Abstract
Due to environmental regulations, antifouling marine coatings must be gradually replaced by biocide-free coatings. Marine organisms weakly adhere to fouling release coatings, presenting a low surface free energy and a high elasticity, so they can be readily removed by the sheer force of water. Currently, these materials are mainly composed of petrochemical polymers, such as silicone or fluoropolymers, with hydrophilic polymers as additives. However, following the ever-increasing environmental concerns, the research on new, alternative, eco-friendly coatings is oriented towards the use of biobased polymers from renewable resources. Two main families have been studied: polyhydroxyalkanoates (PHAs) and polysaccharides. PHAs are produced by bacteria in stressful conditions, while polysaccharides are extracted from plants, animals, or micro-organisms such as bacteria, in which case they are called exopolysaccharides (EPS). Since the use of these polymers is a non-toxic approach to controlling fouling colonization, this review provides an overview of these biobased polymers for their applications in new anti-adhesive marine coatings.
1. Introduction
Marine fouling on immersed surfaces is a natural process initiated by the adsorption of mineral and organic molecules onto the surfaces followed by the attachment of living organisms, leading to microfouling (mainly due to bacteria and microalgae) and macrofouling (macroalgae and invertebrates) processes [1]. Biological fouling (known as biofouling) presents undesirable economic and environmental consequences: it increases ships’ fuel consumption, favors the corrosion of both hulls and marine infrastructures, reduces heat exchanger efficiency, and promotes species dispersion [2,3,4]. Consequently, several strategies against fouling have been developed throughout the history of navigation [4]. Currently, the protection of immersed surfaces (mainly ship’s hulls) is carried out using two main methods: anti-fouling (AF) paints and fouling release (FR) systems [4,5,6]. AF paints are the historical solution, approximately covering 95% of market share. Its working principle is based on the controlled release of a biocide, mostly copper-based, at the rate of approximately 5 µg/cm²/day [7]. The effectiveness can last between 6 months and up to 5 years depending on biocide load, matrix composition, and paint layer thickness. AF paints are governed by the biocide European regulation UE 528/2012, which aims to deliver market authorization for products showing a benefit-over-risk positive balance [8]. This regulation is still under development and no market authorization has been delivered to date.
The second fouling protection strategy relies on FR systems, using either paints or stickers [9]. In both cases, FR is based on a silicone (crosslinked PDMS) matrix doped with 6%–10% of PEG-PDMS copolymer, which forms a hydrogel (i.e., a highly hydrated layer) on the coated surface with both hydrophilic and hydrophobic domains [10]. The aim is to prevent fouling attachment and favor the release of the fouling layer when ships move. It has been demonstrated that a high smoothness, low elastic modulus, and minimal film thickness are essential elements in the overall system efficiency [11]. Consequently, the correlation between surface characteristics and both fouling settlement prevention and fouling release by weak forces was investigated. It was found that PEG-PDMS-based FR systems’ efficiency could be attributed to their highly hydrated surface [12]. On the nanoscale, these coatings combine both low surface energy hydrophobic and highly hydrated hydrophilic areas, favoring fouling release and preventing protein adsorption, i.e., fouling settlement, respectively. Since then, numerous studies have been conducted on the modification of the PDMS matrix with amphiphilic copolymers to improve antifouling and fouling release performance [13]. Hydrophilic polymers such as polyacrylic [14], peptides [15], peptoids [16], polyoxazoline [17], and polyzwitterions [18] have been proposed to replace the PEG counterpart. While PDMS is based on silicon, one of the most abundant elements on earth, its synthesis is petroleum based and its industrial production has noticeable negative environmental impacts. FR systems represent 5% of the market share and can last from 1 to 5 years depending on the system and area of use. They are not regulated (except REACH) despite the toxicity of their leachates, which is comparable to AF paints as demonstrated by a study conducted by Nautisme en Finistère in 2018 (personal communication).
Some alternatives are available on the market but most of them have large limitations either in efficiency or in their user-friendliness (ultrasound systems [19], fur-like stickers, tarpaulin, etc.). As such, while efficient systems do exist, they invariably appear to lead to detrimental consequences on the marine environment. It is therefore necessary to shift the research strategy to new approaches based on surfaces properties without biocide or toxic release. Indeed, another type of solution was recently devised, consisting of hydrophilic networks based on synthetic polymers [20,21,22]. Their mode of action is supposedly linked to the steric barrier effect induced by the structured water layer on the coating surface.
Overall, several biopolymers have been assessed as promising drag-reducing and AF agents to replace synthetic polymers [12,23]. Nevertheless, environmentally friendly approaches based on bio-sourced polymers remain limited and need to be developed. This review mainly highlights anti-adhesive surfaces based on polyhydroxyalkanoates (PHAs) (Section 2) and polysaccharides (Section 3), whose choice as biosourced polymers is motivated by their intrinsic properties.
2. Coatings Based on Poly(hydroxyalkanoates)
2.1. Extraction and Characterization of PHAs
PHAs are naturally produced polyesters stored as intracellular inclusion bodies by a wide variety of prokaryotes, particularly bacteria, in response to excess carbon under nutrient limited conditions [24]. PHAs are energy and carbon storage compounds and have been shown to provide some ecological advantages to cells. Accumulation of PHA enhances the survival and stress tolerance of several producing bacteria when they are exposed to changing environments, including under extreme conditions. Indeed, PHA accumulation provides increased resistance to salinity, thermal and oxidative stress, UV radiation, desiccation, and osmotic pressure shifts [25,26,27]. The ability to withstand these stresses is linked to a cascade of reactions concomitant with the degradation of intracellular PHA and the activation of genes involved in the bacterial stress response [25]. PHA degradation supplies energy cofactors for the activation of antioxidant enzymes that are involved in the protection against damaging agents. Thus, it is now generally agreed upon that PHAs are not only reservoirs of carbon and energy, but they play a central role in metabolism regulation and offer an adaptive advantage for bacteria able to produce them.
Chemically, most PHAs are linear polyesters composed of (R)-3-hydroxyalkanoic acids monomers (Figure 1) featuring an asymmetric carbon in position 3, providing the polymers chiral characteristics [28].
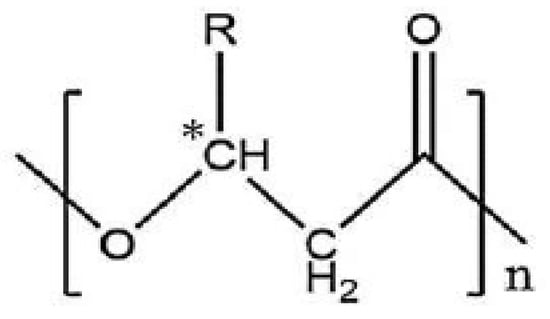
Figure 1.
General structure of poly(3-hydroxyalkanoate). * means assymetric carbon.
Only bacterial production of PHA guarantees complete stereospecificity (i.e., all chiral carbon atoms in the backbone are in the (R)-configuration), which is essential for the biodegradability and biocompatibility of PHA [29,30]. They are classified according to the monomers’ carbon chain length: short chain length (scl-PHA) consists of monomers with three to five carbon atoms (e.g., C4: poly(3-hydroxybutyrate) or PHB) and medium chain length (mcl-PHA) consists of C6 to C14 monomers. While many bacteria can produce scl-PHA, mcl-PHAs are mainly produced by fluorescent pseudomonads strains belonging to the rRNA homology group I [31,32,33]. Due to structural differences, mcl-PHAs physical properties are significantly different from scl-PHAs. Indeed, PHB is a highly crystalline and rigid material, whereas mcl-PHAs present elastomeric properties. Moreover, the polymer monomeric composition is associated with both the nature of the supplied carbon sources and the bacteria itself. Hence, bacterial PHAs comprise a large range of polyesters with more than 150 different monomers described in the literature, and include linear, saturated, unsaturated, and functionalized groups in the side chain [34]. The latter is particularly interesting since these groups can bestow new properties and/or enable further chemical modifications to modulate the PHA properties. For example, mcl-PHAs with vinyl pendant groups feature unique properties. Unsaturation holds the possibility of further additions. Indeed, new functional groups such as epoxy [35], carboxyl [36,37], chlorine [38], hydroxyl [39,40,41], phenoxy, or methyl esters [42] have been introduced to produce polymers with different physico-chemical properties [43]. Thus, chemical modifications of PHA can greatly impact bacterial polyester properties and expand their use in the medical and environmental fields [44].
2.2. Advantages and Applications of PHAs
PHAs exhibit unique and remarkable features: they are biosourced, biodegradable, and show high biocompatibility with the human organism. They have attracted great interest over the past three decades due to their thermoplastic properties being similar to those of petrochemical plastics, including materials resembling polypropylene and others featuring elastomeric characteristics. As such, PHAs are regarded as promising eco-friendly substitutes for petrochemical plastics, and thus can reduce plastic waste moving forward [45]. Additionally, PHAs are naturally produced from renewable carbon sources such as glucose, fructose, or fatty acids, and therefore represent a new means of valorizing waste from low-cost carbon stocks [46,47,48,49]. Furthermore, the most attractive feature of PHAs is their R-configuration-induced biodegradability. They can be fully degraded to water and carbon dioxide under aerobic conditions and to methane and carbon dioxide under anaerobic conditions by microorganisms in soil [50], lakes, marine water [51], and sewage [52,53]. The degrading microorganisms produce extracellular PHA depolymerases which convert the polyesters into water-soluble oligomers and monomers that are used as a carbon source [53]. Factors affecting the degradation of PHAs include environmental conditions and PHAs inherent properties such as their composition, crystallinity, additives, and surface area. Consequently, they are a new generation of environmentally friendly plastic-like materials towards a greener environment via the reduction in our dependency on non-degradable petrochemical plastic.
Moreover, PHAs have been studied for medical applications as biodegradable and biocompatible materials [54,55,56,57], and have been considered as good biosourced candidates for the development of controlled drug delivery systems [58] and tissue engineering [59,60]. Finally, PHAs have recently been investigated as a source of new chiral compounds (synthons) which can be used as precursors in the pharmaceutical and chemical industries, since enantiopure (R)-3-hydroxyalkanoic acids are difficult to obtain by conventional chemical processes [61]. However, studies on the use of PHAs for the preparation of non-adhesive marine coatings are sparse and only one recent study has evaluated the potential of these biopolymers as a matrix for fouling release coatings [62]. Nevertheless, PHAs have been used to evaluate the factors controlling bacterial attachment and/or biofilm formation in polymeric surfaces [63,64,65,66].
2.3. Anti-Adhesive PHA Coatings
The potentiality of PHAs as hydrophobic matrices to replace PDMS matrices has recently been demonstrated [67]. It is currently accepted that anti-adhesion and fouling-released coating (FRC) behaviors are related to several physico-chemical properties, including surface wettability, topography, or mechanical features. In the study by Guennec et al., two PDMS and two PHBHV coatings were compared [62]. The same proportion of PEG (0.1% w/w) as a copolymer was introduced into each modified coating. Figure 2 shows the percentage of bacterial (Bacillus 4J6) and diatomic (Phaeodactylum tricornutum) bioadhesion to the surface of the two coatings containing or not containing a PEG additive.
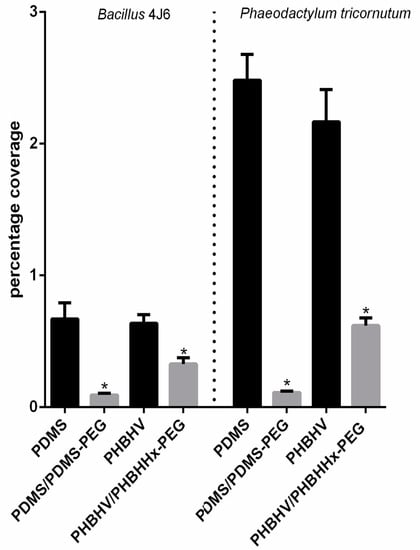
Figure 2.
Percentage coverage of Bacillus sp. 4J6 bacteria (t = 2 h; initial concentration = 108 cells/mL) and and Phaeodactylum tricornutum (t = 48 h; initial concentration = 105 cells/mL), on PHBHV and PDMS coatings with or without PEG in its copolymer form (PHBHHx-PEG or PDMS-PEG) (n = 30). * indicates significant difference from the coatings without PEG (Adapted with permission from Ref. [62]. 2021 Taylor & Francis).
Overall, the percentage coverage of all tested marine microorganisms significantly decreased in the presence of hydrophilic PEG for both coatings; marine bacterial adhesion declined by up to 50% for films containing the PHBHHx-PEG copolymer and 85% for the PDMS/PDMS-PEG one. Similar results were observed for diatom adhesion, with a more than 50% decrease on all surfaces containing the PEG copolymer. This validates the addition of PEG within the matrix as a strategy to improve anti-adhesion performance.
PHBHV coatings are slightly less hydrophobic than PDMS coatings (contact angle of 87° versus 95° and surface energy 42 mJ/m2 versus 22 mJ/m2). Addition of PEG as a copolymer further reduces the water contact angle by approximately 10° for the PHBHV coatings, while it was not statistically modified for PDMS films. Therefore, with a contact angle of approximately 90°, PHBHV coatings are classified as hydrophobic and suitable for limiting the adhesion of microorganisms, although this criterion cannot be considered on its own [6].
It is generally admitted that decreasing the roughness of a surface tends to minimize bacterial adhesion by reducing the anchoring sites [2]. PDMS coatings are smooth, showing a mesh-like networked structure, with pore sizes estimated between 15 and 18 nm [62]. In contrast, PHBHV coatings present macromolecules which are organized in large concentric spherulites [62].
Surface stiffness is yet another factor influencing micro-organism adhesion [67]. Although Arias et al. found that bacterial bioadhesion on PDMS was independent of substrate stiffness [68], Kolewe et al. suggested that material stiffness reduced the initial adhesion of bacteria [68]. There was a significant difference between the indentation modulus of PHBHV and PDMS coatings (2–3 GPa and 1 MPa, respectively) [62]. By simply comparing the PHBHV coatings with and without PEG, the authors highlighted that the adhesion of microorganisms was reduced in the additive’s presence, while the indentation modulus and the RMS remained identical [62]. Despite an indentation modulus in the GPa range and a relatively high RMS due to the presence of a crystallites structure, PHBHV coatings doped with PHBHHx-b-PEG present a hydrophobic character with a presumably good compatibility with PEG polymers, and was found to be an interesting candidate for designing anti-adhesive-based coatings [62].
3. Coatings Based on Polysaccharides
PEG-based materials have a drawback in the fact that they are prone to oxidative degradation in the presence of oxygen and transition metal ions, potentially leading to various issues in marine environments [69]. Consequently, alternative strategies based on hydrophilic polysaccharides have been investigated.
3.1. Anti-Adhesive Polysaccharides
Bioadhesion is a complex process which is affected by many factors, including the physical and chemical characteristics of the surface material, the properties of the biological cells, environmental factors, and flow conditions [70]. For example, adhesion of cells to negatively charged surfaces may be affected by electrostatic repulsion forces since the net electrostatic charge of most bacteria or diatom cell walls are negative at environmental pH [70]. Moreover, it has also been frequently observed that hydrophilic materials are less favorable to bioadhesion than hydrophobic ones [70,71,72,73]. Consequently, polysaccharides (especially anionic) have been considered as possible candidates to coat anti-adhesive surfaces as they present a film-forming ability [70]. Many studies have shown that their hydrophilicity and high hydration are correlated to their resistance against protein adsorption and cell adhesion [74,75,76,77].
Moreover, polysaccharides have attracted attention in the biomedical and environmental fields due to their abundance, biocompatibility, biodegradability, and chemical modifiability [78,79,80]. The structures of the main polysaccharides used in anti-adhesive coatings are presented in Figure 3. They are primarily studied for biomedical applications (implant material in orthopedics/dentistry and intraocular lenses), although their use in the marine environment has also been investigated.
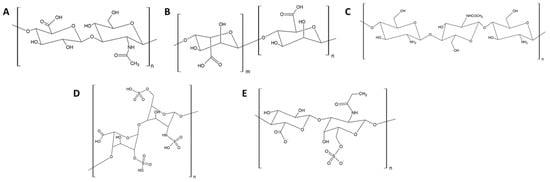
Figure 3.
Main polysaccharides used in anti-adhesive coatings. (A) Hyaluronic acid, (B) alginate, (C) chitosan, (D) heparin, and (E) chondroitin.
For this purpose, hyaluronic acid (HA) is the most studied polysaccharide and consists of alternating N-acetyl-b-D-glucosamine and b-D-glucuronic acid residues linked in (1–3) and (1–4), respectively [70]. It is a naturally occurring anionic polysaccharide present in the extracellular matrix of living organisms. Alginate (AA) consists of (1–4) linked b-D-mannuronic and a-L-glucuronic acid residues and is sourced from brown algae (Laminaria, Macrocystis, Lessonia, Ascophyllum, etc.) and bacterial fermentations (Azotobacter vinelandii, Pseudomonas aeruginosa, and P. mendocina) [70,81]. Chitosan (CH) is the second most abundant polysaccharide found in nature after cellulose and is present in the exoskeletons of arthropods (crustaceans and insects). It is composed of N-acetyl-b-D-glucosamine and b-D-glucosamine linked in (1–4) [70,82,83]. Partial N-desacetylation of chitin yields CH which is known for its antimicrobial activity attributed to its high polycationic nature; the positively charged amine groups of glucosamine interact with negatively charged constituents of microbial cell membranes, causing the linkage of intracellular components [70]. CH can also undergo chemical modifications, notably esterification, to enhance its properties, and modified polymers such as carboxymethyl chitosan are typically used in the design of coatings [84,85]. Heparin (HP) is a polysaccharide of animal origin whose anti-adhesive properties have been extensively investigated in biomedical applications (catheters and stents) [70,86]. Being highly negatively charged due to the presence of numerous carboxylates and sulfates, many physical and chemical strategies enable its immobilization on the surface of materials. Other polysaccharides such as dextran, a branched glucan, or chondroitin sulfate (CS), a sulfated derivative of HA, have also been reported for their use in anti-adhesive coatings [70].
Additionally, polysaccharides have been studied as AF coatings [72]. They have been immobilized on surfaces via various physical and chemical strategies [70], including physical adsorption and covalent attachment, as well as layer-by-layer self-assembly (Figure 4).
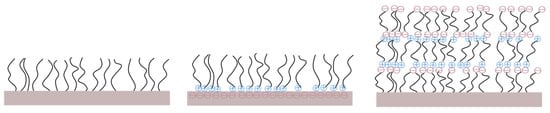
Figure 4.
Various assemblies of polysaccharides onto a substrate.
They have been tested on their own or combined depending on the coating formation process and the desired properties and applications. They have shown good AF performance against proteins (BSA, Fibrinogen, and Lysozyme), bacteria (Cobetia marina, S. aureus, E. coli, P. aeruginosa, and S. epidermidis), diatoms (Navicula incerta, Navicula perminuta, and amphora coffeaeformis), macroalgae spores (Ulva linza), and cypris of the barnacle (Balanus Amphitrite and Balanus improvisus) [74,87,88].
3.1.1. Coordination Bonds
Kim and Kang [89] reported a method for the manufacturing of a marine antifouling coating by crosslinking Zr(IV) ions and carrageenan (CAR), which is a sulfated polysaccharide derived from marine red algae and composed of D-galactose and 3,6-anhydro-galactose joined by alternating a-1,3 and b-1,4-glycosidic linkages [89]. This method enabled the direct grafting of CAR multilayers on metal–polyphenol network (MPN)-coated substrates via coordination bond formation without modification of the natural polymer [89]. As phenolic materials can coordinate with various metals, tannic acid was selected. The thickness of coatings obtained varied from 10 to 25 nm and the coatings resisted exposure to various chemical environments (acidic and alkaline) for short lead times (from minutes to a few hours). They were applied on several substrates (Ti/TiO2, Si/SiO2, polystyrene, nylon, glass, and gold) and showed high hydrophilic properties (contact angle <10°) [89]. Overall, the adhesion of a marine diatom A. coffeaformis significantly inhibited the resulting coatings. The efficiency increased as the film thickness increased from 10.4 to 25.9 nm, while the CAR sulfate content also influenced its performance. Indeed, ι- and κ-CAR contain fewer sulfate groups than λ-CAR and are therefore less effective in creating non-covalent cross links between sulfate moieties. Nevertheless, a decrease in marine antifouling properties was observed for longer periods depending on the substrate’s nature due to its interactions with MPN (hydrogen bond and/or hydrophobic interactions). The multiplicity of interactions enhanced the long-term coating stability and therefore their performance.
3.1.2. Self-Assembled Monolayers
The use of self-assembled monolayers (SAMs) for studies on protein–surface interactions, as well as alga spore and barnacle larvae interactions is widespread in antifouling research [90,91]. SAMs enable tight control of surface properties and chemical composition of the same type of substrate, while also allowing for the monitoring of the monolayer structure. This has been exploited in several marine biofouling studies [92].
Ederth et al. compared the attachment and adhesion strengths of organisms onto single-component monolayers formed from non-methylated, monomethylated, or fully methylated SAMs with galactoside-terminated alkythiols which feature protein-resistant properties that can be tuned via the degree of methylation [92]. More specifically, a partially methylated compound was more resistant to protein adsorption than hydroxylated or fully methylated versions when tested for marine organism adhesion and settlement [92]. The authors suggested that the wettability and surface chemistry were insufficient to explain the discrepancies in the fouling resistance and that differences in the structure of interfacial water may explain the variation in adhesion onto the SAMs [92]. Fyrner et al. showed that the SAM molecular structure was not only affected by the bulkiness of the saccharide moieties but was also dependent on subtle details in the glucoside headgroups in some cases [89]. Moreover, the structure of the carbohydrate moiety affects the packing density, which also alters the alkane chain organization, and bulkier headgroups reduce the structural qualities and increase disorder in both the headgroup and the alkyl chain regions [89]. Regarding trisaccharide, slow reorganization dynamics in response to changes in the environmental polarity were observed.
Nugraha et al. studied the effect of the saccharide chain length (presence of one, two, or three lactose units) and confirmed significant effects on the antifouling properties of SAM surfaces whilst having negligible effects on wettability [90]. Additionally, Ulva spore settlement was higher on bulkier and more hydrated saccharide SAM moieties. As such, the degree of order in the SAMs (and associated differences in crystallinity and stiffness) was a key parameter for the attachment of marine organisms [90].
3.1.3. Covalent Attachment
- Mussel-inspired technology: Synthetic oligomers and polymers inspired by the multifunctional tethering system (byssus) of the common mussel (genus Mytilus) emerged in the 1980s and have since become a very active research domain within the wider bioinspired and biomimetic materials arena [92]. Studies on the remarkable ability of mussels to attach onto virtually any surface underwater has unveiled L-DOPA (3,4-dihydroxy-L-phenylalanine) as the key molecule responsible for this versatile adhesion. Consequently, DOPA or cathecol were conjugated with functional polymers to impart the adhesive properties of mussels to them [69]. An HP-doped coating was developed by combining polysaccharide with mussel-inspired technology [69]. Cathecol-grafted HP was synthetized by an amide bond forming reaction, then the adhesive HP was utilized for surface modification of stainless-steel substrates. A reduction of approximately 75% in the adhesion of A. coffeaformis and N. perminuta was observed and ascribed to the hydration and electrostatic repulsion between the negatively charged HP and the diatom cell walls [92].
- Silanization: The functionalization of surfaces by a self-assembled aminosilane monolayer to introduce an amino function onto a substrate (titanium plates, silicon wafers, and glass slides) has enabled the covalent grafting of polysaccharides [93]. Covalent binding of polysaccharides was achieved by conversion of carboxylic acids into esters functional groups via reaction with N-hydroxysccinimide (NHS) and carbodiimide (EDC) and formation of amide bonds with amine groups on the surface [93] (Figure 5).
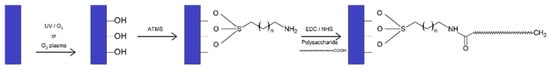
Figure 5.
Reaction scheme of the immobilization of polysaccharide.
Anti-adhesive surfaces modified by HA, AA, or pectic acid (PA) have been obtained using this method [73]. Surfaces were hydrophilic with a sessile water drop contact angle below or equal to 20° depending on the polysaccharide (AA: 20°; PA: 18°; HA: < 10°). All three polysaccharide surfaces reduced the settlement of zoospores of Ulva [74]. HA exhibited lower settlement and attachment strengths of barnacle Ulva zoospores and barnacle cyprids [74]. However, polysaccharides are capable of binding calcium after immersion in seawater because they carry two carboxyl groups per dimer unit (particularly AA and PA) [74]. Calcium bridging contributes to the decreased resistance versus colonization when antifouling polysaccharides are used in the marine environment since their hydration is reduced [73,74].
On the other hand, an enhancement in the antifouling performance was observed when amphiphilic derivatizations were introduced into the polysaccharide matrix [75]. Bauer et al. have investigated the non-fouling properties of HA, AA, and CS against marine fouling organisms [75,87]. The free carboxyl groups of the polysaccharides were post-modified with hydrophobic trifluoroethylamine (TFEA) in order to create amphiphilic surfaces. Against marine bacterium, zoospores of U. linza, diatoms, and after field immersion, TFEA capping increased or maintained the efficiency of the HA and AA coatings, whereas it decreased the efficiency of the normally very good performing CS coatings. TFEA capping did not significantly affect the density and adhesion strength of diatoms, bacteria, or spores [75]. Only the modification of AA reduced spore settlement by 25%, whereas the settlement of cypris larvae of B. Amphitrite was reduced ib both modified polysaccharides [75]. In field immersion (after 24 h), the settlement densities of all unmodified sugars were comparable and modification with TFEA significantly reduced the settlement on AA and HA [75]. Hence, hydrophobic modifications optimized the efficiency of polysaccharides against adhesion of marine fouling organisms, although their nature is of great importance.
Jakobi studied AA-based coatings with different degrees of PFPA modifications (from 0 to 28%) [88]. The introduction of fluorinated compounds increased the coating thickness and the water contact angle from 17° to 47°, and a low to medium degree of PFPA modification showed the greatest inhibition of protein attachment, as well as diatom and zoospore settlement [88].
A sulfated polysaccharide extract from marine green algae Ulva sp., Ulvan, was covalently grafted onto surfaces through amine-terminated SAMs and EDC/NHS chemistry [93,94]. The anti-adhesion effects against bacteria (P. aeruginosa, S. epidermidis) and diatoms (Amphora coffeaformis) were noted. Unfortunately, the stability of these surfaces and their anti-adhesive performances were limited from one to a few days [74,86,93].
3.1.4. Polyelectrolyte Multilayers
Polysaccharides have the required properties to manufacture polyelectrolyte multilayers (PEM) following a layer-by-layer approach because they are charged macromolecules that can be assembled using polymers of opposite charges (Figure 6). However, only a few studies have reported this feature.
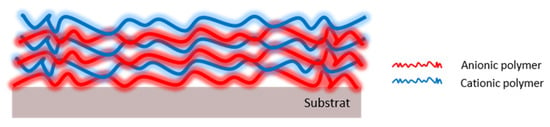
Figure 6.
Polyelectrolyte multilayer structure.
Amongst the challenges associated with the use of PEM coatings in the marine environment, the high salinity of seawater is a primary issue. Indeed, electrostatic interactions are strongly reduced upon immersion due to charge screening and the film rapidly degrades [95]. Thus, PEMs must be cross-linked to covalently bond the layers, increase their stability, and reduce their susceptibility to enzymatic degradation [95,96,97].
Yu et al. proposed PEMs based on HA and CH via layer-by-layer assembly [95,96,97]. Thermal and chemical cross-linking methods were used, which resulted in different roughnesses, moduli, and hydrophobicities, and therefore performances. Indeed, chemically crosslinked PEMs had a higher affinity for water than thermally crosslinked PEMs [97]. Thermal crosslinking produced hydrophobic, less hydrated, and smooth surfaces, whereas chemical crosslinking led to hydrophilic, more hydrated, and comparatively rough coatings [97]. For example, the static water contact angles of HA/CH PEM blends characterized before and after thermal and chemical cross-linking were 18°, 73°, and 20°, respectively, while their RMS roughness increased from 1.9 to 3.3 nm [97]. Nevertheless, this stability enhancement against degradation in seawater affected the coating efficiency against protein resistance and diatom and algae spore settlement [97]. The hydrophilicity obtained with chemical cross-linking was highly linked to its protein resistance and good FR properties against two marine species: zoospores of the macroalga U. linza and cells of the unicellular diatom N. incerta [97]. However, hydrophilicity was not the only discriminant parameter because the lowest settlement was observed with partially cross-linked PEMs regardless of the crosslinking process. Thus, the crosslinking had to be carefully selected and further optimized since its mechanism played an important role. Moreover, the authors showed an enhancement in FR properties against marine biofouling organisms by introducing degradability into PEM coatings constructed from aqueous solutions of naturally occurring biomacromolecules [97]. Hence, reduced cross-linking and self-degrading properties were recommended to obtain an effective antifouling coating.
Gnanasampanthan et al. showed the effect of the terminal layer on chitosan/alginic acid (CH/AA) PEMs [95]. Coatings were efficient against N. perminuta cells irrespective of the termination. The performance was connected to their hydrophilic nature, resulting from the presence of hydroxyl, carboxylate, and amino groups [95]. Inversely, the multilayer termination had a stronger influence on the barnacle larvae settlement. Settlement on CH-terminated coatings (28%) was substantially lower than on AA-terminated coatings (56%), because B. improvisus cyprids favored anionic settling surfaces rather than cationic ones [95]. Moreover, the settlement of barnacle cyprids was positively correlated with the swelling properties of PEMs in seawater. The authors also studied a higher charged PEM based on AA and a synthetic polymer (polyethylenimine) carrying additional positive charges [95]. They speculated that the resistance of alginate-based PEM coatings was affected by the charge density in the bulk of the film.
Xu et al. synthetized a polysaccharide-based self-polishing multilayer coating from dextran aldehyde and carboxymethyl chitosan via layer-by-layer deposition [98]. The chemical cross-linking of the two polymers was made possible thanks to the chemical modification previously performed on the dextran. It was oxidized into dextran aldehyde, which reacted with the amine function of the CH to form an imine linkage. The authors varied the number of layers from 1 to 11. As revealed by static water contact angle and AFM measurements, the surface hydrophilicity progressively increased while the surface roughness continually decreased as more bilayers were added, which improved the antifouling performance against bacteria and diatoms [98]. In addition, the self-polishing ability of the multilayer coatings was achieved via cleavage of pH-responsive imine linkage under acidic environments (pH = 5.5). The authors confirmed the bacterial aggregation-induced self-polishing capability of the coatings in the presence of dense bacterial adhesions [98].
Furthermore, amphiphilic multilayers are promising materials for fouling-release applications [99]. Amphiphilic polysaccharides were fabricated by coupling hydrophobic pentafluoropropylamine (PFPA) to the carboxylate groups of hydrophilic alginic acid [99]. PEM coatings were assembled to explore how different PFPA contents affect the physicochemical properties and antifouling activities against marine bacteria (Cobetia marina) and diatoms (Navicula perminuta) [99]. The amphiphilic multilayers showed similar or even higher swelling in water and exhibited higher resistance toward microfouling marine organisms than multilayers without fluoroalkyl groups [99]. Recently, Lakhan et al. assembled different numbers of layers on films composed of CH and acidic siloxane resin (ASR) [100]. Marine diatom settlement and growth inhibition was tested against Nitzschia Closterium and the results suggested that CH/ASR films presented desirable antifouling properties which increased with the number of ASR layers [100].
Consequently, in contrast to SAM-based model coatings, the physicochemical and antifouling properties of PEM films can easily be controlled by changing the PEM building blocks, number of deposited layers, pH, salt type, ionic strength, solvent, or cross-linking method [95]. Although the applicability of thin film PEMs are limited, they are ideally suited for small structures such as submerged windows or sensors.
3.1.5. Polysaccharide–Silane Hybrid Materials
Hybrid materials (HMs) offer unique properties as they combine inorganic and organic components into a single material [101]. HMs can be divided into two categories based on the bond between the organic and the inorganic species. Class I hybrids show weak interactions between the two elements, such as van der Waals, hydrogen bonding, and electrostatic interactions, while class II hybrids feature covalent bonding. Sol-gel-based silicone coatings have demonstrated highly effective antifouling and fouling release properties in recent years [11]. HMs based on sol-gel chemistry are class II hybrids, where components react by forming strong covalent Si-O bonds (average 440 kcal/mol) [101]. Among the different bioinspired building blocks for hybrid materials, polysaccharides are an interesting class of biocompatible and renewable biopolymers showing promising properties as low-fouling materials [101]. Wanka et al. used polysaccharides with different net charges and carrying different functional groups (CH, HA, AA, CS, and HP) [101]. After a week in saline water, most coatings remained on the substrate. They presented low contact angles (<10°), indicating the hydrophilic properties of the interface, except for CH (45°). The CH-based HM exhibited a low roughness with a nanoscale structure [101]. HA and CS coatings revealed a fine, porous morphology with randomly distributed spots, whereas AA coatings presented a hill-and-valley structure. HP showed a very porous structure reminiscent of micro-donuts.
Initial attachment of the N. incerta diatom was similar for all coatings. However, the removal of all the hybrid coatings was more significant than removal from glass reference surfaces (except for CH) [101]. Attachment was weakest on CS and HP coatings, with removal of up to 77%, followed by AA (65%) and HA (53%). Regarding the settlement of B. improvisus cyprids, the lowest settlement was found for HP (23%), followed by CS and AA (28%), HA (47%), and then CH (60%) [101]. Biofouling coverage after 7 days immersion increased in the following order: AA ~ HA ~ HP < CS ~ CH. Even small changes in the functional groups of the polysaccharides can influence their physico-chemical properties and their antifouling and fouling release properties against marine organisms during short-term field exposure. HP and AA, rich in anionic groups, presented particularly promising results [101].
Another approach to produce HP coatings was proposed by Yu et al. [71]. The authors introduced a method for the spin coating of silane-based sol-gel chemistries using layer-by-layer assembly of polysaccharide-based hybrid polymer coatings (LBLHPs). As mentioned above, the PEMs are unstable in high ionic strength environments and require cross-linkage. Methods commonly used to cross-link polysaccharide-based PEMs are thermal or chemical cross-linking. These optimized treatments allow the hydrated and antiadhesive polysaccharides to be stable in seawater while retaining their antifouling properties, although harsh reaction conditions were required [96]. The silane sol-gel chemistry enabled films to be cross-linked under water-based and mild reaction conditions [96]. This allowed silane hydrolysis and condensation reactions between hydroxyl groups to take place, leading to siloxane bond formation. The results showed that this cross-linking method outperformed both thermal and chemical ones with excellent antifouling performance against the attachment of the diatom N. perminuta and settlement of zoospores of the macroalgae U. linza [96]. While the stability of the multilayer coatings in seawater improved, the high hydration and antiadhesion nature of the polysaccharides was also preserved.
3.2. The Particular Case of Exopolysaccharides
Fully natural, bacteria-produced exopolysaccharides are extremely complex sugars that cannot be recreated by chemical syntheses. Bacterial exopolysaccharides (EPSs) are biomolecules secreted in the extracellular space and have various biological functionalities, such as environmental protection, surface adherence, and cellular interactions [102]. EPSs have been found to be biocompatible and eco-friendly, thus making them attractive for applications in many areas of study and in an array of industrial products [103]. Compared to vegetal and algal polysaccharides (gums), they present significant advantages: they are not dependent on climate or geopolitics; their production under controlled fermentation conditions guaranties extreme reproducibility; and, most of all, their chemical composition diversity has almost no limits thanks to the huge micro-organism biodiversity [102,103].
Microbial EPS producers include both eukaryotes (phytoplankton, fungi, and algae) and prokaryotes (eubacteria and archaebacteria) [103,104,105,106,107]. However, since EPSs from eubacteria have considerably shorter production times and simpler extraction procedures, they are the most attractive from an industrial viewpoint [108,109].
Although still not completely understood, several key physiological functions have been suggested for microbial EPS, including protection against environmental pressures (e.g., osmotic stress, temperature or pH variations, damage by UV light, presence of heavy metals and oxidants, and desiccation), cell adherence to surfaces, and carbon or water storage [104,105,106,107,108,110].
Bacterial EPSs are biopolymers that are secreted by the cells and can form either a capsule that remains associated with the cell surface or a slime that is loosely bound to the cell surface [108]. These biopolymers are mainly composed of neutral carbohydrates; D-glucose, D-galactose, and D-mannose are the most common, whereas D-fucose and D-rhamnose are less common and generally confer some biological activities to the EPS. Other examples are acid sugars (mainly glucuronic and galacturonic acid) and hexosamines such as N-acetylamino sugars (mainly glucose or galactose) [109]. In addition, EPSs can also feature non-sugar components such as sulphate, succinate, pyruvate, or acetate, which confers anionic properties to the EPSs [110].
Dextran, xanthan, gellan, levan, alginate, hyaluronic acid, and FucoPol are the most commercialized bacterial EPSs for food, feed, medical, pharmaceutical, and chemical industry applications [111,112]. However, in recent decades, several new EPSs have been shown to exhibit some promising characteristic and properties. Indeed, certain vibrio species are able to produce hyaluronic-acid-like EPSs with promising bone and tissue regenerating activity [113,114]. Moreover, marine Alteromonas species are now known to generate highly viscous EPS. Deep sea hydrothermal vent exploration led to the discovery of innovative EPSs [102], leading to the report of the first marine EPS from Alteromonas to be used in cosmetics [115]. More recently, [116] described six new EPSs from marine Alteromonas with noteworthy rheological properties. More interestingly, several new EPSs showed good antibiofilm or antifouling properties [117,118,119].
The biotechnological production of EPSs offers advantages over chemical- and plant-derived production such as energy efficiency, fast production (a few days) regardless of the location and season, and the possibility to use industrial and agricultural wastes as substrates [120]. However, some limitations, for example, production costs, may hinder the process [120].
EPSs are generally produced in large scale bioreactors containing a sterile medium inoculated with a 10% (v/v) preculture. Culture media typically consist of a nitrogen (N) source (e.g., Trypton or yeast extract), a carbon (C) source (e.g., glucose or sucrose), and minerals. The optimum C/N ratio (depending on the bacteria) must be maintained during fermentation. At the end of production, EPS is extracted by centrifugation, desalted by ultra- or dia-filtration (with a 10 to 100 kD cut-off, typically), and then purified by filtration (from 1 to 0.22 µm). Depending on the application and the required purity, further precipitation (in ethanol or methanol) or enzymic treatment (proteases) can be performed.
Analytical methods for the characterization of the chemistry of EPSs differ depending on laboratories. However, most utilize the following: the global composition is established by colorimetric methods; the monosaccharide composition and relative ratio are determined by gas chromatography; the non-osidic components are assessed by NMR and HPLC; the linkage is characterized by 2D NMR; and the molecular weight range is determined by HP-SEC [113,116,121]
3.2.1. Antiadhesive Activity of Bacterial Exopolysaccharides
Although the mode of action of antibiofilm polysaccharides is not completely understood, these molecules act by modifying the physical properties of both biotic and abiotic surfaces [122,123]. Some exopolysaccharides can perform functions that inhibit or destabilize the biofilms without exhibiting biocidal or bacteriostatic activities [122,124]. Their antibiofilm activity is likely to be mediated by mechanisms other than growth inhibition [125,126,127,128,129,130,131,132,133,134,135,136,137,138,139]. Most antibiofilm polysaccharides act as surfactant molecules that modify the physical characteristics of bacterial cells and abiotic surfaces [122]. Studies utilizing culture supernatants or purified polysaccharides as surface coatings have provided further evidence that antibiofilm polysaccharides modify the physical properties of abiotic surfaces [69,131,132]. Equally, evidence suggests that polysaccharides not only modify abiotic surfaces but also alter the physical properties of both Gram-negative and Gram-positive bacterial cell surfaces [122]. Many authors have highlighted a direct interaction between the EPS and the cell surface, leading to a reduction in surface hydrophobicity, cell interactions, and antibiotic resistance patterns [126,128]. Hence, polysaccharides act not only in initial adhesion by weakening cell–surface contacts but also in biofilm maturation by reducing cell–cell interactions [129]. Moreover, several anti-biofilm polysaccharides have been shown to enhance or trigger biofilm dispersal [122,130].
Polysaccharides may act as signaling molecules that modulate gene expression of bacteria and may also act as an interspecies cell-to-cell signal to downregulate biofilm formation in other species [122,129]. Wu [131] showed that an EPS (EPS273) from a marine bacterium, P. stutzeri, may inhibit the biofilm formation of P. aeruginosa PAO1 by decreasing the production of pyocyanin, which consequently inhibits the generation of H2O2 and the release of eDNA, which is essential for biofilm formation and stability [131]. Moreover, EPS273 may exert its anti-biofilm activity by downregulating the gene expression of the two-component system PhoP-PhoQ and QS systems LasI/LasR and RhlI/RhlR of P. aeruginosa, which modulate the expression of genes involved in biofilm formation [131]. EPS273 showed great antifouling effects on both bacteria and diatoms after up to 2 weeks in the ocean [131].
Competitive inhibition of multivalent carbohydrate–protein interactions is another possible mode of action. Indeed, anti-biofilm polysaccharides might block lectins or sugar binding proteins present on the surface of bacteria or block tip adhesion of fimbriae and pili [124].
A hyaluronan-like EPS produced by a bacterium from the Vibrio genus isolated from bacterial Mats in French Polynesia showed interesting anti-adhesive activity [113]. Its action on the adhesion of the bacterium P. Aeruginosa and two marine bacteria, Bacillus sp. and V. harveyi, was evaluated by conditioning the surface of adhesion of the EPS [132]. On average, an inhibition of 50% in the adhesion of all bacterial strains was induced in comparison to the control (Figure 7). The overlap percentage (%) of bacteria onto the glass surface decreased from 3.8 to 1.8 µm3/µm2, from 2.1 to 0.9 µm3/µm2, and from 2.4 to 1.7 µm3/µm2 for P. aeruginosa, Bacillus sp., and V. harveyi, respectively.
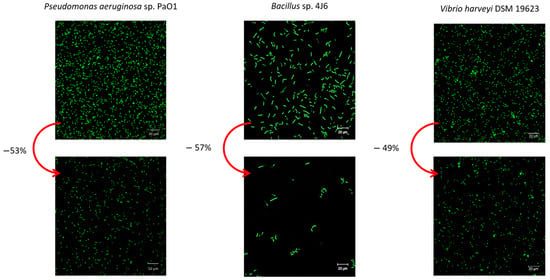
Figure 7.
Prevention of bacterial adhesion (P. aeruginosa, Bacillus sp., and V. harveyi) with hyaluronan-like EPS in static conditions. Confocal laser scanning microscopy observations of the bacterial adhesion with syto9® after 2 h with addition of 125 µg/mL hyaluronan-like EPS at 20 °C. The biomasses of the biofilms in flow cells were analyzed using COMSTAT software and biofilm percentages (%) were determined compared to the control (Adapted with permission from Ref. [132]. 2022 MDPI ).
The impact of hyaluronan-like EPS was also observed on biofilm formation. The results were compared to those observed for HA. Considering the strain V. harveyi, observations obtained in CLSM are shown in Figure 8. The addition of hyaluronan-like EPS or HA to the growth medium induced an inhibition of the biofilm (53% and 38%, respectively) which was significantly different from the control. Additionally, the evaluation of the interferences of both EPS with the biofilm’s behavior was studied. Biofilms were observed after a contact time of two hours with EPS. The ones which had already been formed were degraded by the addition of the EPS in the channel flow cell (Figure 8). Additionally, a significant inhibition (28%) was noted between the biovolume of the control and the biovolume after contact with the hyaluronan-like EPS. The inhibition was lower and less significant for HA, as 16% inhibition was observed. Such inhibitions of bacterial adhesion, biofilm development, and biofilm disruption are based on a mechanism unrelated to bacterial growth as no decease in bacterial growth was observed at the tested concentration (125 µg/mL).
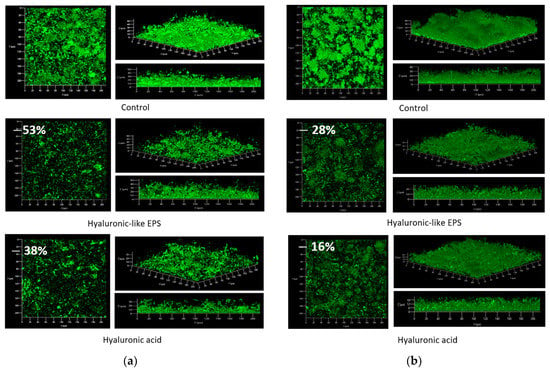
Figure 8.
Prevention (a) and disruption (b) of V. harveyi biofilms (syto9®) with hyaluronan-like EPS or HA in a flow cell. Flow cells irrigated by a medium were inoculated at 28 °C for 24 h with and without (control) 125 µg/mL of hyaluronan-like EPS or HA. Images and structures of biofilms in flow cells were detected by CLSM. The biomasses of biofilms in flow cells were analyzed using COMSTAT software and biofilm percentages (%) were determined compared to the control (Adapted with permission from Ref. [132]. 2022 MDPI).
3.2.2. Antiadhesive EPS Coatings
Hydrogels have good hydrophilicity, biocompatibility, stimuli responsiveness, and functionality, which make them potentially great candidates in marine antifouling applications [133,134]. Mo245 and its HA homologue are particularly interesting in obtaining non-adhesive coatings and have been incorporated into a chemically cross-linked coating as hydrogels. Glass substrates were covered with a polydopamine layer to improve hydrogel adhesion, and then coated with a crosslinked blend of CH (80% wt) and Mo245 or HA (20% wt) in the presence of citric acid as a natural crosslinker. After curing, biobased coatings adhered firmly to the substrate. The immersion in artificial seawater resulted in the hydrophilic crosslinked coatings showing a hydrated thickness of about 60 µm. They revealed different mechanical properties, as the storage modulus of CH/Mo (1250 Pa) and CH/HA (1120 Pa) were significatively different from CH (680 Pa), although this order of magnitude was an indication of an ultra-soft matrix compared to PDMS (of the order of MPa) [135]. The introduction of Mo or HA increased the stiffness of this ultra-soft matrix. The coatings were tested against the adhesion of one bacterial strain (V. harveyi) and a diatom (C. closterium) (Figure 9). Bacterial adhesion was carried out under a controlled flow to avoid bacterial sedimentation (size ~ 1 µm) inside the highly hydrated coatings. In contrast, diatom adhesion was realized in static conditions because the diatom size (~65 µm) did not permit sedimentation. The incorporation of 20% Mo245 or HA significantly reduced the bacterial adhesion; the percent coverage decreased from 13.2% to 6.5% and 9.7%, respectively (p < 0.001).
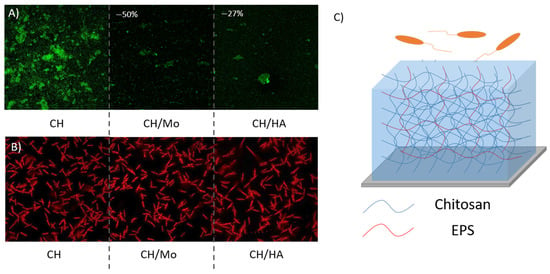
Figure 9.
Confocal laser microscopy observations of CH, CH/Mo, and CH/HA coatings and the adhesion of (A) V. harveyi at a flow of at 1 mL/min at 108 cells/mL for 4 h in ASW at 20 °C, values represent the percentage of adhesion inhibition (B) C. closterium at 105 cells/mL in static conditions for 72 h in ASW medium complemented with F/2 silica nutrients at 20 °C, (C) schematic representation of anti-adhesive coatings. Bacteria were stained with Syto9®.
Many factors alter bacterial attachment, including the chemistry, topography, and mechanical properties of a surface [68,74,136]. Specifically, an increase in attachment was observed with an increase in the hydrogel stiffness from 20 to 1000 kPa [68] and with a decrease in the thickness from 150 µm to 25 µm [137]. The variation in bacterial adhesion could be due to the thickness of the hydrogel, associated with very low modulus values. However, other factors are probably involved and remain to be uncovered.
Contrastingly, no effect on diatom adhesion was observed. The percentage coverage was not significantly different at 9.6%, 9.1%, and 10.1% for CH, CH/Mo, and CH/MA, respectively (p > 0.05), showing that diatoms adhered regardless of the coating composition. Diatoms are not motile in the water column and reach the surface though current and gravity transport [74]. As such, flow may be required to evaluate the fouling release effect of these hydrogels [138,139].
These results showed the potential anti-adhesive effect of these coatings, albeit organism dependent. To understand these differences, it will be necessary to carry out (i) more specific physicochemical characterizations of coatings (e.g., swelling and roughness) and (ii) studies on organism–coating interactions.
3.2.3. Marine Paints Based on EPSs
Not only do low concentrations of EPSs affect the formation of bacterial biofilms without causing toxicity, they also decrease barnacle larval settlement [140]. Thus, EPSs could be potential candidates as the active ingredient in marine coatings and their incorporation in antifouling paints has been the subject of some studies [141,142]. An EPS from Bacillus sp. was mixed with varnish and coated onto acrylic coupons. An antifouling assay conducted in coastal waters showed that the EPS had inhibitory activity against the settlement of biofouling organisms [141]. In another study, an EPS from Phormidium sp. was used as an auxiliary biocide along with copper-oxide-based paint [142]. EPS incorporation inhibited bacterial establishment, indicating a delay in the coating colonization process which could be attributed to the biological activity of the EPS. However, no information regarding the mechanisms of EPS activity (biocidal or anti-adhesive) was provided.
4. Conclusions and Future Trends
The advantages of substituting petrochemical polymers, which are responsible for environmental pollution (such as PDMS), with biodegradable and biocompatible polymers are obvious. The use of PHAs or EPSs provides a route to amphiphilic and/or hydrophilic coatings that exhibit promising antifouling properties. Indeed, coatings featuring various structures can be manufactured for fouling release using marine biosourced materials with promising efficiency. Such materials represent environmentally friendly antifouling alternatives. However, the development of these new bio-based antifouling and antifouling coatings remains a challenge for the future.
In addition to the need for a larger-scale production of these polymers to reduce its cost, the current techniques used to characterize and highlight the physicochemical properties of surface coatings remain particularly limited, especially in the hydrated state. Moreover, significant discrepancies in the accumulation of marine organisms on these coatings can be observed between laboratory and field assays [142], highlighting, amongst other things, the need for increasing the robustness of coatings in general. For example, soft hydrogel layers are easily destroyed and scarcely withstand mechanical deformations and friction during service life [143]. Moving forward, enhancements in the mechanical properties of these coatings are required to prevent both adhesive and cohesive failures.
Author Contributions
Investigation, F.F., M.C., A.G., X.M. and C.S.-C.; writing—original draft preparation, F.F.; writing—review and editing, F.F. and M.E.; project administration, F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by financial support from the Région Bretagne (ARED BIOPS), the Université de Bretagne Sud, and Pacific Biotech (OV 2020_00200).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wahl, M. Marine Epibiosis.1. Fouling and antifouling—Some basic aspects. Mar. Ecol. Prog Ser. 1989, 58, 175–189. Available online: https://www.jstor.org/stable/24842178. (accessed on 11 February 2023). [CrossRef]
- Nurioglu, A.G.; Catarina, A.; Esteves, C.; de With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Song, S.; Degiuli, N.; Martić, I.; Demirel, Y.K. Impact of biofilm on the ship propulsion characteristics and the speed reduction. Ocean. Eng. 2020, 1999, 107033. [Google Scholar] [CrossRef]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Donelly, B.; Sammut, K.; Tang, Y. Materials selection for antifouling systems in marine structures. Molecules 2022, 27, 3408. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Amara, I.; Miled, W.; Ben Slama, R.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Env. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef]
- de Campos, B.G.; Figueiredo, J.; Perina, F.; Abessa, D.M.D.S.; Loureiro, S.; Martins, R. Occurrence, effects and environmental risk of antifouling biocides (EU PT 21); Are marine ecostyems threatened? Crit. Rev. Envir. Sci. Technol. 2022, 52, 3179–3210. [Google Scholar] [CrossRef]
- Murthy, P.S.; Venugopalan, V.; Krishna Mohan, V.P.; Nachariah, Y.V.; Das, A.; Venkatnarayanan, S.; Stahya, S.; Subba Rao, T. Adavancements and modifications to polydimethylsiloxane foul release antifouling coatings. In A Treatise on Corrosiion Sciene, Engineering and Technology; Indian Institute of Metals Series; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Holberg, S.; Losada, R.; Blaikie, F.; Hanse, H.H.W.B.; Soreau, S.; Onderwater, R.C.A. Hydrophilic silicone coatings as fouling release: Simple synthesis, comparison to commercial, marine coatings and application on fresh water-cooled heat exchangers. Mater. Today Commun. 2022, 22, 100750. [Google Scholar] [CrossRef]
- Hu, P.; Xie, Q.; Ma, C.; Zhang, G. Silicone-Based Fouling-Release Coatings for Marine Antifouling. Langmuir 2020, 36, 2170–2183. [Google Scholar] [CrossRef]
- Lu, G.; Tian, S.; Li, J.; Xu, X.; Liu, S.; Pu, J. Fabrication of bio-based amphiphilic hydrogel coating with excellent antifouling and mechanical properties. Chem. Eng. J. 2021, 409, 128134. [Google Scholar] [CrossRef]
- Qiu, H.; Feng, K.; Gapeeva, A.; Meurisch, K.; Kaps, S.; Li, X.; Yu, L.; Mishra, Y.K.; Adelung, R.; Baum, M. Functional polymer materials for modern marine biofouling control. Prog. Polym. Sci. 2022, 127, 101516. [Google Scholar] [CrossRef]
- Kuliasha, C.A.; Finlay, J.A.; Franco, S.C.; Clare, A.S.; Stafslien, S.J.; Brennan, A.B. Marine anti-biofouling efficacy of amphiphilic poly(coacrylate) grafted PDMSe: Effect of graft molecular weight. Biofouling 2017, 33, 252–267. [Google Scholar] [CrossRef]
- Sakala, G.P.; Reches, M. Peptide-Based Approaches to Fight Biofouling. Adv. Mater. Interfaces 2018, 5, 1800073. [Google Scholar] [CrossRef]
- Barry, M.E.; Davidson, E.C.; Zhang, C.; Patterson, A.L.; Yu, B.; Leonardi, A.K.; Duzen, N.; Malaviya, K.; Clarke, J.L.; Finlay, J.A.; et al. The Role of Hydrogen Bonding in Peptoid-Based Marine Antifouling Coatings. Macromolecules 2019, 52, 1287–1295. [Google Scholar] [CrossRef]
- Portier, É.; Azemar, F.; Benkhaled, B.T.; Bardeau, J.-F.; Faÿ, F.; Réhel, K.; Lapinte, V.; Linossier, I. Poly(oxazoline) for the design of amphiphilic silicone coatings. Prog. Org. Coat. 2021, 153, 106116. [Google Scholar] [CrossRef]
- Jensen, M.J.; Peel, A.; Horne, R.; Chamberlain, J.; Xu, L.; Hansen, M.R.; Guymon, C.A. Antifouling and Mechanical Properties of Photografted Zwitterionic Hydrogel Thin-Film Coatings Depend on the Cross-Link Density. ACS Biomater. Sci. Eng. 2021, 7, 4494–4502. [Google Scholar] [CrossRef]
- Aktij, S.A.; Taghipour, A.; Rahimpour, A.; Mollahosseini, A.; Tiraferri, A. A critical review on ultrasonic-assisted fouling control and cleaning of fouled membranes. Ultrasonics 2020, 108, 106228. [Google Scholar] [CrossRef]
- Xie, L.; Hong, F.; He, C.; Ma, L.; Liu, J.; Zang, J.; Wu, C. Coatings with a self-generating hydrogel surface for antifouling. Polymer 2011, 52, 3738–3744. [Google Scholar] [CrossRef]
- Murosaki, T.; Ahmed, N.; Gong, J.P. Antifouling properties of hydrogels. Sci. Technol. Adv. Mater. 2011, 12, 064706. [Google Scholar] [CrossRef]
- Su, X.; Hao, D.; Xu, X.; Guo, X.; Li, Z.; Jiang, L. Hydrophilic/hydrophobic heterogeneity anti-biofouling hydrogels wirh well-regulated rehydration. ACS Appl. Mater. Interfaces 2020, 12, 25316–25323. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.Y.; Pan, J.; Ma, C.; Qian, P.Y. Combining a bio-based polymer and a natural antifoulant into an eco-friendly antifouling coating. Biofouling 2020, 36, 200–2009. [Google Scholar] [CrossRef] [PubMed]
- Simon-Colin, C.; Gouin, C.; Lemencko, P.; Schmitt, S.; Senant, A.; Kervarec, N.; Guezennec, J. Biosynthesis and characterization of polyhydroxyalkanoates by Pseudomonas guezennei from alkanoates and glucose. Int. J. Biol. Macromol. 2012, 51, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sowinski, S.; Burdman, S.; Matan, O.; Okon, Y. Natural Functions of Bacterial Polyhydroxyalkanoates. In Plastics from Bacteria, Chen, G.G.-Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 39–61. [Google Scholar] [CrossRef]
- Kadouri, D.; Jurkevitch, E.; Okon, Y.; Castro-Sowinski, S. Ecological and Agricultural Significance of Bacterial Polyhydroxyalkanoates. Crit. Rev. Microbiol. 2005, 31, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Slaninova, E.; Fritz, I.; Daffert, C.; Meixner, K.; Sedrlova, Z.; Koller, M. Novel unexpected functions of PHA granules. Appl. Microbiol. Biotechnol. 2020, 104, 4795–4810. [Google Scholar] [CrossRef]
- Prieto, M.A.; Bühler, B.; Jung, K.; Witholt, B.; Kessler, B. PhaF, a Polyhydroxyalkanoate-Granule-Associated Protein of Pseudomonas oleovorans GPo1 Involved in the Regulatory Expression System for pha Genes. J. Bacteriol. 1999, 181, 858–868. [Google Scholar] [CrossRef]
- Bachmann, B.M.; Seebach, D. Investigation of the Enzymatic Cleavage of Diastereomeric Oligo(3-hydroxybutanoates) Containing Two to Eight HB Units. A Model for the Stereoselectivity of PHB Depolymerase from Alcaligenes faecalis T1. Macromolecules 1999, 32, 1777–1784. [Google Scholar] [CrossRef]
- Hocking, P.J.; Marchessault, R.H.; Timmins, M.R.; Lenz, R.W.; Fuller, R.C. Enzymatic Degradation of Single Crystals of Bacterial and Synthetic Poly(β-hydroxybutyrate). Macromolecules 1996, 29, 2472–2478. [Google Scholar] [CrossRef]
- Huisman, G.W.; de Leeuw, O.; Eggink, G.; Witholt, B. Synthesis of poly-3-hydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl. Environ. Microbiol 1989, 55, 1949–1954. [Google Scholar] [CrossRef]
- Lageveen, R.G.; Huisman, G.W.; Preusting, H.; Ketelaar, P.; Eggink, G.; Witholt, B. Formation of Polyesters by Pseudomonas oleovorans: Effect of Substrates on Formation and Composition of Poly-( R )-3-Hydroxyalkanoates and Poly-(R)-3-Hydroxyalkenoates. Appl. Environ. Microbiol. 1988, 54, 2924–2932. [Google Scholar] [CrossRef]
- Timm, A.; Steinbüchel, A. Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent pseudomonads. Appl. Environ. Microbiol. 1990, 56, 3360–3367. [Google Scholar] [CrossRef]
- Steinbuchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Bear, M.-M.; Leboucher-Durand, M.-A.; Langlois, V.; Lenz, R.W.; Goodwin, S.; Guérin, P. Bacterial poly-3-hydroxyalkenoates with epoxy groups in the side chains. React. Funct. Polym. 1997, 34, 65–77. [Google Scholar] [CrossRef]
- Kurth, N.; Renard, E.; Brachet, F.; Robic, D.; Guerin, P.; Bourbouze, R. Poly(3-hydroxyoctanoate) containing pendant carboxylic groups for the preparation of nanoparticles aimed at drug transport and release. Polymer 2002, 43, 1095–1101. [Google Scholar] [CrossRef]
- Stigers, D.J.; Tew, G.N. Poly(3-hydroxyalkanoate)s Functionalized with Carboxylic Acid Groups in the Side Chain. Biomacromolecules 2003, 4, 193–195. [Google Scholar] [CrossRef]
- Arkin, A.H.; Hazer, B.; Borcakli, M. Chlorination of Poly(3-hydroxy alkanoates) Containing Unsaturated Side Chains. Macromolecules 2000, 33, 3219–3223. [Google Scholar] [CrossRef]
- Babinot, J.; Renard, E.; Langlois, V. Preparation of Clickable Poly(3-hydroxyalkanoate) (PHA): Application to Poly(ethylene glycol) (PEG) Graft Copolymers Synthesis: Preparation of Clickable Poly(3-hydroxyalkanoate). Macromol. Rapid Commun. 2010, 31, 619–624. [Google Scholar] [CrossRef]
- Eroğlu, M.S.; Hazer, B.; Ozturk, T.; Caykara, T. Hydroxylation of pendant vinyl groups of poly(3-hydroxy undec-10-enoate) in high yield. J. Appl. Polym. Sci. 2005, 97, 2132–2139. [Google Scholar] [CrossRef]
- Lee, M.Y.; Park, W.H.; Lenz, R.W. Hydrophilic bacterial polyesters modified with pendant hydroxyl groups. Polymer 2000, 41, 1703–1709. [Google Scholar] [CrossRef]
- Tortajada, M.; Ferreira da Silva, L.; Auxiliadora Prieto, M. Second-generation functionalized mediumchain- length polyhydroxyalkanoates: The gateway to high-value bioplastic applications. Int. Microbiol. 2013, 16, 1–15. [Google Scholar] [CrossRef]
- Reddy, V.U.N.; Ramanaiah, S.V.; Reddy, M.V.; Chang, Y.C. Review of the Developments of Bacterial Medium-Chain-Length Polyhydroxyalkanoates (mcl-PHAs). Bioengineering 2022, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Gregory, D.A.; Taylor, C.S.; Fricker, A.T.; Asare, E.; Tetali, S.S.; Haycock, J.W.; Roy, I. Polyhydroxyalkanoates and their advances for biomedical applications. Trends Mol. Med. 2022, 28, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Vicente, D.; Proença, D.N.; Morais, P.V. The Role of Bacterial Polyhydroalkanoate (PHA) in a Sustainable Future: A Review on the Biological Diversity. Int. J. Environ. Res. Public Health 2023, 20, 2959. [Google Scholar] [CrossRef] [PubMed]
- Ashby, R.D.; Solaiman, D.K.Y.; Foglia, T.A. Synthesis of Short-/Medium-Chain-Length Poly(hydroxyalkanoate) Blends by Mixed Culture Fermentation of Glycerol. Biomacromolecules 2005, 6, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Bona, R.; Braunegg, G.; Hermann, C.; Horvat, P.; Kroutil, M.; Martinz, J.; Neto, J.; Pereira, L.; Varila, P. Production of Polyhydroxyalkanoates from Agricultural Waste and Surplus Materials. Biomacromolecules 2005, 6, 561–565. [Google Scholar] [CrossRef]
- Alves, A.A.; Siqueira, E.C.; Barros, M.P.S.; Silva, P.E.C.; Houllou, L.M. Polyhydroxyalkanoates: A review of microbial production and technology application. Int. J. Environ. Sci. Technol. 2023, 20, 3409–3420. [Google Scholar] [CrossRef]
- Tsuge, T. Metabolic improvements and use of inexpensive carbon sources in microbial production of polyhydroxyalkanoates. J. Biosci. Bioeng. 2002, 94, 579–584. [Google Scholar] [CrossRef]
- Chathalingath, N.; Kingsly, J.S.; Gunasekar, A. Biosynthesis and biodegradation of poly(3-hydroxybutyrate) from Priestiaflexa; A promising mangrove halophyte towards the development of sustainable eco-friendly bioplastics. Microbiol. Res. 2023, 267, 127270. [Google Scholar] [CrossRef]
- Ohura, T.; Aoyagi, Y.; Takagi, K.; Yoshida, Y.; Kasuya, K.; Doi, Y. Biodegradation of poly(3-hydroxyalkanoic acids) fibers and isolation of poly(3-hydroxybutyric acid)-degrading microorganisms under aquatic environments. Polym. Degrad. Stab. 1999, 63, 23–29. [Google Scholar] [CrossRef]
- Chou, H.; Chen, C.; Huang, C.; Wang, H.; Hsiung, Y.-C.; Liang, C.-H.; Ou, C.-M.; Guo, G. Screening potential polyhydroxyalkanoate-producing bacteria from wastewater sludge. Arch. Microbiol. 2023, 205, 120. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Che, L.; Jin, W.; Zhou, X.; Han, W.; Chen, Y.; Chen, C.; Jiang, G. Current Status and future perspectives on the biological production of polyhydroxyalkanoates. Asia-Pac. J. Chem. Eng. 2023, e2899. [Google Scholar] [CrossRef]
- Rathbone, S.; Furrer, P.; Lübben, J.; Zinn, M.; Cartmell, S. Biocompatibility of polyhydroxyalkanoate as a potential material for ligament and tendon scaffold material: Biocompatibility of Polyhydroxyalkanoate. J. Biomed. Mater. Res. 2010, 93A, 1391–1403. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Tripathi, A.D. Effect of saturated and unsaturated fatty acid supplementation on bio-plastic production under submerged fermentation. Biotech 2013, 3, 389–397. [Google Scholar] [CrossRef]
- Ladhari, S.; Vu, N.N.; Boisvertn, C.; Saidi, A.; Nguyen-Tri, P. Recent Development of Polyhydroxyalkanoates (PHA)-based Materials for Antibacterial applications: A review. ACS Appl. Bio. Mater. 2023. [Google Scholar] [CrossRef]
- Prakash, P.; Lee, W.-H.; Loo, C.-Y.; Wong, H.S.J.; Parumasivam, T. Advances in Polyhydroxyalkanoate Nanocarriers for Effective Drug Delivery: An Overview and Challenges. Nanomaterials 2022, 12, 175. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef]
- Phuegyod, S.; Pramual, S.; Wattanavichean, N.; Assawajaruwan, S.; Amornsakchai, T.; Sukho, P.; Svasti, J.; Surarit, R.; Niamsiri, N. Microbial Poly(hydroxybutyrate-cohydroxyvalerate) Scaffold for Periodontal Tissue Engineering. Polymers 2023, 15, 855. [Google Scholar] [CrossRef]
- Zhang, J.; Shishatskaya, E.I.; Volova, T.G.; da Silva, L.F.; Chen, G.-Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef]
- Guennec, A.; Brelle, L.; Balnois, E.; Linossier, I.; Renard, E.; Langlois, V.; Faÿ, F.; Chen, G.Q.; Simon-Colin, C.; Vallée-Réhel, K. Antifouling properties of amphiphilic poly(3-hydroxyalkanoate): An environmentally-friendly coating. Biofouling 2021, 37, 894–910. [Google Scholar] [CrossRef]
- Mauclaire, L.; Brombacher, E.; Bünger, J.D.; Zinn, M. Factors controlling bacterial attachment and biofilm formation on medium-chain-length polyhydroxyalkanoates (mcl-PHAs). Colloids Surf. B Biointerfaces 2010, 76, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Piarali, S.; Marlinghaus, L.; Viebahn, R.; Lewis, H.; Ryadnov, M.G.; Groll, J.; Salber, J.; Roy, I. Activated Polyhydroxyalkanoate Meshes Prevent Bacterial Adhesion and Biofilm Development in Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Che, X.M.; Wei, D.X.; Chen, G.Q. Superhydrophobic Polyhydroxyalkanoates: Preparation and applications. Biomacromolecules 2019, 20, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Brelle, L.; Faÿ, F.; Ozturk, T.; Didier, N.; Renard, E.; Langlois, V. Hydrogel based on Polyhydroxyalkanoate Sulfonate: Control of the Swelling Rate by the Ionic Group content. Biomacromolecules 2023, in press. [Google Scholar] [CrossRef]
- Arias, S.L.; Devorkin, J.; Civantos, A.; Allain, J.P. Escherichia coli Adhesion and Biofilm Formation on Polydimethylsiloxane are Independent of Substrate Stiffness. Langmuir 2021, 37, 16–25. [Google Scholar] [CrossRef]
- Kolewe, K.W.; Peyton, S.R.; Schiffman, J.D. Fewer Bacteria Adhere to Softer Hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 19562–19569. [Google Scholar] [CrossRef]
- Kim, S.; Ko, S.; Kang, S.M. Adhesive heparin coating for marine antifouling applications. Macromol. Res. 2016, 24, 645–649. [Google Scholar] [CrossRef]
- Junter, G.-A.; Thébault, P.; Lebrun, L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016, 30, 13–25. [Google Scholar] [CrossRef]
- Wen, C.; Guo, H.; Yang, J.; Li, Q.; Zhang, X.; Sui, X.; Cao, M.; Zhang, L. Zwitteronic hydrogel coated superhydrophilic hierarchical antifouling floater enables unimpeded interfacial steam generation and multi-contamination resistance in complex conditions. Chem. Eng. J. 2021, 421, 130344. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Gnutt, P.; Wanka, R.; Krause, L.M.K.; Finlay, J.A.; Clare, S.A.; Rosenhaln, A. Layer-by-layer deposited hybrid polymer Coatings based on polysaccharides and zwitteronic silanes with marine antifouling properties. ACS Appl. Bio. Mater. 2021, 4, 2385–2397. [Google Scholar] [CrossRef]
- Aghajani, M.; Esmaeili, F. Anti-biofouling assembly strategies for protein & cell repellent surfaces: A mini-review. J. Biomater. Sci. Polym. Ed. 2021, 32, 1770–1789. [Google Scholar] [CrossRef]
- Cao, X.; Pettit, M.E.; Conlan, S.L.; Wagner, W.; Ho, A.D.; Clare, A.S.; Callow, J.A.; Callow, M.E.; Grunze, M.; Rosenhaln, A. Resistance of polysachharide coatings to proteins, hematopoietic cells, and marine organisms. ACS Biomacromol. 2009, 10, 907–915. [Google Scholar] [CrossRef]
- Bauer, S.; Arpa-Sancet, M.P.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Rosenhahn, A. Adhesion of Marine Fouling Organisms on Hydrophilic and Amphiphilic Polysaccharides. Langmuir 2013, 29, 4039–4047. [Google Scholar] [CrossRef]
- Shutava, T.G.; Livanovich, K.S.; Sharamet, A.A. Layer-by-layer films of polysaccharides modified with polyethylene glycol and dextran. Colloids Surf. B Biointerfaces 2019, 173, 412–420. [Google Scholar] [CrossRef]
- Mohan, T.; Cas, A.; Bracic, M.; Plohl, O.; Vesel, A.; Rupnik, M.; Zemljic, L.F.; Rebol, J. Highly protein reppellent and antiadhesive polysaccharide biomaterial coating for urinary catheter applications. ACS Biomater. Sci. Eng. 2019, 5, 5825–5832. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Sajadi, S.M.; Seidi, F.; Rabiee, N.; Fatahi, Y.; Rabiee, M.; Midhun Dominic, C.D.; Zarrintaj, P.; Formela, K.; Reza Saeb, M.; et al. Clockable polysaccharides for biomedical applications: A comprehensive review. Prog. Polym. Sci. 2022, 133, 101590. [Google Scholar] [CrossRef]
- Lee, Y.E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.Y.; Hong Tae Kim, J.; Lee, J. Marine polysaccharides: Therapeutic efficacy and biomedical applications. Arch. Pharm. Res. 2017, 40, 1006–1020. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guareniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef]
- Murugappan, V.; Muthadhi, A. Studies on the influence of alginate as a natural polymer in mechanical and long-lasting properties of concrete-review. Mater. Proc. 2022, 65, 839–845. [Google Scholar] [CrossRef]
- Jiménez-Gomez, C.; Cecilia, J.A. Chitosan: A natural Biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Crini, G. Historical review on chitin and chitosan biopolymers. Env. Chem Lett 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Pokhrel, S.; Yadav, P.N. Functinalization of chitosan polymer and their application. J. Macromol. Sci. A 2019, 56, 450–475. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, Z.; Liang, L.; Zheng, O.; Wang, Y.; Guo, Y.; Zhu, K.; Mehmood, R.; Wang, B. A facile heparin/carboxymethyl chitosan coating mediated by polydopamine on implants for hemocompatibility and antibacterial properties. Appl. Surf. Sci. 2020, 528, 146539. [Google Scholar] [CrossRef]
- Drozd, N.; Lunkov, A.P.; Shagdarova, B.T.; Zhuikova, Y.V.; Il’ina, A.V.; Varlamov, V.P. Chitosan/heparin layer-by-layer coatings for improving thromboresistance of polyurethane. Surf. Interfaces 2022, 28, 101674. [Google Scholar] [CrossRef]
- Bauer, S.; Alles, M.; Arpa-Sancet, M.P.; Ralston, E.; Swain, G.W.; Aldred, N.; Clare, A.S.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; et al. Resistance of Amphiphilic Polysaccharides against Marine Fouling Organisms. Biomacromolecules 2016, 17, 897–904. [Google Scholar] [CrossRef]
- Jakobi, V.; Schwarze, J.; Finlay, J.A.; Nolte, K.A.; Spöllmann, S.; Becker, H.-W.; Clare, A.S.; Rosenhahn, A. Amphiphilic Alginates for Marine Antifouling Applications. Biomacromolecules 2018, 19, 402–408. [Google Scholar] [CrossRef]
- Kim, D.; Kang, S.M. Red Algae-Derived Carrageenan Coatings for Marine Antifouling Applications. Biomacromolecules 2020, 21, 5086–5092. [Google Scholar] [CrossRef]
- Fyrner, T.; Lee, H.-H.; Mangone, A.; Ekblad, T.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Conlan, S.L.; Mutton, R.; Clare, A.S.; et al. Saccharide-Functionalized Alkanethiols for Fouling-Resistant Self-Assembled Monolayers: Synthesis, Monolayer Properties, and Antifouling Behavior. Langmuir 2011, 27, 15034–15047. [Google Scholar] [CrossRef]
- Nugraha, R.; Finlay, J.A.; Hill, S.; Fyrner, T.; Yandi, W.; Callow, M.E.; Callow, J.A.; Ederth, T. Antifouling properties of oligo(lactose)-based self-assembled monolayers. Biofouling 2015, 31, 123–134. [Google Scholar] [CrossRef]
- Ederth, T.; Ekblad, T.; Pettitt, M.E.; Conlan, S.L.; Du, C.-X.; Callow, M.E.; Callow, J.A.; Mutton, R.; Clare, A.S.; D’Souza, F.; et al. Resistance of Galactoside-Terminated Alkanethiol Self-Assembled Monolayers to Marine Fouling Organisms. ACS Appl. Mater. Interfaces 2011, 3, 3890–3901. [Google Scholar] [CrossRef]
- Gadenne, V.; Lebrun, L.; Jouenne, T.; Thebault, P. Antiadhesive activity of ulvan polysaccharides covalently immobilized onto titanium surface. Colloids Surf. B Biointerfaces 2013, 112, 229–236. [Google Scholar] [CrossRef]
- Jeong, Y.; Yoo, J.S.; Kang, S.M. Marine Fouling Resistance of Ulvan-grafted Solid Surface. Bull. Korean Chem. Soc. 2018, 39, 1459–1462. [Google Scholar] [CrossRef]
- Gnanasampanthan, T.; Beyer, C.D.; Yu, W.; Karthäuser, J.F.; Wanka, R.; Spöllmann, S.; Becker, H.-W.; Aldred, N.; Clare, A.S.; Rosenhahn, A. Effect of Multilayer Termination on Nonspecific Protein Adsorption and Antifouling Activity of Alginate-Based Layer-by-Layer Coatings. Langmuir 2021, 37, 5950–5963. [Google Scholar] [CrossRef]
- Yu, W.; Koc, J.; Finlay, J.A.; Clarke, J.L.; Clare, A.S.; Rosenhahn, A. Layer-by-layer constructed hyaluronic acid/chitosan multilayers as antifouling and fouling-release coatings. Biointerphases 2019, 14, 051002. [Google Scholar] [CrossRef]
- Yu, W.; Wanka, R.; Finlay, J.A.; Clarke, J.L.; Clare, A.S.; Rosenhahn, A. Degradable hyaluronic acid/chitosan polyelectrolyte multilayers with marine fouling-release properties. Biofouling 2020, 36, 1049–1064. [Google Scholar] [CrossRef]
- Xu, G.; Liu, P.; Pranantyo, D.; Neoh, K.-G.; Kang, E.-T. Dextran- and Chitosan-Based Antifouling, Antimicrobial Adhesion, and Self-Polishing Multilayer Coatings from pH-Responsive Linkages-Enabled Layer-by-Layer Assembly. ACS Sustain. Chem. Eng. 2018, 6, 3916–3926. [Google Scholar] [CrossRef]
- Gnanasampanthan, T.; Karthäuser, J.F.; Spöllmann, S.; Wanka, R.; Becker, H.-W.; Rosenhahn, A. Amphiphilic Alginate-based layer-by-layer coatings exhibiting resistance against nonspecific protein adsorption and marine biofouling. ACS Appl. Mater. Interfaces 2022, 14, 16062–16073. [Google Scholar] [CrossRef]
- Lakhan, M.N.; Chen, R.; Liu, F.; Shar, A.H.; Soomro, I.A.; Chand, K.; Ahmed, M.; Hanan, A.; Khan, A.; Maitlo, A.A.; et al. Construction of antifouling marine coatings via layer-by-layer assembly of chitosan and acid siloxane resin. J. Polym. Res. 2023, 30, 136. [Google Scholar] [CrossRef]
- Wanka, R.; Koc, J.; Clarke, J.; Hunsucker, K.Z.; Swain, G.W.; Aldred, N.; Finlay, J.A.; Clare, A.S.; Rosenhahn, A. Sol–Gel-Based Hybrid Materials as Antifouling and Fouling-Release Coatings for Marine Applications. ACS Appl. Mater. Interfaces 2020, 12, 53286–53296. [Google Scholar] [CrossRef]
- Guezennec, J. Deep-sea hydrothermal vents: A new source of innovative bacterial exopolysaccharides of biotechnological interest? J. Ind. Microbiol. Biotechnol. 2002, 29, 204–208. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.; Reis, M. Engineering aspects of microbial exopolysaccharide production. Bioresour. Technol. 2017, 245 Pt B, 1674–1683. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Donot, F.; Fontana, A.; Baccou, J.C.; Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Moscovici, M. Present and future medical applications of microbial exopolysaccharides. Front. Microbiol. 2015, 6, 1012. [Google Scholar] [CrossRef] [PubMed]
- Osińska-Jaroszuk, M.; Jarosz-Wilkołazka, A.; Jaroszuk-Ściseł, J.; Szałapata, K.; Nowak, A.; Jaszek, M.; Ozimek, E.; Majewska, M. Extracellular polysaccharides from Ascomycota and Basidiomycota: Production conditions, biochemical characteristics, and biological properties. World J. Microbiol. Biotechnol. 2015, 31, 1823–1844. [Google Scholar] [CrossRef]
- Rehm, B.H. Bacterial polymers: Biosynthesis, modifications and applications. Nature reviews. Microbiology 2010, 8, 578–592. [Google Scholar] [CrossRef]
- Rehm, B.H.; Moradali, M.F. (Eds.) Biopolymers for Biomedical and Biotechnological Applications; John Wiley & Sons: Weinheim, Germany, 2021. [Google Scholar]
- Freitas, F.; Alves, V.D.; Reis, M.A. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Papinutti, L. Effects of nutrients, pH and water potential on exopolysaccharides production by a fungal strain belonging to Ganoderma lucidum complex. Bioresour. Technol. 2010, 101, 1941–1946. [Google Scholar] [CrossRef]
- Barcelos, M.; Vespermann, K.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef]
- Martin-Pastor, M.; Ferreira, A.S.; Moppert, X.; Nunes, C.; Coimbra, M.A.; Reis, R.L.; Guezennec, J.; Novoa-Carballal, R. Structure, rheology, and copper-complexation of a hyaluronan-like exopolysaccharide from Vibrio. Carbohydr. Polym. 2019, 222, 114999. [Google Scholar] [CrossRef]
- Raguenes, G.; Christen, R.; Guezennec, J.; Pignet, P.; Barbier, G. Vibrio diabolicus sp. nov., a new polysaccharide-secreting organism isolated from a deep-sea hydrothermal vent polychaete annelid, Alvinella pompejana. Int. J. Syst. Bacteriol. 1997, 47, 989–995. [Google Scholar] [CrossRef]
- Cambon-Bonavita, M.A.; Raguénès, G.; Jean, J.; Vincent, P.; Guezennec, J. A novel polymer produced by a bacterium isolated from a deep-sea hydrothermal vent polychaete annelid. J. Appl. Microbiol. 2002, 93, 310–315. [Google Scholar] [CrossRef]
- Concórdio-Reis, P.; Alves, V.D.; Moppert, X.; Guézennec, J.; Freitas, F.; Reis, M. Characterization and Biotechnological Potential of Extracellular Polysaccharides Synthesized by Alteromonas Strains Isolated from French Polynesia Marine Environments. Mar. Drugs 2021, 19, 522. [Google Scholar] [CrossRef]
- Sayem, S.A.; Manzo, E.; Ciavatta, L.; Tramice, A.; Cordone, A.; Zanfardino, A.; De Felice, M.; Varcamonti, M. Anti-biofilm activity of an exopolysaccharide from a sponge-associated strain of Bacillus licheniformis. Microb. Cell Factories 2011, 10, 74. [Google Scholar] [CrossRef]
- Spanò, A.; Laganà, P.; Visalli, G.; Maugeri, T.L.; Gugliandolo, C. In Vitro Antibiofilm Activity of an Exopolysaccharide from the Marine Thermophilic Bacillus licheniformis T14. Curr. Microbiol. 2016, 72, 518–528. [Google Scholar] [CrossRef]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Le Costaouëc, T.; Cérantola, S.; Ropartz, D.; Ratiskol, J.; Sinquin, C.; Colliec-Jouault, S.; Boisset, C. Structural data on a bacterial exopolysaccharide produced by a deep-sea Alteromonas macluodii strain. Carbohydr. Polym. 2012, 90, 49–59. [Google Scholar] [CrossRef]
- Rendueles, O.; Kaplan, J.B.; Ghigo, J.-M. Antibiofilm polysaccharides: Bacterial antibiofilm polysaccharides. Environ. Microbiol 2013, 15, 334–346. [Google Scholar] [CrossRef]
- Jha, N.; Madasamy, S.; Prasad, P.; Lakra, A.K.; Tilwani, Y.M.; Arul, V. Physico-chemical and functional characterization of polysaccharide purified from mangrove Rhizophora mucronata leaves having potent biological activity. S. Afr. J. Bot. 2022, 147, 659–669. [Google Scholar] [CrossRef]
- Malakar, C.; Deka, S.; Kalita, M.C. Role of Biosurfactants in Biofilm Prevention and Disruption. In Advancements in Biosurfactants Research; Aslam, R., Mobin, M., Aslam, J., Zehra, S., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Guezennec, J.; Herry, J.M.; Kouzayha, A.; Bachere, E.; Mittelman, M.W.; Bellon Fontaine, M.N. Exopolysaccharides from unusual marine environments inhibit early stages of biofouling. Int. Biodeterior. Biodegrad. 2012, 66, 1–7. [Google Scholar] [CrossRef]
- Jiang, P.; Li, J.; Han, F.; Duan, G.; Lu, X.; Gu, Y.; Yu, W. Antibiofilm Activity of an Exopolysaccharide from Marine Bacterium Vibrio sp. QY101. PLoS ONE 2011, 6, e18514. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Q.; Hao, D.; Jiang, D.; Luo, Y.; Liu, Y.; Zhao, Z. Production, Purification, and Antibiofilm Activity of a Novel Exopolysaccharide from Arthrobacter sp. B4. Prep. Biochem. Biotechnol. 2015, 45, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Pradeepa Shetty, A.D.; Matthews, K.; Hegde, A.R.; Akshatha, B.; Mathias, A.B.; Mutalik, S.; Vidya, S.M. Multidrug resistant pathogenic bacterial biofilm inhibition by Lactobacillus plantarum exopolysaccharide. Bioact. Carbohydr. Diet. Fibre 2016, 8, 7–14. [Google Scholar] [CrossRef]
- Valle, J.; Re, S.D.; Henry, N.; Fontaine, T.; Balestrino, D.; Latour-Lambert, P.; Ghigo, J.-M. Broad-spectrum biofilm inhibition by a secreted bacterial polysaccharide. PNAS 2006, 103, 12558–12563. [Google Scholar] [CrossRef]
- Vishwakarma, J.; Sirisha, V.L. Unraveling the anti-biofilm potential of green algal sulfated polysaccharides against Salmonella enterica and Vibrio harveyi. Appl. Microbiol. Biotechnol. 2020, 104, 6299–6314. [Google Scholar] [CrossRef]
- Wu, S.; Liu, G.; Jin, W.; Xiu, P.; Sun, C. Antibiofilm and Anti-Infection of a Marine Bacterial Exopolysaccharide Against Pseudomonas aeruginosa. Front. Microbiol. 2016, 7, 102. [Google Scholar] [CrossRef]
- Champion, M.; Portier, E.; Vallée-Réhel, K.; Linossier, I.; Balnois, E.; Vignaud, G.; Moppert, X.; Hellio, C.; Faÿ, F. Anti-Biofilm Activity of a Hyaluronan-like Exopolysaccharide from the Marine Vibrio MO245 against Pathogenic Bacteria. Mar. Drugs 2022, 20, 728. [Google Scholar] [CrossRef]
- Su, D.; Bai, X.; He, X. Research progress on hydrogel materials and their antifouling properties. Eur. Polym. J. 2022, 181, 111665. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2015, 8, nwaa254. [Google Scholar] [CrossRef]
- Gokaltun, A.; Kang, Y.B.; Yarmush, M.L.; Usta, O.B.; Asatekin, A. Simple surface modification of poly(dimethylsiloxane) via surface segregating smart polymers for biomicrofluidics. Sci. Rep. 2019, 9, 7377. [Google Scholar] [CrossRef]
- Guégan, C.; Garderes, J.; Le Pennec, G.; Gaillard, F.; Faÿ, F.; Linossier, I.; Herry, J.-M.; Fontaine, M.-N.B.; Réhel, K.V. Alteration of bacterial adhesion induced by the substrate stiffness. Colloids Surf. B Biointerfaces 2014, 114, 193–200. [Google Scholar] [CrossRef]
- Kolewe, K.W.; Zhu, J.; Mako, N.R.; Nonnenman, S.S.; Shiffman, J.D. Bacterial adhesion is affected by the thickness and stiffness of poly(ethylene glycol) hydrogels, ACS Appl. Mater. Interfaces 2018, 10, 2275–2281. [Google Scholar] [CrossRef]
- Finlay, J.A.; Callow, M.E.; Ista, L.K.; Lopez, G.P.; Callow, J.A. The Influence of Surface Wettability on the Adhesion Strength of Settled Spores of the Green Alga Enteromorpha and the Diatom Amphora. Integr. Comp. Biol. 2002, 42, 1116–1122. [Google Scholar] [CrossRef]
- Magin, C.M.; Finlay, J.A.; Clay, G.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Antifouling Performance of cross-linked hydrogel: Refinement of an attachment model. Biomacromolecules 2011, 12, 915–922. [Google Scholar] [CrossRef]
- Rajasree, V.; Satheesh, S.; Vincent, S.P. Antifouling activity of a marine epibiotic bacterium from the seaweed sargassum wightii. Thalassas 2012, 28, 37–43. [Google Scholar]
- Siqueira Melo, R.; Brasil, S.; Carvalho, L.; Limaverde Filho, A.; Pereira, C. Assessment of the Antifouling Effect of Exopolysaccharides Incorporated into Copper Oxide-Based Organic Paint. Int. J. Electrochem. Sci. 2016, 11, 7750. [Google Scholar] [CrossRef]
- Koc, J.; Simovich, T.; Schönemann, E.; Chilkoti, A.; Gardner, H.; Swain, G.W.; Hunsucker, K.; Laschewsky, A.; Rosenhahn, A. Sediment challenge to promising ultra-low fouling hydrophilic surfaces in the marine environment. Biofouling 2019, 35, 454–462. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, L.; Chiao, M.; Yang, W. Antibacterial hydrogel coating: Strategies in surface chemistry. Adv. Colloid Interface Sci. 2020, 285, 102280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).