter | Crude Protein | Ether Extract | Crude Fiber | Non-Nitrogen Extractives |
|---|---|---|---|---|---|---|---|---|---|---|
| (% w w−1a.r.) | (% w w−1a.r.) | (kJ g−1d.m.) | (% w w−1d.b.) | (% w w−1d.b.) | (% w w−1d.b.) | (% w w−1d.b.) | (% w w−1d.b.) | (% w w−1d.b.) | ||
| GT1 | Ethanol | 9.8 de | 90.3 ab | 19.94 bc | 6.1 bc | 93.9 c | 40.7 bc | 1.1 cd | 4.2 bd | 48.0 bc |
| Hexane | 6.8 bc | 93.3 cd | 18.72 ab | 6.0 ab | 94.0 cd | 38.3 b | 0.9 ab | 4.3 cd | 50.5 bc | |
| IRCA 109 | Ethanol | 10.5 e | 89.5 a | 19.10 b | 7.2 e | 92.8 a | 44.4 d | 1.2 d | 4.5 cd | 42.7 a |
| Hexane | 6.8 bc | 93.3 cd | 19.70 bc | 6.2 bc | 93.8 c | 38.9 b | 0.8 a | 3.8 a | 50.4 bc | |
| IRCA 331 | Ethanol | 10.3 e | 89.7 a | 18.03 ab | 6.9 de | 93.1 ab | 42.2 cd | 0.9 ab | 4.1 ac | 46.0 ab |
| Hexane | 8.2 cd | 91.8 bc | 18.48 ab | 6.3 bc | 93.7 c | 32.1 a | 1.0 bc | 4.2 bd | 56.4 d | |
| IRCA 41 | Ethanol | 6.9 bc | 93.1 cd | 20.01 c | 6.7 cd | 93.3 b | 45.4 d | 0.9 ab | 4.9 e | 50.7 c |
| Hexane | 2.8 a | 97.2 e | 19.86 bc | 5.7 a | 94.3 d | 38.4 b | 0.9 ab | 4.4 cd | 42.3 a | |
| PB 260 | Ethanol | 7.8 c | 92.2 c | 18.40 ab | 6.9 de | 93.1 ab | 45.6 d | 0.9 ab | 4.5 cd | 49.6 bc |
| Hexane | 5.6 b | 94.4 d | 17.87 a | 6.4 c | 93.6 bc | 39.2 b | 1.1 cd | 3.9 ab | 42.0 a | |
| Mean | 7.5 | 92.5 | 19.02 | 6.5 | 93.5 | 40.1 | 1.0 | 4.3 | 47.8 | |
| St.Dev. | 1.8 | 1.8 | 0.87 | 0.4 | 0.4 | 2.6 | 0.1 | 0.3 | 4.6 |
| Variety | Solvent | Acid Number | Iodine Number | Saponification Number | Fatty Acids Composition (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg KOH g−1 | g I2 100 g−1 | mg KOH g−1 | C16:0 | C18:0 | C18:1 n−9 | C18:1 n−7 | C18:2 n−6 | C18:3 n−3 | C20:0 | ||
| GT1 | Ethanol | 39.2 | 129.3 | 185.6 | 10.0 | 8.8 | 23.4 | <0.1 | 36.9 | 20.7 | 0.2 |
| Hexane | 76.2 | 126.4 | 191.2 | 11.0 | 9.3 | 22.1 | <0.1 | 38.0 | 19.5 | 0.1 | |
| IRCA 109 | Ethanol | 28.1 | 127.3 | 191.7 | 12.9 | 12.0 | 36.2 | <0.1 | 24.4 | 14.5 | <0.1 |
| Hexane | 105.7 | 127.9 | 188.4 | 13.0 | 12.3 | 36.0 | <0.1 | 25.2 | 13.5 | <0.1 | |
| IRCA 331 | Ethanol | 32.2 | 125.2 | 188.4 | 13.5 | 13.4 | 35.8 | 0.4 | 23.6 | 13.3 | <0.1 |
| Hexane | 84.9 | 129.5 | 192.3 | 14.0 | 12.4 | 34.6 | <0.1 | 27.1 | 11.8 | <0.1 | |
| IRCA 41 | Ethanol | 29.0 | 126.4 | 194.3 | 11.5 | 9.8 | 32.1 | 0.3 | 31.0 | 15.3 | <0.1 |
| Hexane | 100.4 | 125.2 | 189.7 | 11.9 | 10.2 | 27.3 | 0.5 | 37.6 | 12.4 | 0.1 | |
| PB 260 | Ethanol | 25.3 | 124.6 | 189.9 | 13.6 | 12.8 | 35.7 | <0.1 | 26.5 | 11.3 | 0.1 |
| Hexane | 66.0 | 131.9 | 191.3 | 10.1 | 8.1 | 37.2 | 0.4 | 32.3 | 11.9 | <0.1 | |
| Mean | Ethanol | 30.8 | 126.6 | 190.0 | 12.3 | 11.4 | 32.6 | 0.2 | 28.5 | 15.0 | 0.1 |
| St.dev. | 5.3 | 1.9 | 3.8 | 1.5 | 2.0 | 5.4 | 0.1 | 5.5 | 3.5 | 0.1 | |
| Mean | Hexane | 86.6 | 128.2 | 190.4 | 12.0 | 10.5 | 31.4 | 0.2 | 32.0 | 13.8 | 0.1 |
| St.dev. | 16.5 | 2.6 | 1.7 | 1.6 | 1.9 | 6.5 | 0.1 | 5.9 | 3.2 | <0.1 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzi, A.; Duca, D.; Rossini, G.; Fabrizi, S.; Toscano, G. Biofuel, Bioenergy and Feed Valorization of By-Products and Residues from Hevea brasiliensis Cultivation to Enhance Sustainability. Resources 2020, 9, 114. https://doi.org/10.3390/resources9090114
Pizzi A, Duca D, Rossini G, Fabrizi S, Toscano G. Biofuel, Bioenergy and Feed Valorization of By-Products and Residues from Hevea brasiliensis Cultivation to Enhance Sustainability. Resources. 2020; 9(9):114. https://doi.org/10.3390/resources9090114
Chicago/Turabian StylePizzi, Andrea, Daniele Duca, Giorgio Rossini, Sara Fabrizi, and Giuseppe Toscano. 2020. "Biofuel, Bioenergy and Feed Valorization of By-Products and Residues from Hevea brasiliensis Cultivation to Enhance Sustainability" Resources 9, no. 9: 114. https://doi.org/10.3390/resources9090114
APA StylePizzi, A., Duca, D., Rossini, G., Fabrizi, S., & Toscano, G. (2020). Biofuel, Bioenergy and Feed Valorization of By-Products and Residues from Hevea brasiliensis Cultivation to Enhance Sustainability. Resources, 9(9), 114. https://doi.org/10.3390/resources9090114







