Effect of Micromolecules and Macromolecules on the Environmentally Friendly Impregnation Solution for High-Performance Rubber Composites Compared with Traditional RFL Impregnation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
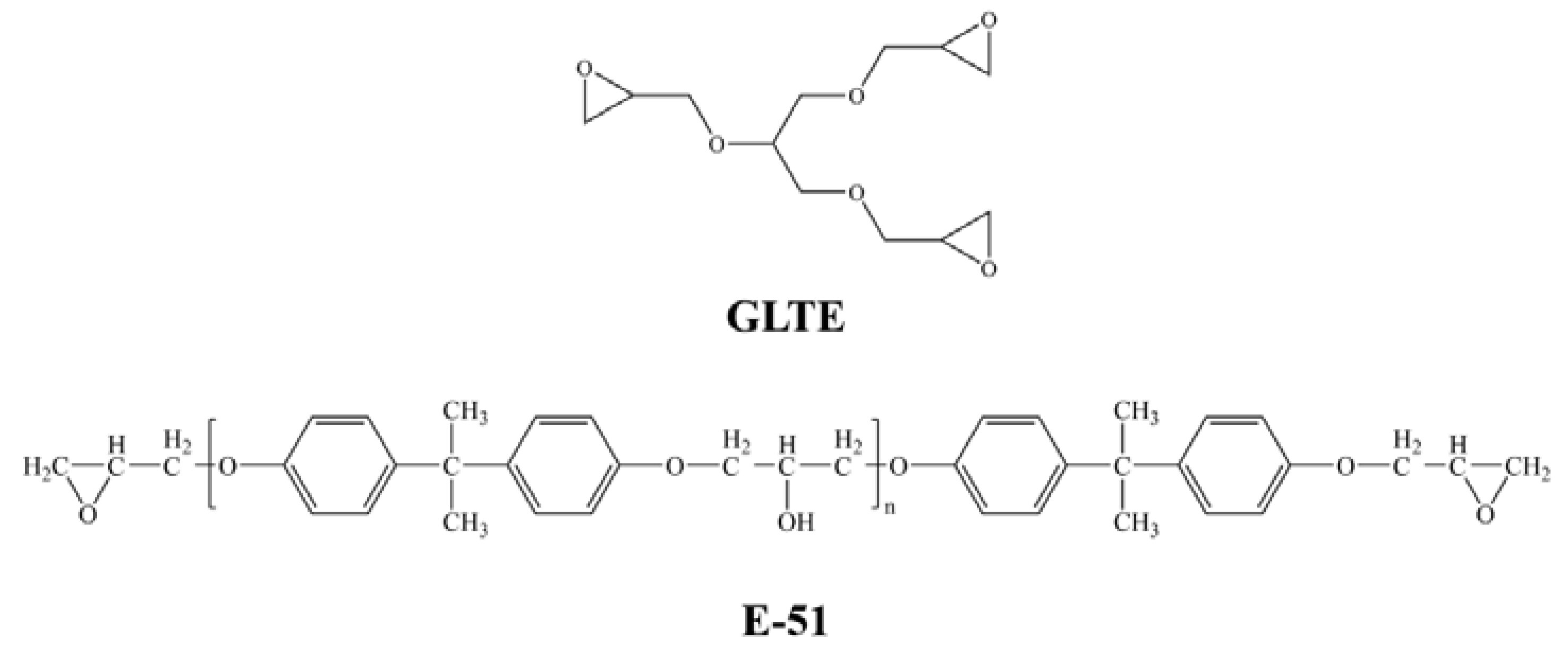
2.2. Preparation of Different Impregnation Solutions
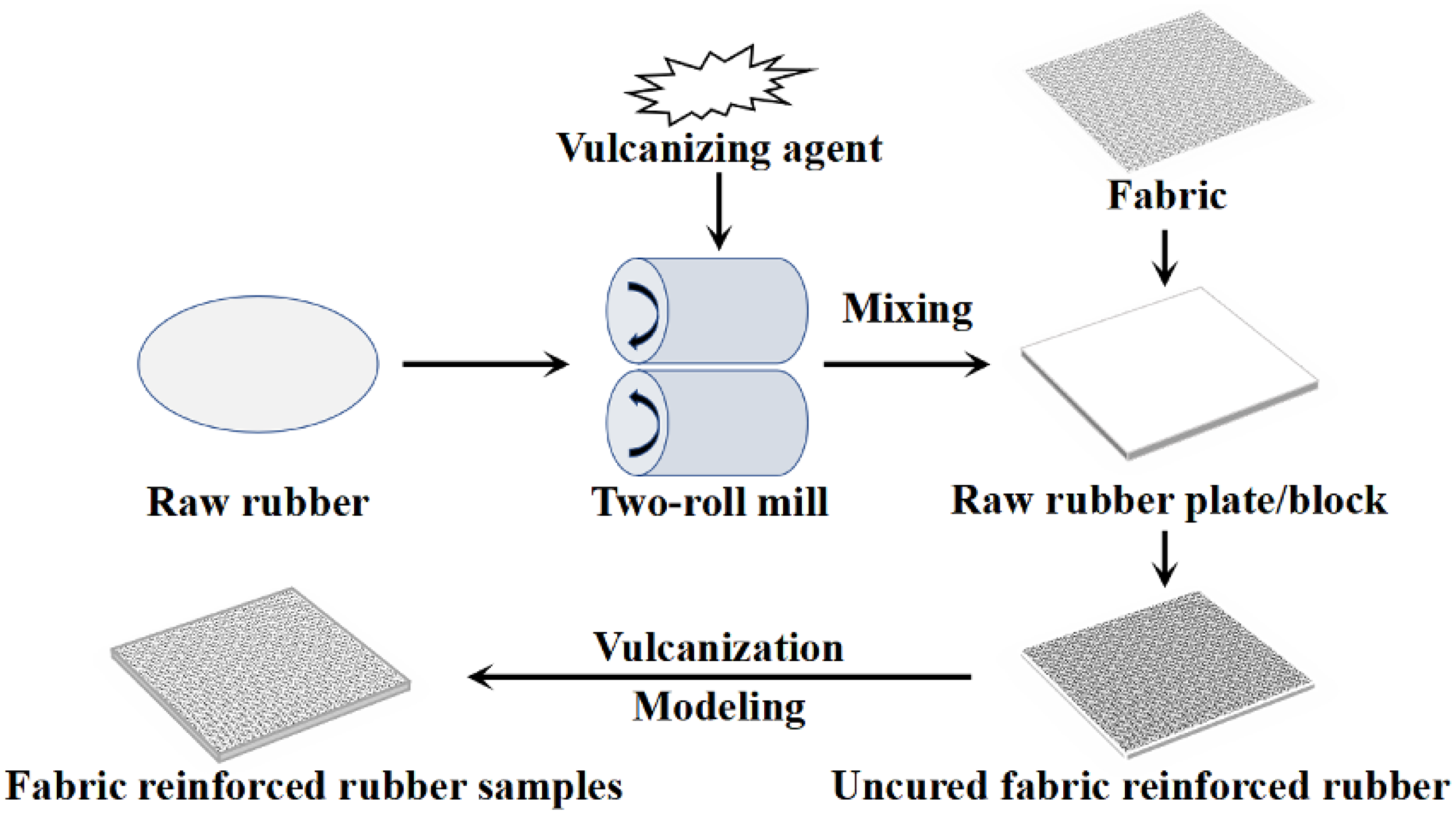
2.3. Impregnated PET Fabrics with Different Impregnation Solutions
2.4. Preparation of PET/Rubber Composites with Different Impregnation Solutions
2.5. Characterization
3. Results and Discussion
3.1. Reaction Conditions of GLTE/TETA and E-51/TETA
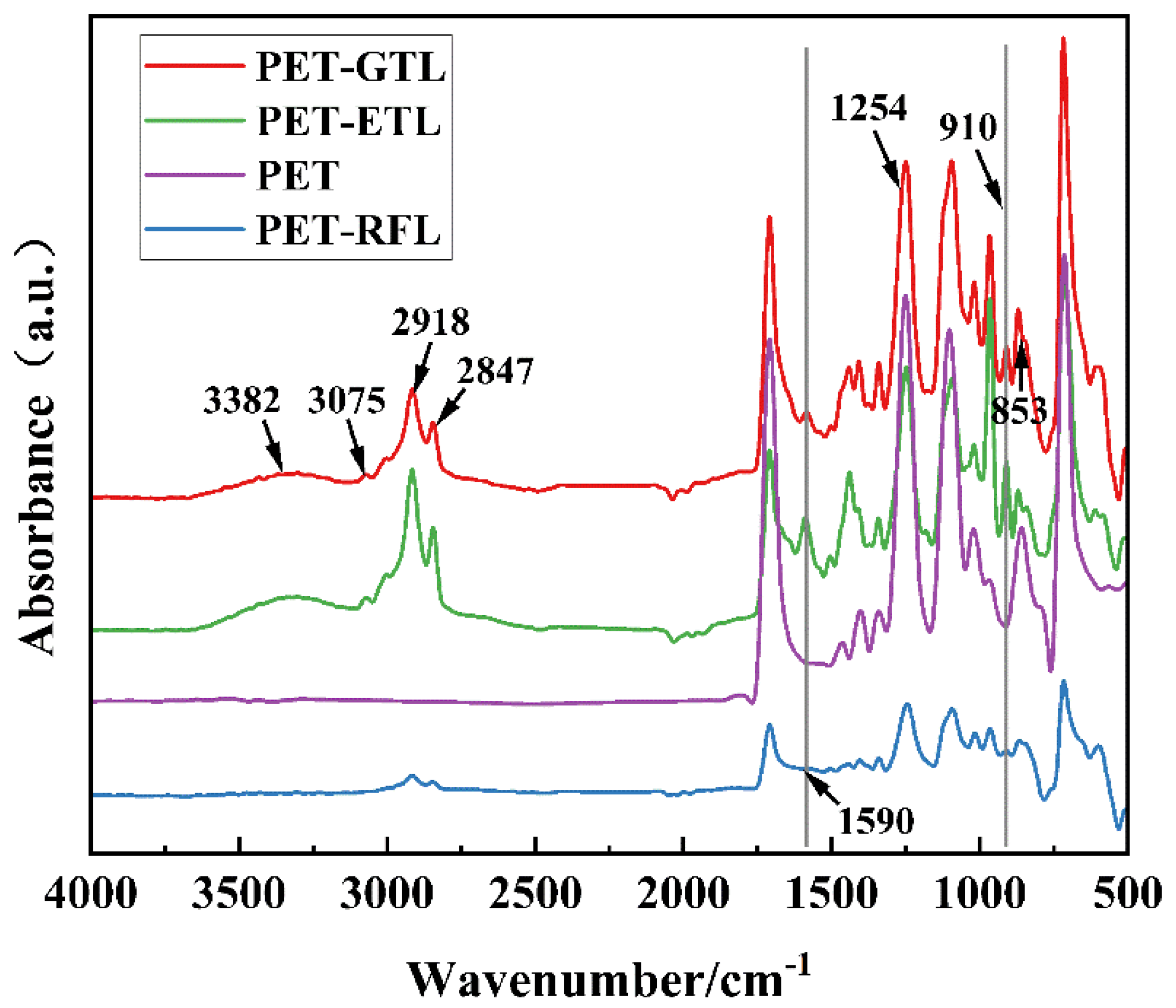
3.2. Chemical Composition of PET Fabric Surface
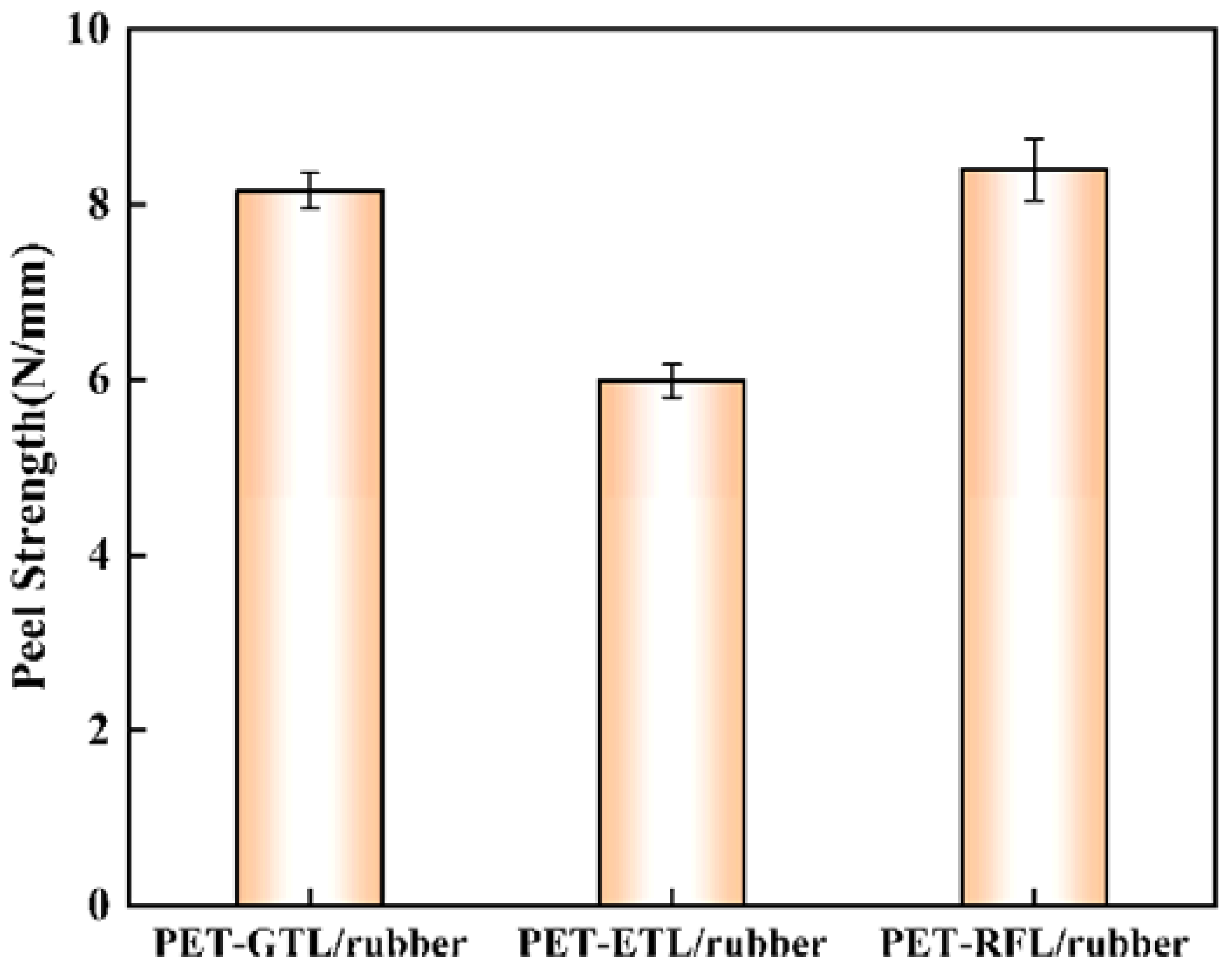
3.3. Peeling Strength of PET/Rubber Composites Impregnated by GTL, ETL and RFL Impregnation Solutions
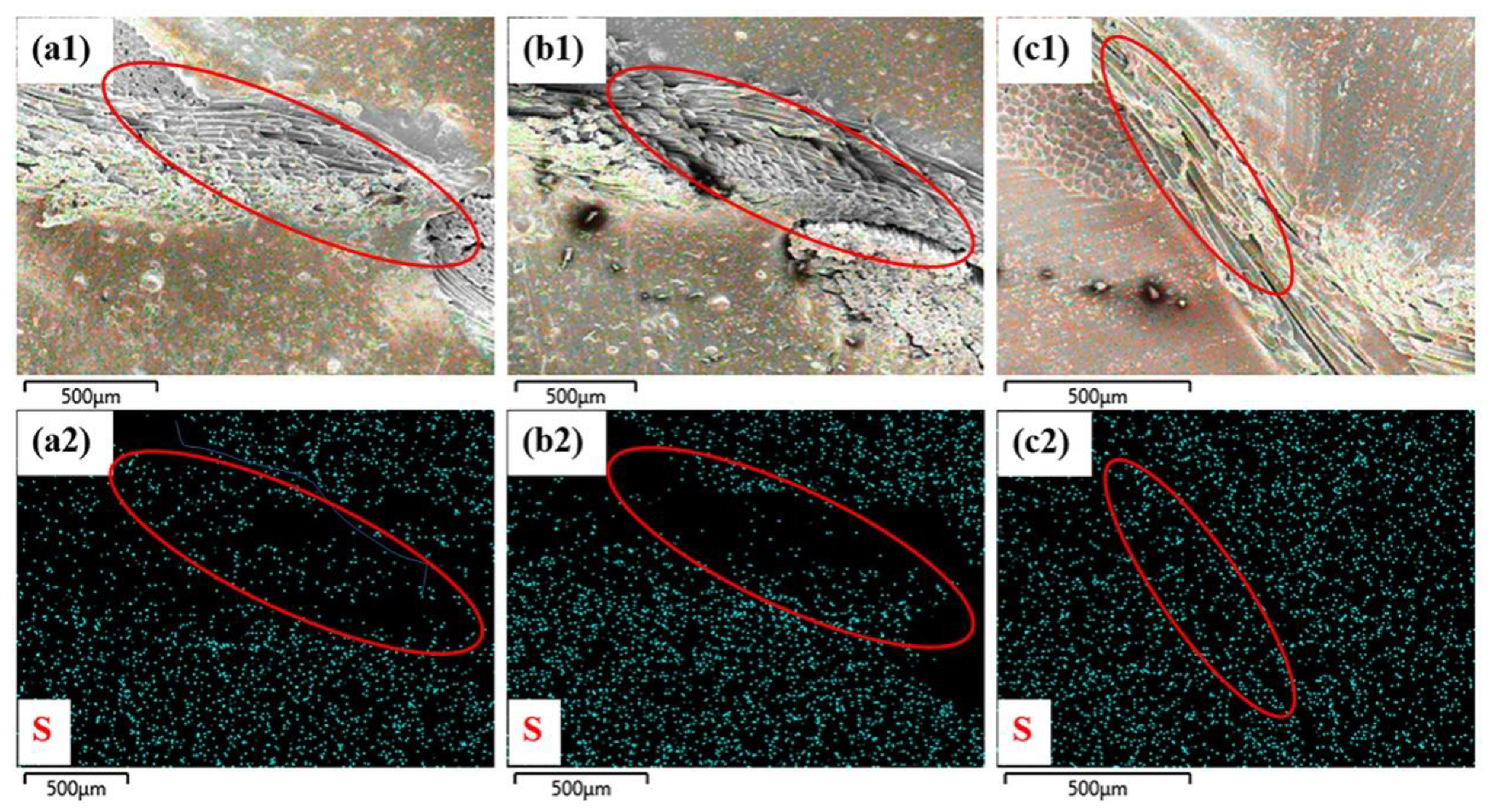
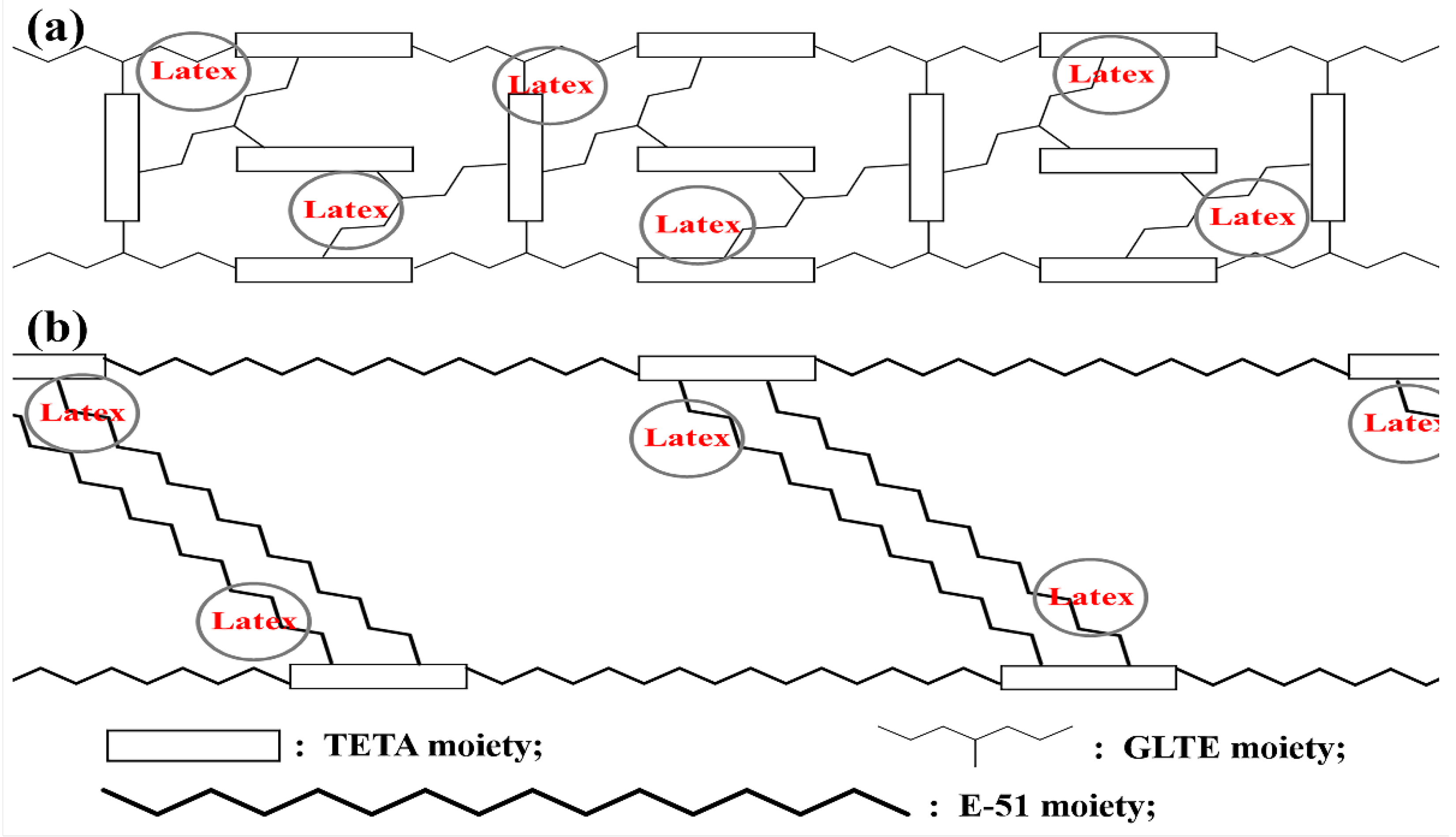
3.4. Interfacial Adhesion Mechanism of Fabrics/Rubber Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shahzad, A. Hemp fiber and its composites-A review. J. Compos. Mater. 2012, 46, 973–986. [Google Scholar] [CrossRef]
- Shen, Z.; Song, W.; Li, X.; Yang, L.; Wang, C.; Hao, Z.; Luo, Z. Enhancing performances of hemp fiber/natural rubber composites via polyhydric hyperbranched polyester. J. Polym. Eng. 2021, 41, 404–412. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, S.; Wang, W.; Tian, M.; Ning, N.; Zhang, L. Polyester (PET) fabrics coated with environmentally friendly adhesive and its interface structure and adhesive properties with rubber. Compos. Sci. Technol. 2020, 195, 108171. [Google Scholar] [CrossRef]
- Teng, C.; Wang, T.; Qin, M.; Kong, H.; Zhang, J.; Yu, M. Facile dispersion of aramid pulp by matrix sizing agent and reinforced rubber composites. Polym. Compos. 2020, 41, 4583–4592. [Google Scholar] [CrossRef]
- Doganci, E. Improving adhesion between polyester cord and rubber by using glycidyl-POSS. J. Appl. Polym. Sci. 2021, 138, 49681. [Google Scholar] [CrossRef]
- Shao, Y.R.; Han, Z.J.; Wang, G.F. In situ grafting coupling agent on polyester fabric to significantly improve the interfacial adhesion to silicone rubber. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125837. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, L.; Jiang, C.; Dai, Y.; Meng, C.; Luo, L.; Liu, X. Aramid fiber with excellent interfacial properties suitable for resin composite in a wide polarity range. Chem. Eng. J. 2018, 347, 483–492. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Chen, S.; Wang, W.; Tian, M.; Ning, N.; Zhang, L. Highly efficient mussel-like inspired modification of aramid fibers by UV-accelerated catechol/polyamine deposition followed chemical grafting for high-performance polymer composites. Chem. Eng. J. 2017, 314, 583–593. [Google Scholar] [CrossRef]
- Zhang, J.M.; Cortés-Ballesteros, B.; Peijs, T. All-aramid composites by partial fiber dissolution in mixed solvents. Polym. Compos. 2018, 39, 3013–3021. [Google Scholar] [CrossRef]
- Lin, T.K.; Wu, S.J.; Lai, J.G.; Shyu, S.S. The effect of chemical treatment on reinforcement/matrix interaction in Kevlar-fiber/bismaleimide composites. Compos. Sci. Technol. 2000, 60, 1873–1878. [Google Scholar] [CrossRef]
- Sari, J.M.; Spatenka, P.; Jeníková, Z.; Grohens, Y.; Thomas, S. New type of thermoplastic bio composites: Nature of the interface on the ultimate properties and water absorption. RSC Adv. 2015, 5, 97536–97546. [Google Scholar]
- Peng, H.; Guo, Z.; Yu, S.; Wang, R. Surface modification of polysulfonamide fiber treated by air plasma. RSC. Adv. 2015, 5, 78172–78179. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, D.H.; Yang, K.S.; Lee, B.-C.; Kim, Y.A.; Endo, M. Electron beam irradiation-enhanced wettability of carbon fibers. ACS Appl. Mater. Interfaces 2011, 3, 119–123. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, C.; Chen, P.; Yu, Q.; Li, W. Modification of carbon fiber by air plasma and its adhesion with BMI resin. RSC Adv. 2014, 4, 26881–26887. [Google Scholar] [CrossRef]
- Kondo, Y.; Miyazaki, K.; Yamaguchi, Y.; Sasaki, T.; Irie, S.; Sakurai, K. Mechanical properties of fiber reinforced styrene–butadiene rubbers using surface-modified UHMWPE fibers under EB irradiation. Eur. Polym. J. 2006, 42, 1008–1014. [Google Scholar] [CrossRef]
- Slouf, M.; Synkova, H.; Baldrian, J.; Marek, A.; Kovarova, J.; Schmidt, P.; Dorschner, H.; Stephan, M.; Gohs, U. Structural changes of UHMWPE after e-beam irradiation and thermal treatment. J. Biomed. Mater. Res. B. Appl. Biomater. 2008, 85, 240–251. [Google Scholar] [CrossRef]
- Mao, L.; Wang, Y.; Zang, Z.; Zhu, S.; Zhang, H.; Zhou, H. Amino-functionalization of carbon fibers through electron-beam irradiation technique. J. Appl. Polym. Sci. 2014, 131, 376–418. [Google Scholar] [CrossRef]
- Xing, L.; Liu, L.; Xie, F.; Huang, Y. Mutual irradiation grafting on indigenous aramid fiber-3 in diethanolamine and epichlorohydrin and its effect on interfacially reinforced epoxy composite. Appl. Surf. Sci. 2016, 375, 65–73. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, G.; Wu, Y.; Kong, F.; Huang, J. Surface functional modification of ultrahigh molecular weight polyethylene fiber by atom transfer radical polymerization. Appl. Surf. Sci. 2018, 427, 410–415. [Google Scholar] [CrossRef]
- Liu, X.D.; Sheng, D.K.; Gao, X.M.; Li, T.-B.; Yang, Y.-M. UV-assisted surface modification of PET fiber for adhesion improvement. Appl. Surf. Sci. 2013, 264, 61–69. [Google Scholar] [CrossRef]
- Sa, R.; Yan, Y.; Wang, L.; Li, Y.; Zhang, L.; Ning, N.; Wang, W.; Tian, M. Improved adhesion properties of poly-p-phenyleneterephthamide fibers with rubber matrix via UV-initiated grafting modification. RSC Adv. 2015, 5, 94351–94360. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Q.; Shen, X.; Jiang, C.; Pan, M.; Zhu, P.; Li, Y.; Hu, C.; Zhang, Q. Study on interfacial adhesion of the aramid fibers/rubber matrix by grafting mercapto hyperbranched polysiloxane. Polym. Test. 2019, 81, 106259. [Google Scholar] [CrossRef]
- Randall, J.D.; Eyckens, D.J.; Servinis, L.; Stojcevski, F.; O’Dell, L.A.; Gengenbach, T.R.; Demir, B.; Walsh, T.R.; Henderson, L.C. Designing carbon fiber composite interfaces using a ‘graft-to’ approach: Surface grafting density versus interphase penetration. Carbon 2019, 146, 88–96. [Google Scholar] [CrossRef]
- Holloway, J.L.; Lowman, A.M.; Vanlandingham, M.R.; Palmese, G.R. Chemical grafting for improved interfacial shear strength in UHMWPE/PVA-hydrogel fiber-based composites used as soft fibrous tissue replacements. Compos. Sci. Technol. 2013, 85, 118–125. [Google Scholar] [CrossRef]
- Li, S.; Gu, A.; Liang, G.; Yuan, L.; Xue, J. A facile and green preparation of poly(glycidyl methacrylate) coated aramide fibers. J. Mater. Chem. 2012, 22, 8960–8968. [Google Scholar] [CrossRef]
- Shibulal, G.S.; Naskar, K. RFL coated aramid short fiber reinforced thermoplastic elastomer: Mechanical, rheological and morphological characteristics. J. Polym. Res. 2011, 18, 2295–2306. [Google Scholar] [CrossRef]
- Shirazi, M.; Rooij, M.B.D.; Talma, A.G.; Noordermeer, J. Adhesion of RFL-coated aramid fibres to elastomers: The role of elastomer-latex compatibility. J. Adhes. Sci. Technol. 2013, 27, 1886–1898. [Google Scholar] [CrossRef]
- Jaššo, M.; Hudec, I.; Alexy, P.; Kováčik, D.; Krump, H. Grafting of maleic acid on the polyester fibres initiated by plasma at atmospheric pressure. Int. J. Adhes. Adhes. 2006, 26, 274–284. [Google Scholar] [CrossRef]
- Bibi, A.N.; Boscott, D.A.; Butt, T.; Lehrle, R.S. Improving the adhesion between rubber and nylon by either epoxidation of the rubber or chemical pre-treatment of the nylon. Eur. Polym. J. 1988, 24, 1127–1131. [Google Scholar] [CrossRef]
- Caster, K.C.; Walls, R.D. Adhesion of fibers to natural rubber elastomer using ring-opening metathesis polymerization (ROMP). Adv. Synth. Catal. 2002, 344, 764–770. [Google Scholar] [CrossRef]
- He, H.; Wu, P.; Yang, Z.; Shi, Z.; Yu, W.; Liu, F.; Zhu, F.; Zheng, Q.; Zhang, D.; Li, S. A facile way to modify polyester fabric to enhance the adhesion behavior to rubber. Coatings 2022, 12, 1344. [Google Scholar] [CrossRef]
- Wennekes, W.B.; Datta, R.N.; Noordermeer, J. Mechanistic Investigations into the Adhesion between RFL-Treated Cords and Rubber. Part II: The Influence of the Vinyl-Pyridine Content of the RFL-Latex. Rubber. Chem. Technol. 2007, 80, 565–579. [Google Scholar] [CrossRef]



| Component | Content (phr) |
|---|---|
| NR | 30 |
| SBR1502 | 70 |
| Zinc oxide | 5 |
| Stearic acid | 2 |
| Carbon black N330 | 30 |
| Antioxidant (4010NA) | 1.5 |
| Aromatic oil 840 | 15 |
| Silica | 15 |
| 2,4,6-Tri [double (methoxy methyl) amino]-1,3,5-triazine (RA) | 1.5 |
| Compound of resorcinol and stearic acid (RS) | 1.5 |
| N-Cyclohexyl-2-truxene thiazole sulfonamide (CZ) | 4 |
| Coumarone resin | 10 |
| Sulfur | 1 |
| Total | 186.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; He, H.; Wu, P.; Yang, Z.; Yu, W.; Liu, F.; Zhu, F.; Zhang, Z.; Zheng, Q. Effect of Micromolecules and Macromolecules on the Environmentally Friendly Impregnation Solution for High-Performance Rubber Composites Compared with Traditional RFL Impregnation. Coatings 2023, 13, 765. https://doi.org/10.3390/coatings13040765
Shi Z, He H, Wu P, Yang Z, Yu W, Liu F, Zhu F, Zhang Z, Zheng Q. Effect of Micromolecules and Macromolecules on the Environmentally Friendly Impregnation Solution for High-Performance Rubber Composites Compared with Traditional RFL Impregnation. Coatings. 2023; 13(4):765. https://doi.org/10.3390/coatings13040765
Chicago/Turabian StyleShi, Zhihao, Hongwei He, Pengfeng Wu, Zeguang Yang, Wenwen Yu, Fuyong Liu, Fengbo Zhu, Zhiyi Zhang, and Qiang Zheng. 2023. "Effect of Micromolecules and Macromolecules on the Environmentally Friendly Impregnation Solution for High-Performance Rubber Composites Compared with Traditional RFL Impregnation" Coatings 13, no. 4: 765. https://doi.org/10.3390/coatings13040765
APA StyleShi, Z., He, H., Wu, P., Yang, Z., Yu, W., Liu, F., Zhu, F., Zhang, Z., & Zheng, Q. (2023). Effect of Micromolecules and Macromolecules on the Environmentally Friendly Impregnation Solution for High-Performance Rubber Composites Compared with Traditional RFL Impregnation. Coatings, 13(4), 765. https://doi.org/10.3390/coatings13040765






