Ultra-Fast Growth of ZnO Nanorods on Cotton Fabrics and Their Self-Cleaning and Physiological Comfort Properties
Abstract
:1. Introduction
2. Materials and Processes
2.1. Materials
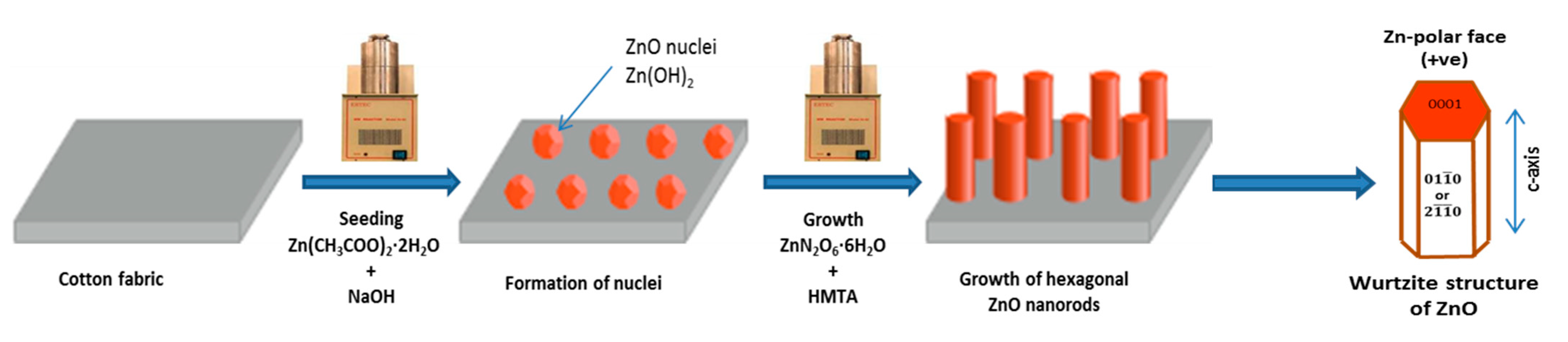
2.2. Seeding and Growth of Nanorods
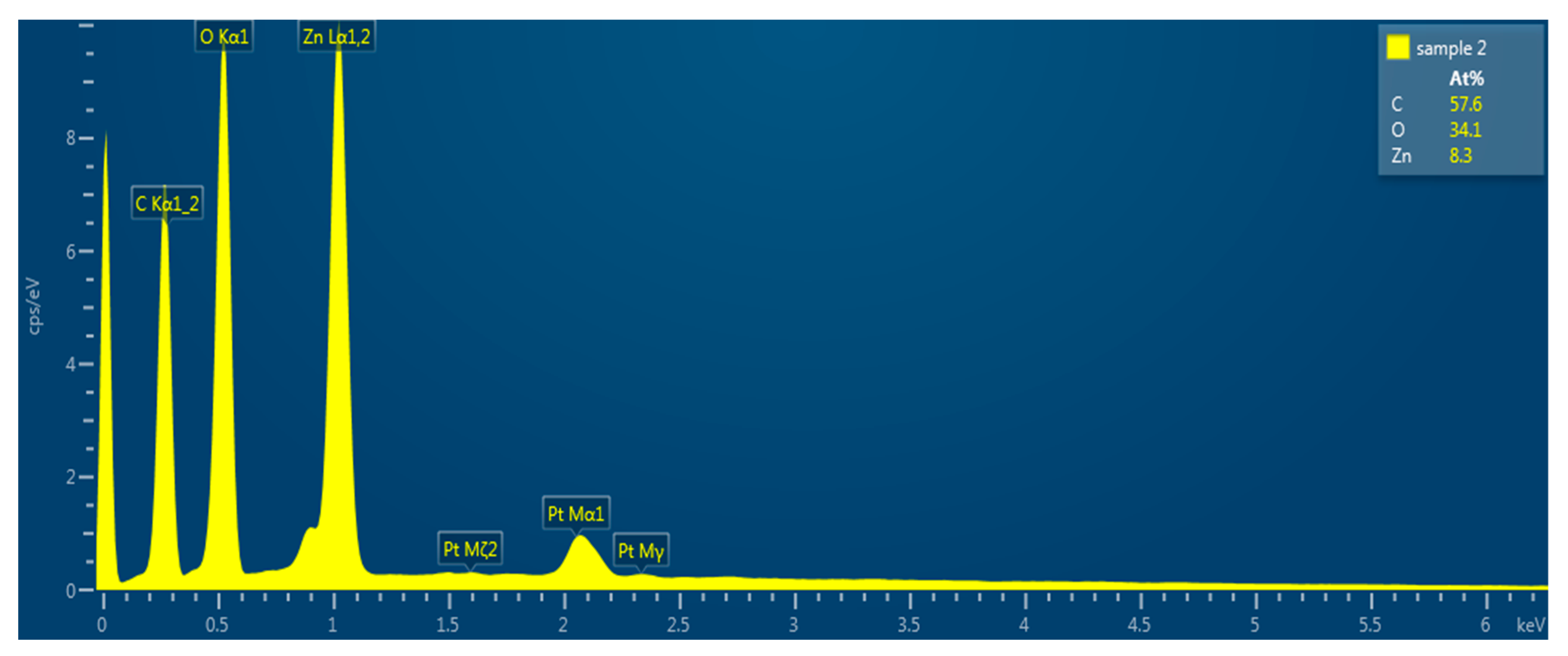
2.3. Characterization of ZnO Nanorods
2.4. Photocatalytic Activity
2.5. Characterization of Physiological Comfort Properties
2.6. Thermal Conductivity
2.7. Thermal Absorptivity
2.8. Relative Water Vapor Permeability
2.9. Air Permeability
2.10. Stiffness
2.11. Washing Durability (Reusability)
3. Results and Discussion
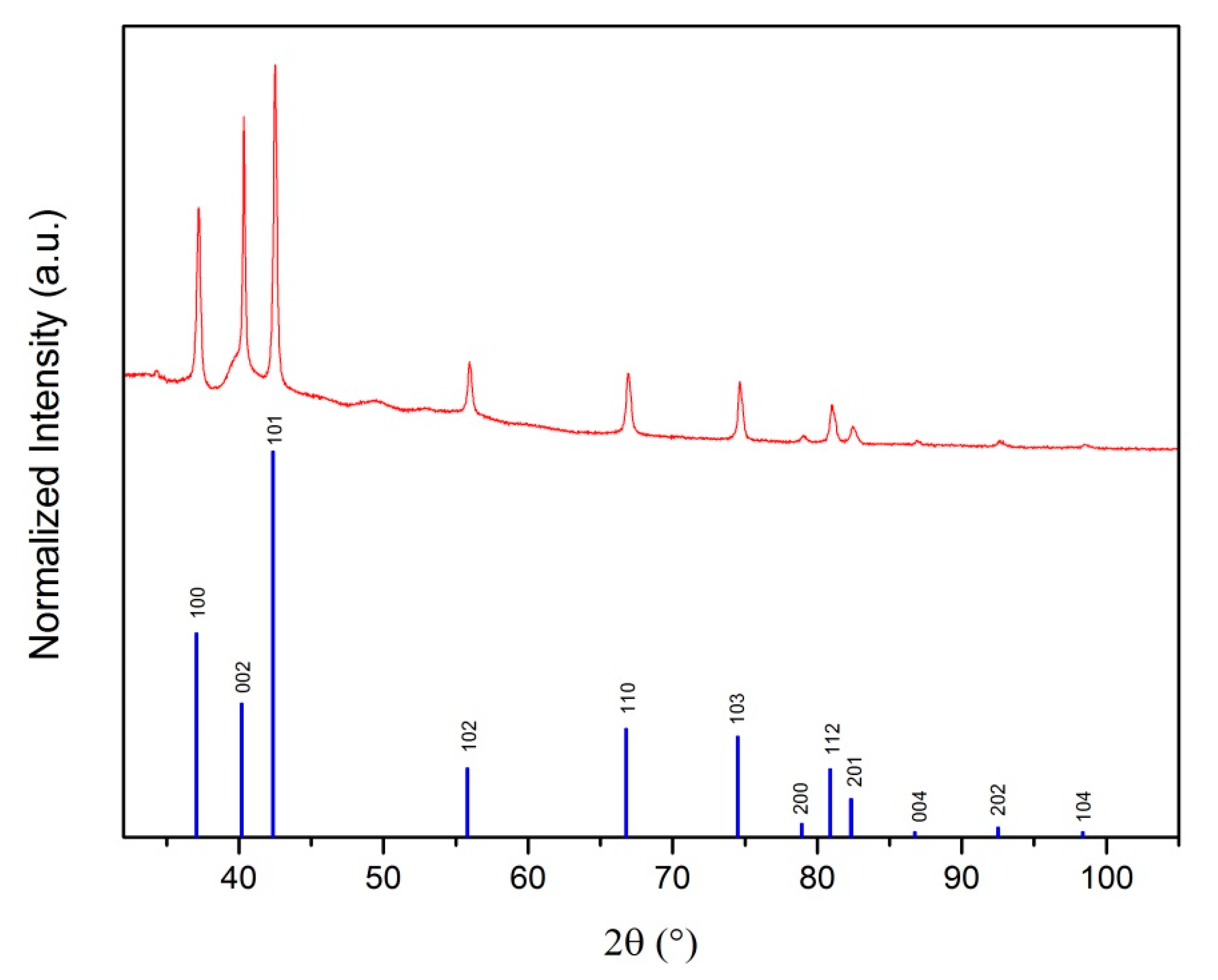
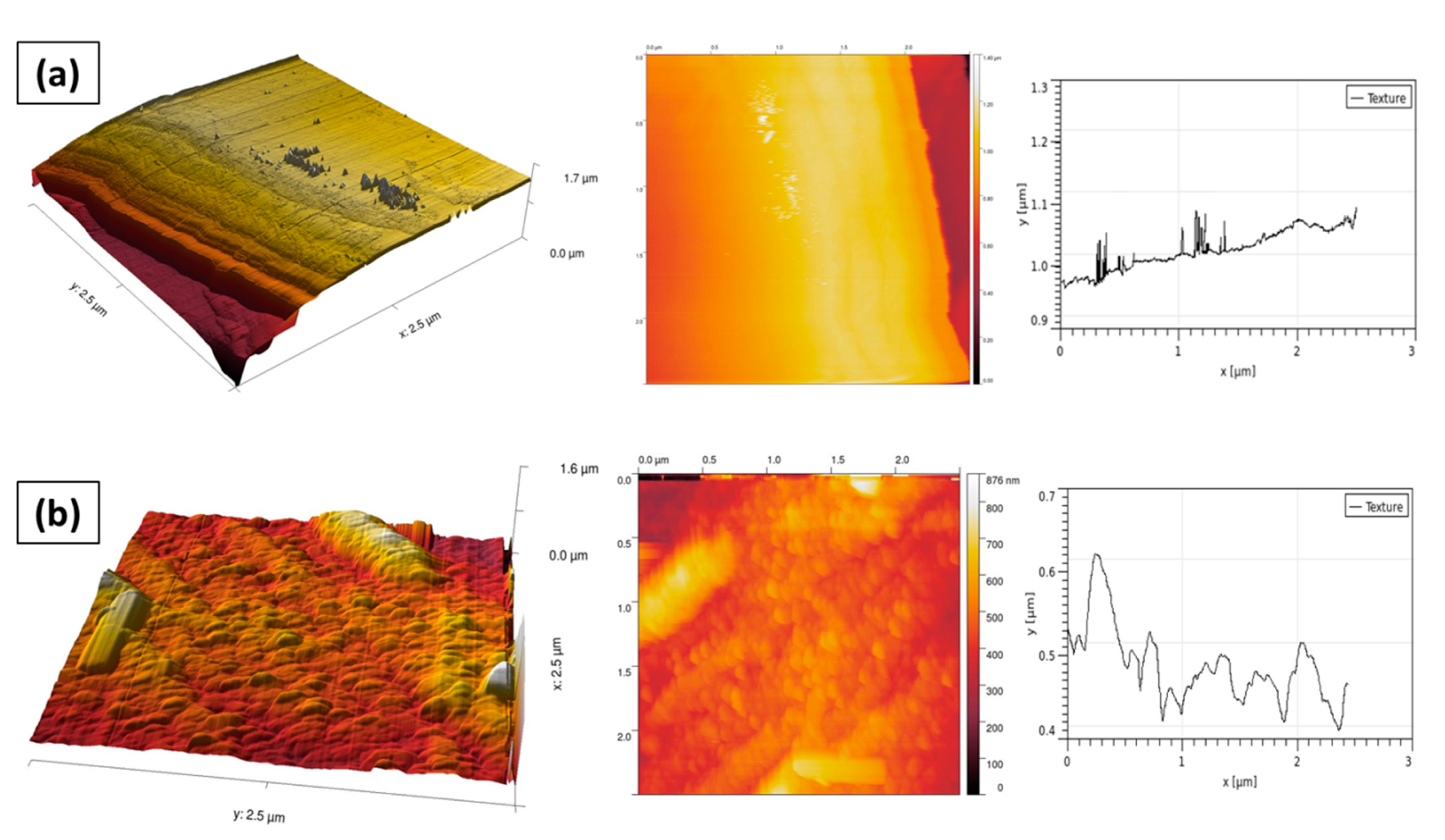
3.1. XRD Analysis
3.2. AFM Analysis
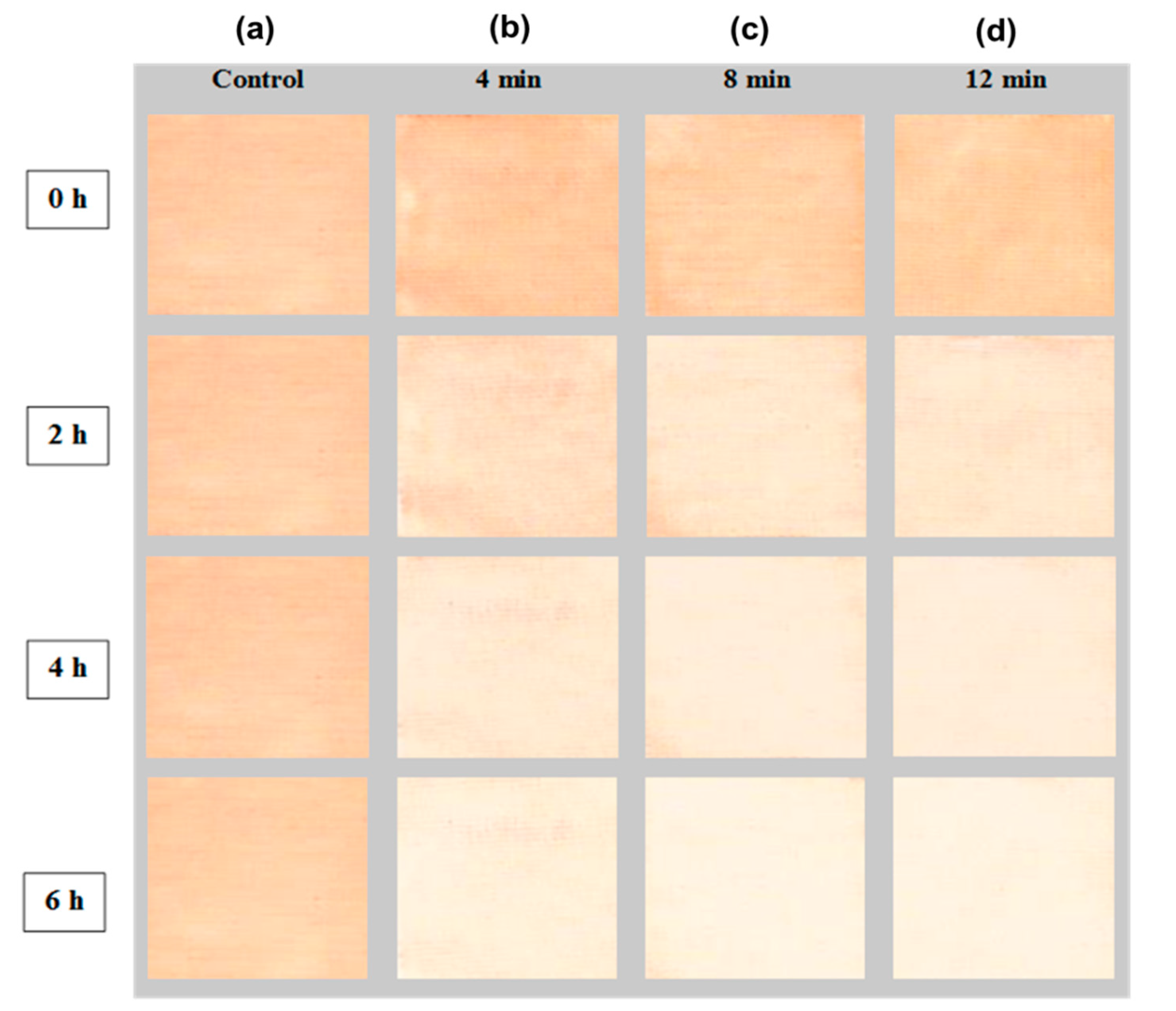
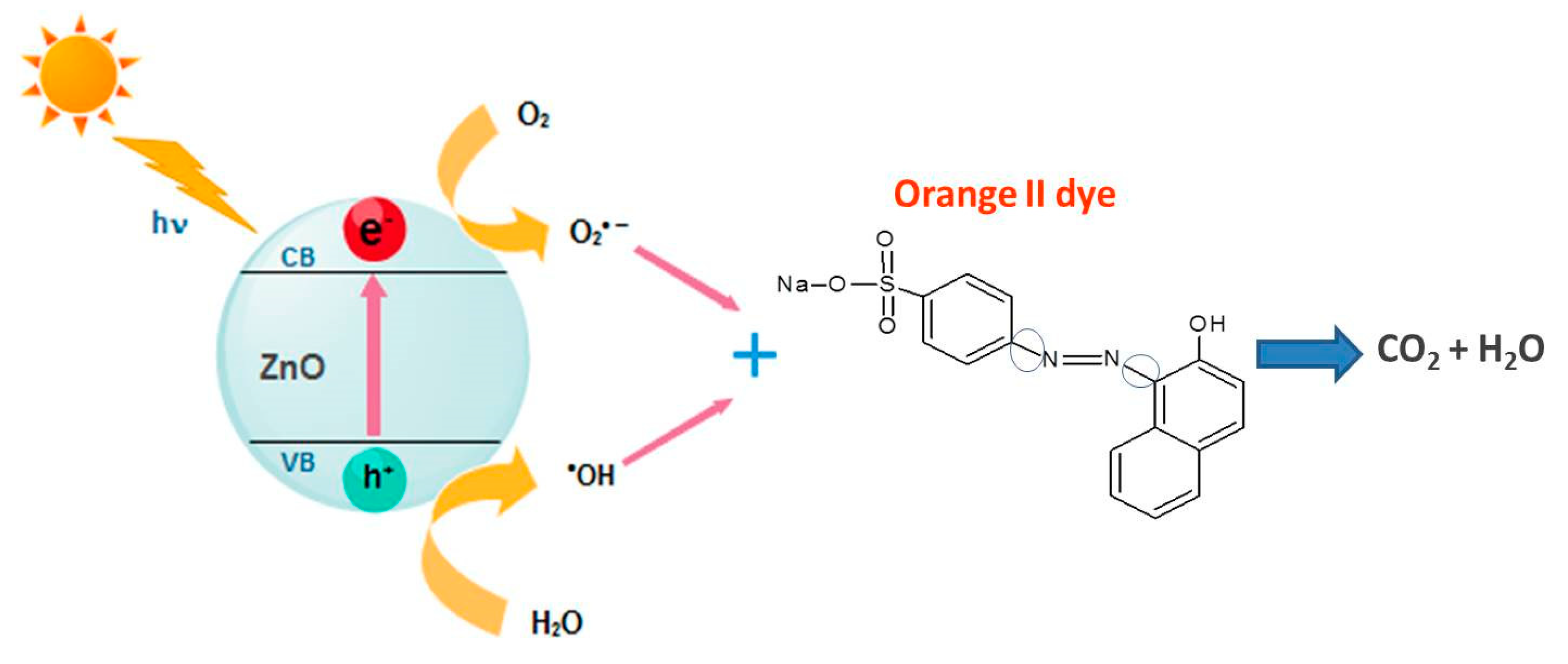
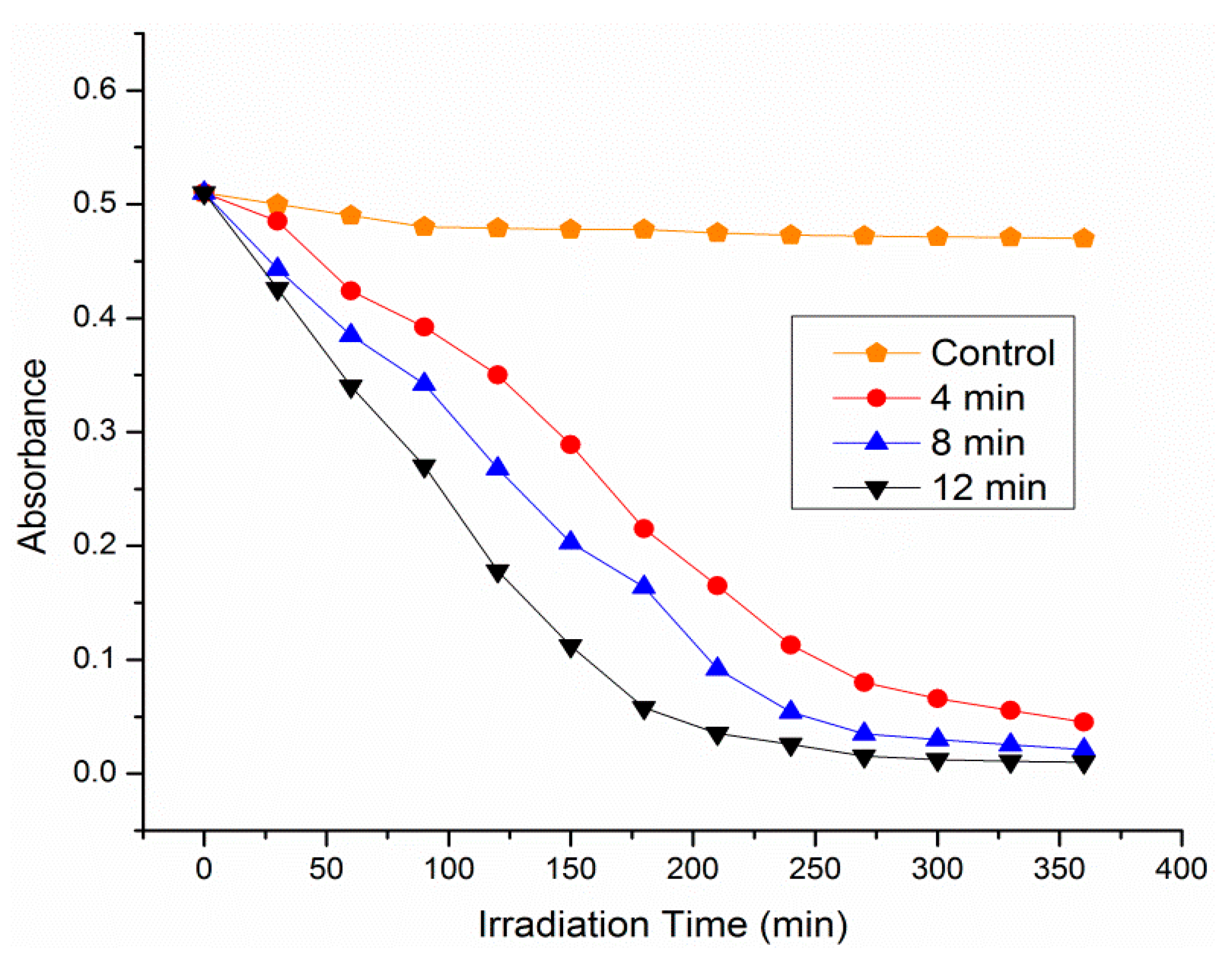
3.3. Photocatalytic Activity
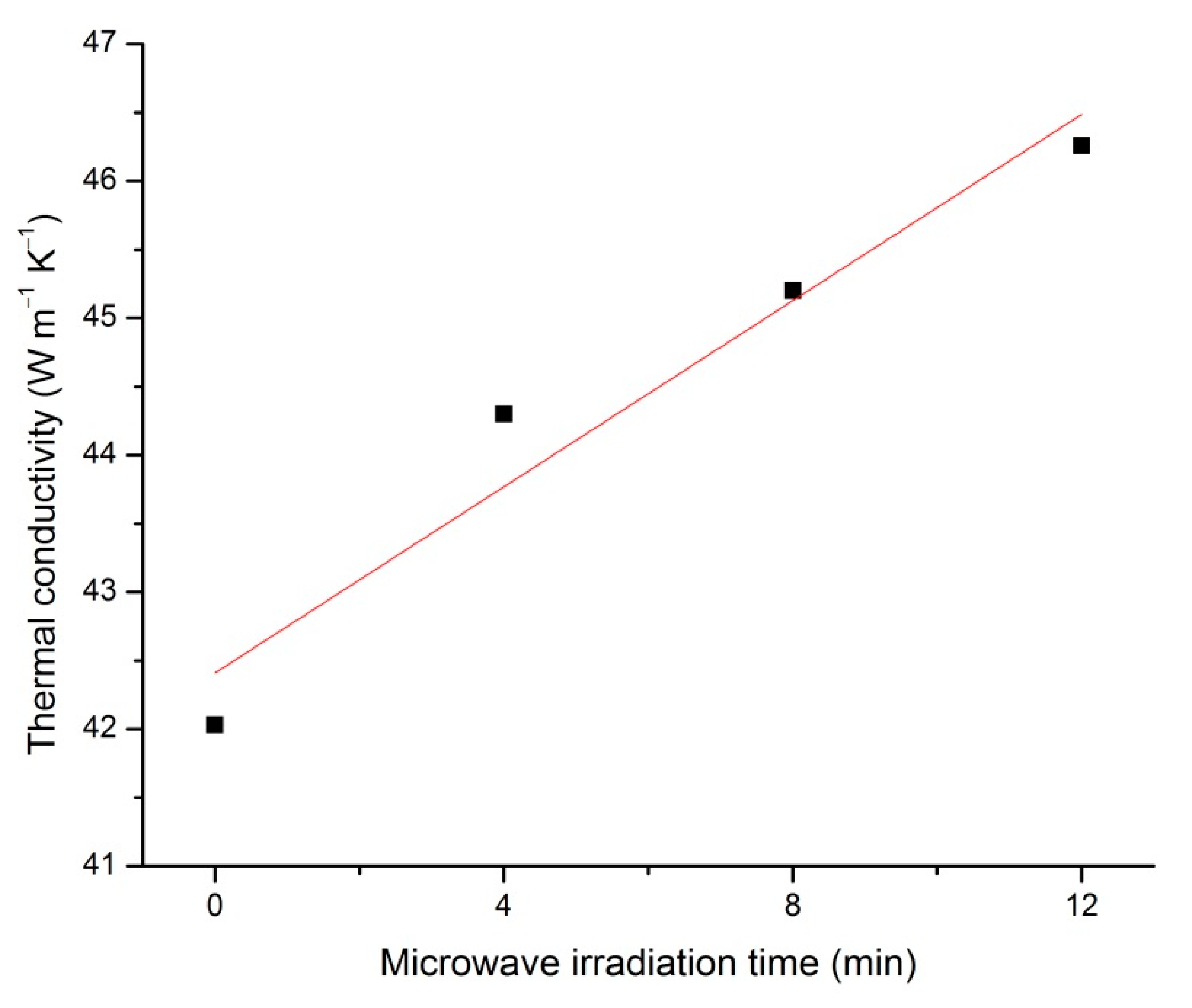
3.4. Thermal Conductivity
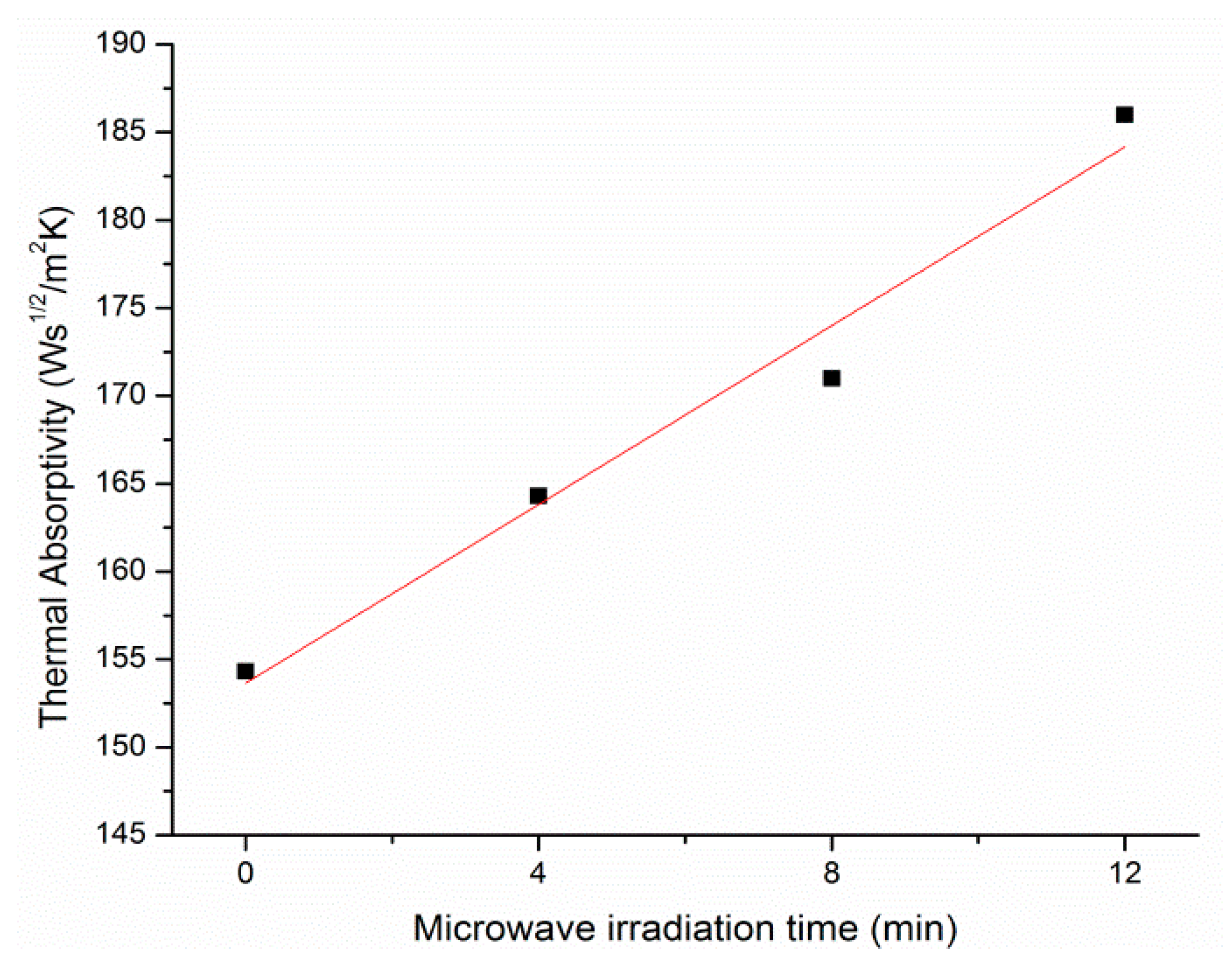
3.5. Thermal Absorptivity
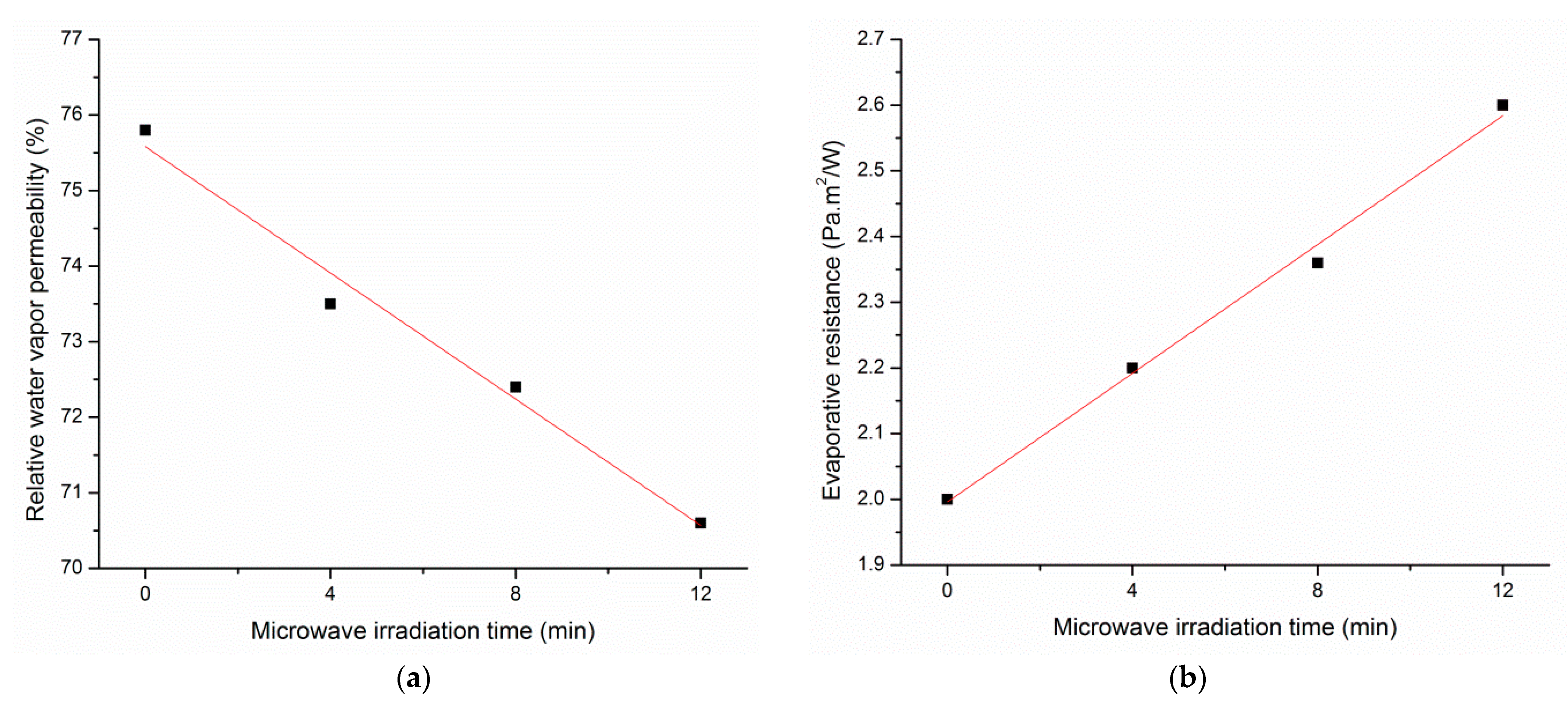
3.6. Water Vapor Permeability
3.7. Air Permeability
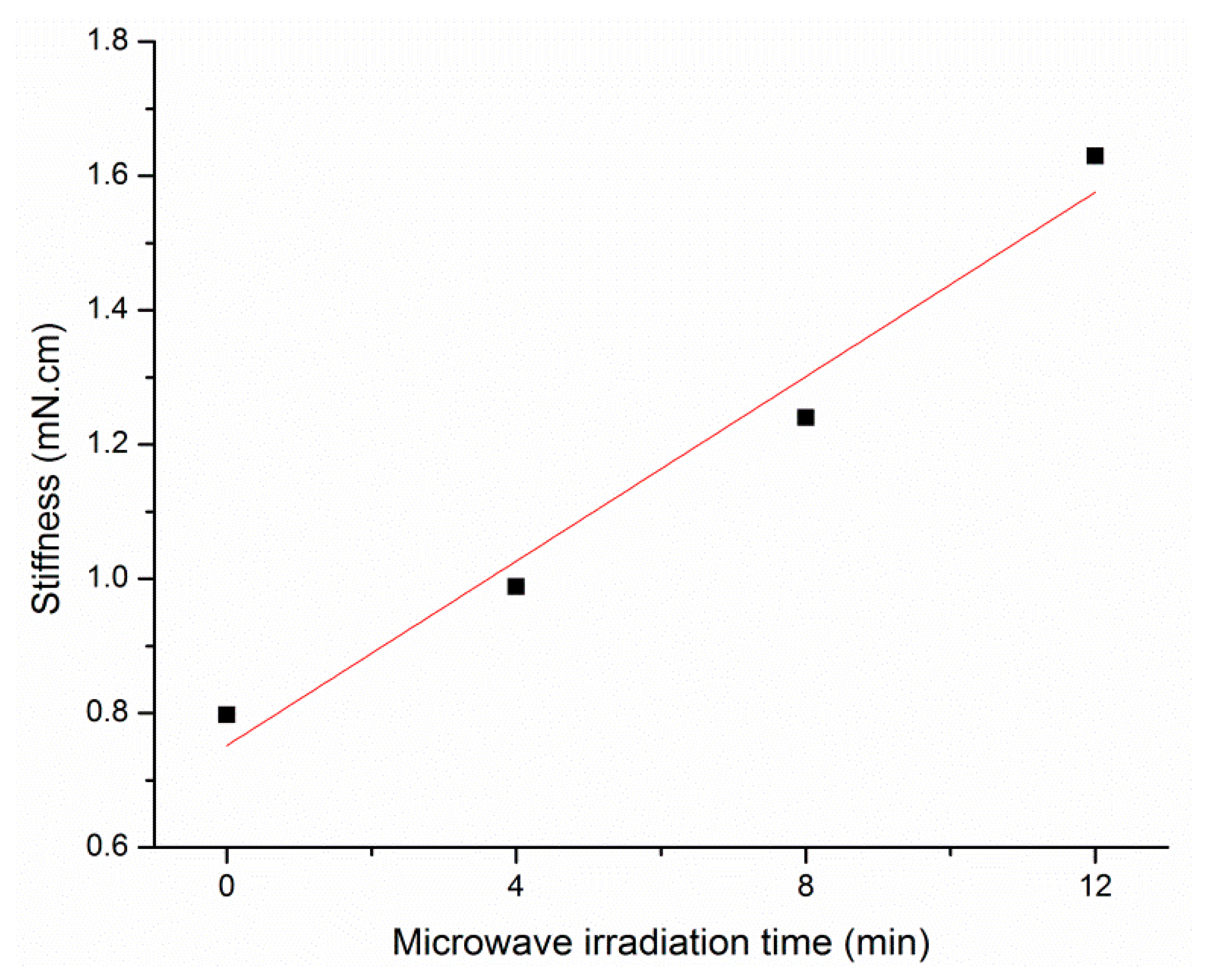
3.8. Stiffness
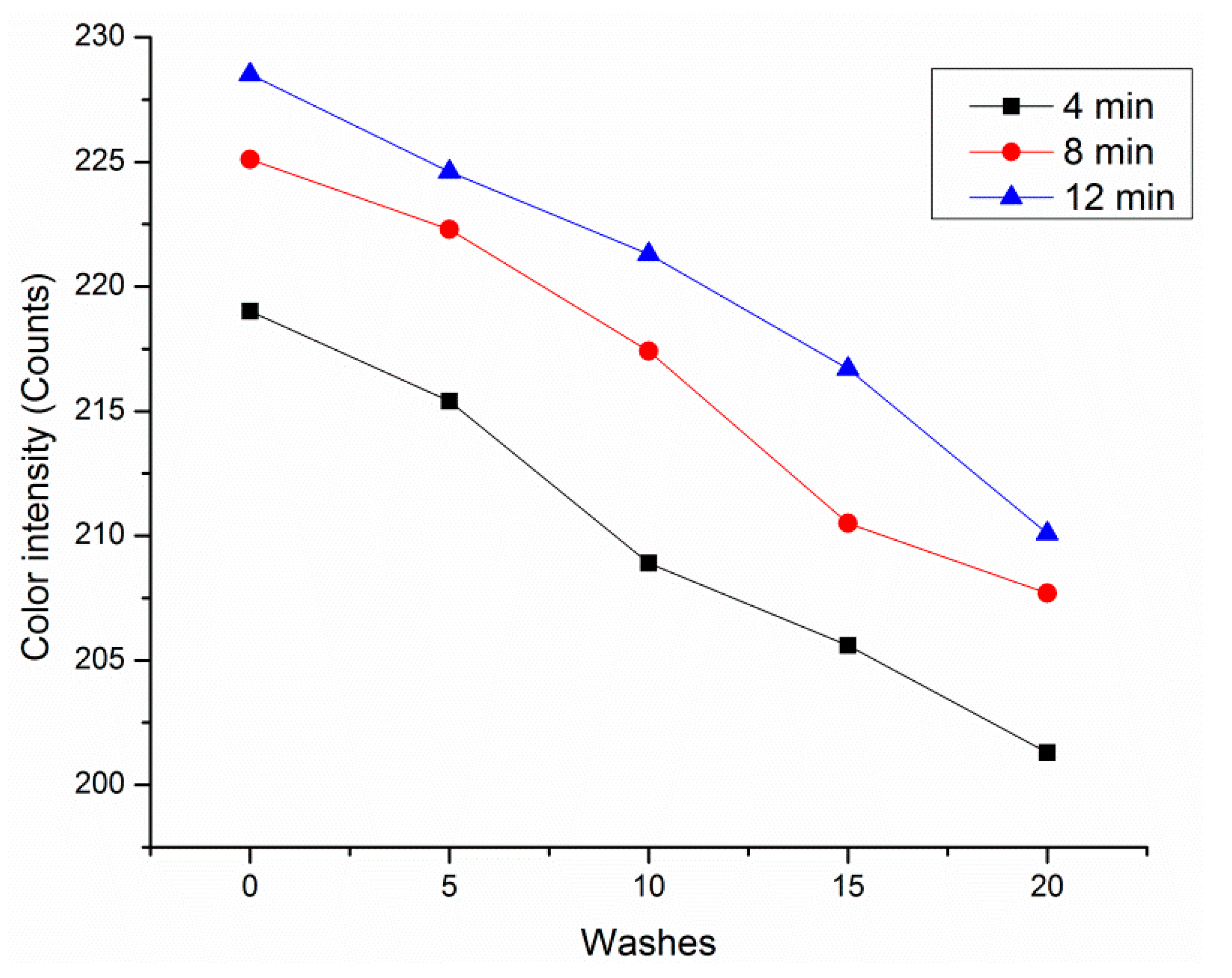
3.9. Washing Durability (Reusability)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasanpoor, M.; Aliofkhazraei, M.; Delavari, H. Microwave assisted synthesis of zinc oxide nanoparticles. Procedia Mater. Sci. 2015, 8, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Czyżowska, A.; Barbasz, A. A review: Zinc oxide nanoparticles–Friends or enemies? Int. J. Environ. Health Res. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc oxide for functional textile coatings: Recent advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Idrees, F.; Idrees, F. Recent advancements in microwave-assisted synthesis of NiO nanostructures and their supercapacitor properties: A comprehensive review. Curr. Nanomater. 2018, 3, 5–17. [Google Scholar] [CrossRef]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Pillai, S.K.; Sinha Ray, S.; Jalama, K.; Krause, R.W.M. Recent trends in the microwave-assisted synthesis of metal oxide nanoparticles supported on carbon nanotubes and their applications. J. Nanomater. 2012, 2012, 15. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Tiwari, S.; Thakur, V.; Pratap, R. Growth of ultrafast, super dense ZnO nanorods using microwaves for piezoelectric MEMS applications. Mater. Chem. Phys. 2020, 255, 123607. [Google Scholar] [CrossRef]
- Kajbafvala, A.; Samberg, J.P.; Ghorbani, H.; Kajbafvala, E.; Sadrnezhaad, S.K. Effects of initial precursor and microwave irradiation on step-by-step synthesis of zinc oxide nano-architectures. Mater. Lett. 2012, 67, 342–345. [Google Scholar] [CrossRef]
- Brahma, S.; Rao, K.J.; Shivashankar, S. Rapid growth of nanotubes and nanorods of würtzite ZnO through microwave-irradiation of a metalorganic complex of zinc and a surfactant in solution. Bull. Mater. Sci. 2010, 33, 89–95. [Google Scholar] [CrossRef]
- Pimentel, A.; Samouco, A.; Nunes, D.; Araújo, A.; Martins, R.; Fortunato, E. Ultra-fast microwave synthesis of ZnO nanorods on cellulose substrates for UV sensor applications. Materials 2017, 10, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajbafvala, A.; Zanganeh, S.; Kajbafvala, E.; Zargar, H.R.; Bayati, M.R.; Sadrnezhaad, S.K. Microwave-assisted synthesis of narcis-like zinc oxide nanostructures. J. Alloy. Compd. 2010, 497, 325–329. [Google Scholar] [CrossRef]
- Fujita, S.; Bhanage, B.M.; Arai, M.; Tambade, P.; Bhatte, K.D. Microwave-assisted additive free synthesis of nanocrystalline zinc oxide. Powder Technol. 2010, 203, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.W.; Peng, S.M.; Wu, J.S.; Shih, W.S.; Wu, C.Z.; Tang, I.T. Effect of seed layer on the growth of well-aligned ZnO nanowires. J. Phys. Chem. Solids 2009, 70, 1359–1362. [Google Scholar] [CrossRef]
- Preda, N.; Enculescu, M.; Zgura, I.; Socol, M.; Matei, E.; Vasilache, V.; Enculescu, I. Superhydrophobic properties of cotton fabrics functionalized with ZnO by electroless deposition. Mater. Chem. Phys. 2013, 138, 253–261. [Google Scholar] [CrossRef]
- Tran Thi, V.H.; Lee, B.K. Development of multifunctional self-cleaning and UV blocking cotton fabric with modification of photoactive ZnO coating via microwave method. J. Photochem. Photobiol. A Chem. 2017, 338, 13–22. [Google Scholar] [CrossRef]
- Ennaceri, H.; Wang, L.; Erfurt, D.; Riedel, W.; Mangalgiri, G.; Khaldoun, A.; El Kenz, A.; Benyoussef, A.; Ennaoui, A. Water-resistant surfaces using zinc oxide structured nanorod arrays with switchable wetting property. Surf. Coat. Technol. 2016, 299, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, M.; Bezverkhyy, I.; Skompska, M. ZnO Nanorods covered with a TiO2 layer: Simple sol-gel preparation, and optical, photocatalytic and photoelectrochemical properties. J. Mater. Chem. A 2015, 3, 12748–12760. [Google Scholar] [CrossRef]
- Ashraf, M.; Champagne, P.; Perwuelz, A.; Campagne, C.; Leriche, A. Photocatalytic solution discoloration and self-cleaning by polyester fabric functionalized with ZnO nanorods. J. Ind. Text. 2015, 44, 884–898. [Google Scholar] [CrossRef]
- Zuliani, A.; Cano, M.; Calsolaro, F.; Puente Santiago, A.R.; Giner-Casares, J.J.; Rodríguez-Castellón, E.; Berlier, G.; Cravotto, G.; Martina, K.; Luque, R. Improving the electrocatalytic performance of sustainable Co/carbon materials for the oxygen evolution reaction by ultrasound and microwave assisted synthesis. Sustain. Energy Fuels 2021, 5, 720–731. [Google Scholar] [CrossRef]
- Chin, C.D.W.; Treadwell, L.J.; Wiley, J.B. Microwave synthetic routes for shape-controlled catalyst nanoparticles and nanocomposites. Molecules 2021, 26, 3647. [Google Scholar] [CrossRef]
- Caglar, Y.; Gorgun, K.; Aksoy, S. Effect of deposition parameters on the structural properties of ZnO nanopowders prepared by microwave-assisted hydrothermal synthesis. Spectrochim. Acta.—Part A Mol. Biomol. Spectrosc. 2015, 138, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xin, J.H.; Tao, X.M.; Daoud, W.A. ZnO nanorods grown on cotton fabrics at low temperature. Chem. Phys. Lett. 2004, 398, 250–255. [Google Scholar] [CrossRef]
- Özen, İ.; Çinçik, E.; Şimşek, S. Thermal comfort properties of simulated multilayered diaper structures in dry and wet conditions. J. Ind. Text. 2016, 46, 256–278. [Google Scholar] [CrossRef]
- Khan, M.Z.; Hussain, S.; Siddique, H.F.; Baheti, V.; Militky, J.; Azeem, M.; Ali, A. Improvement of liquid moisture management in plaited knitted fabrics. Tekst. Konfeksiyon 2018, 28, 182–188. [Google Scholar]
- Khan, M.Z.; Militky, J.; Baheti, V.; Fijalkowski, M.; Wiener, J.; Voleský, L.; Adach, K. Growth of ZnO nanorods on cotton fabrics via microwave hydrothermal method: Effect of size and shape of nanorods on superhydrophobic and UV-blocking properties. Cellulose 2020, 27, 10519–10539. [Google Scholar] [CrossRef]
- Khan, M.Z.; Baheti, V.; Militky, J.; Wiener, J.; Ali, A. Self-cleaning properties of polyester fabrics coated with flower-like TiO2 particles and trimethoxy (octadecyl)silane. J. Ind. Text. 2020, 50, 543–565. [Google Scholar] [CrossRef]
- Tang, X.N.; Berman, A.E.; Swanson, R.A.; Yenaris, M.A. Digitally quantifying cerebral hemorrhage using Photoshop® and Image J. J. Neurosci. Methods 2010, 190, 240–243. [Google Scholar] [CrossRef] [Green Version]
- Hes, L. Non-destructive determination of comfort parameters during marketing of functional garments and clothing. Indian J. Fibre Text. Res. 2008, 33, 239–245. [Google Scholar]
- Mangat, M.M.; Hes, L.; Bajzík, V. Thermal resistance models of selected fabrics in wet state and their experimental verification. Text. Res. J. 2015, 85, 200–210. [Google Scholar] [CrossRef]
- Hes, L.; Loghin, C. Heat, moisture and air transfer properties of selected woven fabrics in wet state. J. Fiber Bioeng. Inform. 2009, 2, 141–149. [Google Scholar]
- Bogusławska-Baczek, M.; Hes, L. Effective water vapour permeability of wet wool fabric and blended fabrics. Fibres Text. East. Eur. 2013, 97, 67–71. [Google Scholar]
- ISO 11092:2014: Textiles—Physiological Effects—Measurement of Thermal and Water-Vapour Resistance under Steady-State Conditions (Sweating Guarded-Hotplate Test); International Organization for Standardization: Geneva, Switzerland, 2014.
- Hes, L.; de Araujo, M. Simulation of the effect of air gaps between the skin and a wet fabric on resulting cooling flow. Text. Res. J. 2010, 80, 1488–1497. [Google Scholar] [CrossRef]
- ISO 9237:1995: Textiles—Determination of the Permeability of Fabrics to Air; International Organization for Standardization: Geneva, Switzerland, 1995.
- Naeem, J.; Mazari, A.; Volesky, L.; Mazari, F. Effect of nano silver coating on thermal protective performance of firefighter protective clothing. J. Text. Inst. 2019, 110, 847–858. [Google Scholar] [CrossRef]
- Fridrichová, L. A new method of measuring the bending rigidity of fabrics and its application to the determination of the their anisotropy. Text. Res. J. 2013, 83, 883–892. [Google Scholar] [CrossRef]
- ISO 105-C06:2010: Textiles—Tests for Colour Fastness—Part C06: Colour Fastness to Domestic and Commercial Laundering; International Organization for Standardization: Geneva, Switzerland, 2010.
- Ul Hassan Sarwar Rana, A.; Kang, M.; Kim, H.S. Microwave-assisted facile and ultrafast growth of ZnO nanostructures and proposition of alternative microwave-assisted methods to address growth stoppage. Sci. Rep. 2016, 6, 24870. [Google Scholar] [CrossRef] [PubMed]
- Shojaee, N.; Ebadzadeh, T.; Aghaei, A. Effect of concentration and heating conditions on microwave-assisted hydrothermal synthesis of ZnO nanorods. Mater. Charact. 2010, 61, 1418–1423. [Google Scholar] [CrossRef]
- Wahab, R.; Kim, Y.S.; Lee, K.; Shin, H.S. Fabrication and growth mechanism of hexagonal zinc oxide nanorods via solution process. J. Mater. Sci. 2010, 45, 2967–2973. [Google Scholar] [CrossRef]
- Polsongkram, D.; Chamninok, P.; Pukird, S.; Chow, L.; Lupan, O.; Chai, G.; Khallaf, H.; Park, S.; Schulte, A. Effect of synthesis conditions on the growth of ZnO nanorods via hydrothermal method. Phys. B Condens. Matter 2008, 403, 3713–3717. [Google Scholar] [CrossRef]
- Ates, E.S.; Unalan, H.E. Zinc oxide nanowire enhanced multifunctional coatings for cotton fabrics. Thin Solid Films 2012, 520, 4658–4661. [Google Scholar] [CrossRef]
- Cheng, D.; He, M.; Li, W.; Wu, J.; Ran, J.; Cai, G.; Wang, X. Hydrothermal growing of cluster-like ZnO nanoparticles without crystal seeding on PET films via dopamine anchor. Appl. Surf. Sci. 2019, 467–468, 534–542. [Google Scholar] [CrossRef]
- Achouri, F.; Merlin, C.; Corbel, S.; Alem, H.; Mathieu, L.; Balan, L.; Medjahdi, G.; Ben Said, M.; Ghrabi, A.; Schneider, R. ZnO nanorods with high photocatalytic and antibacterial activity under solar light irradiation. Materials 2018, 11, 2158. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Kajbafvala, A.; Ghorbani, H.; Paravar, A.; Samberg, J.P.; Kajbafvala, E.; Sadrnezhaad, S.K. Effects of morphology on photocatalytic performance of zinc oxide nanostructures synthesized by rapid microwave irradiation methods. Superlattices Microstruct. 2012, 51, 512–522. [Google Scholar] [CrossRef]
- Yuldashev, S.U.; Yalishev, V.S.; Cho, H.D.; Kang, T.W. Thermal conductivity of ZnO single nanowire. J. Nanosci. Nanotechnol. 2016, 16, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, J. Thermal conductivity of nanowires. Chin. Phys. B 2018, 27, 035101. [Google Scholar] [CrossRef]
- Mamand, S.M. Thermal conductivity calculations for nanoparticles embedded in a base fluid. Appl. Sci. 2021, 11, 1459. [Google Scholar] [CrossRef]
- Dal, V.; Şimşek, R.; Hes, L.; Akçagün, E.; Yilmaz, A. Investigation of thermal comfort properties of zinc oxide coated woven cotton fabric. J. Text. Inst. 2016, 108, 337–340. [Google Scholar] [CrossRef]
- Zhu, G.; Kremenakova, D.; Wang, Y.; Militky, J.; Mishra, R. Study on air permeability and thermal resistance of textiles under heat convection. Text. Res. J. 2015, 85, 1681–1690. [Google Scholar] [CrossRef]
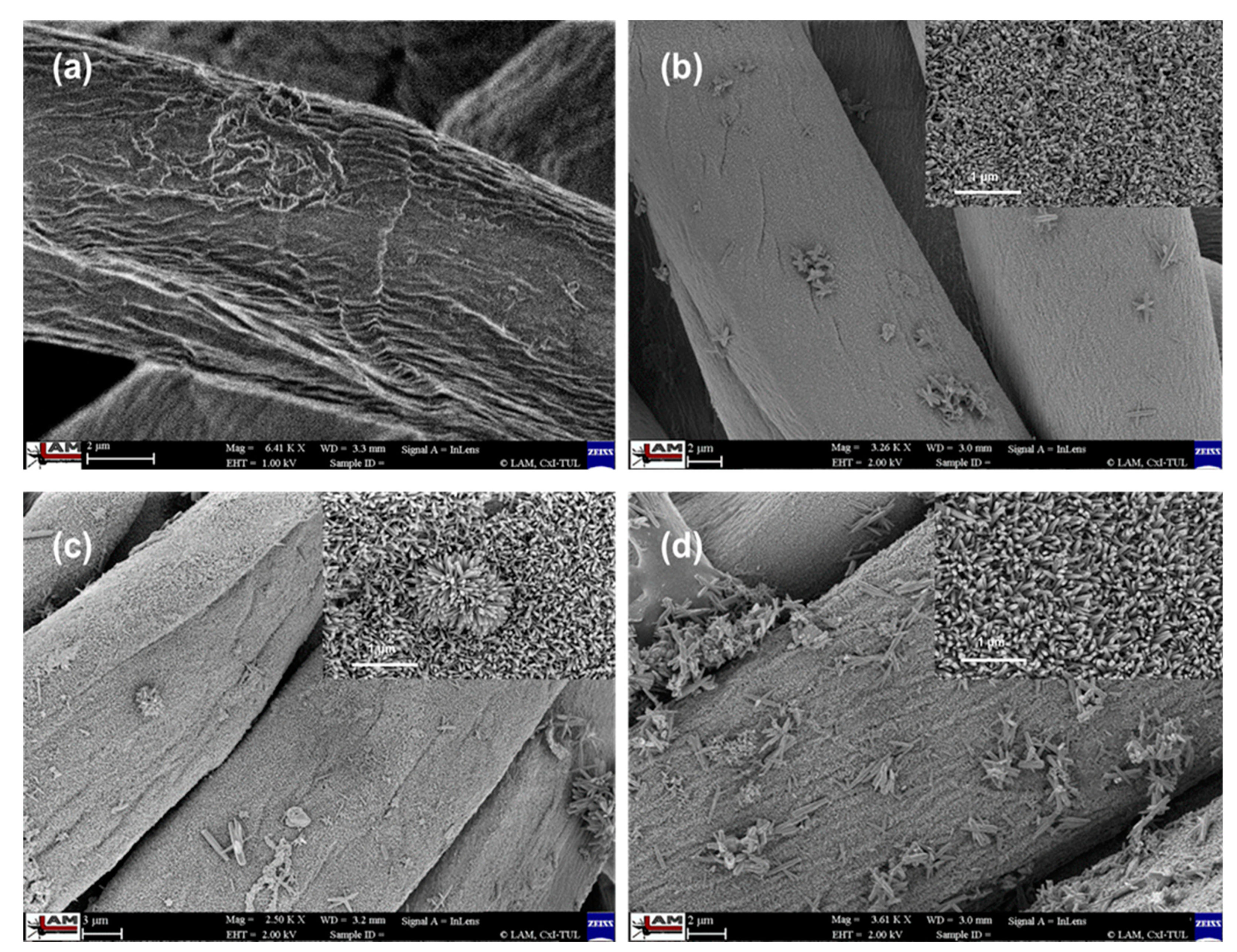


| Microwave Irradiation Time (min) | Mean Diameter (D) of ZnO Nanorods (nm) | Mean Length (L) of ZnO Nanorods (nm) | Zn Content (ppm) |
|---|---|---|---|
| 4 | 32.9 ± 3.1 | 192.7 ± 13.3 | 15,604 |
| 8 | 43.6 ± 2.1 | 259.9 ± 21.8 | 19,361 |
| 12 | 58.1 ± 5.9 | 289.9 ± 19.4 | 26,829 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.Z.; Militky, J.; Petru, M.; Tomková, B.; Ali, A.; Javed, A.; Azeem, M.; Křemenáková, D. Ultra-Fast Growth of ZnO Nanorods on Cotton Fabrics and Their Self-Cleaning and Physiological Comfort Properties. Coatings 2021, 11, 1309. https://doi.org/10.3390/coatings11111309
Khan MZ, Militky J, Petru M, Tomková B, Ali A, Javed A, Azeem M, Křemenáková D. Ultra-Fast Growth of ZnO Nanorods on Cotton Fabrics and Their Self-Cleaning and Physiological Comfort Properties. Coatings. 2021; 11(11):1309. https://doi.org/10.3390/coatings11111309
Chicago/Turabian StyleKhan, Muhammad Zaman, Jiri Militky, Michal Petru, Blanka Tomková, Azam Ali, Asif Javed, Musaddaq Azeem, and Dana Křemenáková. 2021. "Ultra-Fast Growth of ZnO Nanorods on Cotton Fabrics and Their Self-Cleaning and Physiological Comfort Properties" Coatings 11, no. 11: 1309. https://doi.org/10.3390/coatings11111309
APA StyleKhan, M. Z., Militky, J., Petru, M., Tomková, B., Ali, A., Javed, A., Azeem, M., & Křemenáková, D. (2021). Ultra-Fast Growth of ZnO Nanorods on Cotton Fabrics and Their Self-Cleaning and Physiological Comfort Properties. Coatings, 11(11), 1309. https://doi.org/10.3390/coatings11111309








