Abstract
Understanding how rising temperatures, ocean acidification, and hypoxia affect the performance of coastal fishes is essential to predicting species-specific responses to climate change. Although a population’s habitat influences physiological performance, little work has explicitly examined the multi-stressor responses of species from habitats differing in natural variability. Here, clearnose skate (Rostaraja eglanteria) and summer flounder (Paralichthys dentatus) from mid-Atlantic estuaries, and thorny skate (Amblyraja radiata) from the Gulf of Maine, were acutely exposed to current and projected temperatures (20, 24, or 28 °C; 22 or 30 °C; and 9, 13, or 15 °C, respectively) and acidification conditions (pH 7.8 or 7.4). We tested metabolic rates and hypoxia tolerance using intermittent-flow respirometry. All three species exhibited increases in standard metabolic rate under an 8 °C temperature increase (Q10 of 1.71, 1.07, and 2.56, respectively), although this was most pronounced in the thorny skate. At the lowest test temperature and under the low pH treatment, all three species exhibited significant increases in standard metabolic rate (44–105%; p < 0.05) and decreases in hypoxia tolerance (60–84% increases in critical oxygen pressure; p < 0.05). This study demonstrates the interactive effects of increasing temperature and changing ocean carbonate chemistry are species-specific, the implications of which should be considered within the context of habitat.
1. Introduction
Marine climate change is multidimensional and includes (but is not limited to) rising temperatures, increasing severity and frequency of hypoxic events, and ocean acidification (OA) [1,2,3,4]. These three environmental changes result in interactive, yet poorly understood impacts on both individuals and populations [5,6]. Increases in temperature alone are associated with reduced aerobic scope, and thereby reduced fitness [7,8]. Hypoxia likewise reduces fitness and can cause mortality events [9], while OA can impact various aspects of a species’ biology from behavior to growth rates [10]. A comprehensive understanding of the impacts of climate change on species and populations is, therefore, required both to manage fisheries effectively and to conserve ecologically and economically important resources [11,12,13].
Because it is impossible to fully incorporate the complexity of ecological interactions (e.g., interspecific interactions, regional population distributions, seasonal variability) in models designed to predict the effects of climate change, researchers have attempted to assess vulnerability or resilience using other methods. Testing environmental tolerances, and thus potential for resilience in the face of climate change, is commonly done using aerobic metabolic rate as a proxy for fitness [2,7,14]. Intermittent-flow respirometry measures rates of oxygen consumption that can be used to calculate a range of metabolic parameters including standard metabolic rate (SMR), maximum metabolic rate (MMR), absolute aerobic scope (ASa), factorial aerobic scope (ASf), and critical oxygen level [15]. Critical oxygen level (Scrit) is the lowest oxygen level at which SMR remains stable, and below which metabolic rate declines in step with decreases in ambient oxygen [16]. The critical oxygen level can be measured in terms of percent saturation, oxygen content, or partial pressure and is denoted Scrit, Ccrit, or Pcrit respectively depending on the units used [17]. Combined, metabolic parameters can assess species- or population-specific tolerances to environmental perturbations, such as those associated with climate change [11,18,19,20]. According to the theory of oxygen- and capacity-limited thermal tolerance (OCLTT) [21], for example, aerobic scope will decline at sub-optimal temperatures because the ability of the cardio-respiratory system to supply oxygen to tissues is reduced. The applicability of this theory is still debated [22], measuring aerobic scope likely can provide information on species-specific physiological abilities and tolerances necessary to predict the effects of shifting environmental conditions [23,24].
Most research concerning the effects of environmental stressors on marine organisms has focused on temperature or pH changes projected to occur over the next 50–100 years in the open ocean [25]. Such studies must, however, reconcile the uncertainty in environmental forecasting with the difficulty of accounting for transgenerational effects [26], the ability of species to alter their distributions [27], and localized adaptation [5]. Additionally, while many perturbation experiments have focused on the specifics of various laboratory treatments, less attention has been given to the natural short-term fluctuations in environmental conditions encountered by coastal species throughout their range or over ontogeny [12,28,29].
Estuarine environments exhibit regular, acute hypoxia and pH variability [30,31], and species inhabiting these environments likely possess the physiological abilities necessary to withstand a broad range of environmental conditions [32], potentially providing some degree of resiliency to the environmental shifts associated with climate change. While there is evidence that species living in variable habitats (such as rocky intertidal pools) are already living near the limits of their physiological capabilities [33], little research has been done to explicitly compare the tolerances of fishes from variable estuarine environments to those from more stable habitats (e.g., higher latitudes or deeper waters) [28]. Species (or populations) inhabiting variable temperate environments tend to be eurythermal, whereas species (or populations) occupying high latitudes tend to be stenothermal [34,35], likely because of the relatively narrow range of temperatures encountered by any given individual [36]. As temperature has a large impact on the metabolism of ectotherms [37,38,39], a comparison between the thermal tolerances of species inhabiting variable and stable habitats may provide insight into species-specific physiological abilities [6,28].
The east coast of the United States includes habitats that differ greatly with respect to their environmental variability [29,31,40,41]. In the mid-Atlantic, inshore species often utilize salt marsh lagoons during the summer, where daily oscillations of ±5 °C are frequently accompanied by fluctuations in pH (±0.5 pH units) and dissolved oxygen (±4.5 mg L−1) [31,42]. As climate change effects continue to manifest, these environments are likely to experience even greater swings in temperature, pH, and dissolved oxygen (DO) [43,44,45], which are likely to affect fish species such as the clearnose skate (Rostaraja eglanteria) and summer flounder (Paralichthys dentatus). Clearnose skate range from the Gulf of Mexico to Cape Cod, USA [46], and are common in the tidal lagoons along the mid-Atlantic. They occur over a temperature range from ~9–30 °C, but prefer ~9–21 °C [47,48]. Summer flounder utilize near-shore regions during the summer, but migrate offshore to spawn in the fall and prefer temperatures between 9 and 24 °C [49,50]. In contrast, the Gulf of Maine is a more stable environment [40], with benthic temperature fluctuations limited to ~3 °C over the entire year [51]. Thorny skate (Amblyraja radiata) inhabit the Gulf of Maine and are most abundant between 1 and 5 °C [52], thus occupying a habitat that is very different from that (at least during the summer months) of clearnose skate or summer flounder.
We thus sought to quantify the effects of acute temperature change and elevated pCO2 levels on the aerobic metabolic rates and hypoxia tolerances of clearnose skate, summer flounder, and thorny skate. This approach allowed us to compare the physiological abilities of sympatric elasmobranch and teleost species (clearnose skate and summer flounder, respectively), as well as allopatric elasmobranch species (clearnose and thorny skates).
2. Materials and Methods
All capture, handling, and experimental protocols were approved by the College of William and Mary and University of New England Institutional Animal Care and Use Committee (IACUC-2017-03-14-11935-rwbril and IACUC-012418-003, respectively). Clearnose skate and summer flounder were collected from the Eastern Shore of Virginia using rod and reel during the summers of 2016 and 2017, and maintained in recirculating systems at the Virginia Institute of Marine Science (VIMS) Eastern Shore Laboratory at 20–22 °C. Thorny skate were collected from the Gulf of Maine using a commercial otter trawl [53] in February 2018, and maintained in flow through systems at the seawater laboratory at the University of New England at 5, 9, or 13 °C. All individuals were given at least two weeks to acclimate to captivity before use in experimental trials and were fed ad libitum every 2–3 days. Individuals were fasted for 48 h prior to use in an experiment to ensure they were in a post-absorptive state [54].
A total of 24 clearnose skates and 17 thorny skates were subjected to three to four trials each, resulting in eight trials at each of the three temperatures representing the mid- to upper-range of thermal tolerances (20, 24, 28 °C for clearnose skate; 5, 9, 13 °C for thorny skate), under two CO2 conditions representing the present day and that predicted to occur by the end of the century (pH of 7.8 and 7.4, respectively) [55,56]. A total of eight summer flounder were subjected to two trials each at 22 and 30 °C under elevated pCO2 conditions (pH 7.4). Because the most extreme treatment (28 °C and elevated pCO2) resulted in 40% mortality in preliminary trials on clearnose skate, this treatment was discontinued. The equivalent treatment (13 °C and elevated pCO2) of thorny skate experiments was likewise excluded.
Clearnose and thorny skates were acclimated to trial conditions for 48 h and one week, respectively. For clearnose skate, MMR was obtained using an established chase protocol involving enforced exercise (i.e., chasing and turning individuals over to induce swimming until they no longer responded to tactile stimulus) followed by one minute of air exposure [5]. Thorny skate respond to being handled by curling into a ball, and thus could not be chased. Instead, this species was air exposed for eight minutes. Respirometry protocols used with summer flounder were modified from those of Capossela et al. [54], in that fish were not fitted with additional sensors to measure exhalent oxygen. As Capossela et al. [54] only measured SMR and Pcrit, those were the only variables measured from the summer flounder here. These were calculated as described below. We thus re-analyzed the data from Capossela et al. [54] along with the elevated pCO2 data we collected for this study.
Following the chase and/or air exposure protocols, individuals were placed in custom-built Plexiglas respirometers constructed to ensure that the volume to animal mass ratio fell between 30:1 and 50:1. The chambers were equipped with fiber optic oxygen sensors and a recirculating pump, as recommended by Rogers et al. [57]. The respirometry chambers were placed in an outer water bath from which water was used to flush the respirometer. A computer program (developed in Dasylab 13.1; National Instruments, www.ni.com) logged data and continuously controlled temperature and oxygen levels for 36 h. Each measurement cycle lasted 10 min and began with a 5 min flush cycle, which was terminated when the flush pump was turned off. After allowing 2 min for the water in the measurement system to mix, the decline in oxygen over a 5 min period was recorded before the flush pump was turned on again. At no time during normoxic trials was the chamber oxygen level allowed to fall below 80%. At the end of each data recording interval, the Dasylab software executed a call to an Excel macro routine that calculated the rate of change of O2 content (converted from percent saturation) with time (Δ[O2] × t−1) based on a linear regression of the recorded oxygen levels against elapsed time (t). The Excel macro routine subsequently calculated MO2 as follows:
where V—respirometer volume (l) corrected for fish volume and W—fish mass (kg). To estimate microbial oxygen consumption, rates of O2 depletion were measured both before and after the trial (i.e., when the fish was not in the chamber). We used linear regression to estimate rates of oxygen depletion due to microbial respiration occurring over the time course of an experiment. These values were then subtracted from the measured rates of oxygen decline.
MO2 = (Δ [O2] × t−1) × V × W−1
MMR was taken as the single highest metabolic rate measured during the first 12 h following the chase and/or air exposure protocols. For all three species, SMR was taken as the mean of the lowest 10 metabolic rates during the middle 12 h of the trial. Aerobic scope was calculated in two ways (ASa = MMR − SMR; ASf = MMR × SMR−1). Following SMR measurements, oxygen was reduced in a step-wise fashion, with three measurements taken at 80, 60, 40, 30, 20, and 10% O2 saturation. Trials were terminated when MO2 dropped to zero and individuals were then allowed to recover in fully oxygenated seawater for 1 h before being returned to holding tanks. Scrit was defined as the point at which an individual could no longer maintain SMR. This was done following Schurmann and Steffensen [58], where metabolic rate measurements below SMR were evaluated to determine if all subsequent values were also below SMR. When this was true, this subset of points was fit with a linear regression. The oxygen saturation where this regression line and SMR intersected was defined as Scrit (Figure 1). From these, we calculated the critical oxygen content (Ccrit) by converting the percent saturation to mg L−1 using known temperature and salinity values. We also calculated critical oxygen partial pressure (Pcrit) by determining the partial pressure of oxygen at 1 atmosphere on the day of the start of the trial, calculating the percent saturation of seawater, and multiplying that by the temperature- and salinity-specific oxygen content of seawater at full saturation.
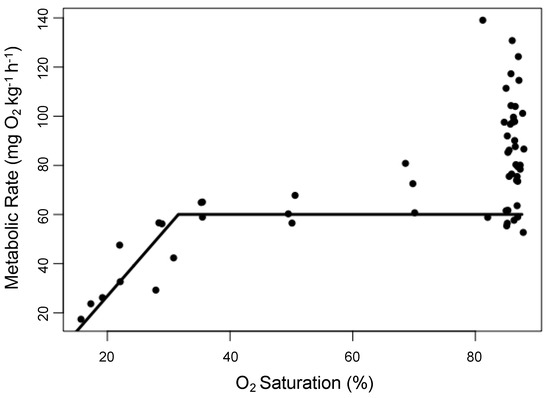
Figure 1.
Data from a single respirometry trial. Scrit is the intersection of the two linear regression lines.
To better compare across different temperature ranges, we calculated Q10 values for SMR as follows:
where Q10 is the temperature coefficient for SMR, R1 is the SMR at T1, and R2 is the SMR at T2.
To increase pCO2, and thus reduce pH, we used the standard method of bubbling CO2 gas [59]. A stand-alone system (TUNZE 7074; Penzberg, Germany), connected to a laboratory-grade glass pH probe in the outer water bath, controlled an electronic solenoid valve connected to a cylinder of CO2. The system injected a slow stream of CO2 into the outer water bath whenever pH of the seawater rose above the set point. Using this method, it was possible to maintain pH within ±0.05 units of the desired level, and it was unlikely that there was a biologically significant gradient in seawater pH within the tanks, as the outer water bath was continuously mixed by a submersible pump.
The pH of each outer water bath in the low pH treatment was independently validated at the start and end of each trial using a SDL100 pH meter (Extech Instruments, Nashua, NH, USA) calibrated daily with fresh pH buffers (Tunze, Penzberg, Germany). Additionally, water samples were taken at the start and end of each trial for dissolved inorganic carbon (DIC) and total alkalinity (TA) Automated InfraRed Inorganic Carbon Analyzer (AIRICA) and Metrohm analyses, or for spectrophotometric determination of pH and TA Metrohm analysis [60]. All pH values were subsequently calculated from these additional measurements using the CO2SYS software [61] with the constants K1 from Mehrbach et al. [62] (refit by Dickson and Millero [63]), and KHSO4 from Dickson et al. [60]. Data on the seawater chemistry of present day pCO2 trials were obtained from 10 samples taken from the water inflow to the seawater labs in Virginia and Maine (Table 1). Equivalent seawater chemistry data for the summer flounder experiments performed by Capossela et al. [54] were not available.

Table 1.
The carbonate chemistry parameters during the high and low pH (i.e., present day and elevated pCO2) experiments. Values are averaged across all temperature treatments. All values represent mean ± SD.
All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA). Data were analyzed using a multivariate repeated measures analysis of variance (ANOVA) using the MIXED procedure to account for the correlation between metabolic indices, with individual being the random factor upon which multiple measures were made [64]. SMR, MMR, ASa, and Pcrit were considered response variables, and temperature, pCO2 level, and a dummy variable representing the number of repetitions being measured on a single individual were considered factors. We modeled the heterogeneity in responses among temperature treatments and specified the Kenward–Roger method for calculating the degrees of freedom [65]. Model selection between different variance/covariance structures was performed using Bayesian information criterion (BIC,) and significant differences were determined using 95% confidence intervals derived using the least squares means (LSM) estimate statement in SAS. Model data are presented from model structures using compound symmetry correlation structures. All statistics were evaluated with a significance level of α = 0.05.
3. Results
Our water chemistry values (Table 1) were largely consistent with published values for both the Chesapeake Bay [66,67] and the Gulf of Maine [68,69]. The elevated pCO2 treatment had higher calculated pCO2 values than expected [66,69].
We collected data from 24 clearnose skate (1.3 ± 0.06 kg; mean mass ± standard error, 17 thorny skate (1.4 ± 0.2 kg), and 9 summer flounder (0.36 ± 0.01 kg), and re-analyzed data from 9 summer flounder reported by Capossela, et al. [53]. Despite high variability within any given parameter, our models revealed differences among the metabolic response to changing environmental parameters. Model-derived estimates for all parameters can be found in Table S1.
3.1. SMR and Q10
We observed an increase in SMR with increasing temperature in both skate species during the present day pCO2 (i.e., at high pH) experiments (Figure 2A,C; Table 1). In clearnose skate, SMR was significantly higher under the elevated pCO2 at 20 °C and 24 °C (Figure 2A). SMR of summer flounder significantly increased between 22 °C and 30 °C (Figure 2B; p < 0.01), and elevated pCO2 caused SMR to increase at 22 °C (p < 0.01). There was no significant effect of temperature on SMR under elevated pCO2 (Figure 2B). In contrast, thorny skate did not demonstrate any significant differences in SMR elevated pCO2 at either test temperature (Figure 2C).
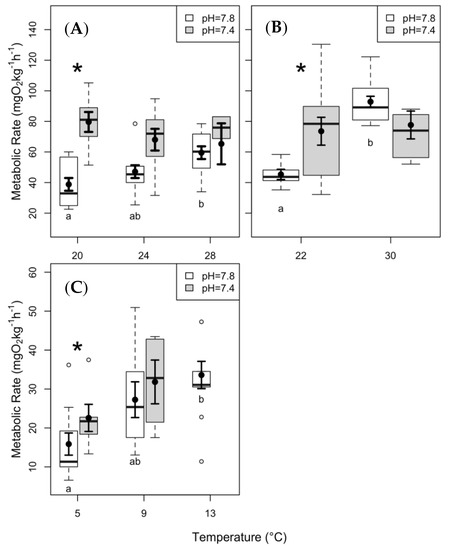
Figure 2.
Standard metabolic rate (SMR) data from (A) clearnose skate, (B) summer flounder, and (C) thorny skate. Box and whisker plots represent raw data, with whiskers representing maximum and minimum points within 1.5 times the interquartile range above the upper quartile and below the lower quartile. Open circles denote points outside of this range, while the filled circles and lines indicate the model-derived estimates and standard errors for each treatment condition. The asterisks above the boxplots represent significant differences between pH treatments within a given temperature. The letters below the boxes represent significant differences among temperatures within a given pH level.
To facilitate comparisons across species, we compiled Q10 values for SMR for all three species (Table 2). Clearnose skate and summer flounder had relatively low Q10 values at the present day pCO2 treatment, while thorny skate Q10 was more similar to the expected value between 2 and 3 [8,14]. Under elevated pCO2, we observed that clearnose skate and summer flounder Q10 values more than doubled, while thorny skate Q10 increased to a lesser degree.

Table 2.
The effects of temperature on standard metabolic rate measured as Q10 values. For both skate species, the values are reported for two different temperature ranges because of the small or non-existent sample size at the highest temperatures and lowered pH level.
3.2. Maximum Metabolic Rate and Aerobic Scope
The differences in mean MMR at 20 °C in clearnose skate were nearly significant between the two pCO2 conditions (Figure 3A; p = 0.051). Thorny skate showed an increasing trend in MMR within a given temperature (at 9 °C) at the elevated pCO2 (Figure 3B; p = 0.07).
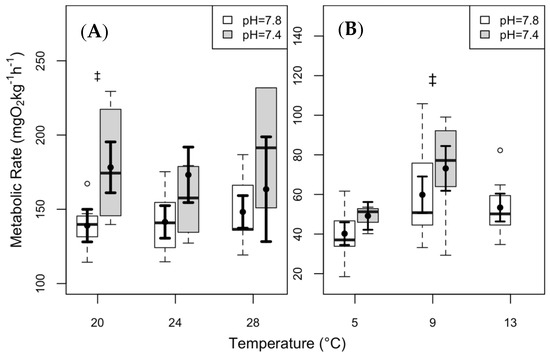
Figure 3.
Maximum metabolic rate (MMR) of (A) clearnose skate and (B) thorny skate. Box and whisker plots represent raw data, with whiskers representing maximum and minimum points within 1.5 times the interquartile range above the upper quartile and below the lower quartile. Open circles denote points outside of this range, while the filled circles and lines indicate the model-derived estimates and standard errors for each treatment condition. There were no significant differences in any of the pairwise comparisons, but the “‡” symbol denotes near significance (p = 0.051 in clearnose skate, and p = 0.07 in thorny skate).
The ASa of clearnose skate did not vary significantly under any of the treatment conditions. The ASa of thorny skate was significantly higher at 5 °C under elevated pCO2. To compare results between species, and following the recommendations of Clark et al. [7] and Lapointe et al. [64], we have included plots for ASa and ASf in Figure 4. Trends between the two different metrics are similar, although no statistical analysis was performed on ASf, as the two metrics were too similar to be fitted by the model.
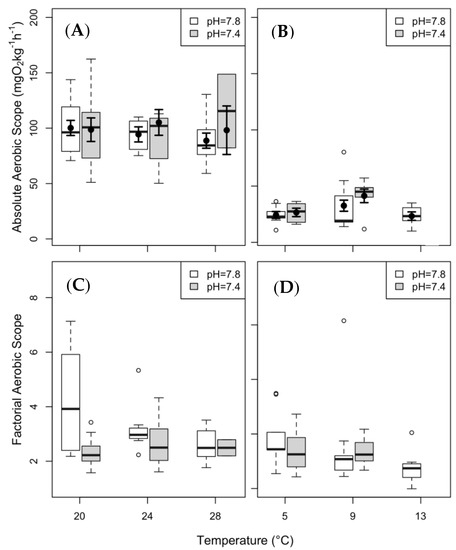
Figure 4.
Aerobic scope (AS) of clearnose skate (panels (A,C) and thorny skate (panels (B,D). ASa is presented in panels (A,B), and ASf in panels (C,D)). Box and whisker plots represent raw data, with whiskers representing maximum and minimum points within 1.5 times the interquartile range above the upper quartile and below the lower quartile. Open circles denote points outside of this range, while the filled circles and lines indicate the model-derived estimates and standard errors for each treatment condition. ASa was analyzed for model analysis, but ASf was not.
3.3. Hypoxia Tolerance
The hypoxia tolerance of clearnose skate under present day pCO2 (i.e., high pH) was reduced at increased temperature, as shown by a significantly higher Pcrit at 28 °C compared with 20 °C and 24 °C (p < 0.01 for both). Under elevated pCO2, we observed a significant increase in Pcrit between 20 °C and 24 °C (p = 0.04). Clearnose skate exhibited marked reductions in hypoxia tolerance under elevated pCO2 (Figure 5A), with significant elevations in Pcrit at 20 °C and 24 °C (p < 0.01 for both). Summer flounder showed the expected significant increase in Pcrit under elevated temperatures at present day pCO2 (p < 0.01), as well as a significant increase under elevated pCO2 at 22 °C (p < 0.01; Figure 5B). The Pcrit of thorny skate at 13 °C was significantly higher compared with that measured at 5 °C under present day pCO2 (p = 0.04); and Pcrit at 5 °C was significantly higher at elevated pCO2 than under present day pCO2 (p < 0.01; Figure 5C).
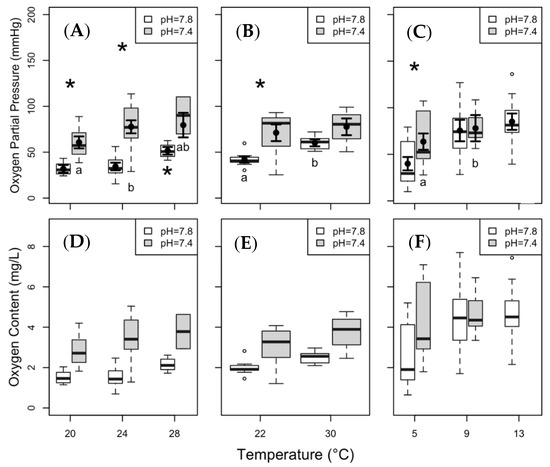
Figure 5.
Critical oxygen partial pressure (Pcrit) and critical oxygen content (Ccrit) of clearnose skate (panels (A,D)), summer flounder (panels (B,E)), and thorny skate (panels (C,F)). Box and whisker plots represent raw data with whiskers representing maximum and minimum points within 1.5 times the interquartile range above the upper quartile and below the lower quartile. Open circles denote points outside of this range, while the filled circles and lines indicate the model-derived estimates and standard errors for each treatment condition. Asterisks above the boxes indicate significant differences between the pH treatments within a given temperature, while asterisks and letters below the boxes indicate significance across temperatures within a pH treatment. No statistical analyses were run on Ccrit and these graphs are included only as an aid for comparison to other studies.
4. Discussion
Our study compares the environmental tolerances of species in two disparate environments under projected conditions (elevated temperature and elevated pCO2), focusing on how observed tolerances are impacted by multiple, concurrent stressors. In general, the physiological abilities to withstand acute exposure to environmental stress were more similar between the sympatric species (clearnose skate and summer flounder) than the abilities of the allopatric species (thorny skate).
Although the pCO2 values used for the elevated pCO2 treatment were somewhat variable, targeted pH values were maintained. For the purposes of interspecific comparison, moreover, the difference between the present day and elevated pCO2 treatments is likely more important than the actual values. The variability observed in the present-day conditions is likely a result of the natural conditions in near-shore water pumped in the seawater facilities; additional manipulation of the carbonate chemistry of the seawater was deemed cost-prohibitive, and likely unwarranted as the modified seawater would not mimic estuarine conditions.
The SMR values measured at present-day pCO2 levels (38.8 ± 4.2 mg O2 kg−1 h−1 at 20 °C for clearnose skate; 45.3 ± 3.4 mg O2 kg−1 h−1 at 22 °C for summer flounder; 15.9 ± 2.8 mg O2 kg−1 h−1 at 5 °C for thorny skate; Figure 2) were lower than other studies at similar temperatures (e.g., 100–150 mg O2 kg−1 h−1 at 20 °C [70]; 68–84 mg O2 kg−1 h−1 at 10 °C [71]), although this could be attributed to the demersal nature of these study species. The increase in SMR under elevated pCO2 within the lowest test temperatures for all three species (105%, 64%, and 43% increase for clearnose skate, summer flounder, and thorny skate, respectively), and the declining difference in SMR between pCO2 treatments at elevated temperatures (down to 10%, −16%, and 17% for clearnose skate, summer flounder, and thorny skate, respectively; Figure 2) matches trends from little skate (Leucoraja erinacea) exposed to elevated temperatures and pCO2 [5], but differs from similar studies on other species [72,73,74,75]. This suggests there may be conserved physiological mechanisms driving this response, but much is still unknown regarding the mechanisms underpinning the observed patterns. The results presented here may be due to bradycardia, increased ventilatory rates, and increased blood pressure [76,77], or to the increased metabolic cost of buffering against plasma pH changes [14,78] and increased ion transport [24]. These known physiological stresses are unlikely, however, to increase the metabolic rate to the extent we observed in this study. An alternative explanation is that individuals are more active under elevated pCO2 (i.e., low pH) conditions [79].
The effect of elevated pCO2 on SMR masks the temperature effect observed under present-day conditions, indicating that the responses to these stressors are not additive. This may be the result of an alternative version of the OCLTT hypothesis, where the physiological consequences of elevated pCO2 (rather than temperature) are predicted to limit oxygen delivery [21,80]. Different responses to temperature under present-day conditions compared to under elevated pCO2 suggest that there are interactive mechanisms regulating oxygen delivery in fishes [8,10,24,81]. Elevated plasma levels of CO2 (with concomitant reductions in plasma pH) reduce hemoglobin oxygen affinity (Bohr effect) and maximum blood oxygen content (Root effect); although the extent of these is unknown in the study species. Alternatively, the effects of one stressor could be compensating the effects of the other [24], resulting in the masking effects. For example, increased metabolic costs of acid-base regulation under ocean acidification could be offset by reduced energetic demand elsewhere. This phenomenon has been demonstrated with low pH-induced metabolic depression in isolated gill cells [82]. Given the large knowledge gaps concerning the mechanisms underpinning our results, we argue—as have others—that more multi-stressor studies are needed [12,13,83,84,85].
Clearnose skate and summer flounder exhibited lower Q10 values (Q10 = 1.62 and 1.07, respectively) at present day pCO2 than the thorny skate (Q10 = 3.87). While Q10 values lower than 2 have been associated with a decreasing ability to function [86], for the two Mid-Atlantic estuarine species studied here (i.e., clearnose skate and summer flounder), the low Q10 values are rather indicative of the ability to maintain a consistent level of aerobic ATP production over a relatively broad range of temperatures [37,87], potentially signifying resilience to the coastal warming predicted under climate change [23]. High Q10 values, in contrast, have been attributed to species from stable environments [88]. The thorny skate, therefore, may not possess isozymes (or the genetic plasticity to produce isozymes) that reduce the effects of temperature on metabolic rate over a broad range of temperatures [32,86,89], and may thus be more sensitive to temperature increases than the other two study species. The idea that the effects of temperature on metabolic rate are closely associated with native thermal range [37], has received mixed support from other studies looking specifically at different populations or species. For example, Di Santo [5] found increased sensitivity to temperature in more northern populations of little skate. According to the evolutionary trade-off hypothesis [90], the resting metabolic rate of a species (or population) at over its normal environmental temperature range represents an evolutionary optimization. In other words, species- or population-specific optimization of metabolic rates to a given temperature (or range of temperatures) might not be explained purely through the kinetic energy of sub-cellular constituents, but may rather be a suite of complex tradeoffs [86,90,91]. This becomes evident as all three species exhibited a decrease in Q10 under elevated pCO2, driven by increases in SMR and emphasizing the masking impact of this additional stressor. Under projected climate change scenarios, elevated temperatures and ocean acidification are likely to have interactive effects on cellular processes [37,39,92,93]. While the Q10 values presented here offer some insight into species-specific sensitivity, more research on the interactive effects of multiple, concurrent stressors on metabolism is needed.
We did not observe any significant trends in MMR between the two skate species with either temperature or pCO2 (Figure 3). This may be attributable to an insufficient stressor prior to the respirometry trial. Although the values presented here are lower than published values for other fish species measured at similar temperatures [20,70,94], this could also be attributed to the more sedentary life style of our study species. Alternatively, the Fry paradigm [38] for diminishing MMR values above an optimal temperature may not hold in these species [14]. There is widespread dissent in the literature regarding the appropriate methods to obtain MMR [14,95] and whether the standard Fry paradigm is valid, which make us hesitant to draw more definitive conclusions.
The aerobic scope data do not support the existence of a bell-shaped curve centered on a single optimal temperature (Topt), but rather AS being relatively temperature-independent. These results may be driven by multiple Topt values for different physiological processes [7,36], and are consistent with other studies [14,26,74,96,97,98]. The lack of significant reduction in aerobic scope under high stress conditions suggests that clearnose and thorny skates, may exhibit resilience to climate change in their respective environments. Given that there have been conflicting reports on the capacity of elasmobranch species to acclimate to climate change conditions [99,100], the findings of this study represent an important step in understanding the physiological tolerances of this understudied group [101]. An important caveat is that because we used wild-caught adults, any early life history detriments to condition and survival [100,102,103] remain unmeasured.
Our most significant finding, however, may be that clearnose skate (Pcrit 32 ± 2 mmHg at 20 °C; mean ± SE) are as hypoxia tolerant as epaulette shark (Pcrit 38 mmHg at 28 °C); and the latter have been deemed to have exceptional hypoxia tolerance [104,105]. While the physiological mechanisms underlying the hypoxia tolerance of epaulette shark have received considerable attention [73,106,107,108,109,110], there are no equivalent data for clearnose skate, and we encourage studies in this area. Our Pcrit data show, however, that summer flounder are also tolerant to hypoxia (Pcrit = 42 mmHg at 22 °C). Considering the correlations between hypoxia tolerance and the environmental variability of a species’ native habitat, we note that epaulette sharks live in reef and tidal environments that experience large diel and tidal cycle fluctuations in temperature, oxygen, and pH, similar to the changes occurring in estuaries along the U.S. mid-Atlantic [29,30,111]. Other species from variable environments are also hypoxia tolerant, including blue crabs (Calinectus sapidus) [112] and crucian carp (Carassius carassius), as well as many rocky tidepool fishes [33]. These results, however, are not ubiquitous. Sandbar shark (Carcharhinus plumbeus) have a markedly higher Pcrit value than clearnose skate or summer flounder [17], despite being a sympatric species. As sandbar shark are an obligate ram-ventilating species [39], this difference is unsurprising and is supported by findings on bonnethead shark (Sphyrna tiburo), which live in seagrass meadows likely to experience large diel cycles in dissolved oxygen. In contrast to clearnose skate and summer flounder, thorny skate are relatively intolerant to hypoxia (Pcrit = 75 mmHg at 9 °C), most likely because this species occupies the Gulf of Maine, an environment that does not exhibit wide swings in temperature and oxygen levels [40,51]. Similarly, the shovelnose ray (Aptychotrema rostrata) that occupies an environment where it rarely encounters hypoxia [113] has a Pcrit = 54 mmHg at 28 °C [104].
The increases in Pcrit under elevated pCO2 (84%, 69%, and 60% increases in Pcrit for clearnose skate, summer flounder, and thorny skate, respectively) may be the result of the inability of the non-bicarbonate blood buffering capacity of all three study species to limit reductions in plasma pH (and subsequently the intracellular environment) under elevated pCO2. To the best of our knowledge, there is no information regarding intracellular pH (pHi) of elasmobranch red blood cells following exposure to simulated OA. Studies on brain, white muscle, and liver tissue isolated from teleost fishes and exposed to elevated pCO2 have, however, found either no change or an increases in pHi [24,72,81,114], suggesting OA may not have a negative impact blood oxygen transport. This is supported by a lack of increase in hematocrit following exposure to elevated pCO2 [74,114,115]. As the differences in Pcrit were most apparent in the mid-Atlantic species, further work on in vivo blood pH levels under changing pCO2 conditions, as well as quantification of the changes in blood oxygen affinity (Bohr shift) and maximum oxygen carrying capacity (Root effect), in these species would help elucidate the mechanisms underpinning our observed reductions in hypoxia tolerance. Because of the high hypoxia tolerance of clearnose skate, we predict that this species may also demonstrate a high blood oxygen affinity and a large Bohr effect, similar to that seen in the hypoxia tolerant bat ray (Myliobatis californica) [104,116]. We also expect summer founder blood to have similar physiological characteristics to those of blood from European flounder (Platichthys flesus) [117].
These mechanisms are largely speculative, however, as there are conflicting reports of the effects of elevated pCO2 on hypoxia tolerance. For example, epaulette sharks do not exhibit decreases in hypoxia tolerance under elevated pCO2 conditions [73]. This may be attributed to the chronic (60-day) exposure of epaulette shark to elevated pCO2 conditions, compared with the acute exposures we employed. Other studies have reported increases in Pcrit under elevated pCO2 in European eel (Anguilla Anguilla) [118] and European flounder [119]; and in acidified water for rainbow trout (Salmo gairdneri) and carp (Cyprinus carpio) [120]. These results are, however, not universal [57], as croaker (Leiostomus xanthurus) and mummichog (Fundulus heteroclitus) exhibit no change in Pcrit under elevated pCO2 [43,57]. We note, however, these two species are common occupants of mid-Atlantic estuaries, and thus regularly experience elevated pCO2 conditions [43].
From an ecological perspective, the observed effect of elevated pCO2 on hypoxia tolerance is concerning. Currently, clearnose skate and summer flounder are unlikely to encounter waters below their Pcrit, assuming the water is at a pH of 7.8 [12,28,30]. Because of the effects of climate change, however, individuals in coastal waters are more likely to experience concurrent hypoxia and elevated pCO2 [31,56,121,122]. While estuarine and coastal species may be able to tolerate current conditions, further extremes of these parameters may force populations to move to alternative habitats. While at present, it is unlikely that thorny skate regularly encounter hypoxia, warming shelf waters could induce changes in dissolved oxygen distribution, resulting in unfavorable habitats in areas such as the Gulf of Maine [40,51,123]. Activity patterns observed in dogfish (Scyliorhinus canicula) suggest that sluggish benthic elasmobranch species do not increase activity under hypoxic conditions [124], although the more active bonnethead shark does [125]. The sedentary strategy of non-obligate ram ventilating species could, therefore, limit their ability to exploit novel habitats under unfavorable environmental conditions.
Recently, Wood [126] argued that Pcrit as a metric of hypoxia tolerance is of limited utility owing to numerous factors including the lack of repeatability and consistency and an insufficient theoretical underpinning. He proposed several alternative metrics that could be used in place of Pcrit, including loss of equilibrium or measurements of ventilation. While we agree that alternative measure of hypoxia tolerance can provide useful information, for the purposes of our study of benthic flatfishes, the loss of equilibrium is not a useful metric. Further, because the calculation of Pcrit was standardized across all three species, concerns regarding different methodology were alleviated. We agree with Regan et al. [127], that “Pcrit contributes to a more complete picture of an animal’s total hypoxic response by capturing the suite of aerobic contributions to hypoxic survival in a single value”, and hope the data presented here can help further our understanding of hypoxia tolerance in a range of coastal species.
5. Conclusions
Understanding the species- and population-specific response to the multiple environmental stressors associated with climate change is essential for managing marine sources in a changing environment. The results presented here quantify the physiological limits of clearnose skate, summer flounder, and thorny skate with respect to acute changes in temperature and elevated pCO2. All three species exhibited increases in SMR (105%, 42%, and 22% for clearnose skate, summer flounder, and thorny skate, respectively) at the lowest test temperature under elevated pCO2, and this increases masked increases in SMR at the high test temperatures. All three species also showed decreased hypoxia tolerance (150%, 85%, and 113% increases in Pcrit) under the most extreme combined stressors. While the clearnose skate did exhibit remarkable hypoxia tolerance under the least stressful treatment, as climate change impacts continue to increase in severity, even this species may be pushed towards or past the limits of their physiological capabilities. Incorporating multi-stressor studies into future climate change research is essential to predicting how species will respond to changing environmental conditions. If conditions are near the limits of physiological abilities, individuals may choose to seek out more favorable habitats, resulting in shifting distributions, fecundities, and food web dynamics with cascading ecological and economic implications.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-7737/8/3/56/s1, Table S1: The estimated values ± standard error based on model output.
Author Contributions
Conceptualization, G.D.S. and R.W.B.; methodology, D.P.C., R.W.B., and P.G.B.; formal analysis, G.D.S. and D.P.C.; software, R.W.B.; validation, G.D.S. and R.W.B.; investigation, G.D.S., D.R.L., and B.N.A.; resources, R.W.B., P.G.B., and J.A.S.; data curation, G.D.S.; writing—original draft preparation, G.D.S.; writing—review and editing, G.D.S. and R.W.B.; visualization, G.D.S.; supervision, R.W.B., P.G.B., and J.A.S.; project administration, G.D.S.; funding acquisition, G.D.S. and J.A.S.
Funding
This research was funded by the National Science Foundation Graduate Research Fellowship Program, grant number #DGE-1444317 and the National Oceanographic and Atmospheric Administration, Hollings Fellow Program.
Acknowledgments
We would like to thank Karen Capossela for sharing her raw data. Elizabeth Shadwick, Olivia De Meo, Annie Shatz, and Emily Rivest assisted with carbonate chemistry analysis. We would like to thank the staff of the Virginia Institute of Marine Science Eastern Shore Laboratory and of the University of New England Marine Science Center for their help facilitating this work. Two anonymous reviewers provided useful suggestions on a previous version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Melzner, F.; Thomsen, J.; Koeve, W.; Oschlies, A.; Gutowska, M.A.; Bange, H.W.; Hansen, H.P.; Körtzinger, A. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 2013, 160, 1875–1888. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Axelsson, M.; Chabot, D.; Claireaux, G.; Cooke, S.J.; Corner, R.A.; De Boeck, G.; Domenici, P.; Guerreiro, P.M.; Hamer, B.; et al. Conservation physiology of marine fishes: State of the art and prospects for policy. Conserv. Physiol. 2016, 4, cow046. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; García Molinos, J.; Halpern, B.S.; Hoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, W.J. Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 2016, 3, 62. [Google Scholar] [CrossRef]
- Di Santo, V. Intraspecific variation in physiological performance of a benthic elasmobranch challenged by ocean acidification and warming. J. Exp. Biol. 2016, 219, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Duarte, C.M.; Marba, N.; Wernberg, T. Integrating within-species variation in thermal physiology into climate change ecology. Philos. Trans. R. Soc. B 2019, 374, 20180550. [Google Scholar] [CrossRef]
- Clark, T.D.; Sandblom, E.; Jutfelt, F. Aerobic scope measurements of fishes in an era of climate change: Respirometry, relevance and recommendations. J. Exp. Biol. 2013, 216, 2771–2782. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Lefevre, S. Physiological Challenges to Fishes in a Warmer and Acidified Future. Physiology 2016, 31, 409–417. [Google Scholar] [CrossRef]
- Claireaux, G.; Chabot, D. Responses by fishes to environmental hypoxia: Integration through Fry’s concept of aerobic metabolic scope. J. Fish Biol. 2016, 88, 232–251. [Google Scholar] [CrossRef]
- Heuer, R.M.; Grosell, M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1061–R1084. [Google Scholar] [CrossRef]
- Horodysky, A.Z.; Cooke, S.J.; Brill, R.W. Physiology in the service of fisheries science: Why thinking mechanistically matters. Rev. Fish Biol. Fish. 2015, 25, 425–447. [Google Scholar] [CrossRef]
- Baumann, H. Experimental assessments of marine species sensitivities to ocean acidification and co-stressors: How far have we come? Can. J. Zool. 2019, 97, 399–408. [Google Scholar] [CrossRef]
- Lyons, K.; Bigman, J.S.; Kacev, D.; Mull, C.G.; Carlisle, A.B.; Imhoff, J.L.; Anderson, J.M.; Weng, K.C.; Galloway, A.S.; Cave, E.; et al. Bridging disciplines to advance elasmobranch conservation: Applications of physiological ecology. Conserv. Physiol. 2019, 7, coz011. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, S. Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO2 and their interaction. Conserv. Physiol. 2016, 4, cow009. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, M.B.; Bushnell, P.G.; Steffensen, J.F. Design and setup of intermittent-flow respirometry system for aquatic organisms. J. Fish Biol. 2016, 88, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Speers-Roesch, B.; Richards, J.G.; Brauner, C.J.; Farrell, A.P.; Hickey, A.J.; Wang, Y.S.; Renshaw, G.M. Hypoxia tolerance in elasmobranchs. I. Critical oxygen tension as a measure of blood oxygen transport during hypoxia exposure. J. Exp. Biol. 2012, 215, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Crear, D.P.; Brill, R.W.; Bushnell, P.G.; Latour, R.J.; Schwieterman, G.D.; Steffen, R.M.; Weng, K.C.; Clark, T. The impacts of warming and hypoxia on the performance of an obligate ram ventilator. Conserv. Physiol. 2019, 7, coz026. [Google Scholar] [CrossRef]
- Brill, R.W. Selective advantages conferred by the high performance physiology of tunas, billfishes, and dolphin fish. Comp. Biochem. Physiol. 1996, 113, 3–15. [Google Scholar] [CrossRef]
- Teal, L.R.; Marras, S.; Peck, M.A.; Domenici, P. Physiology-based modeling approaches to characterize fish habitat suitability: Their usefulness and limitations. Estuar. Coast. Shelf Sci. 2015, 201, 56–63. [Google Scholar] [CrossRef]
- Claireaux, G.; Lagardère, J.-P. Influence of temperature, oxygen and salinity on the metabolism of the European sea bass. J. Sea Res. 1999, 42, 157–168. [Google Scholar] [CrossRef]
- Portner, H.O. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Jutfelt, F.; Norin, T.; Ern, R.; Overgaard, J.; Wang, T.; McKenzie, D.J.; Lefevre, S.; Nilsson, G.E.; Metcalfe, N.B.; Hickey, A.J.R.; et al. Oxygen- and capacity-limited thermal tolerance: Blurring ecology and physiology. J. Exp. Biol. 2018, 221, jeb169615. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; Dubash, N.K. IPCC Fifth Assessment Synthesis Report-Climate Change 2014 Synthesis Report; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014. [Google Scholar]
- Esbaugh, A.J. Physiological implications of ocean acidification for marine fish: Emerging patterns and new insights. J. Comp. Physiol. B 2018, 188, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Munday, P.L.; McCormick, M.I.; Nilsson, G.E. Impact of global warming and rising CO2 levels on coral reef fishes: What hope for the future? J. Exp. Biol. 2012, 215, 3865–3873. [Google Scholar] [CrossRef] [PubMed]
- Rummer, J.L.; Stecyk, J.A.; Couturier, C.S.; Watson, S.A.; Nilsson, G.E.; Munday, P.L. Elevated CO2 enhances aerobic scope of a coral reef fish. Conserv. Physiol. 2013, 1, cot023. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.W.; Selden, R.L.; Latour, R.J.; Frölicher, T.L.; Seagraves, R.J.; Pinsky, M.L. Projecting shifts in thermal habitat for 686 species on the North American continental shelf. PLoS ONE 2018, 13, e0196127. [Google Scholar] [CrossRef] [PubMed]
- Morash, A.J.; Neufeld, C.; MacCormack, T.J.; Currie, S. The importance of incorporating natural thermal variation when evaluating physiological performance in wild species. J. Exp. Biol. 2018, 221, jeb164673. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.; Smith, E.M. Quantifying Metabolically Driven pH and Oxygen Fluctuations in US Nearshore Habitats at Diel to Interannual Time Scales. Estuar. Coasts 2017, 41, 1102–1117. [Google Scholar] [CrossRef]
- Baumann, H.; Wallace, R.B.; Tagliaferri, T.; Gobler, C.J. Large Natural pH, CO2 and O2 Fluctuations in a Temperate Tidal Salt Marsh on Diel, Seasonal, and Interannual Time Scales. Estuar. Coasts 2014, 38, 220–231. [Google Scholar] [CrossRef]
- Breitberg, D. Near-shore Hypoxia in the Chesapeake Bay: Patterns and Relationships among Physical Factors. Estuar. Coast. Shelf Sci. 1990, 30, 593–609. [Google Scholar] [CrossRef]
- Norin, T.; Metcalfe, N.B. Ecological and evolutionary consequences of metabolic rate plasticity in response to environmental change. Philos. Trans. R. Soc. B 2019, 374, 20180180. [Google Scholar] [CrossRef] [PubMed]
- Fangue, N.A.; Flaherty, K.E.; Rummer, J.L.; Cole, G.; Hansen, K.S.; Hinote, R.; Noel, B.L.; Wallman, H.; Bennett, W.A. Temperature and hypoxia tolerance of selected fishes from a hyperthermal rockpool in the Dry Torgugas, with notes on diversity and behavior. Carribean J. Sci. 2001, 37, 81–87. [Google Scholar]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Nye, J.A.; Link, J.S.; Hare, J.A.; Overholtz, W.J. Changing spatial distribution of fish stocks in relation to climate and population size on the Northeast United States continental shelf. Mar. Ecol. Prog. Ser. 2009, 393, 111–129. [Google Scholar] [CrossRef]
- Schulte, P.M. The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 2015, 218, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Johnston, N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- Fry, F.E.J. Effect of the environment on animal activity. Publ. Out. Fish. Res. Lab. 1947, 68, 1–62. [Google Scholar]
- Bernal, D.; Lowe, C.G. Field Studies of Elasmobranch Physiology. In Physiology of Elasmobranch Fishes: Structure and Interaction with Environment; Shadwich, R.E., Farrell, A.P., Brauner, C.J., Eds.; Elsevier Inc.: San Diego, CA, USA, 2016; Volume 34, pp. 311–377. [Google Scholar]
- Saba, V.S.; Griffies, S.M.; Anderson, W.G.; Winton, M.; Alexander, M.A.; Delworth, T.L.; Hare, J.A.; Harrison, M.J.; Rosati, A.; Vecchi, G.A.; et al. Enhanced warming of the Northwest Atlantic Ocean under climate change. J. Geophys. Res. Ocean. 2016, 121, 118–132. [Google Scholar] [CrossRef]
- Atkinson, L.P.; Lee, T.N.; Blanton, J.O.; Chandler, W.S. Climatology of the southeastern United States continental shelf waters. J. Geophys. Res. Ocean. 1983, 88, 4705–4718. [Google Scholar] [CrossRef]
- Breitberg, D.; Salisbury, J.; Bernhard, J.; Cai, W.-J.; Dupont, S.; Doney, S.; Kroeker, K.; Levin, L.; Long, C.; Milke, L.; et al. And on Top of All That: Coping with Ocean Acidification in the Midst of Many Stressors. Oceanography 2015, 28, 48–61. [Google Scholar] [CrossRef]
- Cochran, R.E.; Burnett, L.E. Respiratory responses of the salt marsh animals, Fundulus heteroclitus, Leiostomus xanthurus, and Palaemonetes pugio to environmental hypoxia and hypercapnia and to the organophosphate pesticide, azinphosmethyl. J. Exp. Mar. Biol. Ecol. 1996, 195, 125–144. [Google Scholar] [CrossRef]
- Diaz, R.J.; Breitburg, D.L. The hypoxic environment. Fish Physiol. 2009, 27, 1–23. [Google Scholar]
- Breitberg, D.L.; Craig, J.K.; Fulford, R.S.; ROse, K.A.; Boynton, W.R.; Brady, D.C.; Ciotti, B.J.; Diaz, R.J.; Friedland, K.D.; Hagy III, J.D.; et al. Nutrient enrichment and fisheries exploitation: Interactive effects on estuarine living resources and their managment. Hydrobiologia 2009, 629, 31–47. [Google Scholar] [CrossRef]
- Packer, D.B.; Zetlin, C.A.; Vitaliano, J.J. Clearnose Skate, Raja Eglanteria, Life History and Habitat Characteristics; NOAA, N., Northeast Fisheries Science Center, Eds.; Northeast Fisheries Science Center, NOAA: Woods Hole, MA, USA, 2003.
- McEachran, J.D.; Musick, J.A. Distribution and relative abundance of seven species of skates (Pisces: Rajidae) which occur between Nova Scotia and Cape Hatteras. Fish. Bull. 1975, 73, 110–1336. [Google Scholar]
- Schwartz, F.J. Biology of the clearnose skate, raja eglanteria, from North Carolina. Fla. Sci. 1996, 59, 82–95. [Google Scholar]
- Buchheister, A.; Latour, R.J. Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Can. J. Fish. Aquat. Sci. 2010, 67, 445–461. [Google Scholar] [CrossRef]
- Scott, W.; Scott, M. Atlantic Fishes of Canada Canadian Bulletin of Fisheries and Aquatic Science, 219; University of Toronto Press: Toronto, ON, Canada, 1988. [Google Scholar]
- Petrie, B.; Drinkwater, K. Temperature and salinity variability on the Scotian Shelf and in the Gulf of Maine 1945–1990. J. Geophys. Res. 1993, 98, 20079–20089. [Google Scholar] [CrossRef]
- Swain, D.P.; Benoit, H.P. Change in habitat associations and geographic distribution of thorny skate (Amblyraja radiata) in the southern Gulf of St Lawrence: Density-dependent habitat selection or response to environmental change? Fish. Oceanogr. 2006, 15, 166–182. [Google Scholar] [CrossRef]
- Cicia, A.M.; Schlenker, L.S.; Sulikowski, J.A.; Mandelman, J.W. Seasonal variations in the physiological stress response to discrete bouts of aerial exposure in the little skate, Leucoraja erinacea. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 162, 130–138. [Google Scholar] [CrossRef]
- Capossela, K.M.; Brill, R.W.; Fabrizio, M.C.; Bushnell, P.G. Metabolic and cardiorespiratory responses of summer flounder Paralichthys dentatus to hypoxia at two temperatures. J. Fish Biol. 2012, 81, 1043–1058. [Google Scholar] [CrossRef]
- Najjar, R.; Patterson, L.; Graham, S. Climate simulations of major estuarine watersheds in the Mid-Atlantic region of the US. Clim. Chang. 2008, 95, 139–168. [Google Scholar] [CrossRef]
- Najjar, R.G.; Pyke, C.R.; Adams, M.B.; Breitburg, D.; Hershner, C.; Kemp, M.; Howarth, R.; Mulholland, M.R.; Paolisso, M.; Secor, D.; et al. Potential climate-change impacts on the Chesapeake Bay. Estuar. Coast. Shelf Sci. 2010, 86, 1–20. [Google Scholar] [CrossRef]
- Rogers, N.J.; Urbina, M.A.; Reardon, E.E.; McKenzie, D.J.; Wilson, R.W. A new analysis of hypoxia tolerance in fishes using a database of critical oxygen level (P crit). Conserv. Physiol. 2016, 4, cow012. [Google Scholar] [CrossRef]
- Schurmann, H.; Steffensen, J. Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J. Fish Biol. 1997, 50, 1166–1180. [Google Scholar]
- Michaelidis, B.; Spring, A.; Pörtner, H.O. Effects of long-term acclimation to environmental hypercapnia on extracellular acid–base status and metabolic capacity in Mediterranean fish Sparus aurata. Mar. Biol. 2007, 150, 1417–1429. [Google Scholar] [CrossRef]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean CO2 Measurements; North Pacific Marine Science Organization: Sidney, BC, Canada, 2007. [Google Scholar]
- Pierrot, D.; Lewis, E.; Wallace, D. MS Excel program developed for CO2 system calculations. In ORNL/CDIAC-105a; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy: Oak Ridge, TN, USA, 2006; Volume 3. [Google Scholar]
- Mehrbach, C.; Culberson, C.; Hawley, J.; Pytkowicx, R. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure 1. Limnol. Oceanogr. 1973, 18, 897–907. [Google Scholar] [CrossRef]
- Dickson, A.; Millero, F.J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. Part A Oceanogr. Res. Pap. 1987, 34, 1733–1743. [Google Scholar] [CrossRef]
- Lapointe, D.; Vogelbein, W.K.; Fabrizio, M.C.; Gauthier, D.T.; Brill, R.W. Temperature, hypoxia, and mycobacteriosis: Effects on adult striped bass Morone saxatilis metabolic performance. Dis. Aquat. Org. 2014, 108, 113–127. [Google Scholar] [CrossRef]
- Kenward, M.G.; Roger, J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997, 983–997. [Google Scholar] [CrossRef]
- Cai, W.-J.; Hu, X.; Huang, W.-J.; Murrell, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.-C.; Zhai, W.; Hollibaugh, J.T.; Wang, Y.; et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Tzortziou, M.; Neale, P.J.; Megonigal, J.P.; Pow, C.L.; Butterworth, M. Spatial gradients in dissolved carbon due to tidal marsh outwelling into a Chesapeake Bay estuary. Mar. Ecol. Prog. Ser. 2011, 426, 41–56. [Google Scholar] [CrossRef]
- Signorini, S.R.; Mannino, A.; Najjar, R.G.; Friedrichs, M.A.; Cai, W.J.; Salisbury, J.; Wang, Z.A.; Thomas, H.; Shadwick, E. Surface ocean pCO2 seasonality and sea-air CO2 flux estimates for the North American east coast. J. Geophys. Res. Ocean. 2013, 118, 5439–5460. [Google Scholar] [CrossRef]
- Cai, W.J.; Hu, X.; Huang, W.J.; Jiang, L.Q.; Wang, Y.; Peng, T.H.; Zhang, X. Alkalinity distribution in the western North Atlantic Ocean margins. J. Geophys. Res. Ocean. 2010, 115. [Google Scholar] [CrossRef]
- Marcek, B. Individual- and Population-Level Effects of Temperature and Hypoxia on Two Demersal Fishes in Chesapeake Bay; College of William and Mary, Virginia Institute of Marine Science: Gloucester Point, VA, USA, 2018. [Google Scholar]
- Steinhausen, M.F.; Steffensen, J.F.; Andersen, N.G. Tail beat frequency as a predictor of swimming speed and oxygen consumption of saithe (Pollachius virens) and whiting (Marlangius merlangus) during forced swimming. Mar. Biol. 2005, 148, 197–204. [Google Scholar] [CrossRef]
- Esbaugh, A.J.; Heuer, R.; Grosell, M. Impacts of ocean acidification on respiratory gas exchange and acid–base balance in a marine teleost, Opsanus beta. J. Comp. Physiol. B 2012, 182, 921–934. [Google Scholar] [CrossRef]
- Heinrich, D.D.U.; Rummer, J.L.; Morash, A.J.; Watson, S.A.; Simpfendorfer, C.A.; Heupel, M.R.; Munday, P.L. A product of its environment: The epaulette shark (Hemiscyllium ocellatum) exhibits physiological tolerance to elevated environmnetal CO2. Conserv. Physiol. 2014, 2, cou047. [Google Scholar] [CrossRef]
- Green, L.; Jutfelt, F. Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biol. Lett. 2014, 10, 20140538. [Google Scholar] [CrossRef]
- Bouyoucos, I.A.; Simpfendorfer, C.A.; Rummer, J.L. Estimating oxygen uptake rates to understand stress in sharks and rays. Rev. Fish Biol. Fish. 2019, 29, 297–311. [Google Scholar] [CrossRef]
- Perry, S.; McKendry, J. The relative roles of external and internal CO2 versus H+ in eliciting the cardiorespiratory responses of Salmo salar and Squalus acanthias to hypercarbia. J. Exp. Biol. 2001, 204, 3963–3971. [Google Scholar]
- Perry, S.F.; Gilmour, K.M. Sensing and transfer of respiratory gases at the fish gill. J. Exp. Zool. 2002, 293, 249–263. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Kikkawa, T.; Hayashi, M.; Lee, K.-S.; Kita, J. Effects of CO2 on marine fish: Larvae and adults. J. Oceanogr. 2004, 60, 731–741. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Munday, P.L. Animal behaviour shapes the ecological effects of ocean acidification and warming: Moving from individual to community-level responses. Glob. Chang. Biol. 2016, 22, 974–989. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Knust, R. Climate Change Affects Marine Fishes through the Oxygen Limitation of Thermal Tolerance. Science 2006, 315, 95–97. [Google Scholar]
- Heuer, R.; Welch, M.; Rummer, J.; Munday, P.; Grosell, M. Altered brain ion gradients following compensation for elevated CO2 are linked to behavioural alterations in a coral reef fish. Sci. Rep. 2016, 6, 33216. [Google Scholar] [CrossRef]
- Stapp, L.S.; Kreiss, C.M.; Portner, H.O.; Lannig, G. Differential impacts of elevated CO2 and acidosis on the energy budget of gill and liver cells from Atlantic cod, Gadus morhua. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 187, 160–167. [Google Scholar] [CrossRef]
- Portner, H.O.; Langenbuch, M.; Reipschlager, A. Biological Impact of Elevated Ocean CO2 Concentrations: Lessons from Animal Physiology and Earth History. J. Oceanogr. 2004, 60, 705–718. [Google Scholar] [CrossRef]
- Sackville, M.A.; Shartau, R.B.; Damsgaard, C.; Hvas, M.; Phuong, L.M.; Wang, T.; Bayley, M.; Huong, D.T.T.; Phuong, N.T.; Brauner, C.J. Water pH limits extracellular but not intracellular pH compensation in the CO2 tolerant freshwater fish, Pangasianodon hypophthalmus. J. Exp. Biol. 2018, 221, jeb190413. [Google Scholar] [CrossRef]
- Burnett, L.E. The challenges of living in hypoxic and hypercapnic aquatic environments. Am. Zool. 1997, 37, 633–640. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanisms and Process in Physiological Evolution; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Turingan, R.; Sloan, T. Thermal Resilience of Feeding Kinematics May Contribute to the Spread of Invasive Fishes in Light of Climate Change. Biology 2016, 5, 46. [Google Scholar] [CrossRef]
- Rummer, J.L.; Couturier, C.S.; Stecyk, J.A.; Gardiner, N.M.; Kinch, J.P.; Nilsson, G.E.; Munday, P.L. Life on the edge: Thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Glob. Chang. Biol. 2014, 20, 1055–1066. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Todgham, A.E. Living in the now: Physiological mechanisms to tolerate a rapidly changing environment. Annu. Rev. Physiol. 2010, 72, 127–145. [Google Scholar] [CrossRef]
- Clarke, A.; Fraser, K.P.P. Why does metabolism scale with temperature? Funct. Ecol. 2004, 18, 243–251. [Google Scholar] [CrossRef]
- Clarke, A. Is there a universal temperature dependence of metabolism? Funct. Ecol. 2004, 18, 252–256. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Kultz, D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005, 67, 225–257. [Google Scholar] [CrossRef]
- Claireaux, G.; Webber, D.M.; Lagardère, J.P.; Kerr, S.R. Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua). J. Sea Res. 2000, 44, 257–265. [Google Scholar] [CrossRef]
- Peake, S.; Farrell, A. Fatigue is a behavioural response in respirometer-confined smallmouth bass. J. Fish Biol. 2006, 68, 1742–1755. [Google Scholar] [CrossRef]
- Melzner, F.; Gutowska, M.; Langenbuch, M.; Dupont, S.; Lucassen, M.; Thorndyke, M.C.; Bleich, M.; Pörtner, H.-O. Physiological basis for high CO2 tolerance in marine ectothermic animals: Pre-adaptation through lifestyle and ontogeny? Biogeosciences 2009, 6, 2313–2331. [Google Scholar] [CrossRef]
- Hamilton, S.L.; Logan, C.A.; Fennie, H.W.; Sogard, S.M.; Barry, J.P.; Makukhov, A.D.; Tobosa, L.R.; Boyer, K.; Lovera, C.F.; Bernardi, G. Species-specific responses of juvenile rockfish to elevated pCO2: From behavior to genomics. PLoS ONE 2017, 12, e0169670. [Google Scholar] [CrossRef]
- Couturier, C.S.; Stecyk, J.A.; Rummer, J.L.; Munday, P.L.; Nilsson, G.E. Species-specific effects of near-future CO2 on the respiratory performance of two tropical prey fish and their predator. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2013, 166, 482–489. [Google Scholar] [CrossRef]
- Gervais, C.R.; Nay, T.J.; Renshaw, G.; Johansen, J.L.; Steffensen, J.F.; Rummer, J.L. Too hot to handle? Using movement to alleviate effects of elevated temperatures in a benthic elasmobranch, Hemiscyllium ocellatum. Mar. Biol. 2018, 165, 162. [Google Scholar] [CrossRef]
- Pistevos, J.C.; Nagelkerken, I.; Rossi, T.; Olmos, M.; Connell, S.D. Ocean acidification and global warming impair shark hunting behaviour and growth. Sci. Rep. 2015, 5, 16293. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Reynolds, J.D. Predicting Extinction Vulnerability in Skates. Conserv. Biol. 2002, 16, 440–450. [Google Scholar] [CrossRef]
- Rosa, R.; Baptista, M.; Lopes, V.M.; Pegado, M.R.; Paula, J.R.; Trubenbach, K.; Leal, M.C.; Calado, R.; Repolho, T. Early-life exposure to climate change impairs tropical shark survival. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141738. [Google Scholar] [CrossRef]
- Di Santo, V. Ocean acidification exacerbates the impacts of global warming on embryonic little skate, Leucoraja erinacea (Mitchill). J. Exp. Mar. Biol. Ecol. 2015, 463, 72–78. [Google Scholar] [CrossRef]
- Speers-Roesch, B.; Brauner, C.J.; Farrell, A.P.; Hickey, A.J.; Renshaw, G.M.; Wang, Y.S.; Richards, J.G. Hypoxia tolerance in elasmobranchs. II. Cardiovascular function and tissue metabolic responses during progressive and relative hypoxia exposures. J. Exp. Biol. 2012, 215, 103–114. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Renshaw, G.M. Hypoxic survival strategies in two fishes: Extreme anoxia tolerance in the North European crucian carp and natural hypoxic preconditioning in a coral-reef shark. J. Exp. Biol. 2004, 207, 3131–3139. [Google Scholar] [CrossRef]
- Routley, M.H.; Nilsson, G.E.; Renshaw, G.M. Exposure to hypoxia primes the prespiratory and metabolic responses of the epaulette shark to progressive hypoxia. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2002, 131, 313–321. [Google Scholar] [CrossRef]
- Brill, R.W.; Lai, N.C. Elasmobranch Cardiovascular System; Academic Press: New York, NY, USA, 2016. [Google Scholar]
- Chapman, C.A.; Renshaw, G.M. Hematological responses of the grey carpet shark (Chiloscyllium punctatum) and the epaulette shark (Hemiscyllium ocellatum) to anoxia and re-oxygenation. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2009, 311, 422–438. [Google Scholar] [CrossRef]
- Heinrich, D.D.U.; Watson, S.-A.; Rummer, J.L.; Brandl, S.J.; Simpfendorfer, C.A.; Heupel, M.R.; Munday, P.L. Foraging behaviour of the epaulette shark Hemiscyllium ocellatumis not affected by elevated CO2. ICES J. Mar. Sci. 2016, 73, 633–640. [Google Scholar] [CrossRef]
- Johnson, M.S.; Kraver, D.W.; Renshaw, G.M.; Rummer, J.L. Will ocean acidification affect the early ontogeny of a tropical oviparous elasmobranch (Hemiscyllium ocellatum)? Conserv. Physiol. 2016, 4, cow003. [Google Scholar] [CrossRef]
- Shaw, E.C.; McNeil, B.I.; Tilbrook, B.; Matear, R.; Bates, M.L. Anthropogenic changes to seawater buffer capacity combined with natural reef metabolism induce extreme future coral reef CO2 conditions. Glob. Chang. Biol. 2013, 19, 1632–1641. [Google Scholar] [CrossRef]
- Brill, R.W.; Bushnell, P.G.; Elton, T.A.; Small, H.J. The ability of blue crab (Callinectes sapidus, Rathbun 1886) to sustain aerobic metabolism during hypoxia. J. Exp. Mar. Biol. Ecol. 2015, 471, 126–136. [Google Scholar] [CrossRef]
- Dennison, W.; Carruthers, T.; Thomas, J.; Glibert, P. A comparison of issues and management approaches in Moreton Bay, Australia and Chesapeake Bay, USA. In Wetlands Ecosystems in Asia; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Strobel, A.; Bennecke, S.; Leo, E.; Mintenbeck, K.; Pörtner, H.O.; Mark, F.C. Metaboic shifts in the Antarctic fish Notothenia rossii in response to rising temperature and pCO2. Front. Zool. 2012, 9, 28. [Google Scholar] [CrossRef]
- Esbaugh, A.J.; Ern, R.; Nordi, W.M.; Johnson, A.S. Respiratory plasticity is insufficient to alleviate blood acid-base disturbances after acclimation to ocean acidification in the estuarine red drum, Sciaenops ocellatus. J. Comp. Physiol. B 2016, 186, 97–109. [Google Scholar] [CrossRef]
- Hopkins, T.E.; Cech, J.J., Jr. Effect of temperature on oxygen consumption of the bat ray, Myliobatis californica (Chondrichthyes, Myliobatididae). Copeia 1994, 1994, 529–532. [Google Scholar] [CrossRef]
- Weber, R.E.; de Wilde, J.A.M. Oxygenation properties of haemoglobins from the flatfish plaice (Pleuronectes platessa) and flounder (Platichthys flesus). J. Comp. Physiol. 1975, 101, 99–110. [Google Scholar] [CrossRef]
- Cruz-Neto, A.; Steffensen, J. The effects of acute hypoxia and hypercapnia on oxygen consumption of the freshwater European eel. J. Fish Biol. 1997, 50, 759–769. [Google Scholar] [CrossRef]
- Rogers, N. Chapter 4: Respiratory responses in gut carbonate production during hypoxia and hypercarbia in the European flounder (Platichthys flesus). In The Respiratory and Gut Physiology of Fish: Responses to Environmental Change; University of Exeter: Exeter, UK, 2015; pp. 95–139, University of Exeter: Exeter, UK, 2015. [Google Scholar]
- Ultsch, G.R.; Ott, M.E.; Heisler, N. Standard metabolic rate, critical oxygen tension, and aerobic scope for spontaneous activity of trout (Salmo gairdneri) and carp (Cyprinus carpio) in acidified water. Comp. Biochem. Physio. A 1980, 67, 329–335. [Google Scholar] [CrossRef]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Gregoire, M.; Chavez, F.P.; Conley, D.J.; Garcon, V.; Gilbert, D.; Gutierrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef]
- Miller, S.H.; Breitburg, D.L.; Burrell, R.B.; Keppel, A.G. Acidification increases sensitivity to hypoxia in important forage fishes. Mar. Ecol. Prog. Ser. 2016, 549, 1–8. [Google Scholar] [CrossRef]
- Petrie, B.; Yeats, P. Annual and internannual variability of nutriends and their estimated fluxes in the Scotian Shelf- Gulf of Maine region. Can. J. Fish. Aquat. Sci. 2000, 57, 2536–2546. [Google Scholar] [CrossRef]
- Metcalfe, J.D.; Butler, P.J. Changes in activity and ventilation in response to hypoxia in unrestrained, unoperated dogfish (Scyliorhinus canicula L.). J. Exp. Biol. 1984, 108, 411–418. [Google Scholar]
- Parsons, G.R.; Carlson, J.K. Physiological and behavioral responses to hypoxia in the bonnethead shark, Sphyrna tiburo: Routine swimming and respiratory regulation. Fish Physiol. Biochem. 1998, 19, 189–196. [Google Scholar] [CrossRef]
- Wood, C.M. The fallacy of the Pcrit – are there more useful alternatives? J. Exp. Biol. 2018, 221, jeb163717. [Google Scholar] [CrossRef]
- Regan, M.D.; Mandic, M.; Dhillon, R.S.; Lau, G.Y.; Farrell, A.P.; Schulte, P.M.; Seibel, B.A.; Speers-Roesch, B.; Ultsch, G.R.; Richards, J.G. Don’t throw the fish out with the respirometry water. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).