Intra-Specific Difference in the Effect of Salinity on Physiological Performance in European Perch (Perca fluviatilis) and Its Ecological Importance for Fish in Estuaries
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Holding
2.2. Experiments
2.3. Data Analysis and Statistics
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Remane, A. Die Brackwasserfauna. Verh. Zool. Ges. 1934, 36, 34–74. [Google Scholar]
- Whitfield, A.K.; Elliott, M.; Basset, A.; Blaber, S.J.M.; West, R.J. Paradigms in estuarine ecology—A review of the Remane diagram with a suggested revised model for estuaries. Estuar. Coast. Shelf Sci. 2012, 97, 78–90. [Google Scholar] [CrossRef]
- Kennish, M.J. Ecology of Estuaries; CRC Press: Boca Raton, FL, USA, 1986; ISBN 978-0-8493-5892-0. [Google Scholar]
- Cloern, J.E. Turbidity as a control on phytoplankton biomass and productivity in estuaries. Cont. Shelf Res. 1987, 7, 1367–1381. [Google Scholar] [CrossRef]
- Brennan, R.S.; Hwang, R.; Tse, M.; Fangue, N.A.; Whitehead, A. Local adaptation to osmotic environment in killifish, Fundulus heteroclitus, is supported by divergence in swimming performance but not by differences in excess post-exercise oxygen consumption or aerobic scope. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 196, 11–19. [Google Scholar] [CrossRef]
- Gibbons, T.C.; Metzger, D.C.H.; Healy, T.M.; Schulte, P.M. Gene expression plasticity in response to salinity acclimation in threespine stickleback ecotypes from different salinity habitats. Mol. Ecol. 2017, 26, 2711–2725. [Google Scholar] [CrossRef]
- DeWitt, T.J.; Scheiner, S.M. Phenotypic plasticity—Funtional and Conceptional Approaches; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Nonaka, E.; Svanbäck, R.; Thibert-Plante, X.; Englund, G.; Brännström, Å. Mechanisms by which phenotypic plasticity affects adaptive divergence and ecological speciation. Am. Nat. 2015, 186, E126–E143. [Google Scholar] [CrossRef]
- Pfennig, D.W.; Wund, M.A.; Snell-Rood, E.C.; Cruickshank, T.; Schlichting, C.D.; Moczek, A.P. Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol. Evol. 2010, 25, 459–467. [Google Scholar] [CrossRef]
- Kawecki, T.J.; Ebert, D. Conceptual issues in local adaptation. Ecol. Lett. 2004, 7, 1225–1241. [Google Scholar] [CrossRef]
- Thorpe, J. Synopsis of Biological Data on the Perch Perca fluviatilis Linnaeus, 1758 and Perca flavescens Mitchill, 1814; FAO Fisheries Synopsis; Food and Agriculture Organization: Rome, Italy, 1977; p. 147. [Google Scholar]
- Craig, J.F. Percid Fishes: Systematics, Ecology, and Exploitation; Fish and Aquatic Resources Series; Blackwell Science: Oxford, UK; Malden, MA, USA, 2000; ISBN 978-0-632-05616-3. [Google Scholar]
- Couture, P.; Pyle, G. The Biology of Perch; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Jeppesen, E.; Peder Jensen, J.; SØndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient: A detailed study of Danish lakes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Ljunggren, L.; Sandström, A.; Bergström, U.; Mattila, J.; Lappalainen, A.; Johansson, G.; Sundblad, G.; Casini, M.; Kaljuste, O.; Eriksson, B.K. Recruitment failure of coastal predatory fish in the Baltic Sea coincident with an offshore ecosystem regime shift. ICES J. Mar. Sci. 2010, 67, 1587–1595. [Google Scholar] [CrossRef]
- Morgan, D.L.; Hambleton, S.J.; Gill, H.S.; Beatty, S.J. Distribution, biology and likely impacts of the introduced redfin perch (Perca fluviatilis) (Percidae) in Western Australia. Mar. Freshw. Res. 2002, 53, 1211. [Google Scholar] [CrossRef]
- Skovrind, M.; Pacheco, G.; Christensen, E.A.F.; Feitz, F.; Carl, H.; Andersen, M.K.; Gilbert, M.T.P.; Møller, P.R. Population structure and natural selection of European perch in the Baltic Sea. 2019; in Preparation. [Google Scholar]
- Nilsson, J.; Andersson, J.; Karås, P.; Sandström, O. Recruitment failure and decreasing catches of perch (Perca fluviatilis L.) and pike (Esox lucius L.) in the coastal waters of southeast Sweden. Boreal Environ. Res. 2004, 9, 295–306. [Google Scholar]
- Ådjers, K.; Appelberg, M.; Eschbaum, R.; Lappalainen, A.; Minde, A.; Repe, R. Trends in coastal fish stocks of the Baltic Sea. Boreal Environ. Res. 2006, 11, 13–25. [Google Scholar]
- Christensen, E.A.F.; Grosell, M.; Steffensen, J.F. Maximum salinity tolerance and osmoregulatory capabilities of European perch Perca fluviatilis populations originating from different salinity habitats. Conserv. Physiol. 2019, 7, coz004. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Lutz, P. Ionic and body compartment responses to increasing salinity in the perch Perca fluviatilis. Comp. Biochem. Physiol. A Physiol. 1972, 42, 711–717. [Google Scholar] [CrossRef]
- Brauner, C.J.; Shrimpton, J.M.; Randall, D.J. Effect of short-duration seawater exposure on plasma ion concentration and swimming performance in coho salmon (Oncorhynchus kisutch) parr. Can. J. Fisher Aquat. Sci. 1992, 49, 2399–2405. [Google Scholar] [CrossRef]
- Kolok, A.S.; Sharkey, D. Effect of freshwater acclimation on the swimming performance and plasma osmolarity of the euryhaline gulf killifish. Trans. Am. Fisher Soc. 1997, 126, 866–870. [Google Scholar] [CrossRef]
- Wolter, C.; Arlinghaus, R. Navigation impacts on freshwater fish assemblages: The ecological relevance of swimming performance. Rev. Fish Biol. Fish. 2003, 13, 63–89. [Google Scholar] [CrossRef]
- Kapoor, B.G.; Domenici, P. Escape Responses in Fish: Kinematics, Performance and Behavior. In Fish Locomotion; Science Publishers: Enfield, UK, 2010; pp. 123–170. ISBN 978-1-57808-448-7. [Google Scholar]
- Glova, G.J.; McInerney, J.E. Critical swimming speeds of coho salmon (Oncorhynchus kisutch) fry to smolt stages in relation to salinity and temperature. J. Fish. Res. Board Can. 1977, 34, 151–154. [Google Scholar] [CrossRef]
- Nelson, J.A.; Tang, Y.; Boutilier, R.G. The effects of salinity change on the exercise performance of two Atlantic cod (Gadus morhua) populations inhabiting different environments. J. Exp. Biol. 1996, 199, 1295–1309. [Google Scholar] [PubMed]
- Crespel, A.; Dupont-Prinet, A.; Bernatchez, L.; Claireaux, G.; Tremblay, R.; Audet, C. Divergence in physiological factors affecting swimming performance between anadromous and resident populations of brook charr Salvelinus fontinalis: Swimming performance in S. fontinalis. J. Fish. Biol. 2017, 90, 2170–2193. [Google Scholar] [CrossRef] [PubMed]
- Yetsko, K.; Sancho, G. The effects of salinity on swimming performance of two estuarine fishes, Fundulus heteroclitus and Fundulus majalis: Swimming performance of two estuarine fishes. J. Fish. Biol. 2015, 86, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.A.F.; Illing, B.; Iversen, N.S.; Johansen, J.L.; Domenici, P.; Steffensen, J.F. Effects of salinity on swimming performance and oxygen consumption rate of shiner perch Cymatogaster aggregata. J. Exp. Mar. Biol. Ecol. 2018, 504, 32–37. [Google Scholar] [CrossRef]
- Hvas, M.; Nilsen, T.O.; Oppedal, F. Oxygen uptake and osmotic balance of atlantic salmon in relation to exercise and salinity acclimation. Front. Mar. Sci. 2018, 5, 368. [Google Scholar] [CrossRef]
- Chabot, D.; Steffensen, J.F.; Farrell, A.P. The determination of standard metabolic rate in fishes: Measuring smr in fishes. J. Fish. Biol. 2016, 88, 81–121. [Google Scholar] [CrossRef]
- Rao, M.M.R. Oxygen consumption of rainbow trout (Salmo gairdneri) in relation to activity and salinity. Can. J. Zool. 1968, 46, 781–786. [Google Scholar] [CrossRef]
- Febry, R.; Lutz, P. Energy partitioning in fish: The activity-related cost of osmoregulation in a euryhaline cichlid. J. Exp. Biol. 1987, 128, 63–85. [Google Scholar]
- Fry, F.E.J. The Effect of Environmental Factors on the Physiology of Fish. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 1971; Volume 6, pp. 1–98. ISBN 978-0-12-350406-7. [Google Scholar]
- Farmer, G.J.; Beamish, F.W.H. Oxygen consumption of Tilapia nilotica in relation to swimming speed and salinity. J. Fish. Res. Board Can. 1969, 26, 2807–2821. [Google Scholar] [CrossRef]
- Ern, R.; Huong, D.T.T.; Cong, N.V.; Bayley, M.; Wang, T. Effect of salinity on oxygen consumption in fishes: A review: Salinity and oxygen consumption. J. Fish. Biol. 2014, 84, 1210–1220. [Google Scholar] [CrossRef]
- Sardella, B.A.; Brauner, C.J. The effect of elevated salinity on ‘California’ Mozambique tilapia (Oreochromis mossambicus x O. urolepis hornorum) metabolism. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 148, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Norin, T.; Clark, T.D. Measurement and relevance of maximum metabolic rate in fishes: Maximum metabolic rate in fishes. J. Fish. Biol. 2016, 88, 122–151. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, D.; Claireaux, G. The Effects of Environmental Factors on the Physiology of Aerobic Exercise. In Fish Locomotion; Domenici, P., Kapoor, B., Eds.; Science Publishers: Enfield, UK, 2010; pp. 296–332. ISBN 978-1-57808-448-7. [Google Scholar]
- Brix, K.V.; Grosell, M. Evaluation of pre- and post-zygotic mating barriers, hybrid fitness and phylogenetic relationship between Cyprinodon variegatus variegatus and Cyprinodon variegatus hubbsi (Cyprinodontiformes, Teleostei). J. Evol. Biol. 2013, 26, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.; Roach, J.L.; Zhang, S.; Galvez, F. Genomic mechanisms of evolved physiological plasticity in killifish distributed along an environmental salinity gradient. Proc. Natl. Acad. Sci. USA 2011, 108, 6193–6198. [Google Scholar] [CrossRef] [PubMed]
- Skovrind, M.; Christensen, E.; Carl, H.; Jacobsen, L.; Møller, P. Marine spawning sites of perch Perca fluviatilis revealed by oviduct-inserted acoustic transmitters. Aquat. Biol. 2013, 19, 201–206. [Google Scholar] [CrossRef]
- Skovrind, M.; Olsen, M.T.; Vieira, F.G.; Pacheco, G.; Carl, H.; Gilbert, M.T.P.; Møller, P.R. Genomic population structure of freshwater-resident and anadromous ide (Leuciscus idus) in north-western Europe. Ecol. Evol. 2016, 6, 1064–1074. [Google Scholar] [CrossRef]
- Christensen, E.A.F.; Svendsen, M.B.S.; Steffensen, J.F. Growth, migration, and physio-chemical environment of European perch (Perca fluviatilis L.) in the western Baltic Sea. 2019; in preparation. [Google Scholar]
- Fischer, H.; Matthäus, W. The importance of the Drogden Sill in the Sound for major Baltic inflows. J. Mar. Syst. 1996, 9, 137–157. [Google Scholar] [CrossRef]
- Jacobsen, L.; Bekkevold, D.; Berg, S.; Jepsen, N.; Koed, A.; Aarestrup, K.; Baktoft, H.; Skov, C. Pike (Esox lucius L.) on the edge: Consistent individual movement patterns in transitional waters of the western Baltic. Hydrobiologia 2016, 784, 143–154. [Google Scholar] [CrossRef]
- Fenchel, T.; Sand-Jensen, K. Naturen i Danmark—Havet; Gyldendal: Copenhagen, Denmark, 2017. [Google Scholar]
- Bœuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef]
- Brett, J.R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish. Res. Board Can. 1964, 88, 152–172. [Google Scholar] [CrossRef]
- Steffensen, J.F. Some errors in respirometry of aquatic breathers: How to avoid and correct for them. Fish. Physiol. Biochem. 1989, 6, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, M.B.S.; Bushnell, P.G.; Steffensen, J.F. Design and setup of intermittent-flow respirometry system for aquatic organisms: How to set up an aquatic respirometry system. J. Fish. Biol. 2016, 88, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.A.F.; Svendsen, M.B.S.; Steffensen, J.F. Plasma osmolality and oxygen consumption of perch Perca fluviatilis in response to different salinities and temperatures. J. Fish. Biol. 2017, 90, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, C.; de Boeck, G.; Claireaux, G. Forced and Preferred Swimming Speeds of Fish: A Methodological Approach. In Swimming Physiology of Fish; Palstra, A.P., Planas, J.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 81–108. ISBN 978-3-642-31048-5. [Google Scholar]
- Bushnell, P.G.; Steffensen, J.F.; Schurmann, H.; Jones, D.R. Exercise metabolism in two species of cod in arctic waters. Polar Biol. 1994, 14, 43–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Ojanguren, A.F.; Brana, F. Effects of size and morphology on swimming performance in juvenile brown trout (Salmo trutta L.). Ecol. Freshw. Fish. 2003, 12, 241–246. [Google Scholar] [CrossRef]
- Morgan, J.D.; Iwama, G.K. Energy cost of NaCl transport in isolated gills of cutthroat trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 277, R631–R639. [Google Scholar] [CrossRef]
- Kidder, G.W.; Petersen, C.W.; Preston, R.L. Energetics of osmoregulation: I. oxygen consumption by Fundulus heteroclitus. J. Exp. Zool. 2006, 305A, 309–317. [Google Scholar] [CrossRef]
- Kirschner, L.B. Energetics of osmoregulation in fresh water vertebrates. J. Exp. Zool. 1995, 271, 243–252. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Segal, M.S.; Beem, E. Effect of pH, ionic charge, and osmolality on cytochromec -mediated caspase-3 activity. Am. J. Physiol. Cell Physiol. 2001, 281, C1196–C1204. [Google Scholar] [CrossRef]
- Nordlie, F.G. The influence of environmental salinity on respiratory oxygen demands in the euryhaline teleost, Ambassis interrupta Bleeker. Comp. Biochem. Physiol. A Physiol. 1978, 59, 271–274. [Google Scholar] [CrossRef]
- Larsen, P.F.; Nielsen, E.E.; Koed, A.; Thomsen, D.S.; Olsvik, P.A.; Loeschcke, V. Interpopulation differences in expression of candidate genes for salinity tolerance in winter migrating anadromous brown trout (Salmo trutta L.). BMC Genet. 2008, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, M.B.S.; Bushnell, P.G.; Christensen, E.A.F.; Steffensen, J.F. Sources of variation in oxygen consumption of aquatic animals demonstrated by simulated constant oxygen consumption and respirometers of different sizes: Variation in intermittent-flow respirometry. J. Fish. Biol. 2016, 88, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Norin, T.; Malte, H. Repeatability of standard metabolic rate, active metabolic rate and aerobic scope in young brown trout during a period of moderate food availability. J. Exp. Biol. 2011, 214, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.P.; Sun, C.M.; Wu, S.M. Changes of plasma osmolality, chloride concentration and gill Na/K-ATPase activity in tilapia Oreochromis mossambicus during seawater acclimation. Mar. Biol. 1989, 100, 295–299. [Google Scholar] [CrossRef]
- Morgan, J.D.; Sakamoto, T.; Grau, E.G.; Iwama, G.K. Physiological and respiratory responses of the mozambique tilapia (Oreochromis mossambicus) to salinity acclimation. Comp. Biochem. Physiol. A Physiol. 1997, 117, 391–398. [Google Scholar] [CrossRef]
- Van der Linden, A.; Vanaudenhove, M.; Verhoye, M.; De Boeck, G.; Blust, R. Osmoregulation of the common carp (Cyprinus carpio) when exposed to an osmotic challenge assessed in-vivo and non-invasively by diffusion- and T2-weighted magnetic resonance imaging. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1999, 124, 343–352. [Google Scholar] [CrossRef]
- Morgan, J.D.; Iwama, G.K. Cortisol-induced changes in oxygen consumption and ionic regulation in coastal cutthroat trout (Oncorhynchus clarki clarki) parr. Fish. Physiol. Biochem. 1996, 15, 385–394. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, G.; Vlaeminck, A.; Van der Linden, A.; Blust, R. The energy metabolism of common carp (Cyprinus carpio) when exposed to salt stress: An increase in energy expenditure or effects of starvation? Physiol. Biochem. Zool. 2000, 73, 102–111. [Google Scholar] [CrossRef]
- Killen, S.S. Growth trajectory influences temperature preference in fish through an effect on metabolic rate. J. Anim. Ecol. 2014, 83, 1513–1522. [Google Scholar] [CrossRef]
- Roche, D.G.; Binning, S.A.; Bosiger, Y.; Johansen, J.L.; Rummer, J.L. Finding the best estimates of metabolic rates in a coral reef fish. J. Exp. Biol. 2013, 216, 2103–2110. [Google Scholar] [CrossRef]
- Schurmann, H.; Steffensen, J.F. Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J. Fish. Biol. 1997, 50, 1166–1180. [Google Scholar]
- Reidy, S.P.; Nelson, J.A.; Tang, Y.; Kerr, S.R. Post-exercise metabolic rate in Atlantic cod and its dependence upon the method of exhaustion. J. Fish. Biol. 1995, 47, 377–386. [Google Scholar] [CrossRef]
- Hvas, M.; Oppedal, F. Influence of experimental set-up and methodology for measurements of metabolic rates and critical swimming speed in Atlantic salmon Salmo salar. J. Fish. Biol. 2019, 95, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Hollins, J.; Thambithurai, D.; Koeck, B.; Crespel, A.; Bailey, D.M.; Cooke, S.J.; Lindström, J.; Parsons, K.J.; Killen, S.S. A physiological perspective on fisheries-induced evolution. Evol. Appl. 2018, 11, 561–576. [Google Scholar] [CrossRef]
- Stieglitz, J.D.; Mager, E.M.; Hoenig, R.H.; Benetti, D.D.; Grosell, M. Impacts of Deepwater Horizon crude oil exposure on adult mahi-mahi (Coryphaena hippurus) swim performance: Impacts of Deepwater Horizon exposure on mahi-mahi. Environ. Toxicol. Chem. 2016, 35, 2613–2622. [Google Scholar] [CrossRef]
- Marras, S.; Cucco, A.; Antognarelli, F.; Azzurro, E.; Milazzo, M.; Bariche, M.; Butenschön, M.; Kay, S.; Di Bitetto, M.; Quattrocchi, G.; et al. Predicting future thermal habitat suitability of competing native and invasive fish species: From metabolic scope to oceanographic modelling. Conserv. Physiol. 2015, 3, cou059. [Google Scholar] [CrossRef]
- Peck, M.A.; Arvanitidis, C.; Butenschön, M.; Canu, D.M.; Chatzinikolaou, E.; Cucco, A.; Domenici, P.; Fernandes, J.A.; Gasche, L.; Huebert, K.B.; et al. Projecting changes in the distribution and productivity of living marine resources: A critical review of the suite of modelling approaches used in the large European project VECTORS. Estuar. Coast. Shelf Sci. 2018, 201, 40–55. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Peck, M.A. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish. Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Farrell, A.P. Physiology and climate change. Science 2008, 322, 690–692. [Google Scholar] [CrossRef]
- Evans, T.G.; Diamond, S.E.; Kelly, M.W. Mechanistic species distribution modelling as a link between physiology and conservation. Conserv. Physiol. 2015, 3, cov056. [Google Scholar] [CrossRef]
- Wikelski, M.; Cooke, S.J. Conservation physiology. Trends Ecol. Evol. 2006, 21, 38–46. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, D.J.; Axelsson, M.; Chabot, D.; Claireaux, G.; Cooke, S.J.; Corner, R.A.; De Boeck, G.; Domenici, P.; Guerreiro, P.M.; Hamer, B.; et al. Conservation physiology of marine fishes: State of the art and prospects for policy. Conserv. Physiol. 2016, 4, cow046. [Google Scholar] [CrossRef] [PubMed]
- Sandblom, E.; Clark, T.D.; Gräns, A.; Ekström, A.; Brijs, J.; Sundström, L.F.; Odelström, A.; Adill, A.; Aho, T.; Jutfelt, F. Physiological constraints to climate warming in fish follow principles of plastic floors and concrete ceilings. Nat. Commun. 2016, 7, 11447. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.L.; Overgaard, J.; Wang, T.; Gesser, H.; Malte, H. Temperature effects on aerobic scope and cardiac performance of European perch (Perca fluviatilis). J. Therm. Biol. 2017, 68, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.A.F.; Skovrind, M.; Olsen, M.T.; Carl, H.; Gravlund, P.; Møller, P.R. Hatching success in brackish water of Perca fluviatilis eggs obtained from the western Baltic Sea. Cybium 2016, 6, 133–138. [Google Scholar]
- Nesbø, C.L.; Magnhagen, C.; Jakobsen, K.S. Genetic differentiation among stationary and anadromous perch (Perca fluviatilis) in the Baltic Sea. Hereditas 1999, 129, 241–249. [Google Scholar] [CrossRef]
- George, A.L.; Kuhajda, B.R.; Williams, J.D.; Cantrell, M.A.; Rakes, P.L.; Shute, J.R. Guidelines for propagation and translocation for freshwater fish conservation. Fisher 2009, 34, 529–545. [Google Scholar] [CrossRef]
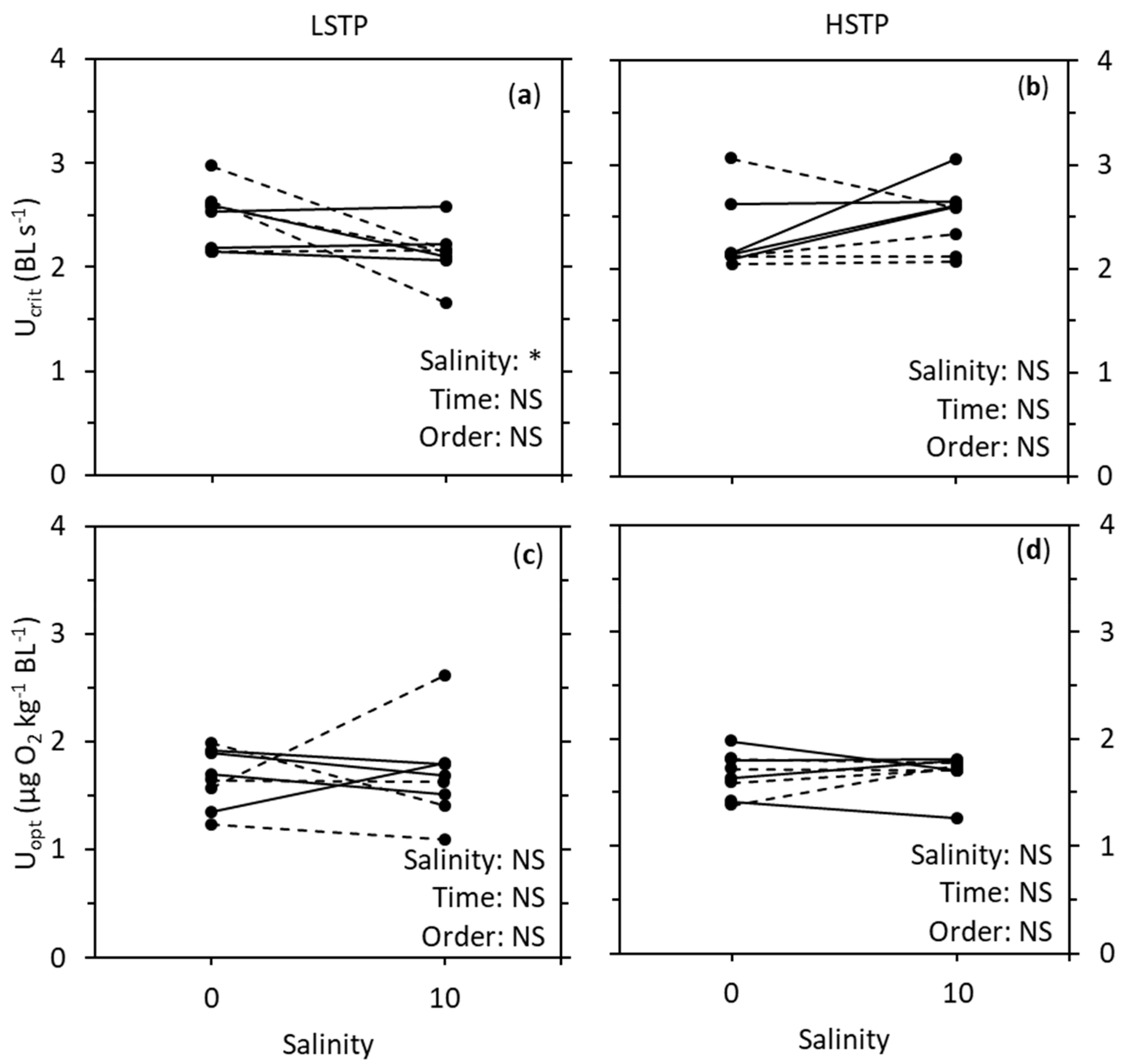
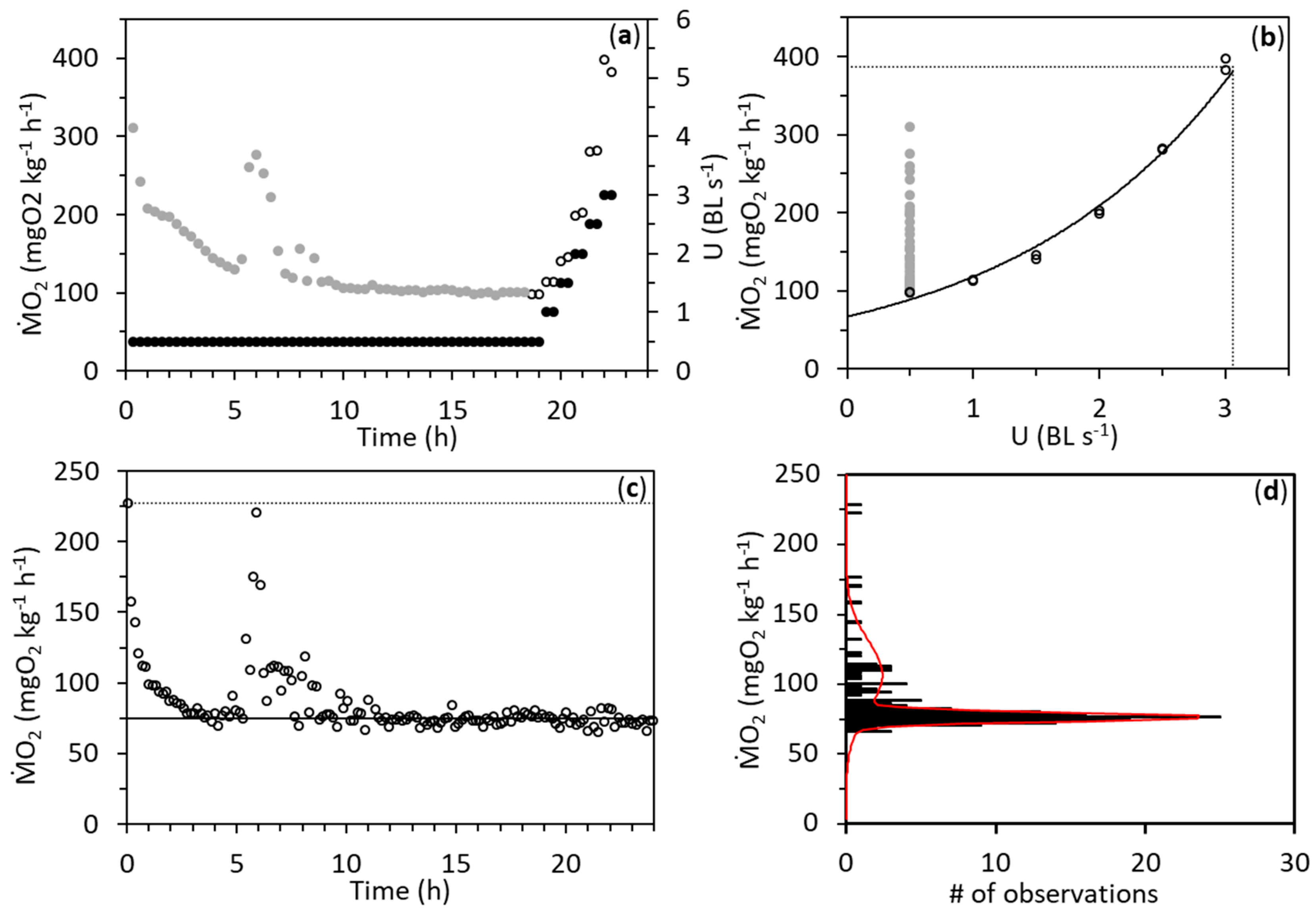
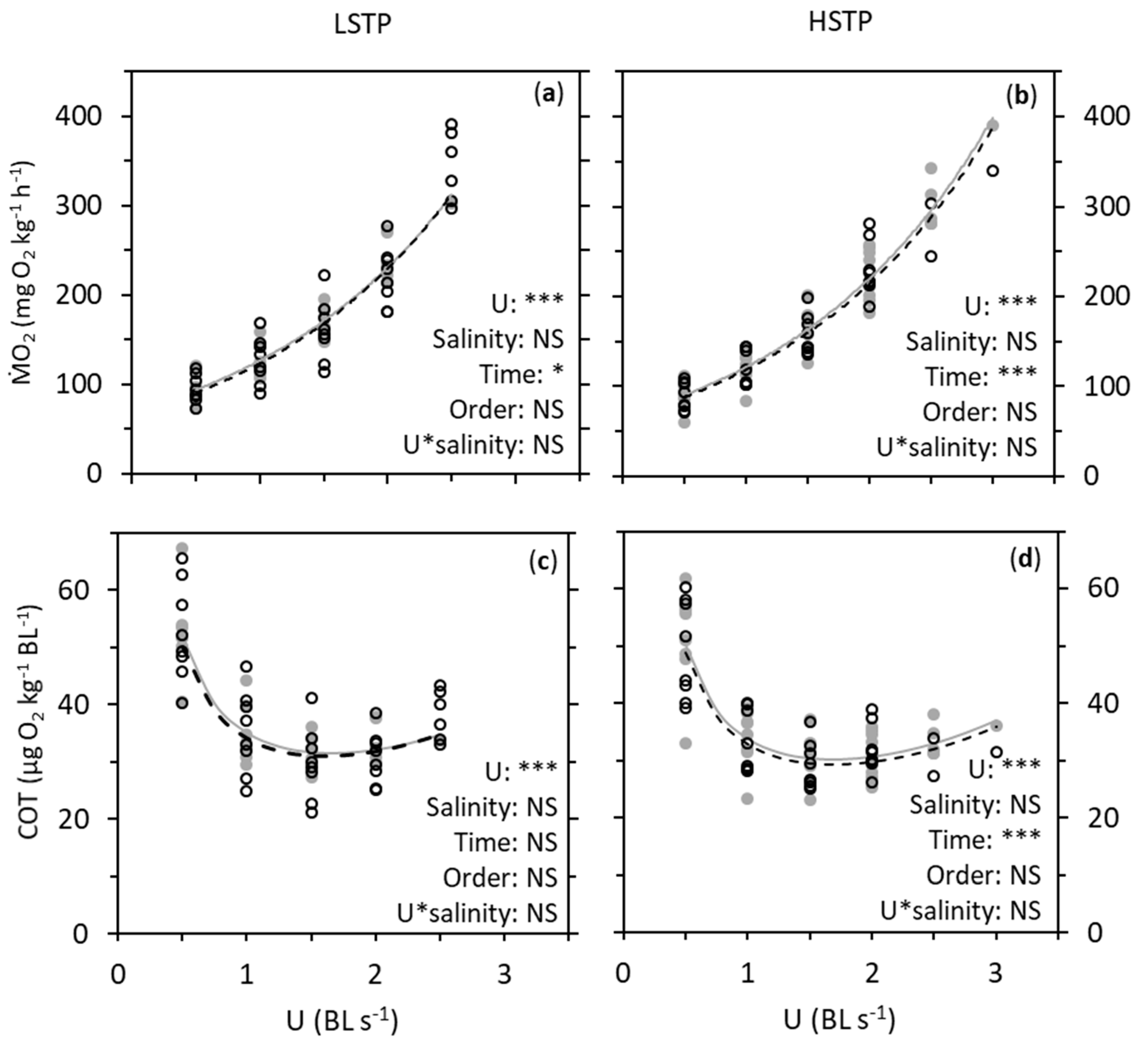
| Metric | Factor | LSTP | HSTP |
|---|---|---|---|
| Ucrit (BL s−1) | 2.30 ± 0.08 | 2.40 ± 0.09 | |
| Salinity 1 | * | NS | |
| Time 2 | NS | NS | |
| Order 3 | NS | NS | |
| Uopt (µg O2 kg−1 BL−1) | 1.68 ± 0.09 | 1.68 ± 0.05 | |
| Salinity | NS | NS | |
| Time | NS | NS | |
| Order | NS | NS | |
| SMR (mg O2 kg−1 h−1) | 70.1 ± 2.5 | 69.4 ± 2.6 | |
| Respirometry method 4 | NS | NS | |
| Salinity | NS | * | |
| Time | * | *** | |
| Order | NS | NS | |
| MMR (mg O2 kg−1 h−1) | 294.8 ± 10.7 | 291.8 ± 9.9 | |
| Respirometry method | NS | NS | |
| Salinity | NS | NS | |
| Time | NS | NS | |
| Order | NS | NS | |
| Aerobic scope(mg O2 kg−1 h−1) | 294.8 ± 10.7 | 222.4 ± 9.7 | |
| Respirometry method | NS | NS | |
| Salinity | NS | NS | |
| Time | NS | NS | |
| Order | NS | NS |
| Population | Respirometry Method | Standard Deviation (%) | Sample Size | Cost of Osmoregulation (in % of SMR) |
|---|---|---|---|---|
| LSTP | Swimming | 8 | 16 | 12 |
| Static | 20 | 16 | 28 | |
| HSTP | Swimming | 7 | 16 | 10 |
| Static | 13 | 8 | 19 |
| Population | Respirometry Method | Standard Deviation (%) | Sample Size |
|---|---|---|---|
| LSTP | Swimming | 8 | 82 |
| Static | 20 | 502 | |
| HSTP | Swimming | 7 | 62 |
| Static | 13 | 212 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, E.A.F.; Stieglitz, J.D.; Grosell, M.; Steffensen, J.F. Intra-Specific Difference in the Effect of Salinity on Physiological Performance in European Perch (Perca fluviatilis) and Its Ecological Importance for Fish in Estuaries. Biology 2019, 8, 89. https://doi.org/10.3390/biology8040089
Christensen EAF, Stieglitz JD, Grosell M, Steffensen JF. Intra-Specific Difference in the Effect of Salinity on Physiological Performance in European Perch (Perca fluviatilis) and Its Ecological Importance for Fish in Estuaries. Biology. 2019; 8(4):89. https://doi.org/10.3390/biology8040089
Chicago/Turabian StyleChristensen, Emil A. F., John D. Stieglitz, Martin Grosell, and John F. Steffensen. 2019. "Intra-Specific Difference in the Effect of Salinity on Physiological Performance in European Perch (Perca fluviatilis) and Its Ecological Importance for Fish in Estuaries" Biology 8, no. 4: 89. https://doi.org/10.3390/biology8040089
APA StyleChristensen, E. A. F., Stieglitz, J. D., Grosell, M., & Steffensen, J. F. (2019). Intra-Specific Difference in the Effect of Salinity on Physiological Performance in European Perch (Perca fluviatilis) and Its Ecological Importance for Fish in Estuaries. Biology, 8(4), 89. https://doi.org/10.3390/biology8040089





