Effect of SiC Particle Contents and Size on the Microstructure and Dissolution of SiC-Hydroxyapatite Coatings
Abstract
:1. Introduction
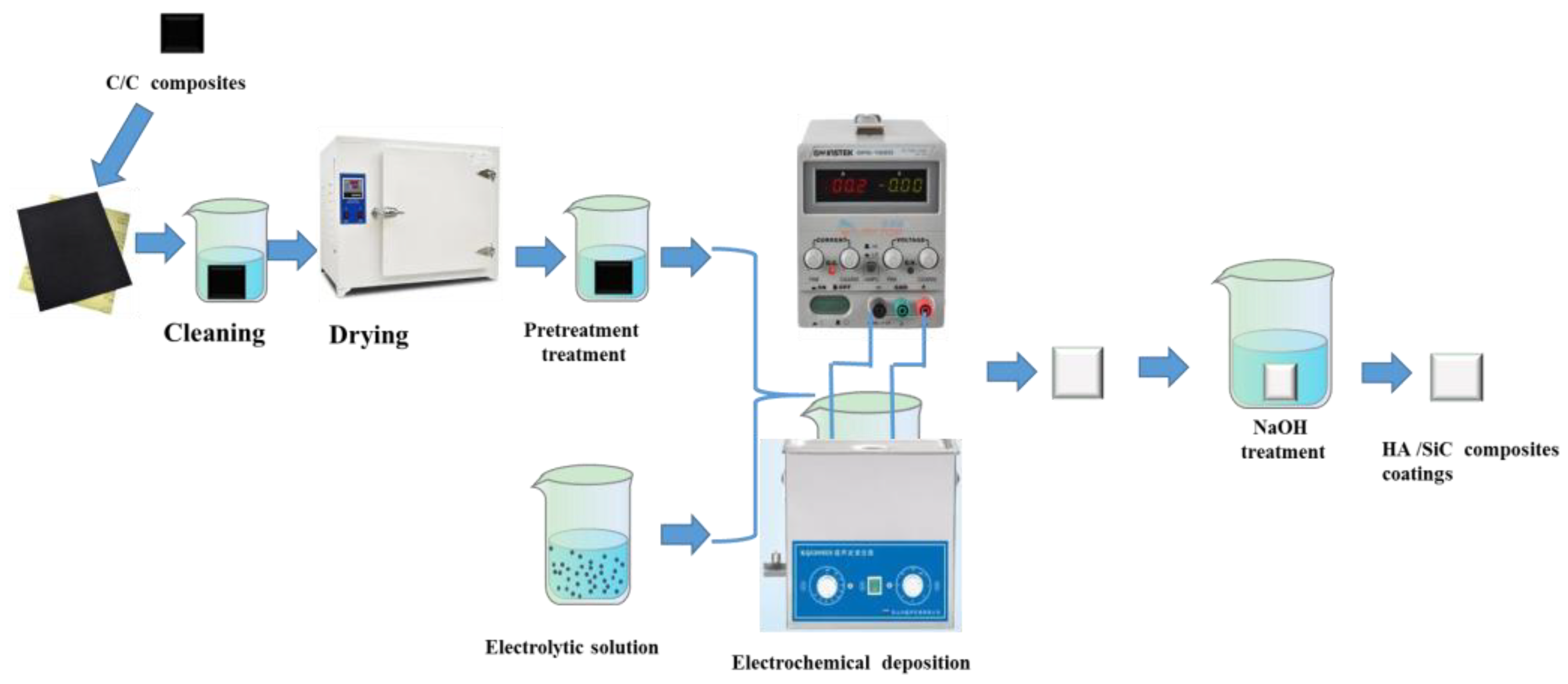
2. Materials and Methods
3. Results
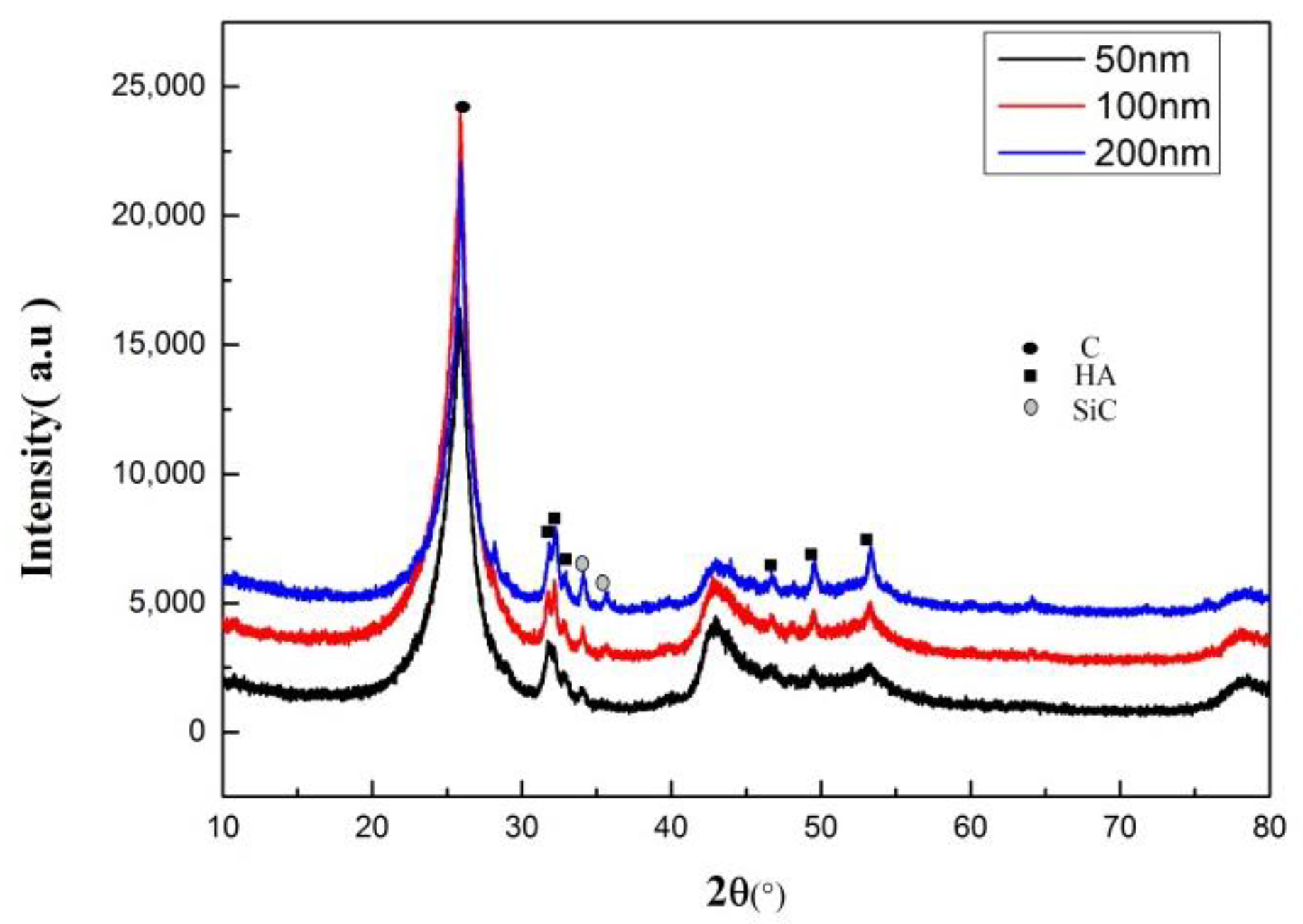
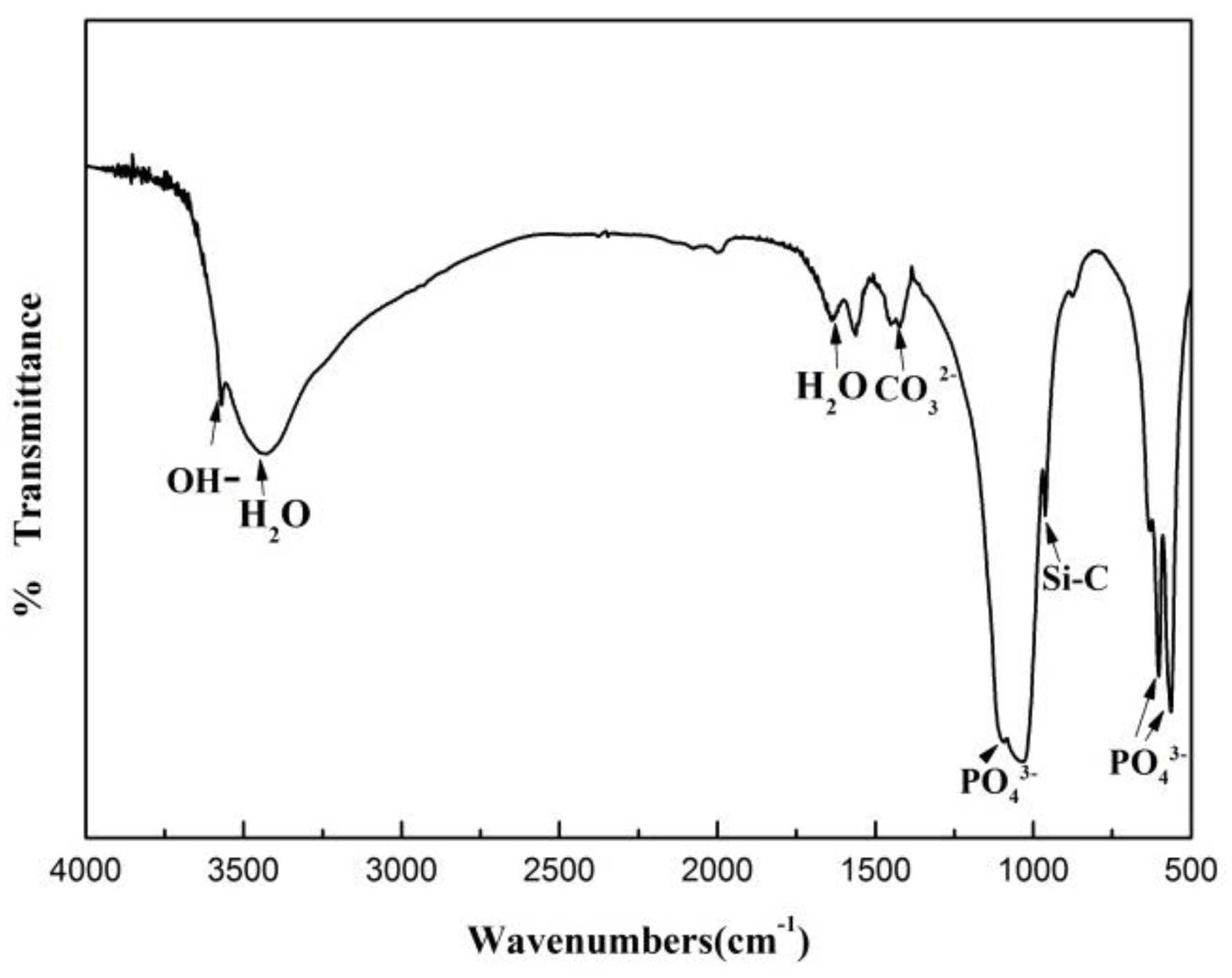
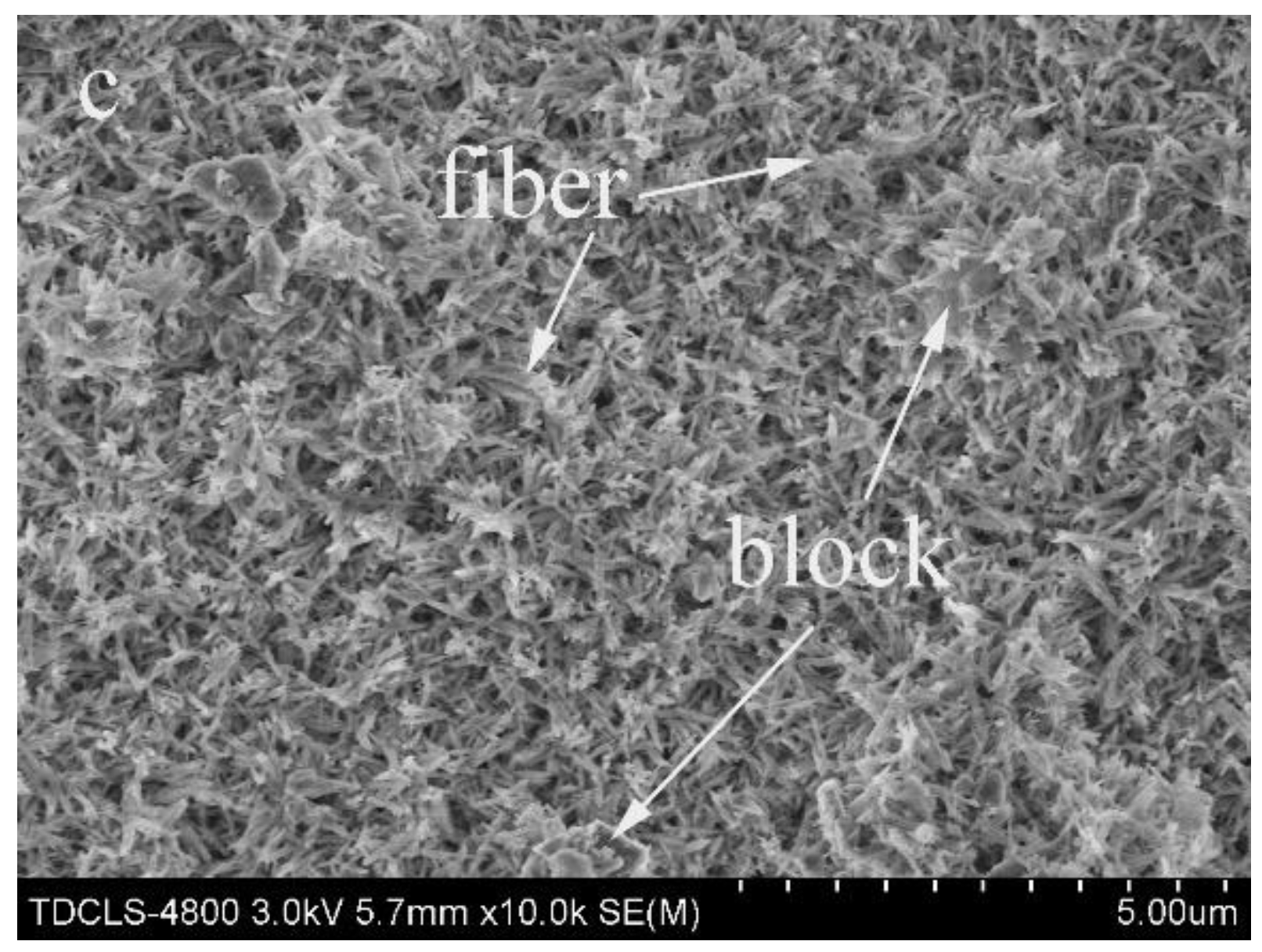
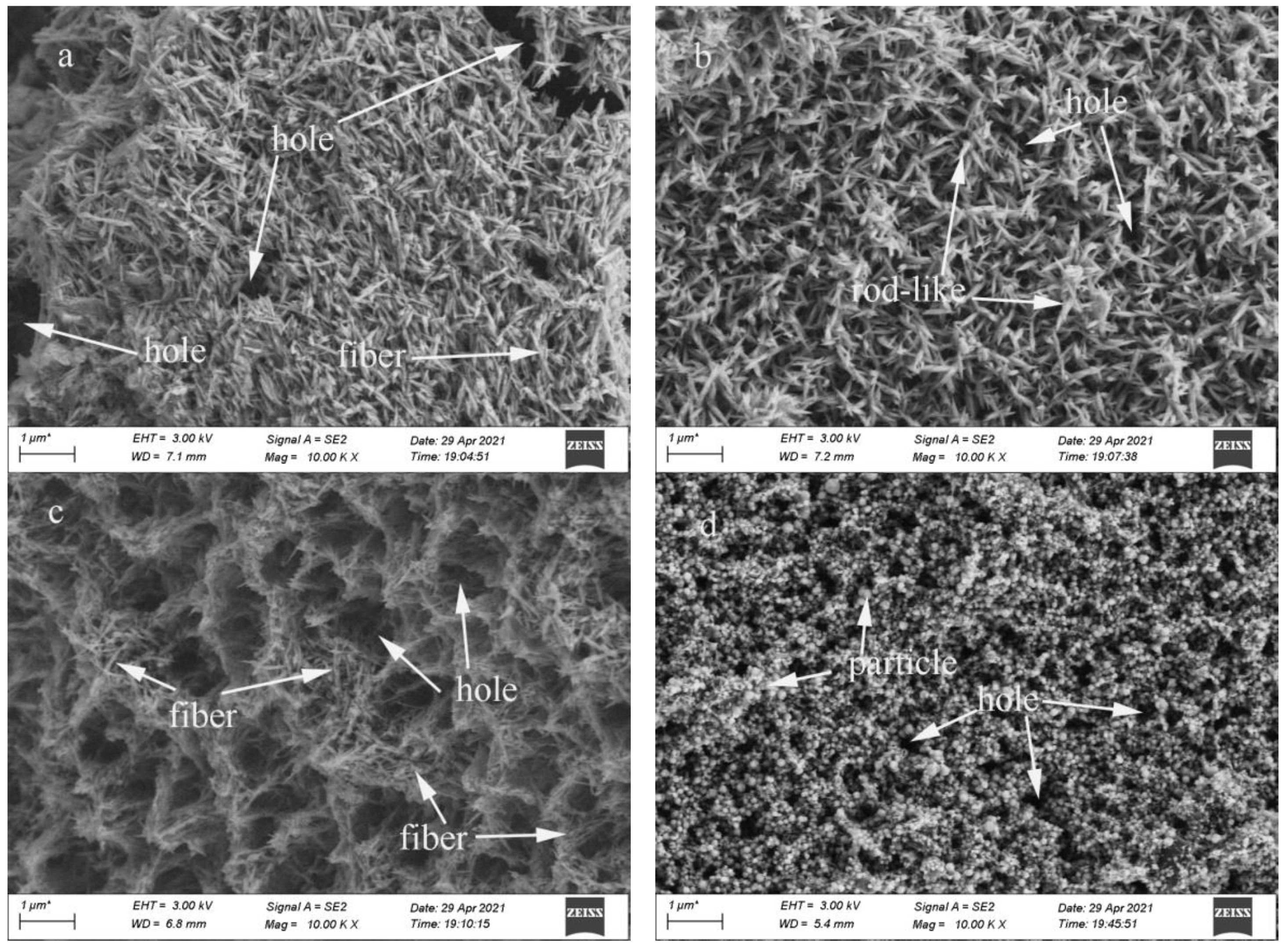
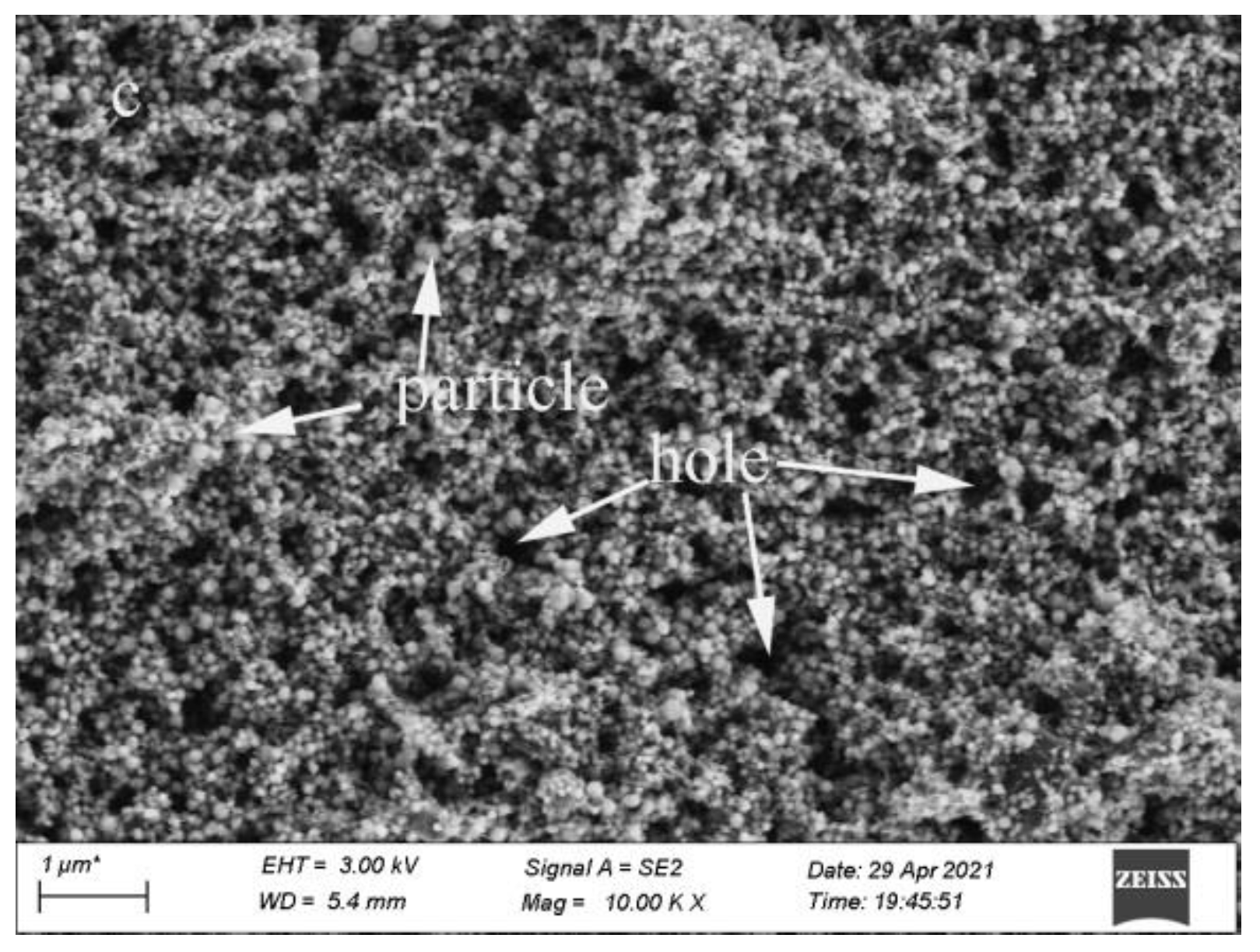
3.1. Effect of SiC Particle Sizes on the Hydroxyapatite Coating Properties
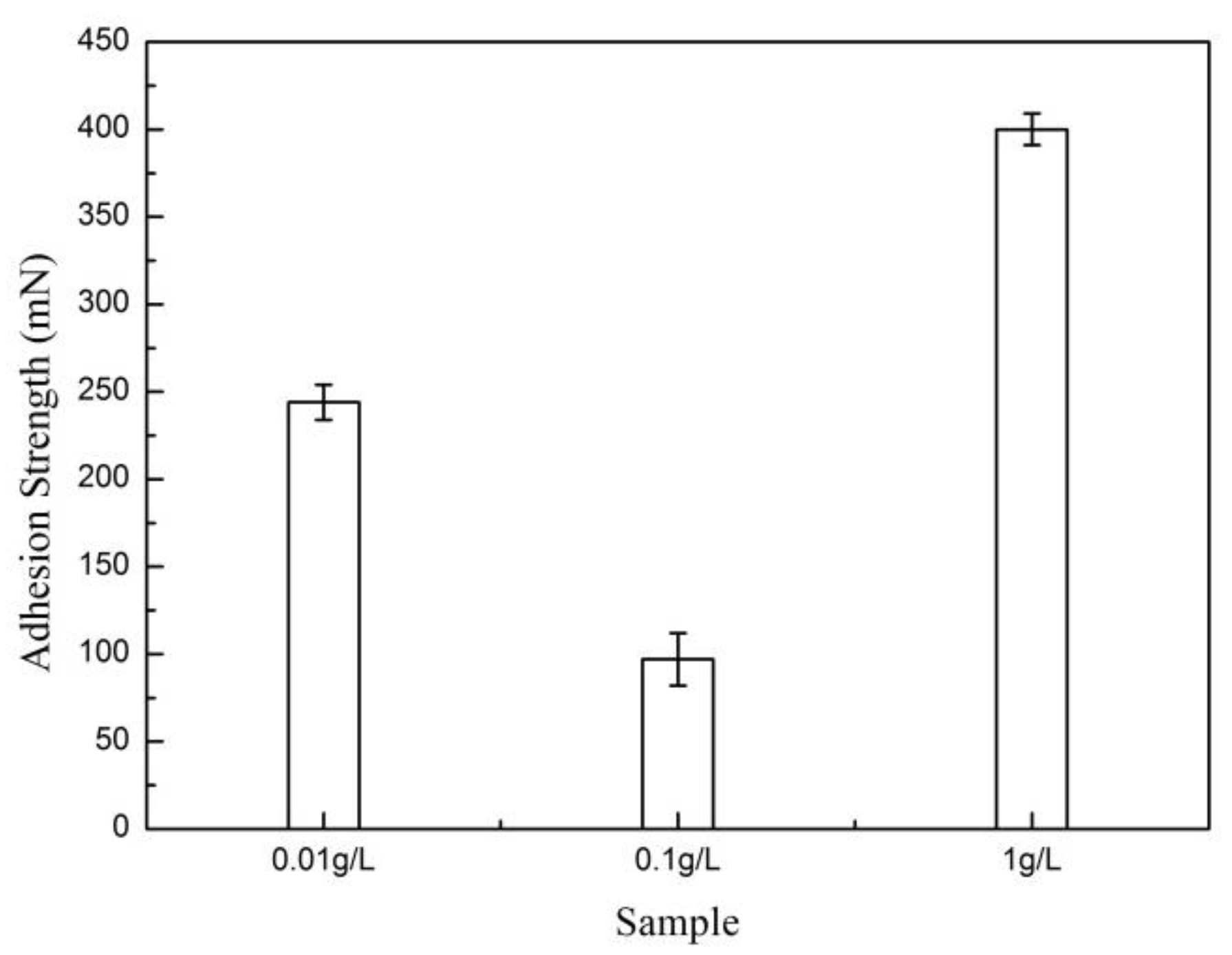
3.2. Effect of SiC Content on HA Coating Properties
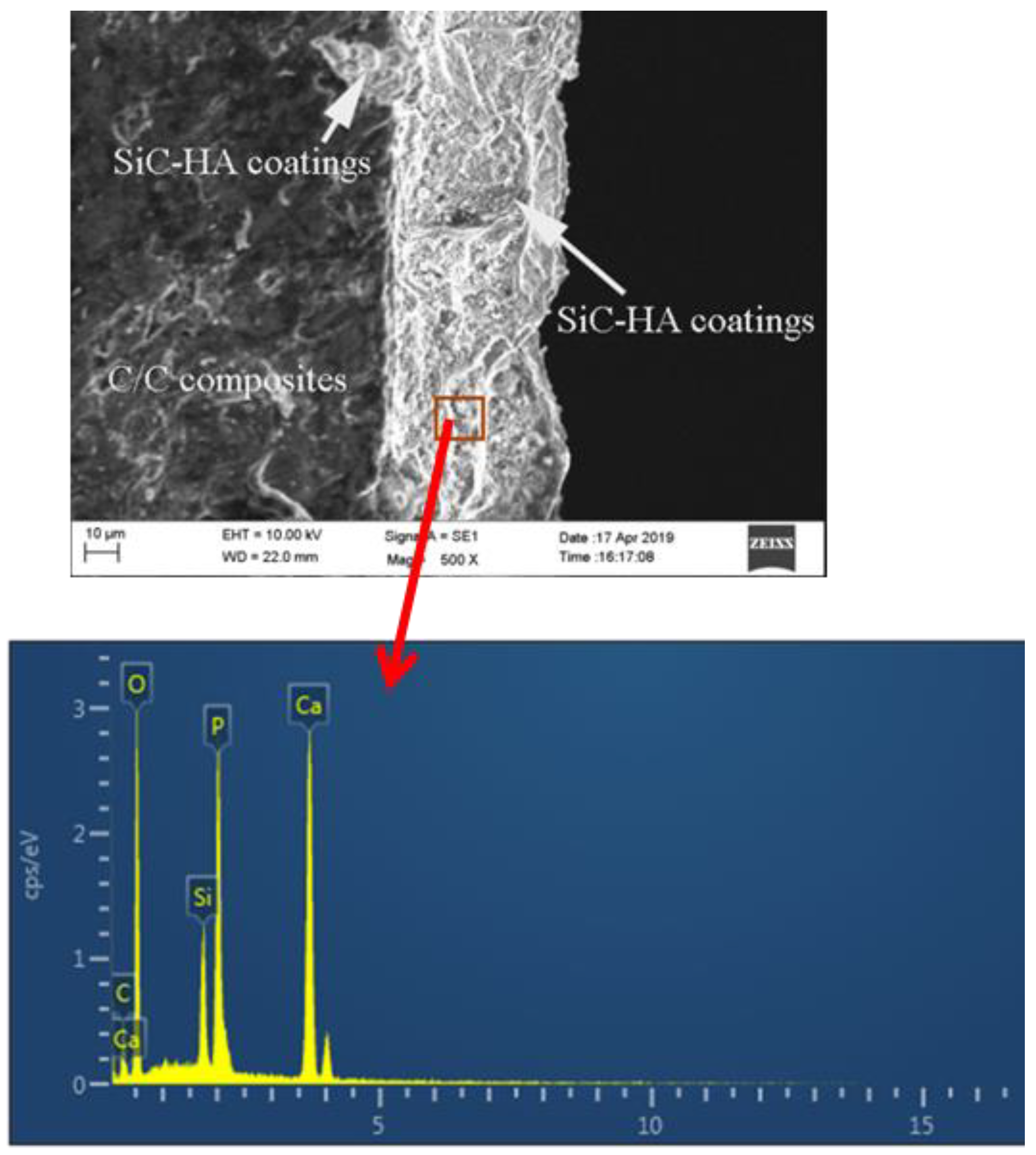
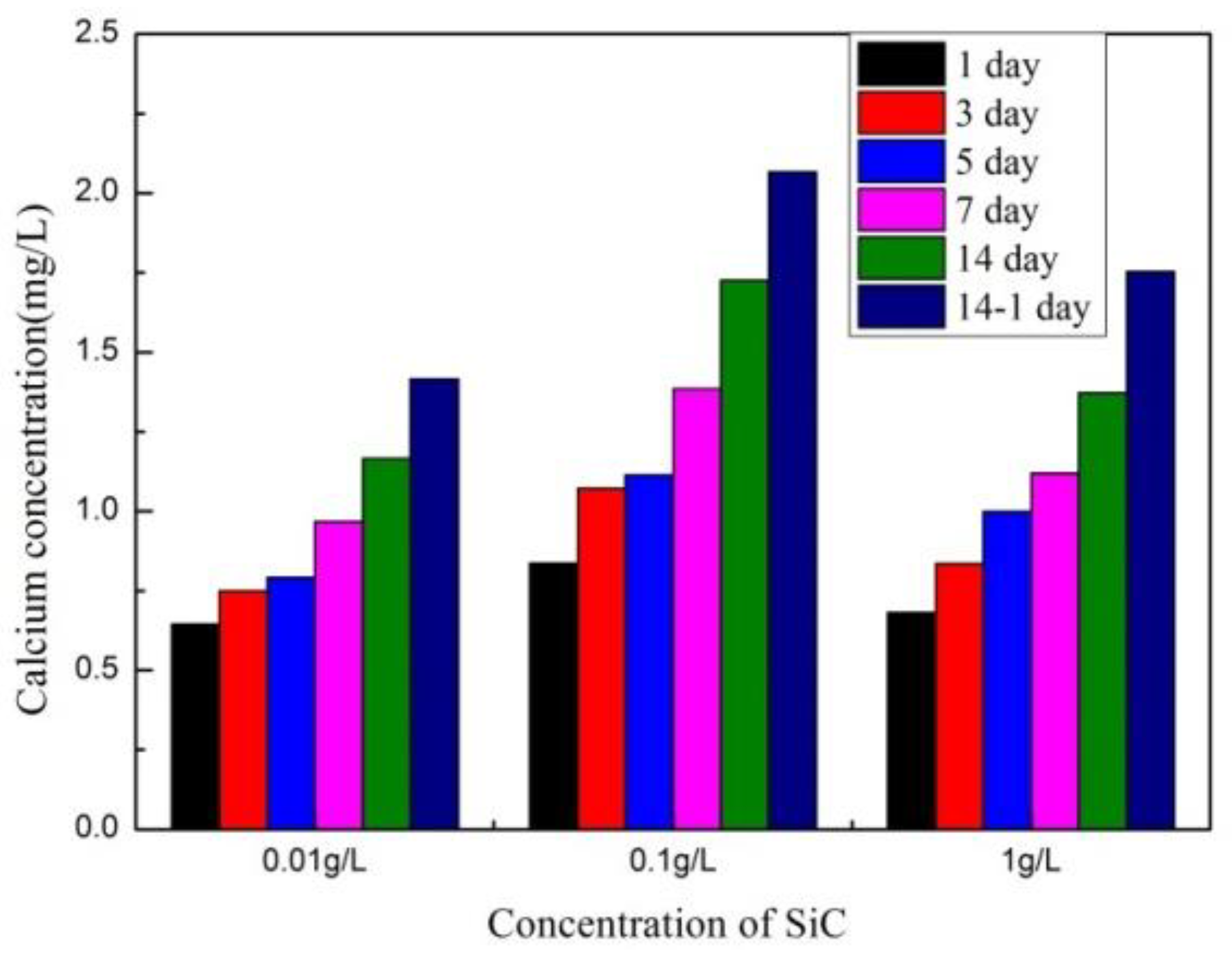
3.3. Dissolution of SiC-Hydroxyapatite Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.L.; Li, H.J.; Li, K.Z.; Zhang, Y.L.; Liu, S.J.; Guo, Q.; Li, S.X. Micro-oxidation treatment to improve bonding strength of Sr and Na co-substituted hydroxyapatite coatings for carbon/carbon composites. Appl. Surf. Sci. 2016, 378, 136–141. [Google Scholar] [CrossRef]
- Cao, N.; Dong, J.W.; Wang, Q.X.; Ma, Q.S.; Wang, F.; Chen, H.Y.; Xue, C.Q.; Li, M.S. Plasma sprayed hydroxyapatite coating on carbon/carbon composite scaffolds for bone tissue engineering and related tests in vivo. J. Biomed. Mater. Res. Part A 2010, 3, 1019–1027. [Google Scholar] [CrossRef]
- Zhai, Y.Q.; Li, K.Z.; Li, H.J.; Wang, C.; Liu, H. Influence of NaF concentration on fluorine-containing hydroxyapatite coating on carbon/carbon composites. Mater. Chem. Phys. 2007, 106, 22–26. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, S.X.; Li, H.J.; Pei, L.N. Bioactive surface modification of carbon/carbon composites with multilayer SiC-SiC nanowire-Si doped hydroxyapatite coating. J. Alloys Compd. 2018, 740, 109–117. [Google Scholar] [CrossRef]
- Ni, X.Y.; Tang, X.B.; Lin, T.; Zhao, Q.D.; Geng, C.R.; Cai, L.M.; Gua, W.D.; Miao, Y.L.; Chen, D. Properties of plasma-spray coated hydroxyapatite on carbon/carbon composites pretreated by an argon plasma. Carbon 2013, 63, 594–595. [Google Scholar] [CrossRef]
- Vilardella, A.M.; Cincaa, N.; Garcia-Giraltb, N.; Dostaa, S.; Canoa, I.G.; Noguésb, X.; Guilemanya, J.M. In-vitro comparison of hydroxyapatite coatings obtained by cold spray and conventional thermal spray technologies. Mater. Sci. Eng. C 2020, 107, 110306. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.W.; Han, G.; Zheng, X.S.; Chen, G.; Zhu, P.Z. Characterization and biocompatibility study of hydroxyapatite coating on the surface of titanium alloy. Surf. Coat. Technol. 2019, 375, 645–651. [Google Scholar] [CrossRef]
- Chambard, M.; Marsan, O.; Charvillat, C.; Grossin, D.; Fort, P.; Rey, C.; Gitzhofer, F.; Bertrand, G. Effect of the deposition route on the microstructure of plasma-sprayed hydroxyapatite coatings. Surf. Coat. Technol. 2019, 371, 68–77. [Google Scholar] [CrossRef]
- Wen, S.F.; Liu, X.L.; Ding, J.H.; Liu, Y.L.; Lan, Z.T.; Zhang, Z.M.; Chen, G.M. Hydrothermal synthesis of hydroxyapatite coating on the surface of medical magnesium alloy and its corrosion resistance. Prog. Nat. Sci. Mater. Int. 2021, 31, 324–333. [Google Scholar] [CrossRef]
- Lo, Y.S.; Chang, C.C.; Lin, P.-C.; Lin, S.P.; Wang, C.L. Direct growth of structurally controllable hydroxyapatite coating on Ti-6Al-4V through a rapid hydrothermal synthesis. Appl. Surf. Sci. 2021, 556, 149672. [Google Scholar] [CrossRef]
- Cao, N.; Yang, Z.G.; Yang, B.; Wang, W.B.; Boukherroubc, R.; Li, M. Construction of a bone-like surface layer on hydroxyl-modified carbon/carbon composite implants via biomimetic mineralization and in vivo tests. RSC Adv. 2016, 6, 9370–9378. [Google Scholar] [CrossRef]
- Cao, N.; Dong, J.W.; Wang, Q.X.; Ma, Q.S.; Xue, C.Q.; Li, M.S. An experimental bone defect healing with hydroxyapatite coating plasma sprayed on carbon/carbon composite implants. Surf. Coat. Technol. 2010, 205, 1150–1156. [Google Scholar] [CrossRef]
- Xiong, X.B.; Liu, L.; Ma, J.; Ni, X.Y.; Li, Y.Y.; Zeng, X.R. A simplified process for preparing adhesive hydroxyapatite coatings on carbon/carbon composites. Surf. Coat. Technol. 2019, 377, 124925. [Google Scholar] [CrossRef]
- Liu, L.; Ni, X.Y.; Xiong, X.B.; Ma, J.; Zeng, X.R. Low temperature preparation of SiO2 reinforced hydroxyapatite coating on carbon/carbon composites. J. Alloys Compd. 2019, 788, 768–778. [Google Scholar] [CrossRef]
- Guan, K.J.; Zhang, L.L.; Zhu, F.Y.; Li, H.J.; Sheng, H.C.; Guo, Y. Multi-layer SiC-graphene oxide-hydroxyapatite bioactive coating for carbon/carbon composites. J. Alloys Compd. 2020, 821, 153543. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, H.J.; Li, K.Z.; Zhang, S.Y.; Fu, Q.G.; Zhang, Y.L.; Lu, J.H.; Li, W. Preparation and characterization of carbon/SiC nanowire/Na-dopedcarbonated hydroxyapatite multilayer coating for carbon/carbon composites. Appl. Surf. Sci. 2014, 313, 85–92. [Google Scholar] [CrossRef]
- Li, H.; Khor, K.A.; Cheang, P. Titanium dioxide reinforced hydroxyapatite coatings deposited by high velocity oxy-fuel (HVOF) spray. Biomaterials 2002, 23, 85–91. [Google Scholar] [CrossRef]
- Natalia, M.; Stan, G.E.; Duta, L.; Mariana, C.C.; Coralia, B.; Sopronyi, M.; Luculescu, C.; Oktar, F.N.; Mihailescu, I.N. Structural, compositional, mechanical characterization and biological assessment of bovine-derived hydroxyapatite coatings reinforced with MgF2 or MgO for implants functionalization. Mater. Sci. Eng. C 2016, 59, 863–874. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, L.L.; Guan, K.J.; Wang, Z.K.; Wang, Y.T.; Li, H.P.; Lei, X.Y.; Zhang, W.X.; Wang, K.; Hu, Y.Y. Boosting bonding strength of hydroxyapatite coating for carbon/carbon composites via applying tree-planting interface structure. Ceram. Int. 2021, 47, 11922–11928. [Google Scholar] [CrossRef]
- Fu, Q.G.; Gu, C.G.; Li, H.J.; Chu, Y.H.; Lu, J.H.; Zhang, L.L. Microstructure and mechanical properties of SiC nanowires reinforced hydroxyapatite coating on carbon/carbon composites. Mater. Sci. Eng. A 2013, 563, 133–137. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, L.L.; Guo, Y.; Sheng, H.C.; Zhang, P.X.; Zhang, Y.X.; Li, Q.; Yang, H.Y.; Mikhalovsky, S.V. Mechanically strong and bioactive carbon fiber-SiC nanowire-hydroxyapatite-pyrolytic carbon composites for bone implant application. Ceram. Int. 2021, 47, 3389–3400. [Google Scholar] [CrossRef]
- Hosseini, M.R.; Ahangari, M.; Johar, M.H.; Allahkaram, S.R. Optimization of nano HA-SiC coating on AISI 316L medical grade stainless steel via electrophoretic deposition. Mater. Lett. 2021, 285, 129097. [Google Scholar] [CrossRef]
- Tao, L.; Li, X.L.; Hu, S.X.; Wu, J. Enhanced osteoporotic effect of silicon carbide nanoparticles combine with nano-hydroxyapatite coated anodized titanium implant on healthy bone regeneration in femoral fracture. J. Photochem. Photobiol. B Biol. 2019, 197, 111515. [Google Scholar] [CrossRef]
- Hnatkoa, M.; Hičáka, M.; Labudováb, M.; Galuskovác, D.; Sedláčeka, J.; Lenčéša, Z.; Šajgalíka, P. Bioactive silicon nitride by surface thermal treatment. J. Eur. Ceram. Soc. 2020, 40, 1848–1858. [Google Scholar] [CrossRef]
- Dodevski, V.; Pagnacco, M.C.; Radović, I.; Rosić, M.; Janković, B.; Stojmenović, M.; Mitić, V.V. Characterization of silicon carbide ceramics obtained from porous carbon structure achieved by plant carbonization. Mater. Chem. Phys. 2020, 245, 122768. [Google Scholar] [CrossRef]
- Xu, L.-L.; Shi, X.-L.; Qian, Q.; Bai, X.-H.; Xu, L.; Wang, Q.-L. Hydrothermal sterilization in silver nitrate solution endows plasma sprayed hydroxyapatite coating with antibacterial property. Mater. Lett. 2020, 263, 127258. [Google Scholar] [CrossRef]
- Xu, M.; Girish, Y.R.; Rakesh, K.P.; Wu, P.; Manukumar, H.M.; Byrappa, S.M.; Udayabhanu; Byrappa, K. Recent advances and challenges in silicon carbide (SiC) ceramic nanoarchitectures and their applications. Mater. Today Commun. 2021, 28, 102533. [Google Scholar] [CrossRef]
- Vladescua, A.; Birlikb, I.; Braica, V.; Toparlib, M.; Celikb, E.; AkAzem, F. Enhancement of the mechanical properties of hydroxyapatite by SiC addition. J. Mech. Behav. Biomed. Mater. 2014, 40, 362–368. [Google Scholar] [CrossRef]
- Leila, C.-K.; Jafar, K.-A.; Amir, M.; Mohamadreza, E. The effect of hydroxyapatite nanoparticles on electrochemical and mechanical performance of TiC/N coating fabricated by plasma electrolytic saturation method. Surf. Coat. Technol. 2020, 394, 125817. [Google Scholar] [CrossRef]
- Mohammad, U.; Colin, H.; Vincent, S. Fabrication, characterisation and corrosion of HA coated AZ31B Mg implant material: Effect of electrodeposition current density. Surf. Coat. Technol. 2020, 385, 125363. [Google Scholar] [CrossRef]
- Li, K.-Z.; Guo, Q.; Zhang, L.-L.; Zhang, Y.-L.; Liu, S.-J.; Guo, K.-B.; Li, S.-X. Synthesis and characterization of Si-substituted hydroxyapatite bioactive coating for SiC-coated carbon/carbon composites. Ceram. Int. 2017, 43, 1410–1414. [Google Scholar] [CrossRef]
- McEntirea, B.-J.; Bala, B.-S.; Rahamanc, M.-N.; Chevalier, J.; Pezzotti, G. Ceramics and ceramic coatings in orthopaedics. J. Eur. Ceram. Soc. 2015, 35, 4327–4369. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Pei, L.-N.; Li, H.-J.; Zhu, F.-Y. Design and fabrication of pyrolytic carbon-SiC fluoridated hydroxyapatite-hydroxyapatite multilayered coating on carbon fibers. Appl. Surf. Sci. 2019, 473, 571–577. [Google Scholar] [CrossRef]
- Batebi, K.; Abbasi Khazaei, B.A.; Afshar, A. Characterization of sol-gel derived silver/ fluor-hydroxyapatite composite coatings on titanium substrate. Surf. Coat. Technol. 2018, 352, 522–528. [Google Scholar] [CrossRef]
- Yuan, Q.H.; Zhang, Z.Q.; Yang, Y.; Jian, Y.L.; Li, R.L.; Dai, X.Y.; Wu, W.S.; Zhong, J.X.; Chen, C. Synthesis, characterization and biological performance study of Sr-doped hydroxyapatite/chitosan composite coatings. Mater. Chem. Phys. 2021, 270, 124752. [Google Scholar] [CrossRef]
- Yao, H.-L.; Hu, X.-Z.; Bai, X.-B.; Wang, H.-T.; Chen, Q.-Y.; Ji, G.-C. Comparative study of HA/TiO2 and HA/ZrO2 composite coatings deposited by high-velocity suspension flame spray (HVSFS). Surf. Coat. Technol. 2018, 351, 177–187. [Google Scholar] [CrossRef]
- Mao, Z.-L.; Yang, X.-J.; Zhu, S.-L.; Cui, Z.-D.; Li, Z.-Y. Effect of Na+ and NaOH concentrations on the surface morphology and dissolution behavior of hydroxyapatite. Ceram. Int. 2015, 41, 3461–3468. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Mao, Z. Effect of SiC Particle Contents and Size on the Microstructure and Dissolution of SiC-Hydroxyapatite Coatings. Coatings 2021, 11, 1166. https://doi.org/10.3390/coatings11101166
Yang L, Mao Z. Effect of SiC Particle Contents and Size on the Microstructure and Dissolution of SiC-Hydroxyapatite Coatings. Coatings. 2021; 11(10):1166. https://doi.org/10.3390/coatings11101166
Chicago/Turabian StyleYang, Li, and Zuli Mao. 2021. "Effect of SiC Particle Contents and Size on the Microstructure and Dissolution of SiC-Hydroxyapatite Coatings" Coatings 11, no. 10: 1166. https://doi.org/10.3390/coatings11101166
APA StyleYang, L., & Mao, Z. (2021). Effect of SiC Particle Contents and Size on the Microstructure and Dissolution of SiC-Hydroxyapatite Coatings. Coatings, 11(10), 1166. https://doi.org/10.3390/coatings11101166




