Antibiotic Resistance of Staphylococci from Bulk-Tank Milk of Sheep Flocks: Prevalence, Patterns, Association with Biofilm Formation, Effects on Milk Quality, and Risk Factors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
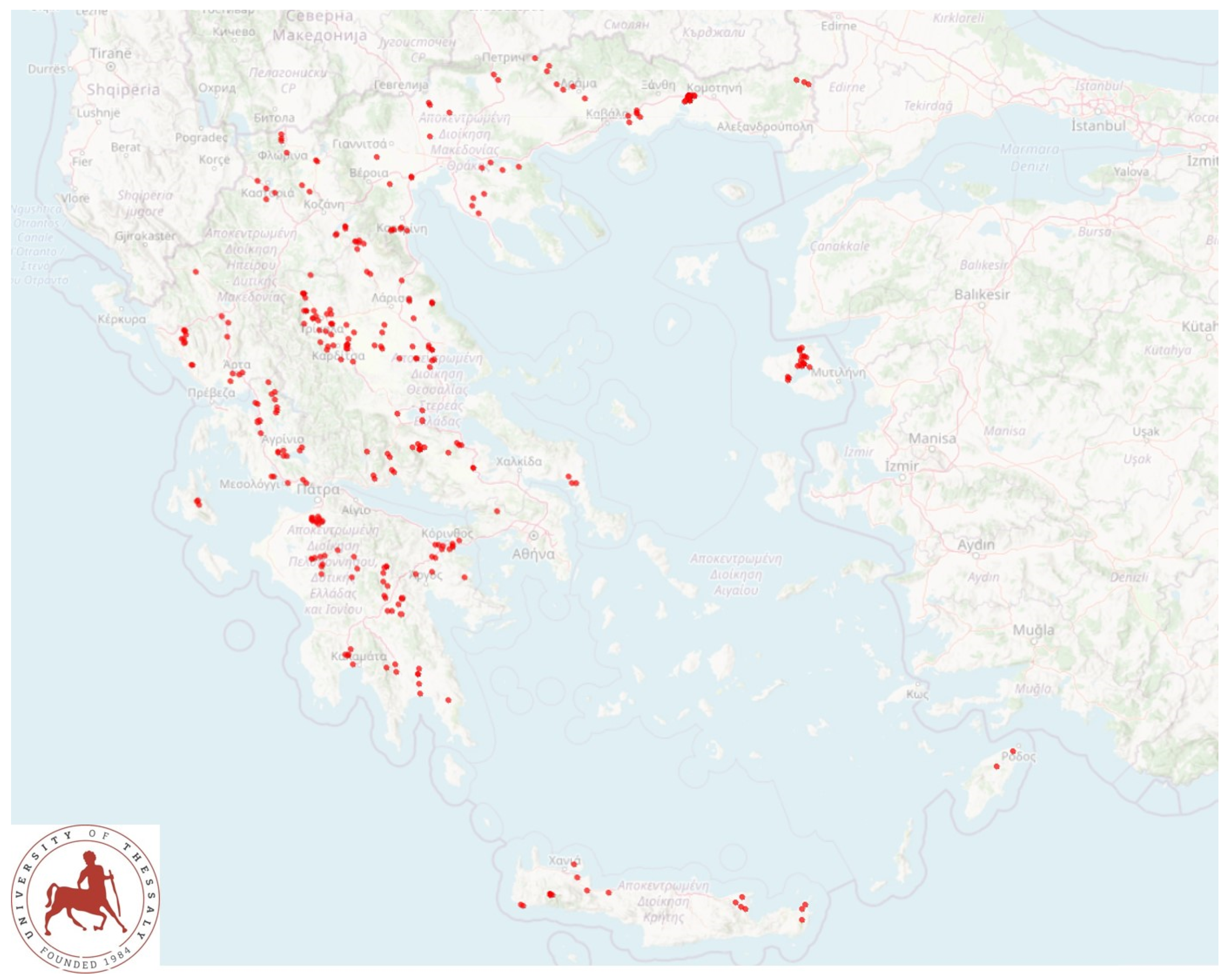
2.1. Sheep Flocks and Sampling
2.2. Laboratory Examinations
2.3. Data Management and Analysis
2.3.1. Data Management
2.3.2. Statistical Analysis
3. Results
3.1. Staphylococcal Recovery and Presence of Antibiotic Resistance
3.2. Biofilm Formation
3.3. Associations with Milk Quality
3.4. Variables Associated with Isolation of Resistant Staphylococcal Isolates from the Bulk-Tank Milk
3.4.1. Isolation of Oxacillin-Resistant Staphylococcal Isolates
3.4.2. Isolation of Staphylococcal Isolates Resistant to at Least One Antibiotic
3.4.3. Isolation of Multi-Resistant Staphylococcal Isolates
4. Discussion
4.1. Presence of Antibiotic Resistance in Staphylococcal Isolates
4.2. Association of Antibiotic Resistance with Biofilm Formation
4.3. Possible Effects on Milk Quality
4.4. Predictors for Antibiotic-Resistant Staphylococcal Isolates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Management system applied in the flock (description according to EFSA classification) [47] |
| Month into the lactation period at sampling (month) |
| Machine- or hand-milking (description) |
| No. of ewes in the flock (no.) |
| Total milk quantity per ewe obtained during the preceding milking period (litres) |
| Average number of lambs born per ewe (no.) |
| Collaboration with a veterinarian (yes/no) |
| Total visits made annually by veterinarians to the flock during the preceding season (no.) |
| Clinical mastitis annual incidence risk in the flock (%) |
| Age of lamb removal from their dams (days) |
| Daily number of milking sessions (no.) |
| Duration of the dry-period (months) |
| Means of calculating live bodyweight for the administration of pharmaceutical products (weighing/estimation) |
| Routine overdosing (compared to dose prescribed) of pharmaceuticals (yes/no) |
| Annual frequency of systemic disinfections in the farm (no. of occasions) |
| Routine administration of antimicrobials in newborns (yes/no) |
| Vaccination against mastitis (yes/no) |
| Administration of ‘dry-ewe’ treatment at the end of the lactation period (yes/no) |
| Use of teat disinfection after milking (yes/no) |
| Age of the farmer (years) |
| Length of previous animal farming experience of the farmer (years) |
| Education of the farmer (description) |
| Farmer by profession (yes/no) |
| Family tradition in farming (yes/no) |
| Presence of working staff in the flock (yes/no) |
References
- Hellenic Agricultural Organisation—Demeter (2020). Deliveries of Ovine and Caprine Milk by Region and Regional Authority and Average Milk Price—Calendar Year 2019. Cumulative Data Updated. Available online: https://www.elgo.gr/images/ELOGAK_files/Statistics/2020/AIGO_Παραδόσεις_Πρόβειου_και_Γίδινου_Γάλακτος_2019.pdf (accessed on 20 December 2020).
- Pulina, G.; Milan, M.J.; Lavin, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Current production trends, farm structures, and economics of the dairy sheep and goat sector. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasileiou, N.G.C.; Chatzopoulos, D.C.; Sarrou, S.; Fragkou, I.A.; Katsafadou, A.I.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Role of staphylococci in mastitis in sheep. J. Dairy Res. 2019, 86, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Lianou, D.T.; Michael, C.K.; Vasileiou, N.G.C.; Petinaki, E.; Cripps, P.J.; Tsilipounidaki, K.; Katsafadou, A.I.; Politis, A.P.; Kordalis, N.G.; Ioannidi, K.S.; et al. Extensive countrywide field investigation of somatic cell counts and total bacterial counts in bulk-tank raw milk in sheep flocks in Greece. Foods 2021, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Macori, G.; Giacinti, G.; Bellio, A.; Gallina, S.; Bianchi, D.M.; Sagrafoli, D.; Marri, N.; Giangolini, G.; Amatiste, S. Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in the ovine dairy chain and in farm-related humans. Toxins 2017, 9, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacinti, G.; Carfora, V.; Caprioli, A.; Sagrafoli, D.; Marri, N.; Giangolini, G.; Amoruso, R.; Iurescia, M.; Stravino, F.; Dottarelli, S.; et al. Prevalence and characterization of methicillin-resistant Staphylococcus aureus carrying mecA or mecC and methicillin-susceptible Staphylococcus aureus in dairy sheep farms in central Italy. J. Dairy Sci. 2017, 100, 7857–7863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulos, P.; Papadopoulos, T.; Angelidis, A.S.; Boukouvala, E.; Zdragas, A.; Papa, A.; Hadjichristodoulou, C.; Sergelidis, D. Prevalence of Staphylococcus aureus and of methicillin-resistant S. aureus (MRSA) along the production chain of dairy products in north-western Greece. Food Microbiol. 2018, 69, 43–50. [Google Scholar] [CrossRef]
- Barrero-Dominguez, B.; Luque, I.; Galan-Relano, A.; Vega-Pla, J.L.; Huerta, B.; Roman, F.; Astorga, R.J. Antimicrobial resistance and distribution of Staphylococcus spp. pulsotypes isolated from goat and sheep bulk tank milk in Southern Spain. Foodb. Pathog. Dis. 2019, 16, 723–730. [Google Scholar] [CrossRef]
- Poveda, J.M.; Jiménez, L.; Perea, J.M.; Arias, R.; Palop, M.L. Farming practices influence antibiotic resistance and biogenic amine capacity of staphylococci from bulk tank ewe’s milk. Animals 2020, 10, 1622. [Google Scholar] [CrossRef]
- Obaidat, M.M.; Roess, A.A.; Mahasneh, A.A.; Al-Hakimi, R.A. Antibiotic-resistance, enterotoxin gene profiles and farm-level prevalence of Staphylococcus aureus in cow, sheep and goat bulk tank milk in Jordan. Int. Dairy J. 2018, 81, 28–34. [Google Scholar] [CrossRef]
- Lianou, D.T.; Chatziprodromidou, I.P.; Vasileiou, N.G.C.; Michael, C.K.; Mavrogianni, V.S.; Politis, A.P.; Kordalis, N.G.; Billinis, C.; Giannakopoulos, A.; Papadopoulos, E.; et al. A detailed questionnaire for the evaluation of health management in dairy sheep and goats. Animals 2020, 10, 1489. [Google Scholar] [CrossRef]
- Laird, D.T.; Gambrel-Lenarz, S.A.; Scher, F.M.; Graham, T.E.; Reddy, R. Microbiological Count Methods. In Standard Methods for the Examination of Dairy Products, 17th ed.; Wehr, H.M., Frank, J.F., Eds.; APHA Press: Washington, DC, USA, 2004; pp. 153–186. [Google Scholar]
- Barrow, G.I.; Feltham, R.K.A. Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Euzeby, J.P. List of bacterial names with standing in nomenclature: A folder available on the Internet. Int. J. Syst. Bacteriol. 1997, 47, 590–592. [Google Scholar] [CrossRef] [Green Version]
- Vasileiou, N.G.C.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Katsafadou, A.I.; Spyrou, V.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Slime-producing staphylococci as causal agents of subclinical mastitis in sheep. Vet. Microbiol. 2018, 224, 93–99. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiggans, G.R.; Shook, G.E. A lactation measure of somatic cell count. J. Dairy Sci. 1987, 70 (Suppl. S13), 2666–2672. [Google Scholar] [CrossRef]
- Franzoi, M.; Manuelian, C.L.; Penasa, M.; De Marchi, M. Effects of somatic cell score on milk yield and mid-infrared predicted composition and technological traits of Brown Swiss, Holstein Friesian, and Simmental cattle breeds. J. Dairy Sci. 2020, 103, 791–804. [Google Scholar] [CrossRef]
- Andreoletti, O.; Baggesen, D.L.; Bolton, D.; Butaye, P.; Cook, P.; Davies, R.; Escamez, P.S.F.; Griffin, J.; Hald, T.; Havellar, A.; et al. Scientific opinion on the public health risks related to the consumption of raw drinking milk. EFSA J. 2015, 13, 3940. [Google Scholar]
- Vasileiou, N.G.C.; Cripps, P.J.; Ioannidi, K.S.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Orfanou, D.C.; Politis, A.; Calvo Gonzalez-Valerio, T.; Argyros, S.; et al. Extensive countrywide field investigation of subclinical mastitis in sheep in Greece. J. Dairy Sci. 2018, 101, 7297–7310. [Google Scholar] [CrossRef] [Green Version]
- Michael, C.K.; Lianou, D.T.; Vasileiou, N.G.C.; Tsilipounidaki, K.; Katsafadou, A.I.; Politis, A.I.; Kordalis, N.G.; Ioannidi, K.S.; Gougoulis, D.A.; Trikalinou, C.; et al. Association of staphylococcal populations on teatcups of milking rarlours with vaccination against staphylococcal mastitis in sheep and goat farms. Pathogens 2021, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Marco Melero, J.C. Mastitis in Laxta Breed Sheep: Epidemiology, Diagnosis and Control. Doctoral thesis, University of Zaragoza, Zaragoza, Spain, 1994. [Google Scholar]
- Ou, Q.T.; Zhou, J.L.; Lin, D.X.; Bai, C.; Zhang, T.; Lin, J.L.; Zheng, H.Q.; Wang, X.J.; Ye, J.P.; Ye, X.H.; et al. A large meta-analysis of the global prevalence rates of S. aureus and MRSA contamination of milk. Crit. Rev. Food Sci. Nutr. 2018, 58, 2213–2228. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, P.O.; Gagnaire, J.; Botelho-Nevers, E.; Grattard, F.; Carricajo, A.; Lucht, F.; Pozzetto, B.; Berthelot, P. Detection and clinical relevance of Staphylococcus aureus nasal carriage: An update. Expert Rev. Anti-Infect. Ther. 2014, 12, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, N.G.C.; Sarrou, S.; Papagiannitsis, C.; Chatzopoulos, D.C.; Malli, E.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Antimicrobial agent susceptibility and typing of staphylococcal isolates from subclinical mastitis in ewes. Microb. Drug. Res. 2019, 25, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Li, Q.; Zhang, Y.; Song, J.; Shi, X.; Shi, C. Biofilm formation and antibiotic resistance pattern of dominant Staphylococcus aureus clonal lineages in China. J. Food Saf. 2016, 37, e12304. [Google Scholar] [CrossRef]
- Ryder, V.J.; Chopra, I.; O’Neill, A.J. Increased mutability of staphylococci in biofilms as a consequence of oxidative stress. PLoS ONE 2012, 7, e476. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.S.; Balfour, J.A.; Bryson, H.M. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 1997, 53, 637–656. [Google Scholar] [CrossRef]
- Castañeda-García, A.; Rodríguez-Rojas, A.; Guelfo, J.R.; Blázquez, J. The glycerol-3-phosphate permease GlpT is the only fosfomycin transporter in Pseudomonas aeruginosa. J. Bacteriol. 2009, 191, 6968–6974. [Google Scholar] [CrossRef] [Green Version]
- Bush, K. Antimicrobial agents targeting bacterial cell walls and cell membranes. Rev. Sci. Tech. 2012, 31, 43–56. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, J.; Wood, T.K. Engineering global regulator Hha of Escherichia coli to control biofilm dispersal. Microb. Biotechnol. 2010, 3, 717–728. [Google Scholar] [CrossRef]
- Hirakawa, H.; Kurabayashi, K.; Tanimoto, K.; Tomita, H. Oxygen limitation enhances the antimicrobial activity of fosfomycin in Pseudomonas aeruginosa following overexpression of glpT which encodes glycerol-3-phosphate/fosfomycin symporter. Fr. Microbiol. 2018, 9, 1950. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Fu, X.; Zhou, Y.; Liu, Y.; Xu, X.; Wang, M. Mutations of the transporter proteins GlpT and UhpT confer fosfomycin resistance in Staphylococcus aureus. Fr. Microbiol. 2017, 8, 914. [Google Scholar] [CrossRef]
- Fragkou, I.A.; Solomakos, N.; Dagleish, M.P.; Cripps, P.J.; Papaioannou, N.; Boscos, C.M.; Ververidis, H.N.; Billinis, C.; Orfanou, D.C.; Govaris, A.; et al. Effects of experimental challenge of ewes with Mannheimia haemolytica on subsequent milk composition. J. Dairy Res. 2008, 75, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Koulenti, D.; Fragkou, P.C.; Tsiodras, S. Editorial for Special Issue ‘Multidrug-Resistant Pathogens’. Microorganisms 2020, 8, 1383. [Google Scholar] [CrossRef] [PubMed]
- Hellenic Veterinary Association. European Day for Information and Awareness for the Use of Antibiotics. Press release (ref. 1631, 15 Nov. 2018), Athens. 2018. [Google Scholar]
- Politis, A.P.; Vasileiou, N.G.C.; Ioannidi, K.S.; Mavrogianni, V.S. Treatment of bacterial respiratory infections in lambs. Small Rumin. Res. 2019, 176, 70–75. [Google Scholar] [CrossRef]
- Mavrogianni, V.S.; Menzies, P.I.; Fragkou, I.A.; Fthenakis, G.C. Principles of mastitis treatment in sheep and goats. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Fthenakis, G.C. Prevalence and aetiology of subclinical mastitis in ewes of Southern Greece. Small Rumin. Res. 1994, 13, 293–300. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Mavrogianni, V.S.; Petridis, I.G.; Vasileiou, N.G.C.; Fthenakis, G.C. Mastitis in sheep-The last 10 years and the future of research. Vet. Microbiol. 2015, 181, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, N.G.C.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Predisposing factors for bacterial mastitis in ewes. Reprod. Dom. Anim. 2019, 54, 1424–1431. [Google Scholar] [CrossRef]
- Templeton, M.R.; Oddy, F.; Leung, W.K.; Rogers, M. Chlorine and UV disinfection of ampicillin-resistant and trimethoprim-resistant Escherichia coli. Can. J. Civ. Eng. 2009, 36, 889–894. [Google Scholar] [CrossRef]
- Khan, S.; Beattie, T.; Knapp, C. Relationship between antibiotic- and disinfectant-resistance profiles in bacteria harvested from tap water. Chemosphere 2016, 152, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; McEntire, J.C.; Zhang, L.; Li, X.; Doyle, M. The transfer of antibiotic resistance from food to humans: Facts, implications and future directions. Rev. Scient. Techn. Off. Internat. Epiz. 2012, 31, 249–260. [Google Scholar] [CrossRef]
- Schwarz, S.; Loeffler, A.; Kadlec, K. Bacterial resistance to antimicrobial agents and its impact on veterinary and human medicine. Vet. Dermatol. 2017, 28, 82-e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority. Scientific opinion on the welfare risks related to the farming of sheep for wool, meat and milk production. EFSA J. 2014, 12, 3933–4060. [Google Scholar]
| Staphylococcal Species | Frequency of Staphylococcal Isolates | ||
|---|---|---|---|
| All Isolates 1 | Resistant Isolates 2,3 | Multi-Resistant Isolates 3,4 | |
| Staphylococcus aureus | 54 (0.233) | 17 (0.315) | 2 (0.037) |
| Staphylococcus simulans | 35 (0.151) | 4 (0.114) | 0 (0.000) |
| Staphylococcus equorum | 23 (0.099) | 17 (0.739) | 14 (0.609) |
| Staphylcoccus haemolyticus | 22 (0.095) | 11 (0.500) | 1 (0.045) |
| Staphylococcus chromogenes | 13 (0.056) | 4 (0.308) | 1 (0.077) |
| Staphylococcus lentus | 12 (0.051) | 11 (0.917) | 8 (0.667) |
| Staphylococcus lugdunensis | 11 (0.047) | 7 (0.636) | 1 (0.091) |
| Staphylococcus warneri | 9 (0.039) | 3 (0.333) | 1 (0.111) |
| Staphylococcus kloosii | 7 (0.030) | 5 (0.714) | 4 (0.571) |
| Staphylococcus capitis | 6 (0.026) | 3 (0.500 | 0 (0.000) |
| Staphylococcus intermedius | 6 (0.026) | 3 (0.500) | 0 (0.000) |
| Staphylococcus cohnii subsp. cohnii | 4 (0.017) | 3 (0.750) | 1 (0.250) |
| Staphylococcus epidermidis | 4 (0.017) | 4 (1.000) | 0 (0.000) |
| Staphylococcus saprophyticus | 4 (0.017) | 2 (0.500) | 2 (0.500) |
| Staphylococcus xylosus | 4 (0.017) | 3 (0.750) | 3 (0.750) |
| Staphylococcus auricularis | 3 (0.013) | 2 (0.667) | 0 (0.000) |
| Staphylococcus cohnii subsp. urealyticum | 3 (0.013) | 2 (0.667) | 1 (0.333) |
| Staphylococcus sciuri | 3 (0.013) | 1 (0.333) | 1 (0.333) |
| Staphylococcus vitulinus | 3 (0.013) | 1 (0.333) | 1 (0.333) |
| Staphylococcus hominis | 2 (0.009) | 1 (0.500) | 0 (0.000) |
| Staphylococcus pasteuri | 2 (0.009) | 2 (1.000) | 0 (0.000) |
| Staphylococcus carnosus | 2 (0.009) | 1 (0.500) | 0 (0.000) |
| Total | 232 (1.000) | 107 (0.461) | 41 (0.177) |
| Variable (n = 1) | Odds Ratio 1 (95% Confidence Intervals) | p |
|---|---|---|
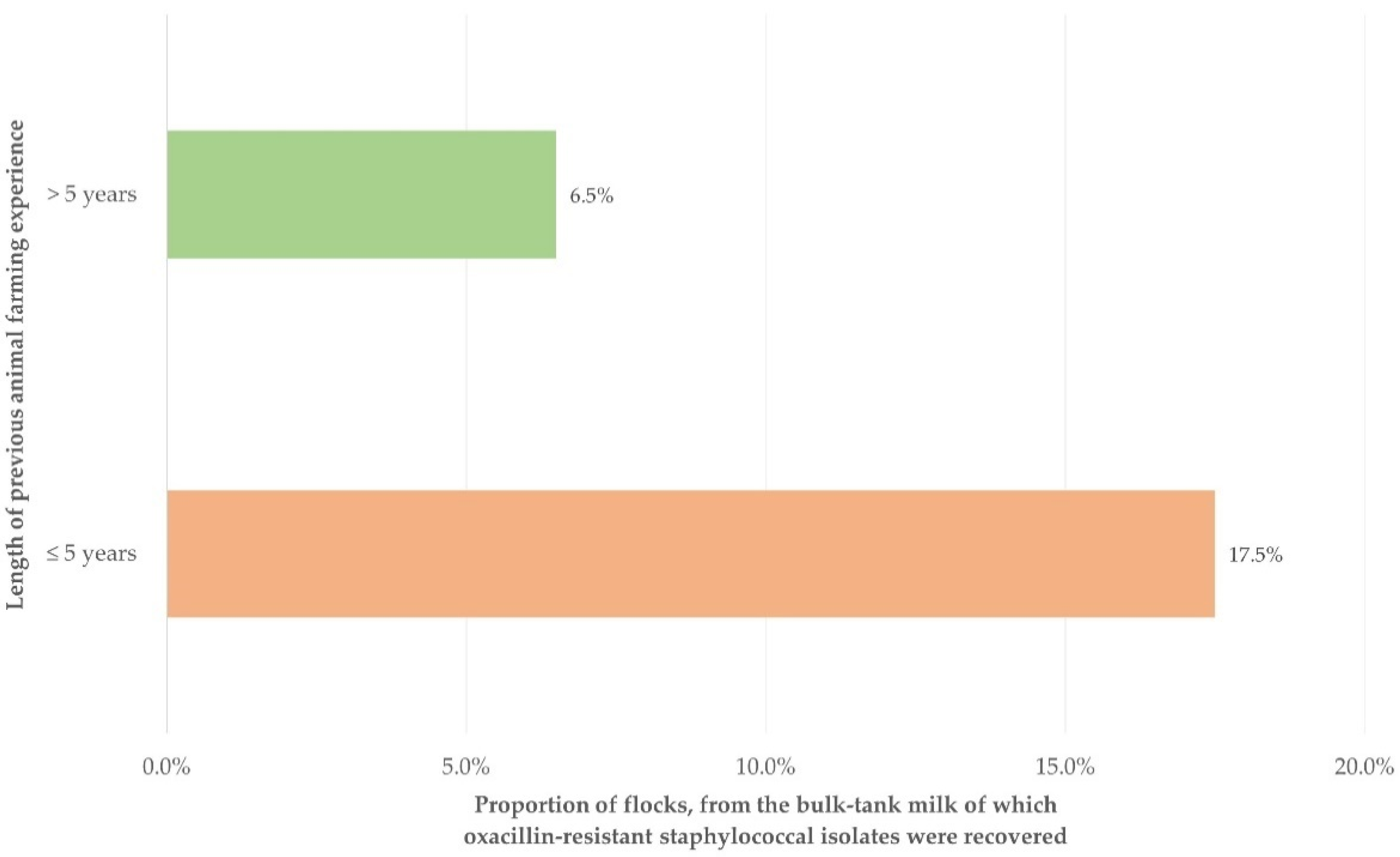
| Length of previous animal farming experience of farmers | 0.018 | |
| ≤5 years (n = 74) | 2.747 (1.202–6.276) | 0.017 |
| >5 years (n = 251) | Reference |
| Variable (n = 1) | Odds Ratios 1 (95% Confidence Intervals) | p |
|---|---|---|
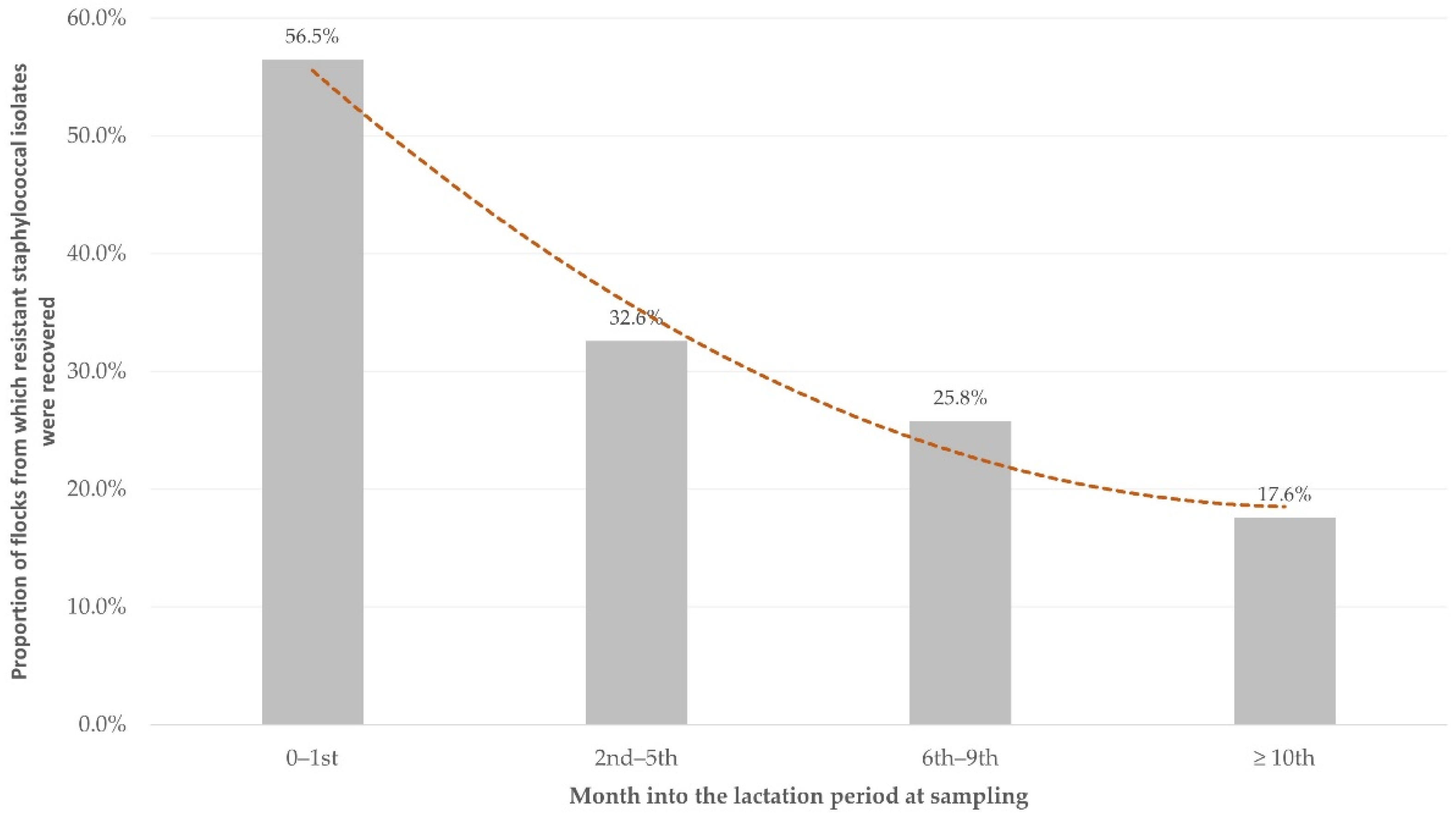
| Month into the lactation period at sampling | 0.003 | |
| 0th to 1st (n = 23) | 6.067 (1.361–27.050) | 0.018 |
| 2nd to 5th (n = 138) | 2.258 (0.617–8.259) | 0.218 |
| 6th to 9th (n = 147) | 1.627 (0.443–5.973) | 0.463 |
| Subsequently to 9th (n = 17) | Reference |
| Variable (n = 1) | Odds Ratios 1 (95% Confidence Intervals) | p |
|---|---|---|
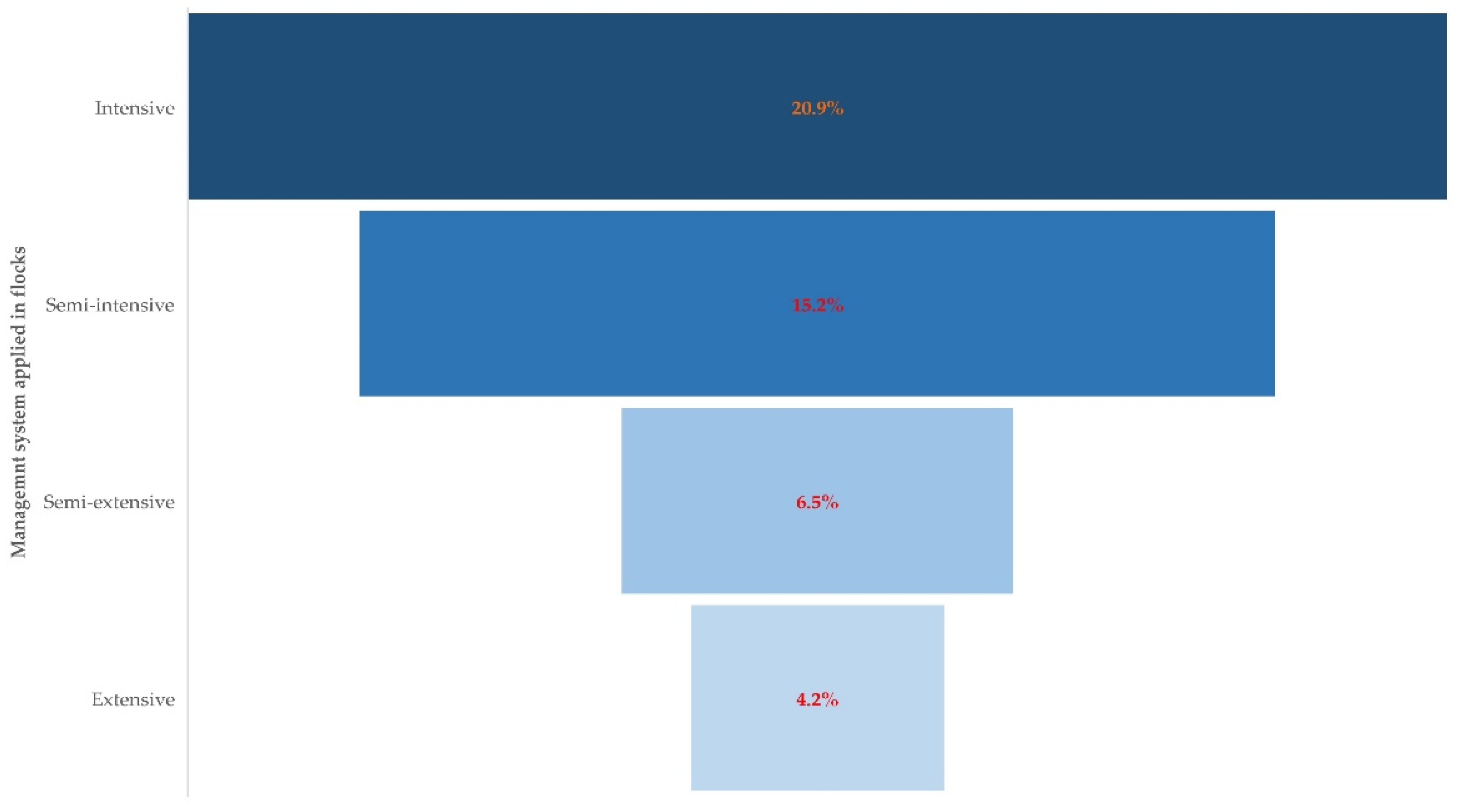
| Management system applied in the flock | 0.015 | |
| Intensive (n = 43) | 13.939 (1.745–111.329) | 0.013 |
| Semi-intensive (n = 151) | 3.512 (0.449–27.460) | 0.23 |
| Semi-extensive (n = 107) | 1.859 (0.221–15.606) | 0.57 |
| Extensive (n = 24) | Reference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lianou, D.T.; Petinaki, E.; Cripps, P.J.; Gougoulis, D.A.; Michael, C.K.; Tsilipounidaki, K.; Skoulakis, A.; Katsafadou, A.I.; Vasileiou, N.G.C.; Giannoulis, T.; et al. Antibiotic Resistance of Staphylococci from Bulk-Tank Milk of Sheep Flocks: Prevalence, Patterns, Association with Biofilm Formation, Effects on Milk Quality, and Risk Factors. Biology 2021, 10, 1016. https://doi.org/10.3390/biology10101016
Lianou DT, Petinaki E, Cripps PJ, Gougoulis DA, Michael CK, Tsilipounidaki K, Skoulakis A, Katsafadou AI, Vasileiou NGC, Giannoulis T, et al. Antibiotic Resistance of Staphylococci from Bulk-Tank Milk of Sheep Flocks: Prevalence, Patterns, Association with Biofilm Formation, Effects on Milk Quality, and Risk Factors. Biology. 2021; 10(10):1016. https://doi.org/10.3390/biology10101016
Chicago/Turabian StyleLianou, Daphne T., Efthymia Petinaki, Peter J. Cripps, Dimitris A. Gougoulis, Charalambia K. Michael, Katerina Tsilipounidaki, Anargyros Skoulakis, Angeliki I. Katsafadou, Natalia G. C. Vasileiou, Themis Giannoulis, and et al. 2021. "Antibiotic Resistance of Staphylococci from Bulk-Tank Milk of Sheep Flocks: Prevalence, Patterns, Association with Biofilm Formation, Effects on Milk Quality, and Risk Factors" Biology 10, no. 10: 1016. https://doi.org/10.3390/biology10101016







