Preparation of High-Efficiency Flame-Retardant and Superhydrophobic Cotton Fabric by a Multi-Step Dipping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Flame-Retardant Cotton Fabric
2.3. Preparation of Hydrophobic CNC-SiO2 Rods
2.4. Preparation of Flame-Retardant and Superhydrophobic Cotton Fabric
2.5. Flammability and Antiabrasion of Cotton Fabric
2.6. Characterization
3. Results and Discussion
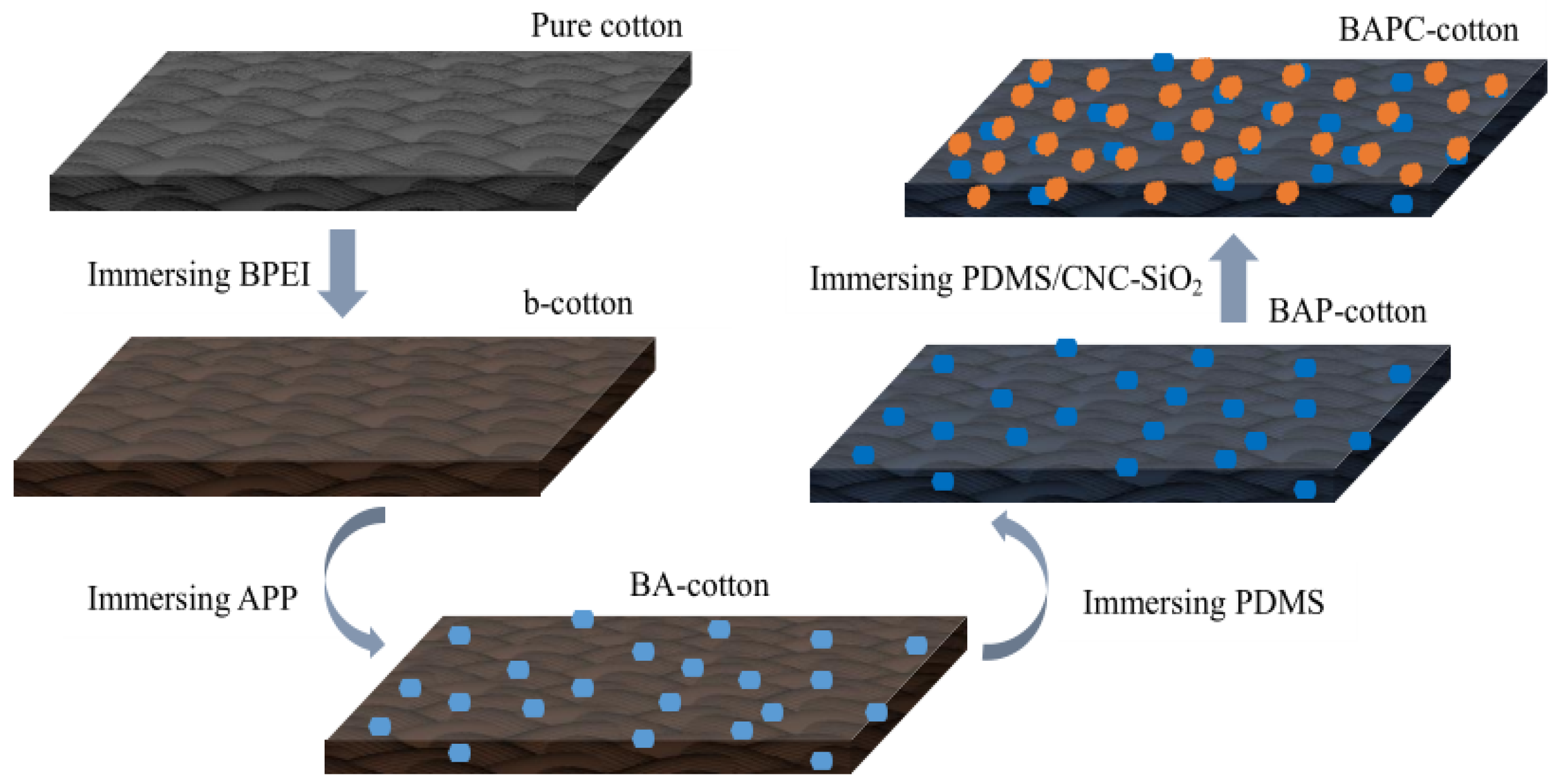
3.1. Formation Mechanism of Flame-Retardant and Superhydrophobic Cotton Fabric
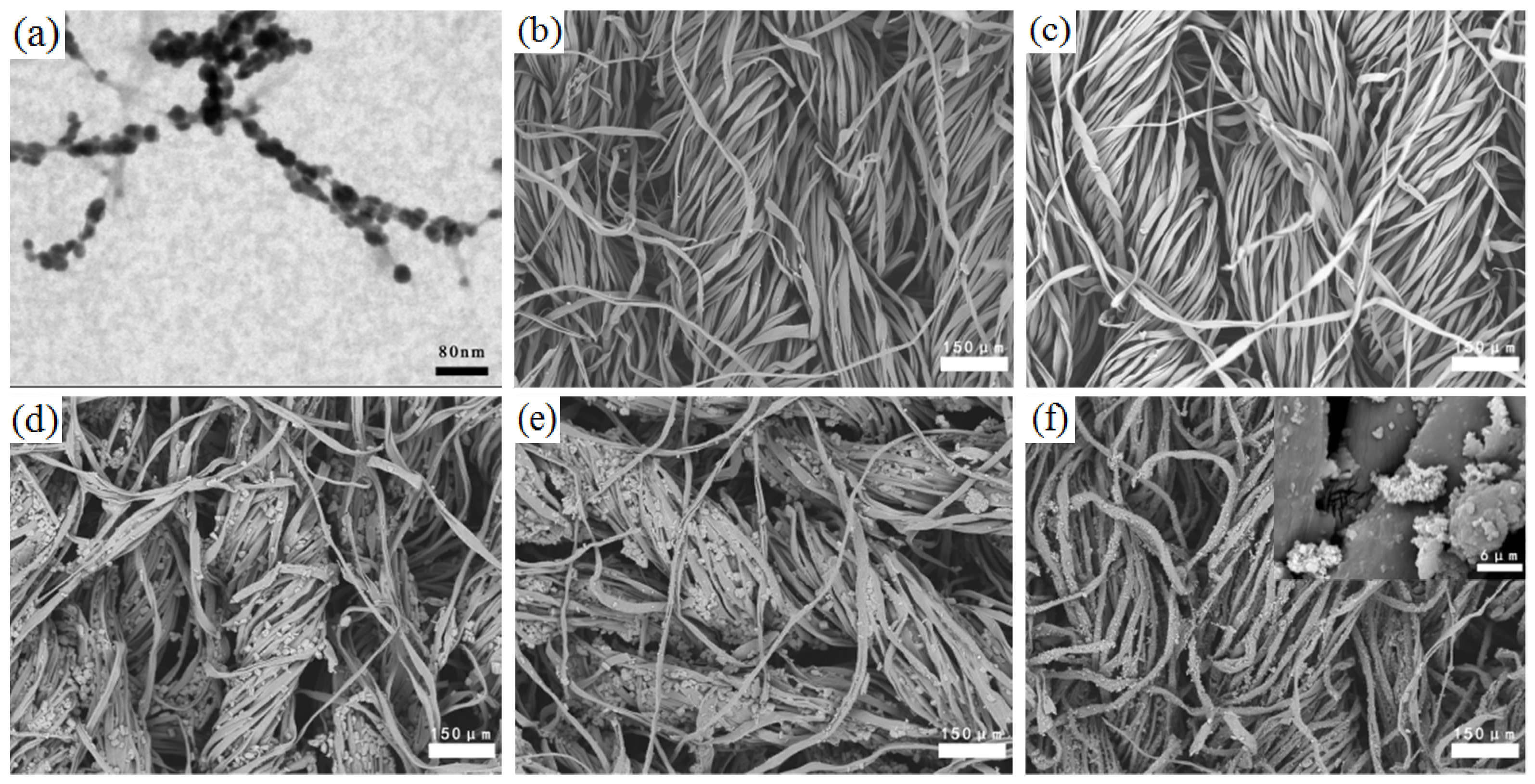
3.2. Surface Morphology
3.3. TGA of Cotton Fabric with Different Treatment
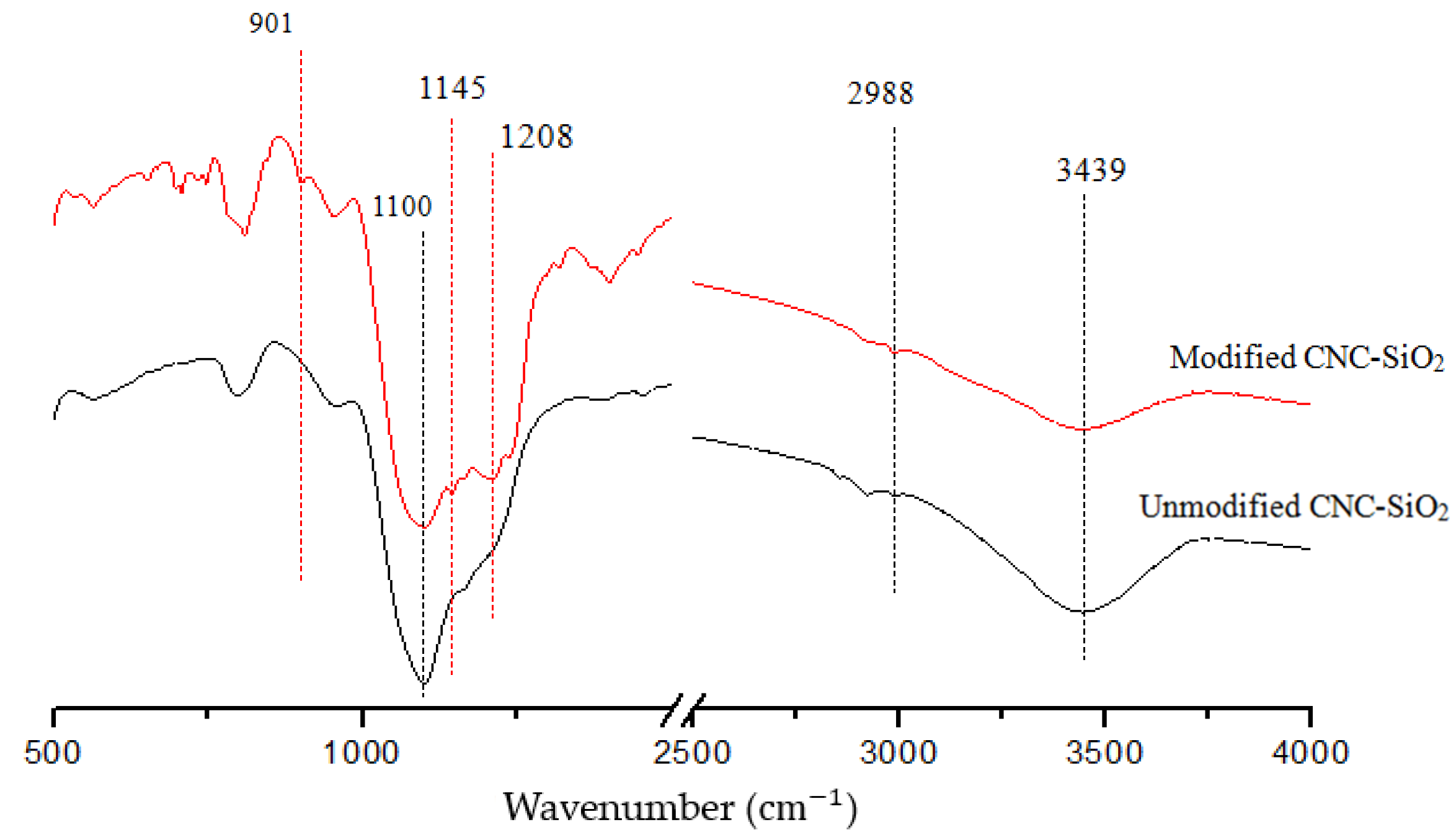
3.4. FTIR Analysis
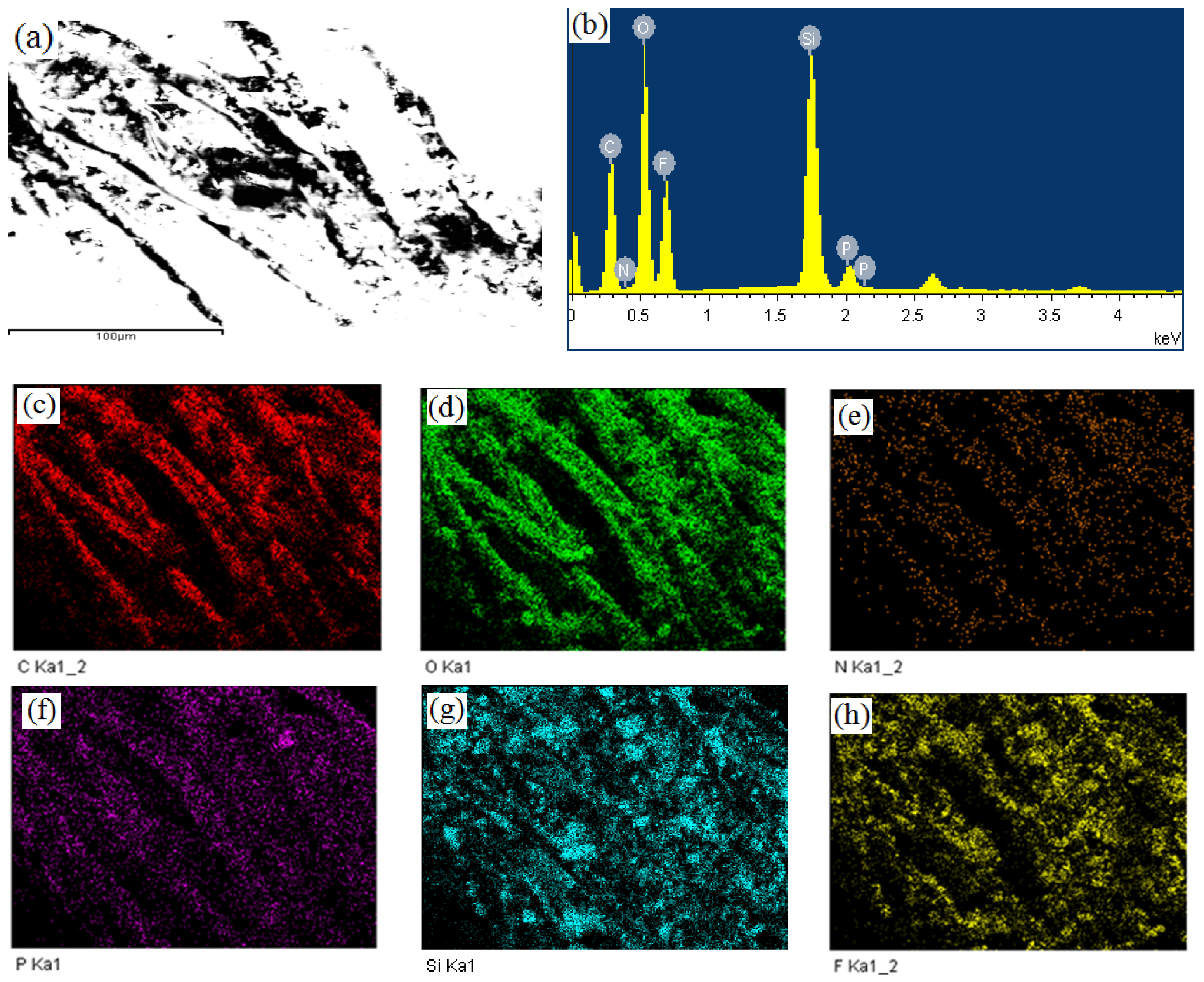
3.5. Mapping Analysis
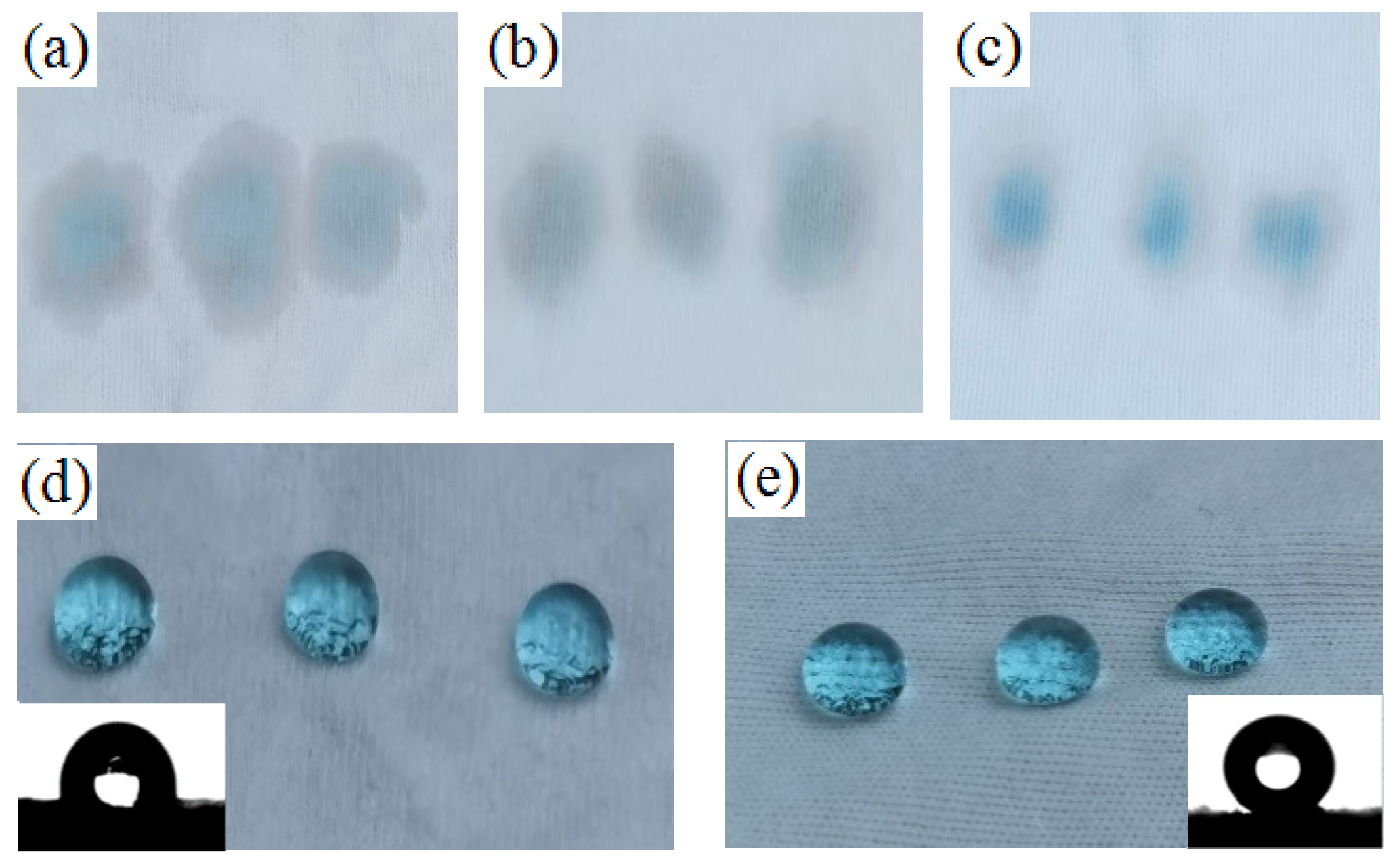
3.6. Wettability
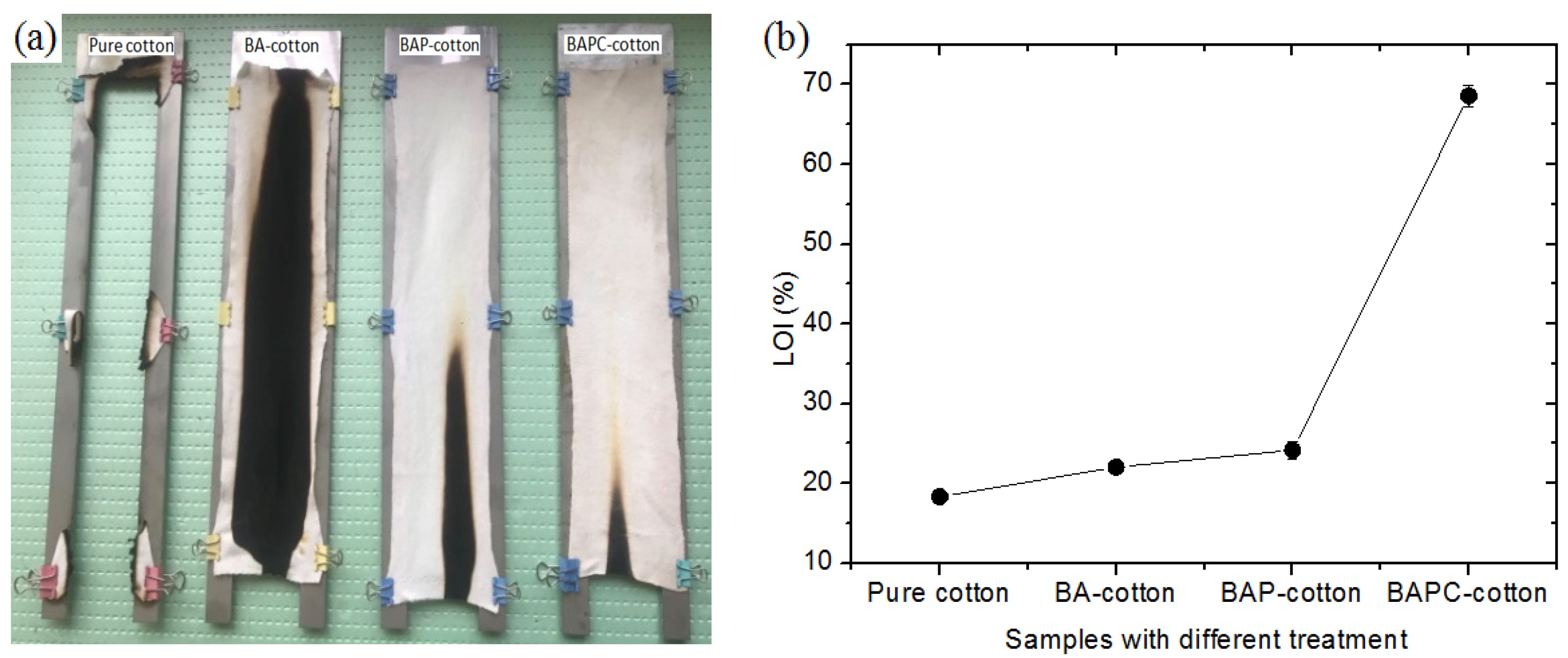
3.7. Flame-Retardant Properties
3.8. Durability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muhammad, K.; Ahmad, J.; Baik, S.W. Early fire detection using convolutional neural networks during surveillance for effective disaster management. Neurocomputing 2018, 288, 30–42. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Qu, L.-Y.; Liu, Y.; Zhu, P. An overview of alginates as flame-retardant materials: Pyrolysis behaviors, flame retardancy, and applications. Carbohydr. Polym. 2021, 260, 117827. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, B.; Xu, Y.-J.; Jiang, Z.; Dong, C.; Liu, Y.; Zhu, P. Ecofriendly Flame-Retardant Cotton Fabrics: Preparation, Flame Retardancy, Thermal Degradation Properties, and Mechanism. ACS Sustain. Chem. Eng. 2019, 7, 19246–19256. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Frache, A.; Malucelli, G. Layer by Layer coatings assembled through dipping, vertical or horizontal spray for cotton flame retardancy. Carbohydr. Polym. 2013, 92, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.S.; Khattab, T.A. Development of One-Step Water-Repellent and Flame-Retardant Finishes for Cotton. ChemistrySelect 2019, 4, 3811–3816. [Google Scholar] [CrossRef]
- Vasiljević, J.; Tomšič, B.; Jerman, I.; Orel, B.; Jakša, G.; Kovač, J.; Simončič, B. Multifunctional superhydrophobic/oleophobic and flame-retardant cellulose fibres with improved ice-releasing properties and passive antibacterial activity prepared via the sol–gel method. J. Sol-Gel Sci. Technol. 2014, 70, 385–399. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C. Fabrication of cotton fabric with superhydrophobicity and flame retardancy. Carbohydr. Polym. 2013, 96, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zheng, J.; Ou, X.; Liu, M. Two in One: Modified polyurethane foams by dip-coating of halloysite nanotubes with acceptable flame retardancy and absorbency. Macromol. Mater. Eng. 2019, 304, 1900213. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Synergistic effects of organically modified montmorillonite on the flame-retardant and smoke suppression properties of transparent intumescent fire-retardant coatings. Prog. Org. Coat. 2018, 122, 107–118. [Google Scholar] [CrossRef]
- Kanat, M.; Eren, T. Synthesis of phosphorus-containing flame-retardants and investigation of their flame-retardant behavior in textile applications. J. Appl. Polym. Sci. 2019, 136, 47935. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Novel flame-retardant finishing systems for cotton fabrics based on phosphorus-containing compounds and silica derived from sol-gel processes. Carbohydr. Polym. 2011, 85, 599–608. [Google Scholar] [CrossRef]
- Reshadi, M.A.M.; Bazargan, A.; McKay, G. A review of the application of adsorbents for landfill leachate treatment: Focus on magnetic adsorption. Sci. Total. Environ. 2020, 731, 138863. [Google Scholar] [CrossRef]
- Fu, Y.; Xiao, C. A facile physical approach to make chitosan soluble in acid-free water. Int. J. Biol. Macromol. 2017, 103, 575–580. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Li, Z.; Zhang, X.; Tian, M.; Zhang, X.; Liu, X.; Qu, L.; Zhu, S. Washable, durable and flame retardant conductive textiles based on reduced graphene oxide modification. Cellulose 2020, 27, 1763–1771. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Hassabo, A.; Mohamed, A.L.; Shaheen, T.I. Surface modification of SiO2 coated ZnO nanoparticles for multifunctional cotton fabrics. J. Colloid Interface Sci. 2017, 498, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, M.; Han, H.; Fan, X.; Liu, Q.; Wang, J. Transparent and abrasion-resistant superhydrophobic coating with robust self-cleaning function in either air or oil. J. Mater. Chem. A 2016, 4, 7869–7874. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Lyu, S.; Fu, F. Preparation of a robust cellulose nanocrystal superhydrophobic coating for self-cleaning and oil-water separation only by spraying. Ind. Crop. Prod. 2018, 122, 438–447. [Google Scholar] [CrossRef]
- Xue, C.-H.; Zhang, L.; Wei, P.; Jia, S.-T. Fabrication of superhydrophobic cotton textiles with flame retardancy. Cellulose 2016, 23, 1471–1480. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Li, Y.; Sun, J. Intumescent flame-retardant and self-healing superhydrophobic coatings on cotton fabric. ACS Nano 2015, 9, 4070–4076. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lyu, S.; Fu, F.; Wu, Y.; Wang, S. Green preparation of a cellulose nanocrystals/polyvinyl alcohol composite superhydrophobic coating. RSC Adv. 2017, 7, 20152–20159. [Google Scholar] [CrossRef] [Green Version]
- Tu, K.; Wang, X.; Kong, L.; Guan, H. Facile preparation of mechanically durable, self-healing and multifunctional superhydrophobic surfaces on solid wood. Mater. Des. 2018, 140, 30–36. [Google Scholar] [CrossRef]
- Huang, J.; Lyu, S.; Chen, Z.; Wang, S.; Fu, F. A facile method for fabricating robust cellulose nanocrystal/SiO2 superhydrophobic coatings. J. Colloid Interface Sci. 2019, 536, 349–362. [Google Scholar] [CrossRef]
- Malucelli, G.; Bosco, F.; Alongi, J.; Carosio, F.; Di Blasio, A.; Mollea, C.; Cuttica, F.; Casale, A. Biomacromolecules as novel green flame retardant systems for textiles: An overview. RSC Adv. 2014, 4, 46024–46039. [Google Scholar] [CrossRef]
- Alongi, J.; Cuttica, F.; Di Blasio, A.; Carosio, F.; Malucelli, G. Intumescent features of nucleic acids and proteins. Thermochim. Acta 2014, 591, 31–39. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Morgan, A.B.; Grunlan, J. Intumescent multilayer nanocoating, made with renewable polyelectrolytes, for flame-retardant cotton. Biomacromolecules 2012, 13, 2843–2848. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.-S.; Deng, C.-L.; Wang, D.-Y.; Song, Y.-P.; Wang, Y.-Z. The synergistic flame-retardant effect of O-MMT on the intumescent flame-retardant PP/CA/APP systems. Polym. Adv. Technol. 2010, 21, 789–796. [Google Scholar] [CrossRef]
- Ortelli, S.; Malucelli, G.; Blosi, M.; Zanoni, I.; Costa, A.L. NanoTiO2@DNA complex: A novel eco, durable, fire retardant design strategy for cotton textiles. J. Colloid Interface Sci. 2019, 546, 174–183. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.-Y.; De Hoop, C.F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef]
- Teixeira, E.D.M.; Corrêa, A.C.; Manzoli, A.; Leite, F.; De Oliveira, C.R.; Mattoso, L.H.C. Cellulose nanofibers from white and naturally colored cotton fibers. Cellulose 2010, 17, 595–606. [Google Scholar] [CrossRef]
- Gao, D.; Wen, X.; Guan, Y.; Czerwonko, W.; Li, Y.; Gao, Y.; Mijowska, E.; Tang, T. Flame retardant effect and mechanism of nanosized NiO as synergist in PLA/APP/CSi-MCA composites. Compos. Commun. 2020, 17, 170–176. [Google Scholar] [CrossRef]
- Shu, F.; Xia, H.H.; Zhou, L.W.; He, Y.H.; Guo, X.L.; Yu, P. Preparation and properties of superhydrophobic silica particles. Chem. Res. Appl. 2016, 28, 512–515. [Google Scholar]
- Hu, Q.; Suzuki, H.; Gao, H.; Araki, H.; Yang, W.; Noda, T. High-frequency FTIR absorption of SiO2/Si nanowires. Chem. Phys. Lett. 2003, 378, 299–304. [Google Scholar] [CrossRef]
- Zheng, Q.; Cai, Z.; Gong, S. Green synthesis of polyvinyl alcohol (PVA)—Cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J. Mater. Chem. A 2014, 2, 3110–3118. [Google Scholar] [CrossRef]
- Bosco, F.; Casale, A.; Mollea, C.; Terlizzi, M.E.; Gribaudo, G.; Alongi, J.; Malucelli, G. DNA coatings on cotton fabrics: Effect of molecular size and pH on flame retardancy. Surf. Coatings Technol. 2015, 272, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Ortelli, S.; Malucelli, G.; Cuttica, F.; Blosi, M.; Zanoni, I.; Costa, A.L. Coatings made of proteins adsorbed on TiO2 nanoparticles: A new flame retardant approach for cotton fabrics. Cellulose 2018, 25, 2755–2765. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Li, M.; Ren, C.; Huang, W.; Wu, Q.; Li, Q.; Zhang, W.; Wang, S. Preparation of High-Efficiency Flame-Retardant and Superhydrophobic Cotton Fabric by a Multi-Step Dipping. Coatings 2021, 11, 1147. https://doi.org/10.3390/coatings11101147
Huang J, Li M, Ren C, Huang W, Wu Q, Li Q, Zhang W, Wang S. Preparation of High-Efficiency Flame-Retardant and Superhydrophobic Cotton Fabric by a Multi-Step Dipping. Coatings. 2021; 11(10):1147. https://doi.org/10.3390/coatings11101147
Chicago/Turabian StyleHuang, Jingda, Mengmeng Li, Changying Ren, Wentao Huang, Qiang Wu, Qian Li, Wenbiao Zhang, and Siqun Wang. 2021. "Preparation of High-Efficiency Flame-Retardant and Superhydrophobic Cotton Fabric by a Multi-Step Dipping" Coatings 11, no. 10: 1147. https://doi.org/10.3390/coatings11101147
APA StyleHuang, J., Li, M., Ren, C., Huang, W., Wu, Q., Li, Q., Zhang, W., & Wang, S. (2021). Preparation of High-Efficiency Flame-Retardant and Superhydrophobic Cotton Fabric by a Multi-Step Dipping. Coatings, 11(10), 1147. https://doi.org/10.3390/coatings11101147






