Mechanical Properties, Corrosion Resistance and Bioactivity of Oxide Layers Formed by Isothermal Oxidation of Ti-6Al-7Nb Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Study of the Surface Geometrical Structure
2.3. Microindentation Studies
2.4. Corrosion Resistance Measurements
2.5. Bioactivity Examination Conditions
2.6. Material Characterization Methods
2.7. Statistical Analysis
3. Results and Discussion
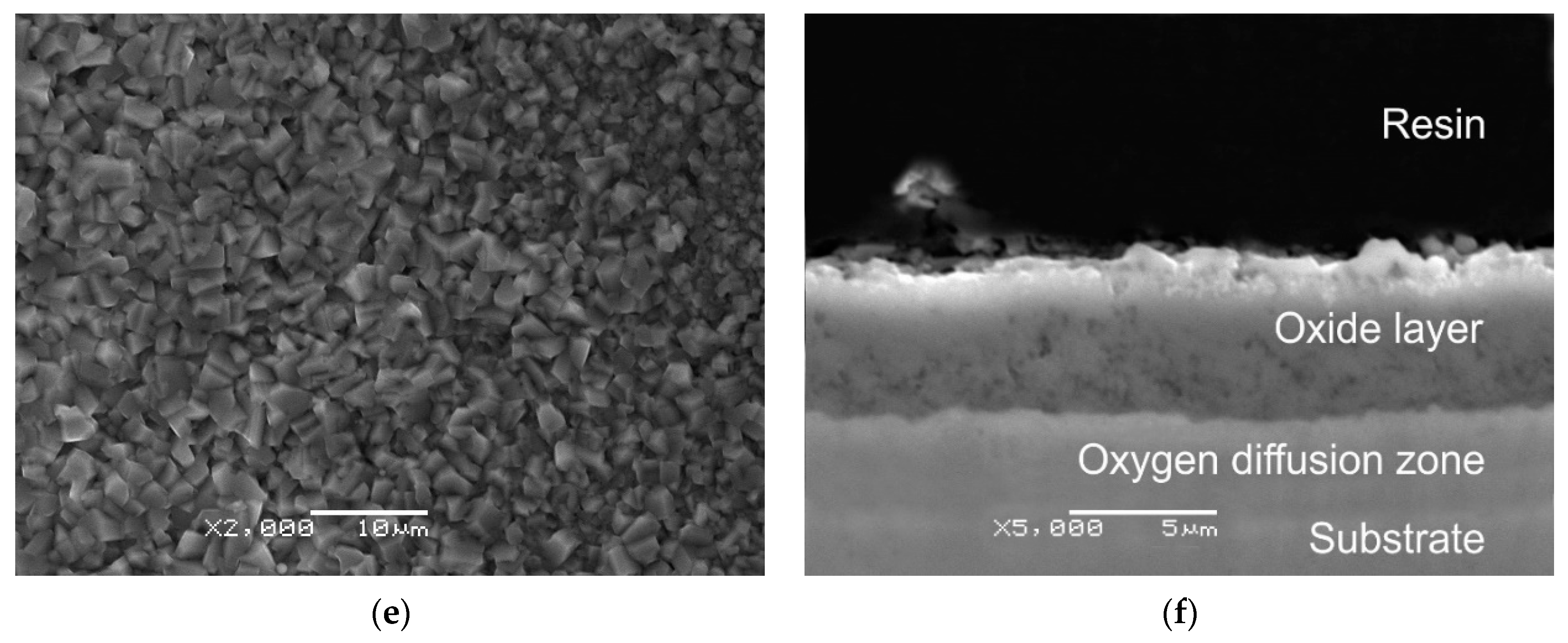
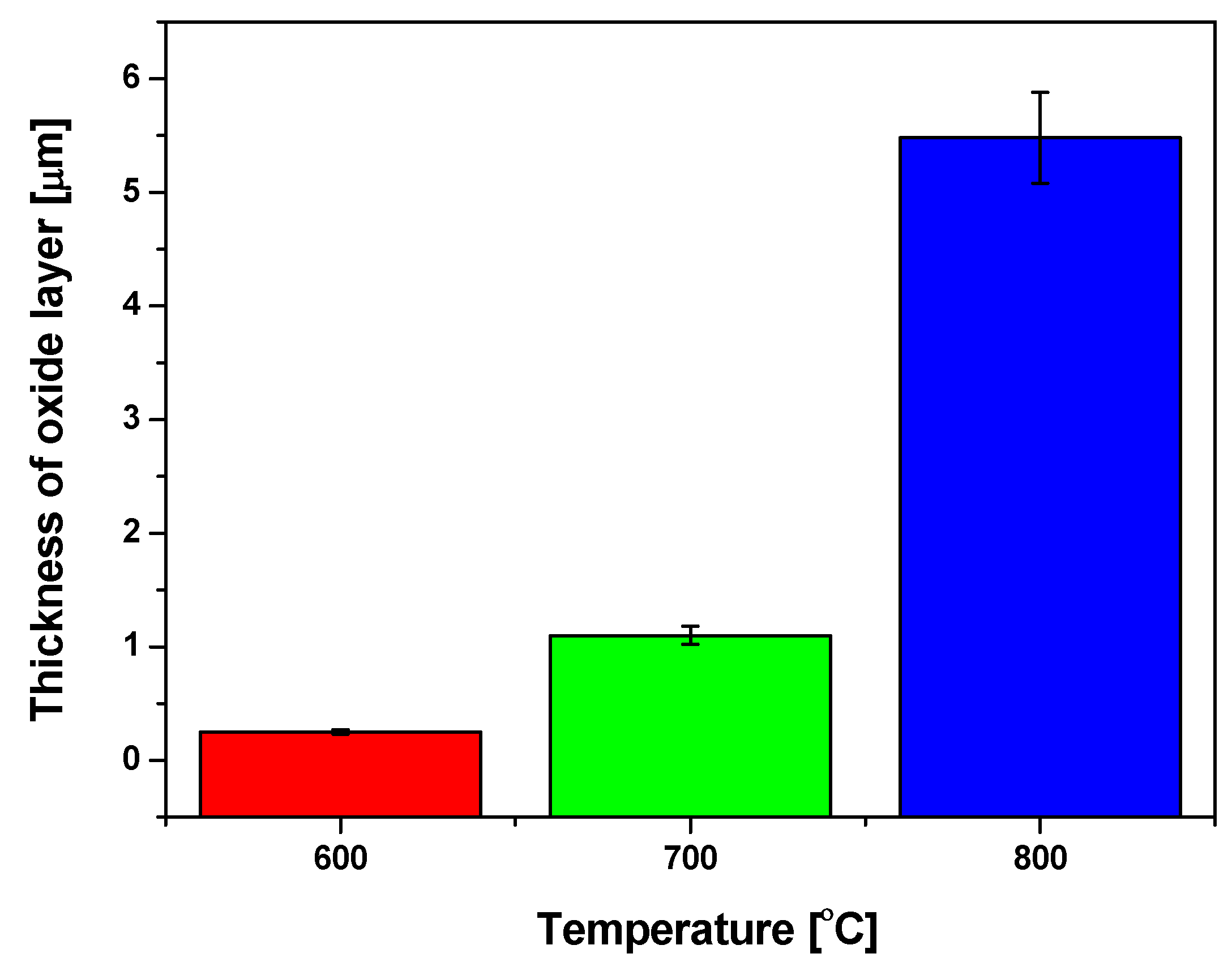
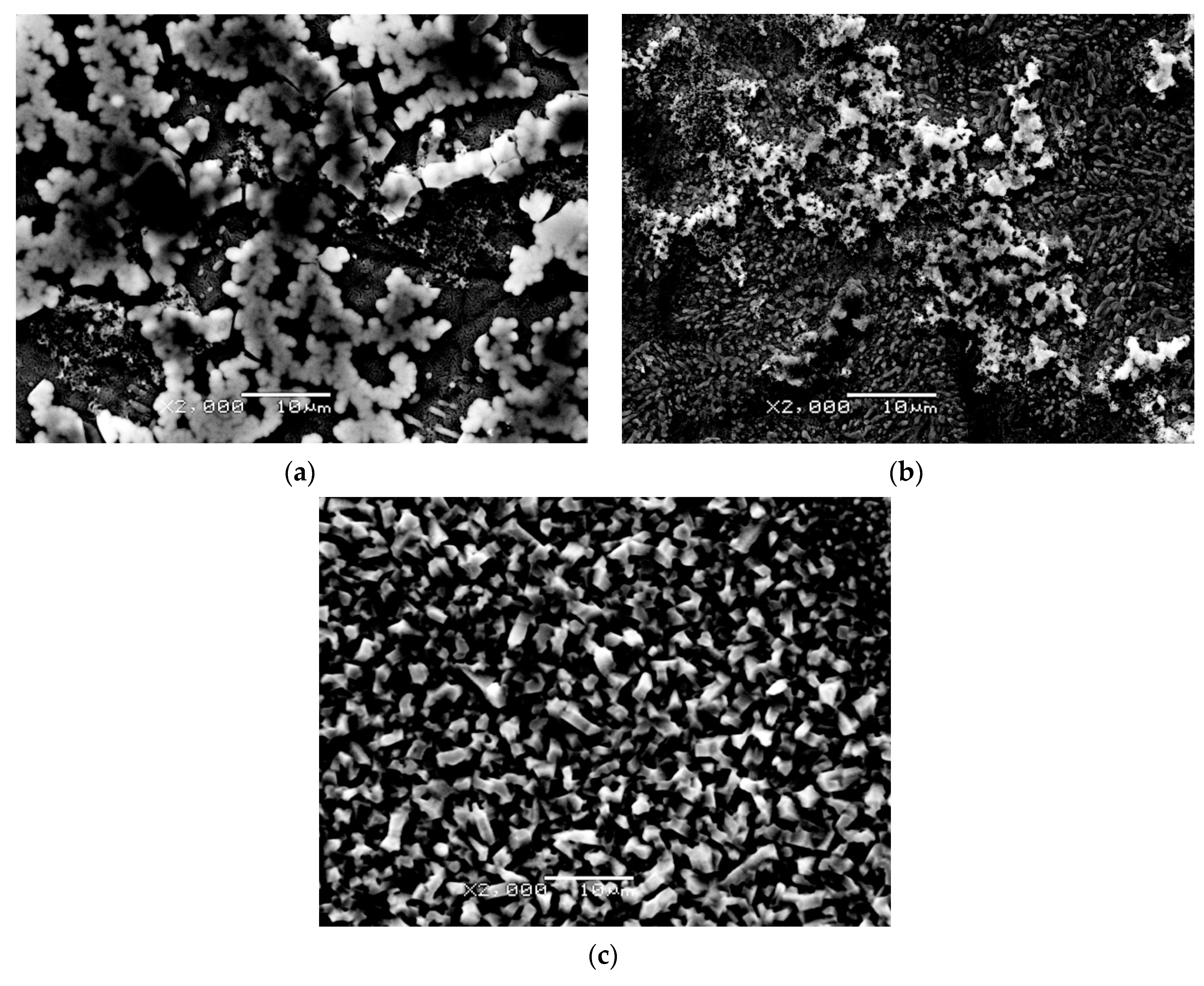
3.1. SEM Study of Microstructure and Thickness of Oxide Layers
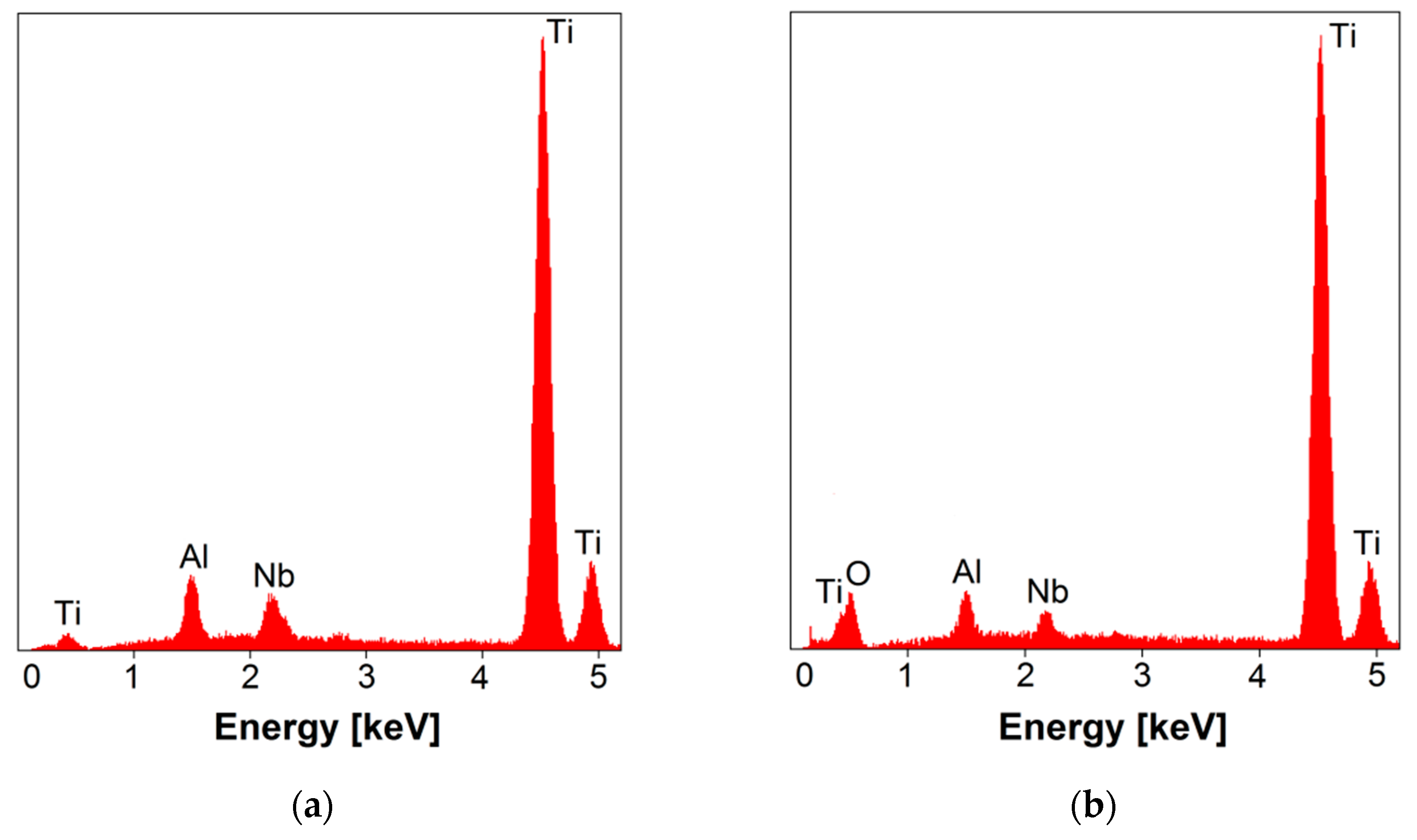
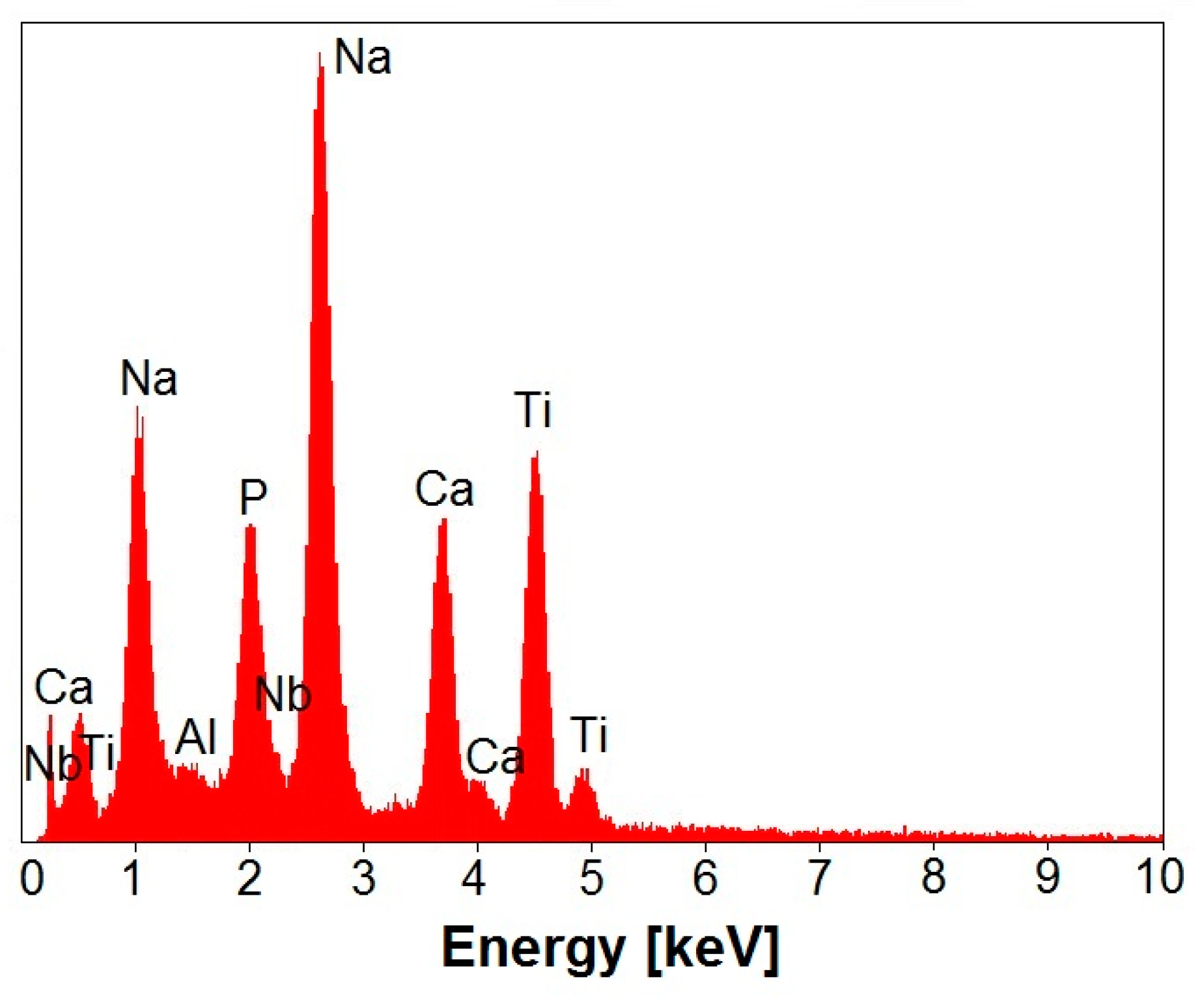
3.2. EDS Study of Chemical Composition
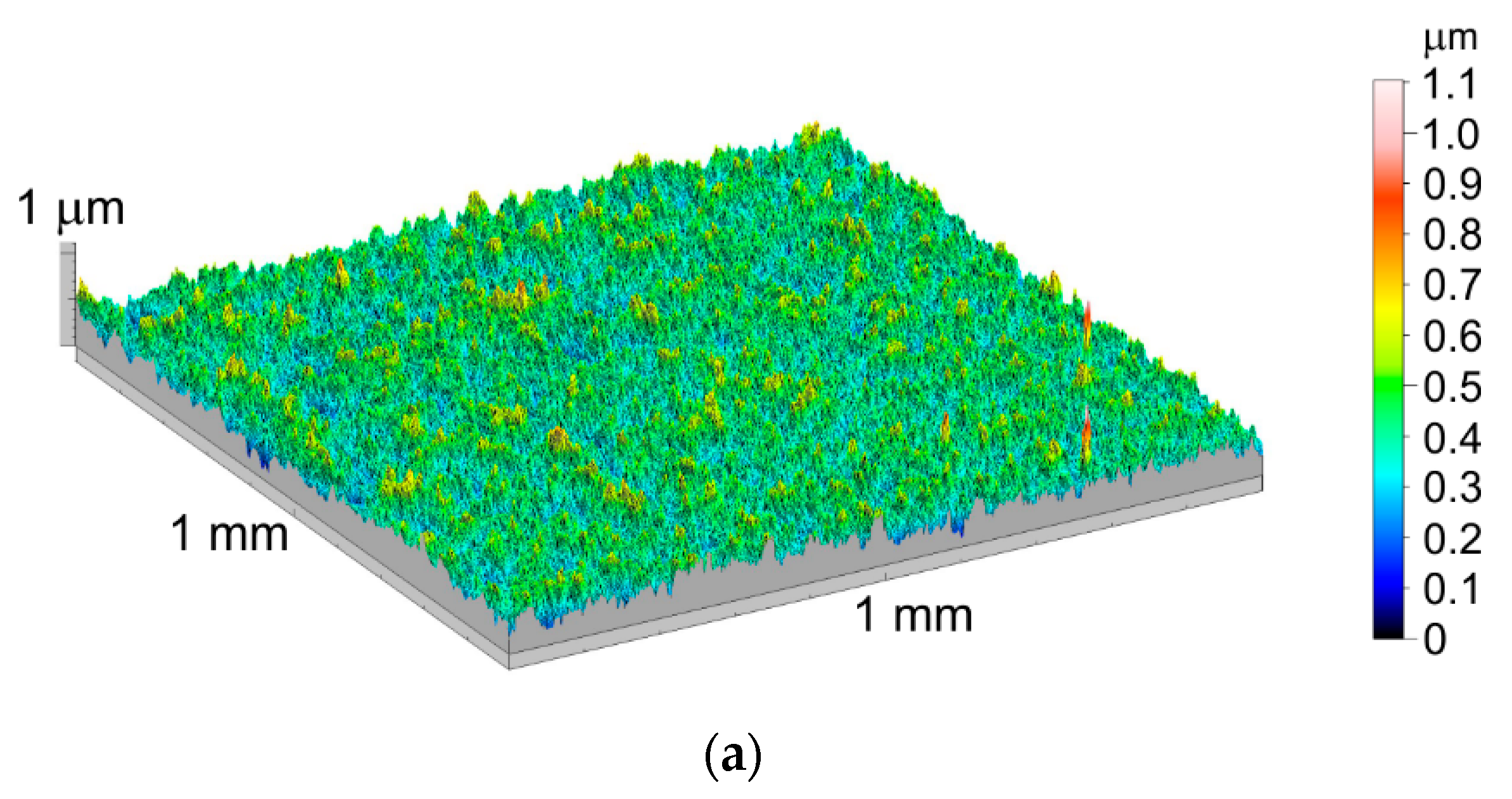
3.3. Geometric Structure of the Ti-6Al-7Nb Alloy Surface after Thermal Oxidation
3.4. Microindentation Tests of Oxide Layers Obtained on the Ti-6Al-7Nb Alloy Surface
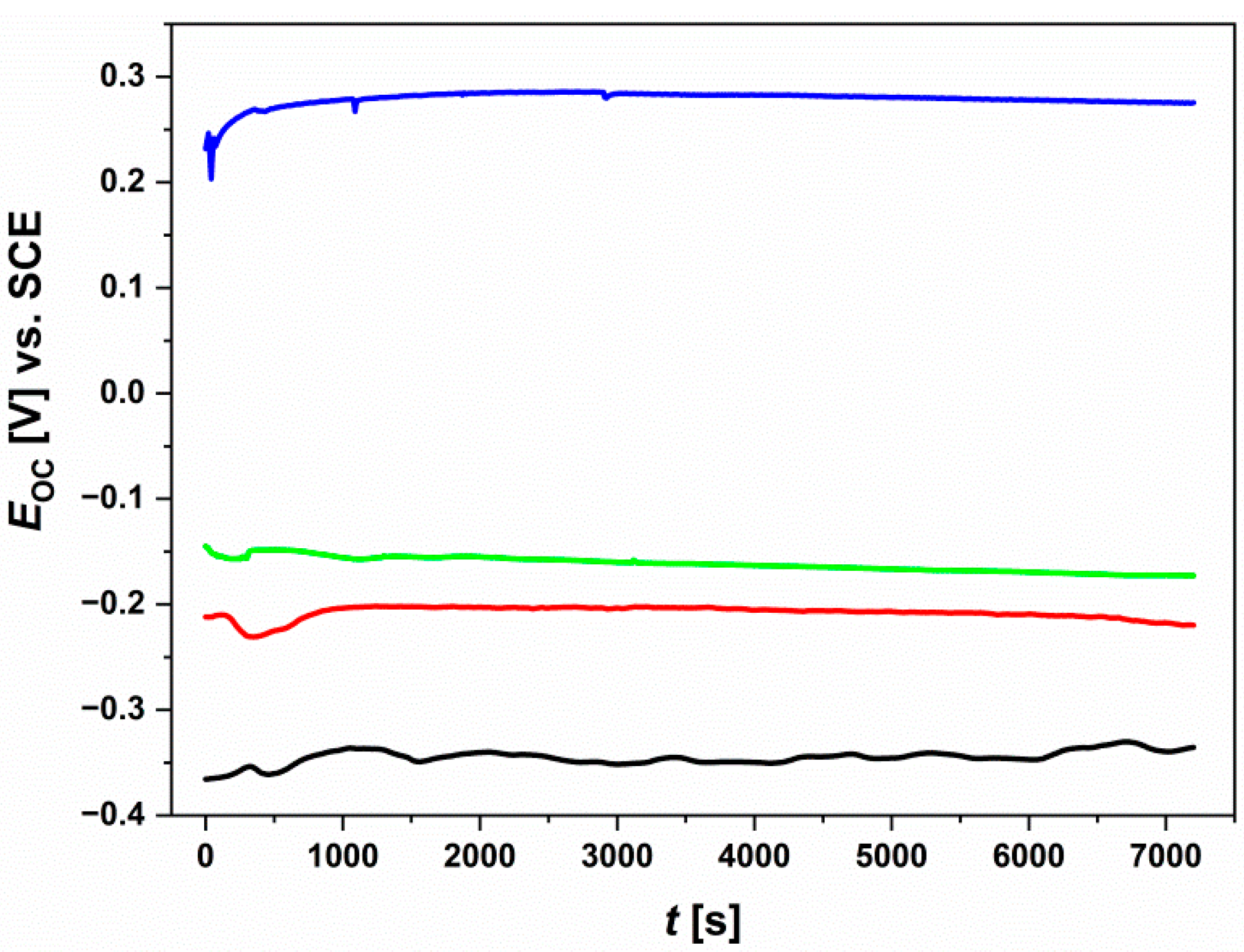
3.5. Open-Circuit Potential Measurements
3.6. Tafel Curves
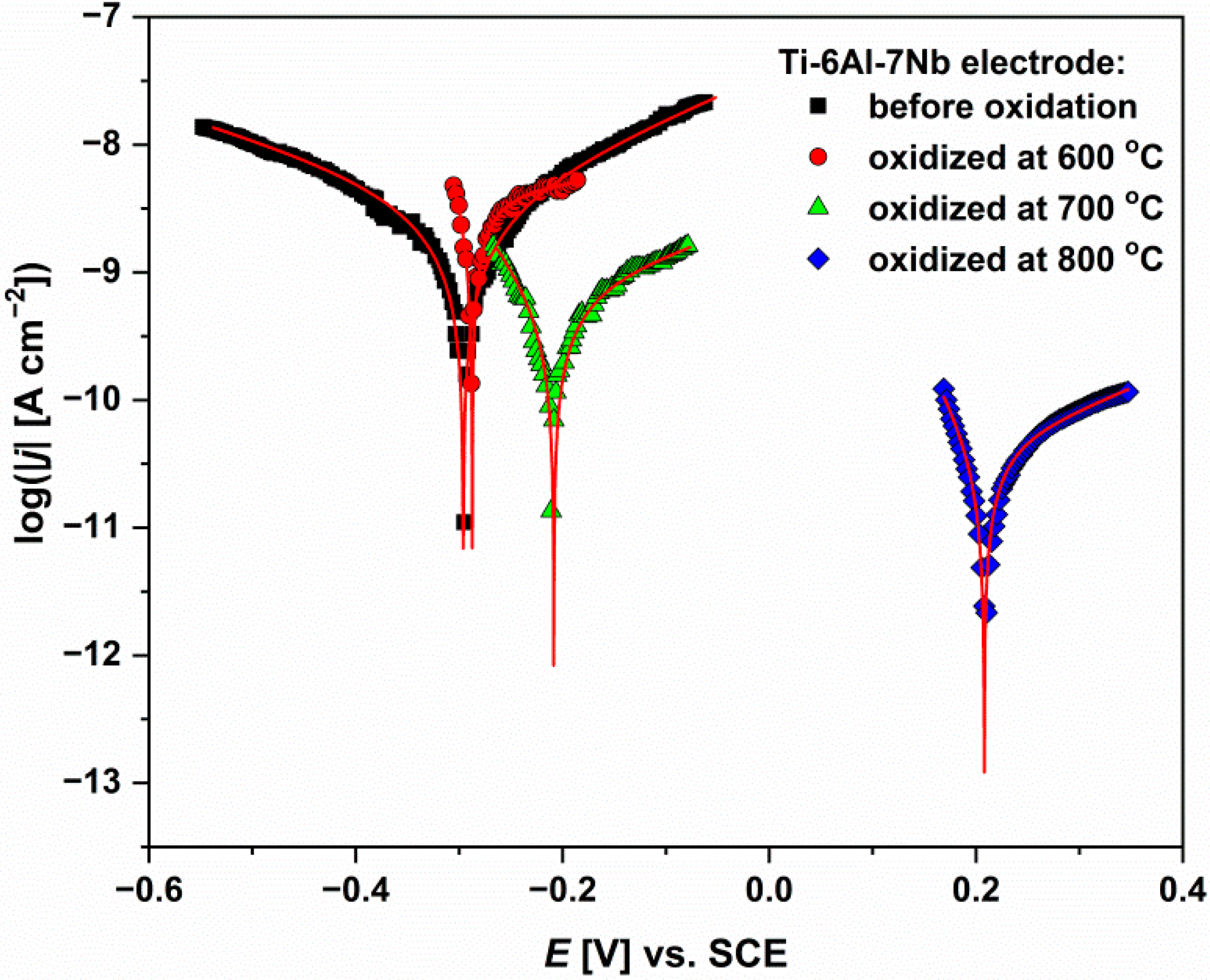
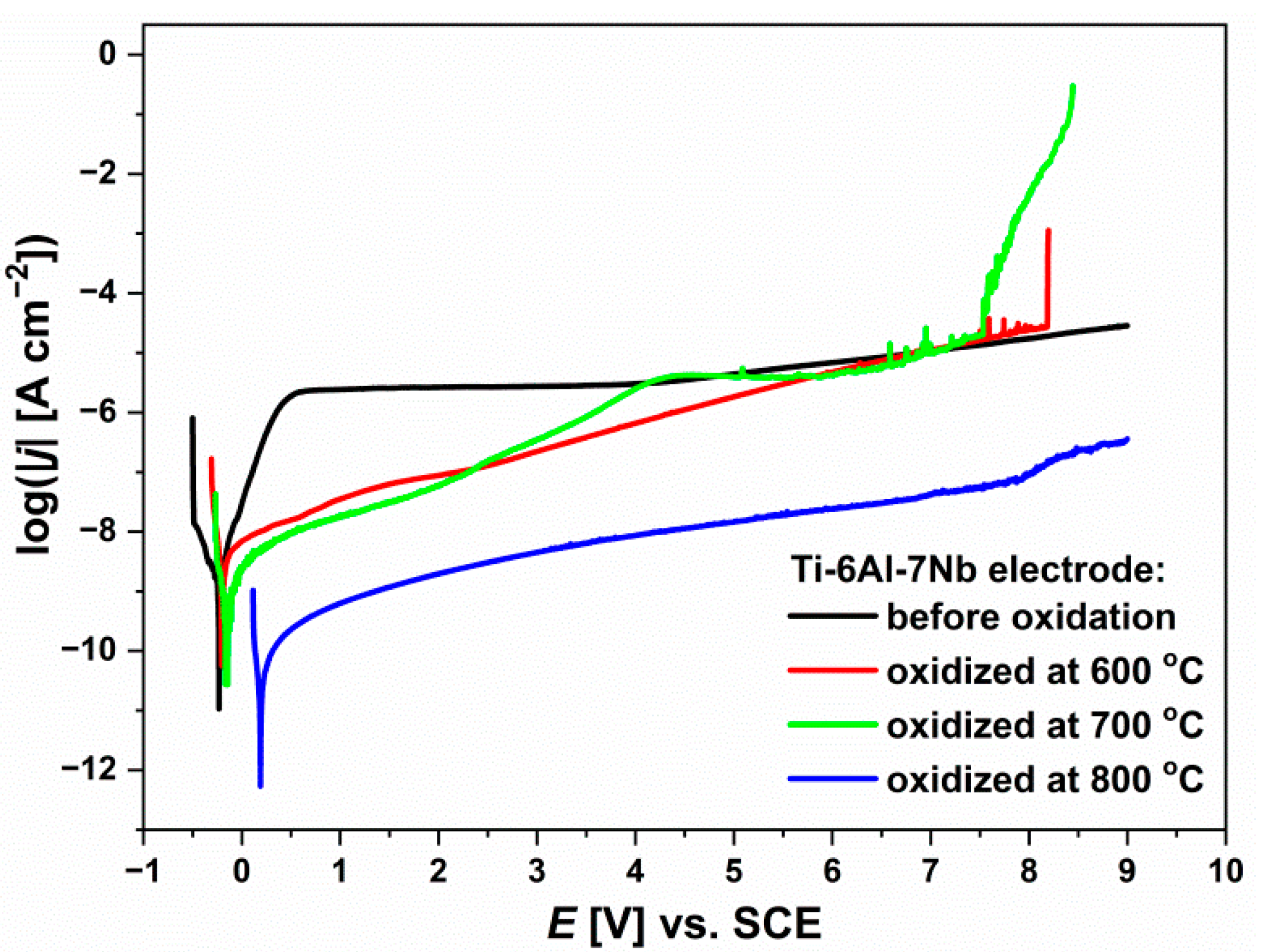
3.7. Anodic Polarization Curves
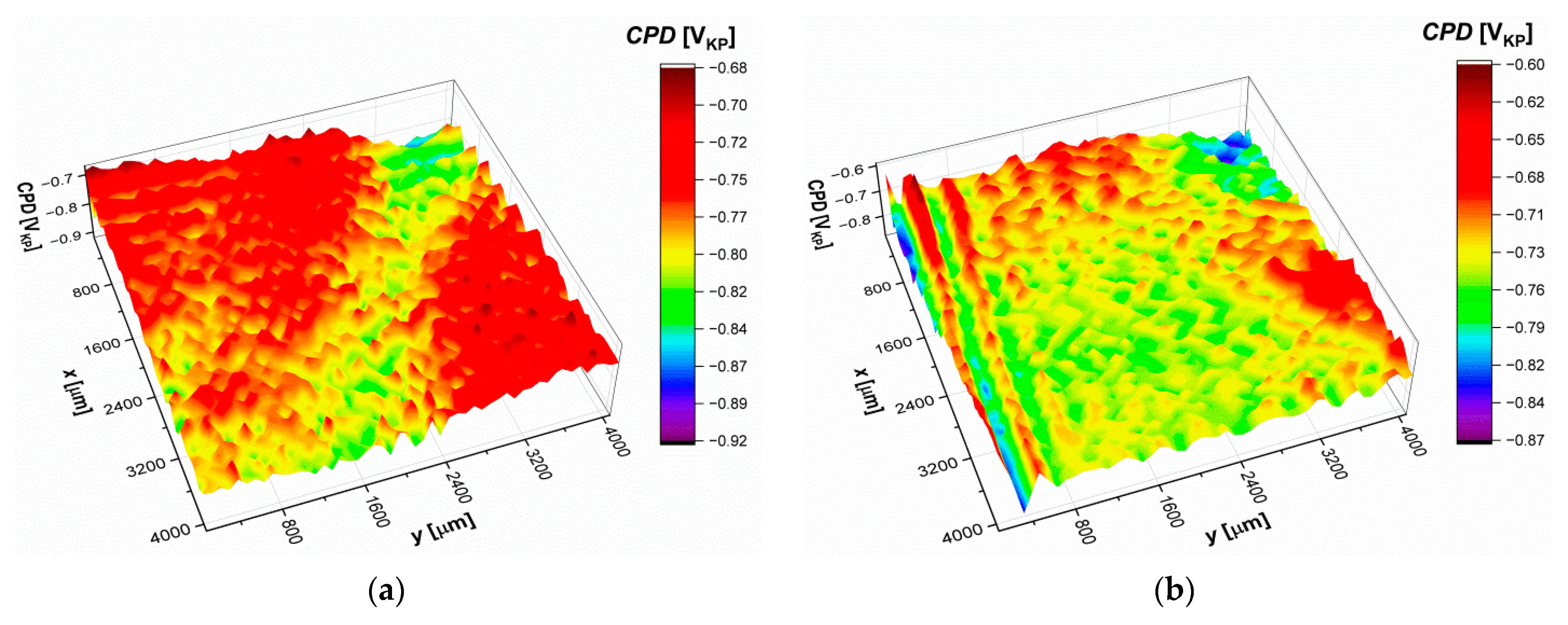
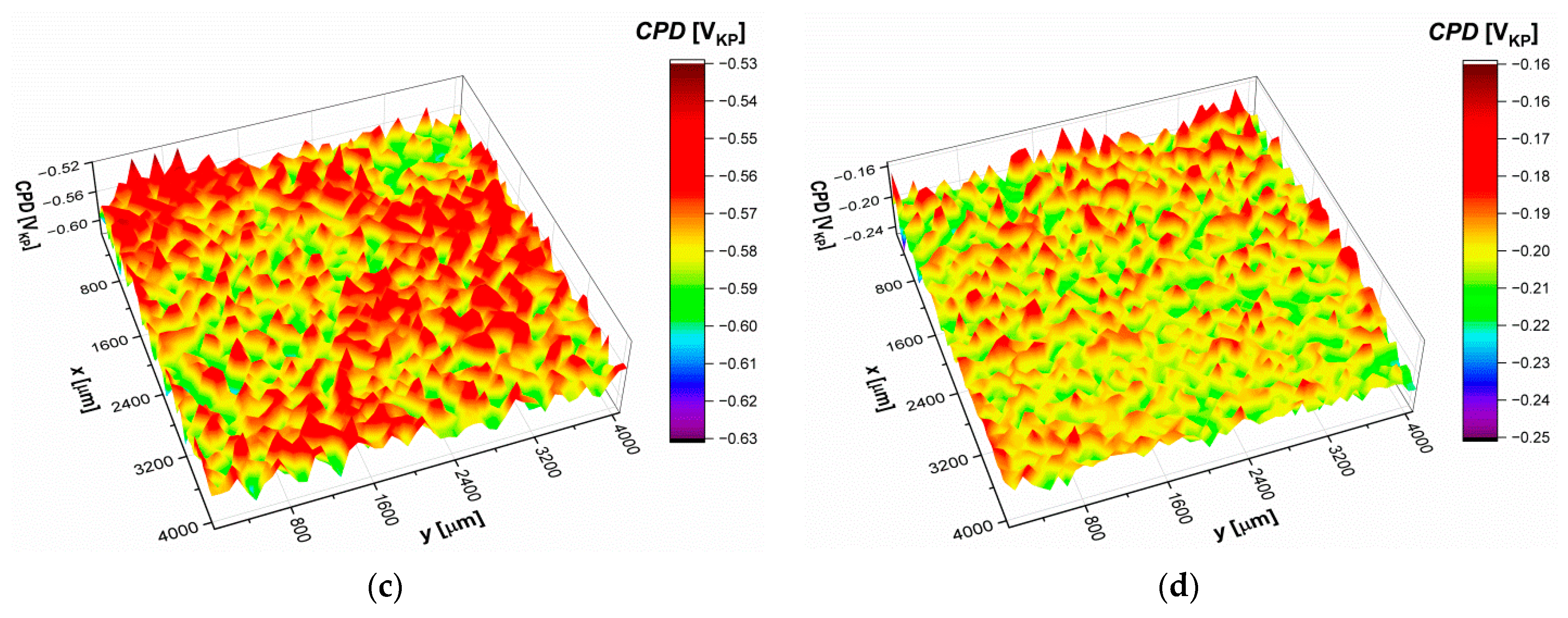
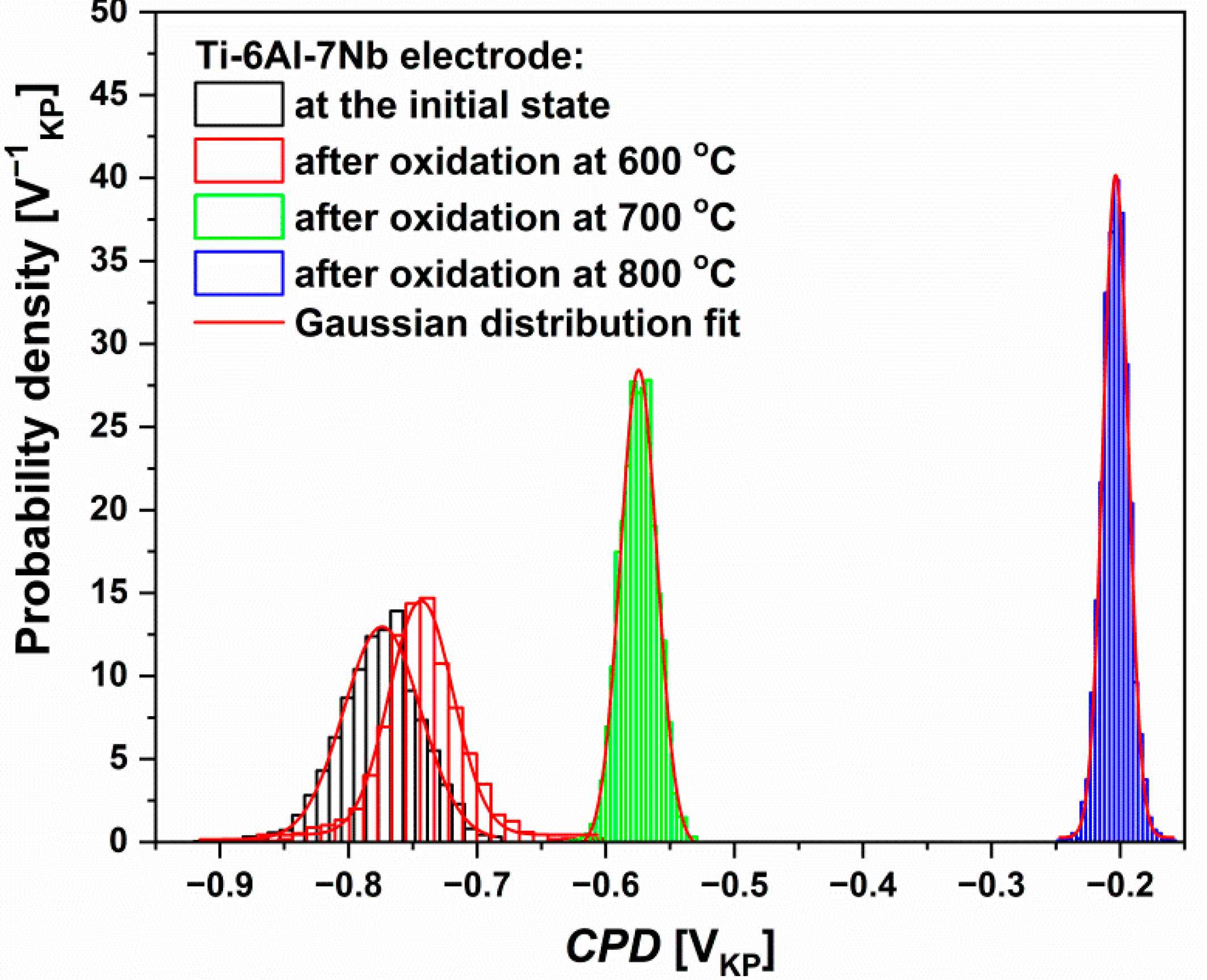
3.8. Scanning Kelvin Probe Measurements
3.9. Bioactivity Study
4. Conclusions
- Based on the obtained results it can be stated that the isothermal oxidation can be proposed to surface modification of the diphase (α + β) Ti-6Al-7Nb biomedical alloy to enhance its mechanical properties, in vitro corrosion resistance and bioactivity in the biological environment.
- SEM/EDS study of the microstructure and local chemical composition of the investigated alloy at the initial state and after isothermal oxidation revealed that with the increase in oxidation temperature from 600 to 800 °C, the mean thickness of the oxide layers well-adhered to the substrate, increased from 0.25 to 5.48 μm, respectively.
- The increase in the oxidation temperature is a factor increasing the surface roughness of the Ti-6Al-7Nb alloy. The hardness of the obtained oxide layers increases with the oxidation temperature growth, regardless of the applied load, in the range 0.1–1 N.
- Corrosion resistance parameters were determined for the obtained materials in the saline solution at 37 °C using the open-circuit potential method and potentiodynamic measurements. The mechanism of the anode process of the Ti-6Al-7Nb alloy before and after isothermal oxidation can be explained by dissolution of the substrate with higher rate in comparison with the dissolution rate of the formed oxide layers including oxides of individual metallic components of the alloy.
- It was found that isothermal oxidation carried out under the proposed conditions increased the contact potential difference, and significantly improved the corrosion resistance of the Ti-6Al-7Nb alloy and reduced its susceptibility to pitting corrosion. The oxide layer grown over Ti-6Al-7Nb alloy at 800 °C for 72 h was characterized by the highest barrier properties to inhibit corrosion process.
- It was ascertained that the alkali- and heat-treated Ti-6Al-7Nb alloy after isothermal oxidation revealed the HAp-forming ability already after one day of soaking in the SBF in the simulated body fluid.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholson, J.W. Titanium alloys for dental implants: A review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Gilbert, J.L. 1.2 Electrochemical Behavior of Metals in the Biological Milieu. In Comprehensive Biomaterials II, 2nd ed.; Ducheyne, P., Healy, K., Hutmacher, D.W., Grainger, D.W., Kirkpatrick, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 19–49. ISBN 9780081006924. [Google Scholar] [CrossRef]
- Verma, R.P. Titanium based biomaterial for bone implants: A mini review. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Kapoor, N.; Nagpal, A.; Verma, R. Surface Treatment of Titanium Implant and Dental Implant Design: Titanium as Biomaterial and Method Used for Surface Treatment to Increase Bone and Soft Tissue Integration; LAP LAMBERT Academic Publishing: Port Louis, Mauritius, 2020; ISBN 978-620-2-55638-5. [Google Scholar]
- Tsai, M.-T.; Chen, Y.-W.; Chao, C.Y.; Jang, J.S.C.; Tsai, C.-C.; Su, Y.-L.; Kuo, C.-N. Heat-treatment effects on mechanical properties and microstructure evolution of Ti-6Al-4V alloy fabricated by laser powder bed fusion. J. Alloys Comp. 2020, 816, 152615. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, W.; Tian, M.; Teng, S. Thermal oxidation of Ti6Al4V alloy and its biotribological properties under serum lubrication. Tribol. Inter. 2015, 89, 67–71. [Google Scholar] [CrossRef]
- Siva Rama Krishna, D.; Brama, Y.L.; Sun, Y. Thick rutile layer on titanium for tribological applications. Tribol. Inter. 2007, 40, 329–334. [Google Scholar] [CrossRef]
- Javadi, A.; Solouk, A.; Nazarpak, M.H.; Bagheri, F. Surface engineering of titanium-based implants using electrospraying and dip coating methods. Mater. Sci. Eng. C 2019, 99, 620–630. [Google Scholar] [CrossRef]
- Kang, J.-I.; Son, M.-K.; Choe, H.-C.; Brantley, W.A. Bone-like apatite formation on manganese-hydroxyapatite coating formed on Ti-6Al-4V alloy by plasma electrolytic oxidation. Thin Solid Films 2016, 620, 126–131. [Google Scholar] [CrossRef]
- Cimenoglu, H.; Meydanoglu, O.; Baydogan, M.; Bermek, H.; Huner, P.; Kayali, S. Characterization of thermally oxidized Ti6Al7Nb alloy for biological applications. Met. Mater. Int. 2011, 17, 765–770. [Google Scholar] [CrossRef]
- Güleryüz, H.; Cimenoğlu, H. Effect of thermal oxidation on corrosion and corrosion–wear behaviour of a Ti-6Al-4V alloy. Biomaterials 2004, 25, 3325–3333. [Google Scholar] [CrossRef]
- Wen, M.; Wen, C.; Hodgson, P.; Li, Y. Improvement of the biomedical properties of titanium using SMAT and thermal oxidation. Colloids Surf. B 2014, 116, 658–665. [Google Scholar] [CrossRef]
- Marsumi, Y.; Pramono, A.W. Influence of niobium or molybdenum in titanium alloy for permanent implant application. Advan. Mater. Res. 2014, 900, 53–63. [Google Scholar] [CrossRef]
- Pambudi, M.J.; Basuki, E.A.; Prajitno, D.H. Improving hot corrosion resistance of two phases intermetallic alloy α2-Ti3Al/γ-TiAl with enamel coating. AIP Conf. Proc. 2017, 1805, 070003. [Google Scholar] [CrossRef]
- Gao, P.F.; Lei, Z.N.; Wang, X.X.; Zhan, M. Deformation in fatigue crack tip plastic zone and its role in crack propagation of titanium alloy with tri-modal microstructure. Mater. Sci. Eng. A 2019, 739, 198–202. [Google Scholar] [CrossRef]
- Chen, Z.X.; Wang, W.X.; Takao, Y.; Matsubara, T.; Ren, L.M. Microstructure and shear fracture characteristics of porous anodic TiO2 layer before and after hot water treatment. App. Surf. Sci. 2011, 257, 7254–7262. [Google Scholar] [CrossRef]
- Sankara Narayan, T.S.N.; Kim, J.; Park, H.W. Fabrication and synthesis of uniform TiO2 nanoporous and nanotubular structures on dual-phase Ti-6Al-4V alloy using electron-beam irradiation. Mater. Chem. Phys. 2020, 242, 122549. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, L.; Chen, D.; Shao, C.; Yi, M.; Zhang, B. Effects of pore size and porosity of surface-modified porous titanium implants on bone tissue ingrowth. Trans. Nonferr. Met. Soc. China 2019, 29, 2534–2545. [Google Scholar] [CrossRef]
- Tallarico, M.; Ceruso, F.M.; Muzzi, L.; Meloni, S.M.; Kim, Y.J.; Gargari, M.; Martinolli, M. Effect of simultaneous immediate implant placement and guided bone reconstruction with ultra-fine titanium mesh membranes on radiographic and clinical parameters after 18 months of loading. Materials 2019, 12, 1710. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Palladino, A.; Inchingolo, A.M.; Dipalma, G. Oral piercing and oral diseases: A short time retrospective study. Int. J. Med. Sci. 2011, 8, 649–652. [Google Scholar] [CrossRef]
- Siwakul, P.; Sirinnaphakorn, L.; Suwanprateep, J.; Hayakawa, T.; Pugdee, K. Cellular responses of histatin-derived peptides immobilized titanium surface using a tresyl chloride-activated method. Dent. Mater. J. 2021. [Google Scholar] [CrossRef]
- Challa, V.S.A.; Mali, S.; Misra, R.D.K. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J. Biomed. Mater. Res. A 2013, 101A, 2083–2089. [Google Scholar] [CrossRef]
- Sasikumar, Y.; Rajendran, N. Effect of acid treatment on the surface modification of Ti-6Al-7Nb and Ti-5Al-2Nb-1Ta and its electrochemical investigations in simulated body fluid. J. Bio Tribo Corros. 2017, 3, 41. [Google Scholar] [CrossRef]
- Tamilselvi, S.; Raghavendran, H.B.; Srinivasan, P.; Rajendran, N. In vitro and in vivo studies of alkali- and heat-treated Ti-6Al-7Nb and Ti-5Al-2Nb-1Ta alloys for orthopedic implants. J. Biomed. Mater. Res. A 2009, 90A, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Xie, L.; Liao, Q.; Gao, A.; Zheng, Y.; Pan, H.; Tong, L.; Yang, D.; Gao, N.; Starink, M.J.; et al. A more defective substrate leads to a less defective passive layer: Enhancing the mechanical strength, corrosion resistance and anti-inflammatory response of the low-modulus Ti-45Nb alloy by grain refinement. Acta Biomater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Aniołek, K.; Kupka, M. Mechanical, tribological and adhesive properties of oxide layers obtained on the surface of the Ti–6Al–7Nb alloy in the thermal oxidation process. Wear 2019, 43–433, 202929. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, G.-R.; Zhang, X.-C.; Li, S.; Tu, S.-T. Thermal oxidation of Ti-6Al–4V alloy and pure titanium under external bending strain: Experiment and modelling. Corr. Sci. 2017, 122, 61–73. [Google Scholar] [CrossRef]
- Li, L.; Yu, K.; Zhang, K.; Liu, Y. Study of Ti–6Al–4V alloy spectral emissivity characteristics during thermal oxidation process. Inter. J. Heat Mass Transf. 2016, 101, 699–706. [Google Scholar] [CrossRef]
- Ravi Shankar, A.; Karthiselva, N.S.; Kamachi Mudali, U. Thermal oxidation of titanium to improve corrosion resistance in boiling nitric acid medium. Surf. Coat. Technol. 2013, 235, 45–53. [Google Scholar] [CrossRef]
- Aniołek, K. The influence of thermal oxidation parameters on the growth of oxide layers on titanium. Vacuum 2017, 144, 94–100. [Google Scholar] [CrossRef]
- Cao, L.; Wan, Y.; Yang, S.; Pu, J. The tribocorrosion and corrosion properties of thermally oxidized Ti6Al4V alloy in 0.9 wt.% NaCl physiological saline. Coatings 2018, 8, 285. [Google Scholar] [CrossRef]
- Aniołek, K. Structure and properties of titanium and the Ti-6Al-7Nb alloy after isothermal oxidation. Surf. Eng. 2020, 36, 847–858. [Google Scholar] [CrossRef]
- Aniołek, K.; Barylski, A.; Kupka, M.; Tylka, J. The influence of thermal oxidation parameters on structural, friction, and wear characteristics of oxide layers produced on the surface of Ti-6Al-7Nb alloy. J. Tribol. 2019, 141, 031605-1–031605-9. [Google Scholar] [CrossRef]
- Hussain, S.N.; Abdullah, A.S.; Hassan, M.A.; Daud, R.; Arafat, A.; Ahmad, M.F. Review on wear and corrosion behavior of thermal oxidation on titanium-based alloy for biomedical application. J. Adv. Res. Fluid Mech. Ther. Sci. 2019, 58, 153–160. [Google Scholar]
- Kumar, S.; Sankara Narayanan, T.S.N.; Ganesh Sundara Raman, S.; Seshadri, S.K. Thermal oxidation of Ti6Al4V alloy: Microstructural and electrochemical characterization. Mater. Chem. Phys. 2010, 119, 337–346. [Google Scholar] [CrossRef]
- Dalili, N.; Edrisy, A.; Farokhzadeh, K.; Li, J.; Lo, J.; Riahi, A.R. Improving the wear resistance of Ti-6Al-4V/TiC composites through thermal oxidation (TO). Wear 2010, 269, 590–601. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Z.; Liu, Y.; Liu, W. Influence of thermal oxidation temperature on the microstructural and tribological behavior of Ti6Al4V alloy. Surf. Coat. Technol. 2014, 240, 470–477. [Google Scholar] [CrossRef]
- Fellah, M.; Labaïz, M.; Assala, O.; Dekhil, L.; Taleb, A.; Rezag, H.; Iost, A. Tribological behavior of Ti-6Al-4V and Ti-6Al-7Nb alloys for total hip prosthesis. Adv. Tribol. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Aniołek, K.; Kupka, M.; Łuczuk, M.; Barylski, A. Isothermal oxidation of Ti-6Al-7Nb alloy. Vacuum 2015, 114, 114–118. [Google Scholar] [CrossRef]
- ISO 14577-4. Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters—Part 4: Test Method for Metallic and Non-Metallic Coatings; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- ISO 10271. Dentistry—Corrosion Test Methods for Metallic Materials; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- Freitag, M.; Łosiewicz, B.; Goryczka, T.; Lelątko, J. Application of EIS to study the corrosion resistance of passivated NiTi shape memory alloy in simulated body fluid. Sol. State Phenom. 2012, 183, 57–64. [Google Scholar] [CrossRef]
- Stróż, A.; Łosiewicz, B.; Zubko, M.; Chmiela, B.; Balin, K.; Dercz, G.; Gawlikowski, M.; Goryczka, T. Production, structure and biocompatible properties of oxide nanotubes on Ti13Nb13Zr alloy for medical applications. Mater. Charact. 2017, 132, 363–372. [Google Scholar] [CrossRef]
- AUTOLAB. Electrochemical Instruments, Description of the Instrument; Eco Chemie, B.V., Ed.; Kanaalweg: Utrecht, The Netherlands, 1998. [Google Scholar]
- Pattanayak, D.K.; Yamaguchi, S.; Matsushita, T.; Kokubo, T. Nanostructured positively charged bioactive TiO2 layer formed on Ti metal by NaOH, acid and heat treatments. J. Mater. Sci. Mater. Med. 2011, 22, 1803–1812. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sankara Narayanan, T.S.N.; Ganesh Sundara Raman, S.; Seshadri, S.K. Thermal oxidation of CP Ti—An electrochemical and structural characterization. Mater. Charact. 2010, 61, 589–597. [Google Scholar] [CrossRef]
- Burnat, B.; Walkowiak-Przybyło, M.; Błaszczyk, T.; Klimek, L. Corrosion behaviour of polished and sandblasted titanium alloys in PBS solution. Acta Bioeng. Biomech. 2013, 15, 87–95. [Google Scholar] [CrossRef] [PubMed]
- ISO 5832-11. Implants for Surgery—Metallic Materials—Part 11: Wrought Titanium 6-Aluminium 7-Niobium Alloy; ISO: Geneva, Switzerland, 2014. [Google Scholar]
- Wang, S.; Liao, Z.; Liu, Y.; Liu, W. Influence of thermal oxidation duration on the microstructure and fretting wear behavior of Ti6Al4V alloy. Mater. Chem. Phys. 2015, 159, 139–151. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Sankara Narayanan, T.S.N. Effect of thermal oxidation on corrosion resistance of commercially pure titanium in acid medium. J. Mater. Eng. Perform. 2012, 21, 900–906. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- ASTM G102—89(2015)e1. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; National Association of Corrosion Engineers: Houston, TX, USA, 2015; Volume 03.02. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd English ed.; NACE International, National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Paszenda, Z.; Walke, W.; Jadacka, S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J. Achiev. Mater. Manuf. Eng. 2010, 38, 24–32. [Google Scholar]
- Kobayashi, E.; Wang, T.J.; Doi, H.; Yoneyama, T.; Hamanaka, H. Mechanical properties and corrosion resistance of Ti±6Al±7Nb alloy dental castings. J. Mater. Sci. Mater. Med. 1998, 9, 567–574. [Google Scholar] [CrossRef]
- Yu, S.Y.; Scully, J.R. Corrosion and passivity of Ti-13% Nb-13% Zr in comparison to other biomedical implant alloys. Corrosion 1997, 53, 965976. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Kwokal, A.; Piljac, J. The influence of niobium and vanadium on passivity of titanium-based implants in physiological solution. Biomaterials 2003, 24, 3765–3775. [Google Scholar] [CrossRef]
- Osak, P.; Goryczka, T.; Łosiewicz, B. Effect of polarization scan rate on the pitting potential of the self-passivated NiTi shape memory alloy in a simulated body fluid. Solid State Phenom. 2015, 227, 443–446. [Google Scholar] [CrossRef]
- Osak, P.; Łosiewicz, B. EIS study on interfacial properties of passivated Nitinol orthodontic wire in saliva modified with Eludril® mouthwash. Prot. Met. Phys. Chem. Surf. 2018, 54, 680–688. [Google Scholar] [CrossRef]
- Handzlik, P.; Fitzner, K. Corrosion resistance of Ti and Ti-Pd alloy in phosphate buffered saline solutions with and without H2O2 addition. Trans. Nonferrous Met. Soc. China 2013, 23, 866–875. [Google Scholar] [CrossRef]
- Kubisztal, J.; Losiewicz, B.; Dybal, P.; Kozik, V.; Bak, A. Temperature-related corrosion resistance of AISI 1010 carbon steel in sulfolane. Materials 2020, 13, 2563. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Losiewicz, B.; Kozik, V.; Kubisztal, J.; Dybal, P.; Swietlicka, A.; Barbusinski, K.; Kus, S.; Howaniec, N.; Jampilek, J. Real-time corrosion monitoring of AISI 1010 carbon steel with metal surface mapping in sulfolane. Materials 2019, 12, 3276. [Google Scholar] [CrossRef] [PubMed]
- Salarian, M.; Solati-Hashjin, M.; Shafiei, S.S.; Goudarzi, A.; Salarian, R.; Nemati, A. Surfactant assisted synthesis and characterization of hydroxyapatite nanorods under hydrothermal condition. Mater. Sci. Pol. 2009, 27, 961–971. [Google Scholar]
- Patel, S.B.; Hamlekhan, A.; Royhman, D.; Butt, A.; Yuan, J.; Shokuhfar, T.; Sukotjo, C.; Mathew, M.T.; Jursich, G.; Takoudis, C.G. Enhancing surface characteristics of Ti–6Al–4V for bio-implants using integrated anodization and thermal oxidation. J. Mater. Chem. B 2014, 2, 3597–3608. [Google Scholar] [CrossRef] [PubMed]
- Pasteris, J.D.; Wopenka, B.; Valsami-Jones, E. Bone and Tooth Mineralization: Why Apatite? Elements 2008, 4, 97–104. [Google Scholar] [CrossRef]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite Biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef]
- Sahin, Y.M.; Orman, Z.; Yucel, S. In Vitro Studies of α-TCP and β-TCP Produced from Clinocardium Ciliatum Seashells. J. Aust. Ceram. Soc. 2020, 56, 477–488. [Google Scholar] [CrossRef]
- Ceruso, F.M.; Ieria, I.; Martelli, M.; Lumbau, A.I.; Xhanari, E.; Gargari, M. New generation of fixture–abutment connection combining soft tissue design and vertical screw-retained restoration: 1-Year clinical, aesthetics and radiographic preliminary evaluation. Dent. J. 2021, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental pulp stem cell mechanoresponsiveness: Effects of mechanical stimuli on dental pulp stem cell behavior. Front. Physiol. 2018, 9, 1685. [Google Scholar] [CrossRef] [PubMed]

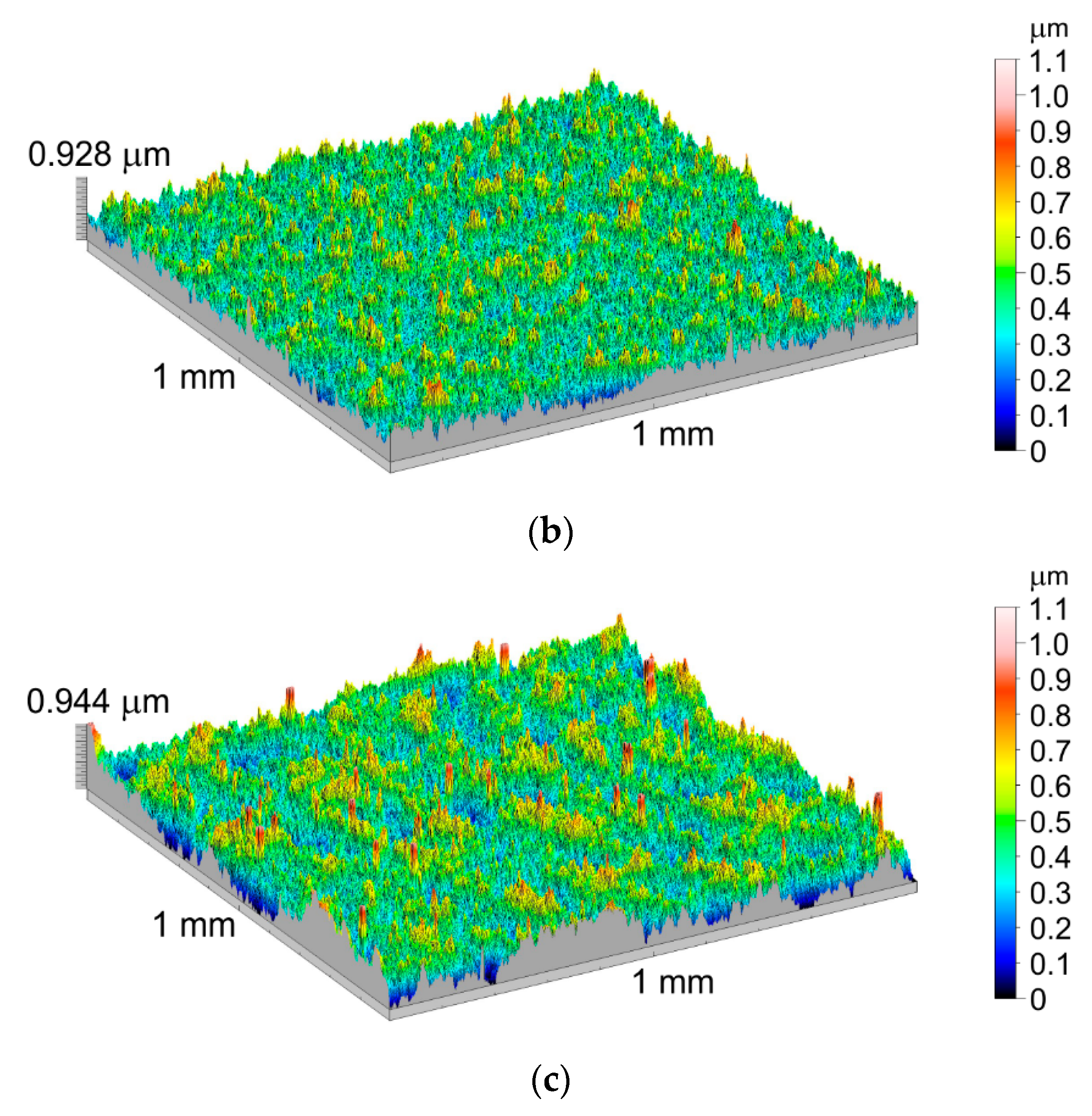
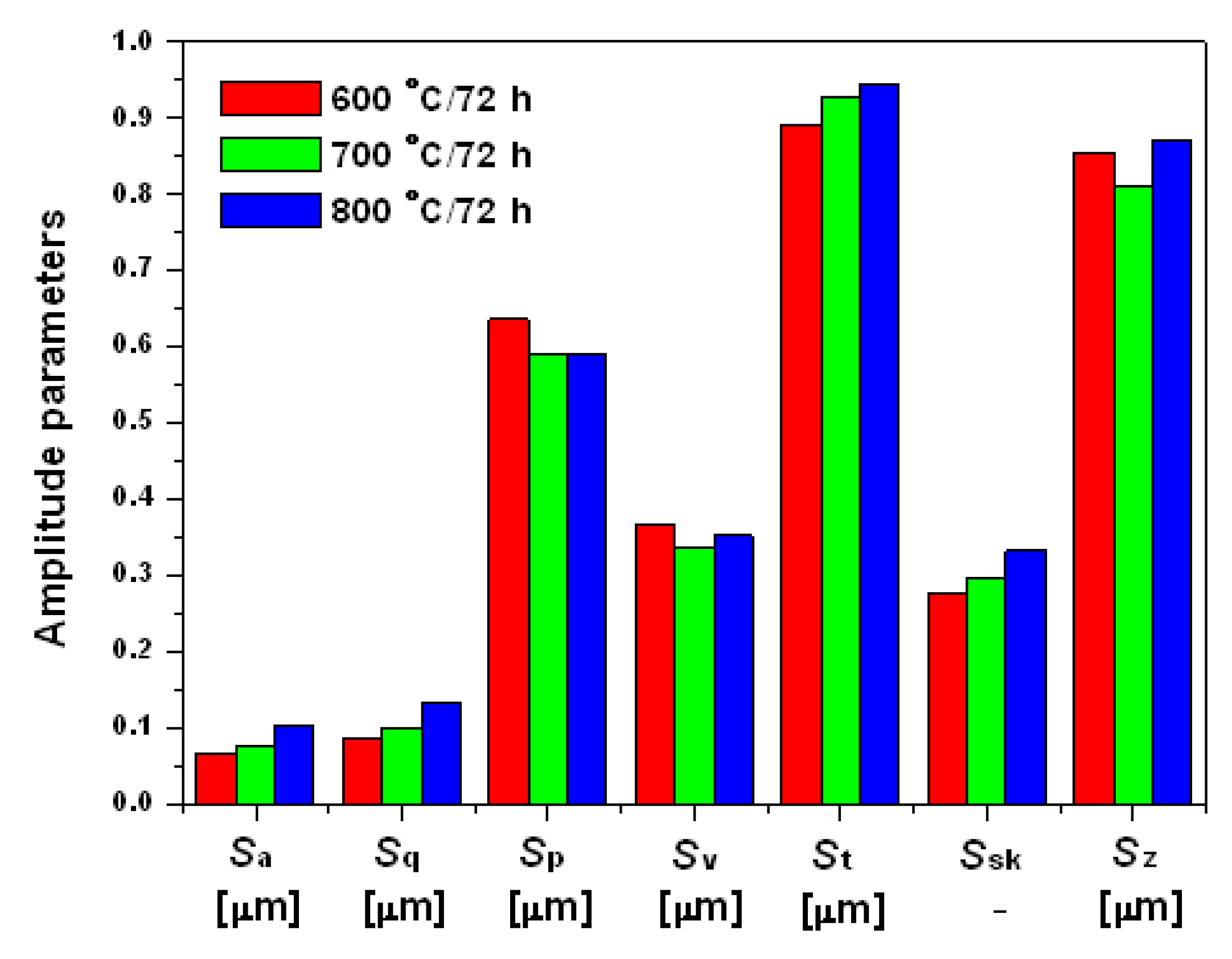


| Symbol | Name of Parameter | Unit |
|---|---|---|
| Sa | Arithmetic mean height | µm |
| Sq | Root mean square height | µm |
| Sp | Maximum peak height | µm |
| Sv | Maximum pit height | µm |
| St | Total height | µm |
| Ssk | Skewness | – |
| Sz | Maximum height | µm |
| Parameter | Before Isothermal Oxidation | After Oxidation at 600 °C | After Oxidation at 700 °C | After Oxidation at 800 °C |
|---|---|---|---|---|
| EOC [mV] | −336 | −220 | −173 | 275 |
| SD [mV] | 17 | 10 | 9 | 14 |
| Element | fi | ni | Mi [g·mol−1] | EW |
|---|---|---|---|---|
| Ti | 0.870 | 4 | 47.87 | 12.06 |
| Al | 0.056 | 3 | 26.98 | |
| Nb | 0.074 | 5 | 92.91 |
| Ti-6Al-7Nb Electrode | Ecor [mV] | jcor [nA·cm−2] | ba [mV] | bc [mV] | B [mV] | Rp [Ω·cm2] | CR at Ecor [mm·yr−1] |
|---|---|---|---|---|---|---|---|
| At the initial state | −296.0(5) | 3.6(2) | 292(8) | 403(17) | 73.5 | 2.0 × 107 | 3.14 × 10−5 |
| Oxidized at 600 °C | −287.3(3) | 3.5(2) | 623(50) | 50(3) | 120.4 | 3.4 × 107 | 3.05 × 10−5 |
| Oxidized at 700 °C | −208.4(6) | 0.67(5) | 343(29) | 117(7) | 37.9 | 5.7 × 107 | 5.85 × 10−6 |
| Oxidized at 800 °C | 207.9(5) | 0.041(2) | 291(12) | 75(3) | 91.0 | 2.2 × 109 | 3.58 × 10−7 |
| Parameter | Before Isothermal Oxidation | After Oxidation at 600 °C | After Oxidation at 700 °C | After Oxidation at 800 °C |
|---|---|---|---|---|
| Ebp [V] | 4.090(13) | 8.098(11) | 7.453(10) | 7.930(14) |
| jbp [A·cm−2] | 3.1(4) × 10−6 | 4.6(5) × 10−5 | 2.4(7) × 10−5 | 7.3(3) × 10−8 |
| Parameter | Before Isothermal Oxidation | After Oxidation at 600 °C | After Oxidation at 700 °C | After Oxidation at 800 °C |
|---|---|---|---|---|
| CPDav [mVKP] | −773.9(7) | −743.5(7) | −574.4(3) | −203.5(1) |
| σ [mVKP] | 29.9(9) | 24.9(9) | 14.3(5) | 9.8(2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniołek, K.; Łosiewicz, B.; Kubisztal, J.; Osak, P.; Stróż, A.; Barylski, A.; Kaptacz, S. Mechanical Properties, Corrosion Resistance and Bioactivity of Oxide Layers Formed by Isothermal Oxidation of Ti-6Al-7Nb Alloy. Coatings 2021, 11, 505. https://doi.org/10.3390/coatings11050505
Aniołek K, Łosiewicz B, Kubisztal J, Osak P, Stróż A, Barylski A, Kaptacz S. Mechanical Properties, Corrosion Resistance and Bioactivity of Oxide Layers Formed by Isothermal Oxidation of Ti-6Al-7Nb Alloy. Coatings. 2021; 11(5):505. https://doi.org/10.3390/coatings11050505
Chicago/Turabian StyleAniołek, Krzysztof, Bożena Łosiewicz, Julian Kubisztal, Patrycja Osak, Agnieszka Stróż, Adrian Barylski, and Sławomir Kaptacz. 2021. "Mechanical Properties, Corrosion Resistance and Bioactivity of Oxide Layers Formed by Isothermal Oxidation of Ti-6Al-7Nb Alloy" Coatings 11, no. 5: 505. https://doi.org/10.3390/coatings11050505
APA StyleAniołek, K., Łosiewicz, B., Kubisztal, J., Osak, P., Stróż, A., Barylski, A., & Kaptacz, S. (2021). Mechanical Properties, Corrosion Resistance and Bioactivity of Oxide Layers Formed by Isothermal Oxidation of Ti-6Al-7Nb Alloy. Coatings, 11(5), 505. https://doi.org/10.3390/coatings11050505








