Influence of the Sputtering Technique and Thermal Annealing on YSZ Thin Films for Oxygen Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystallinity
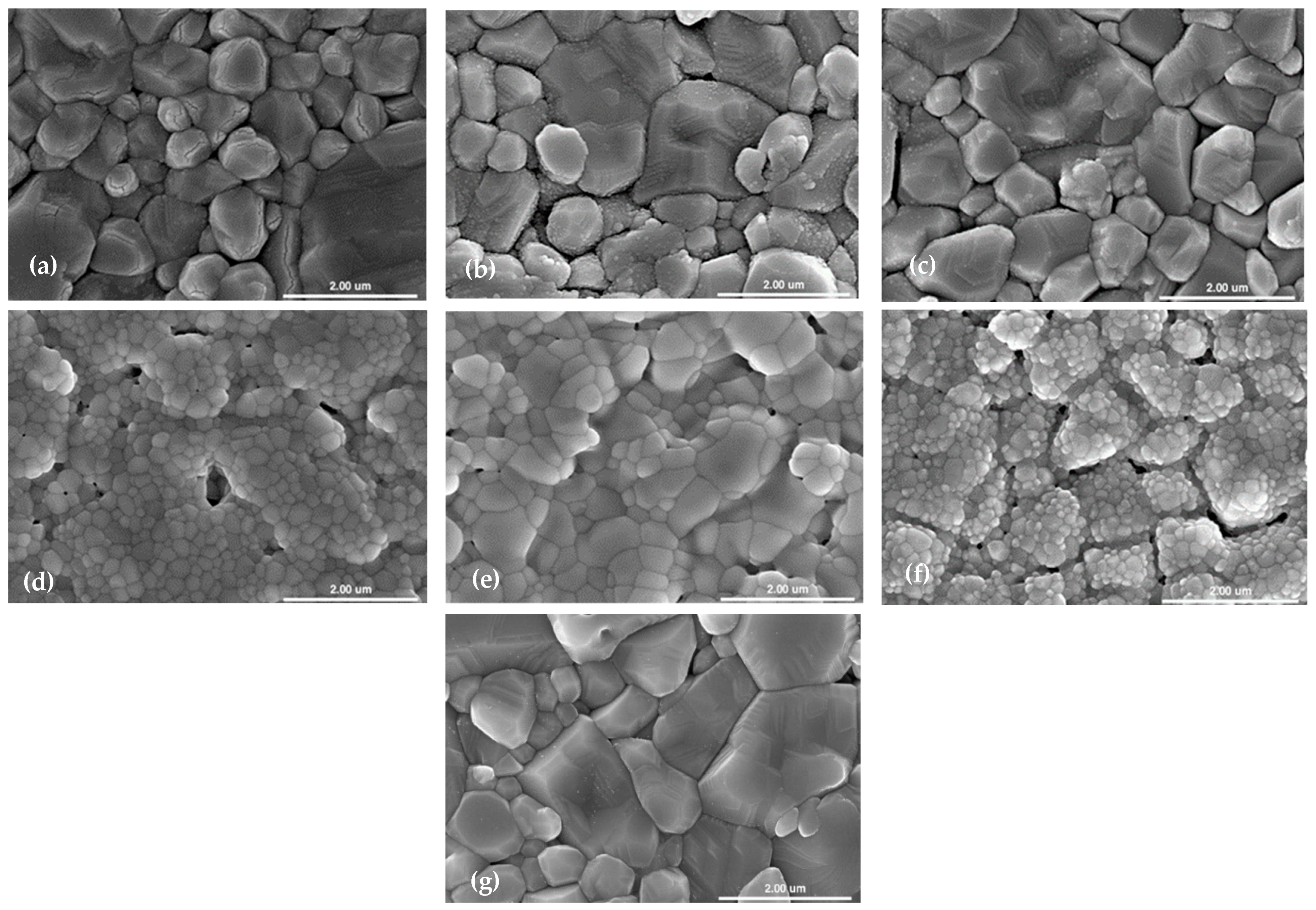
3.2. Thin Film Morphology
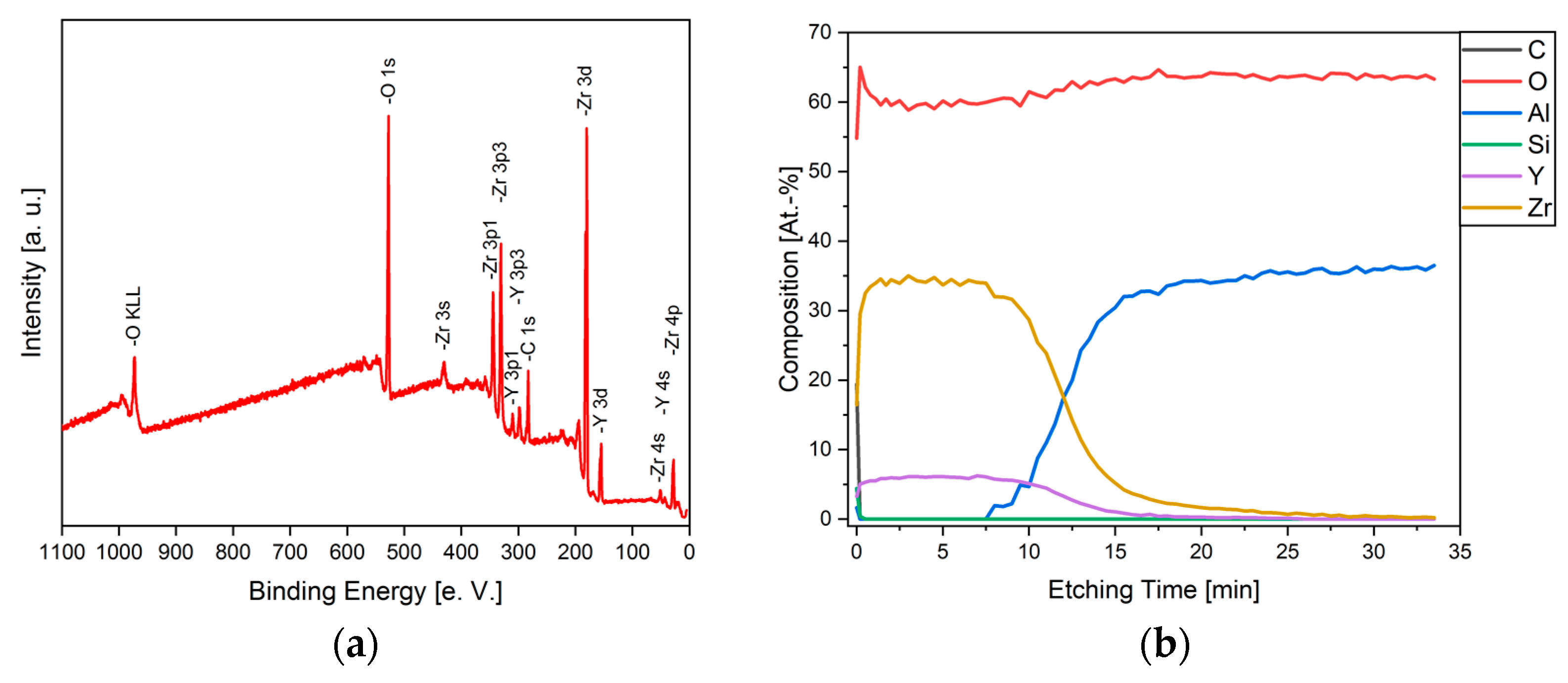
3.3. Elemental Analysis
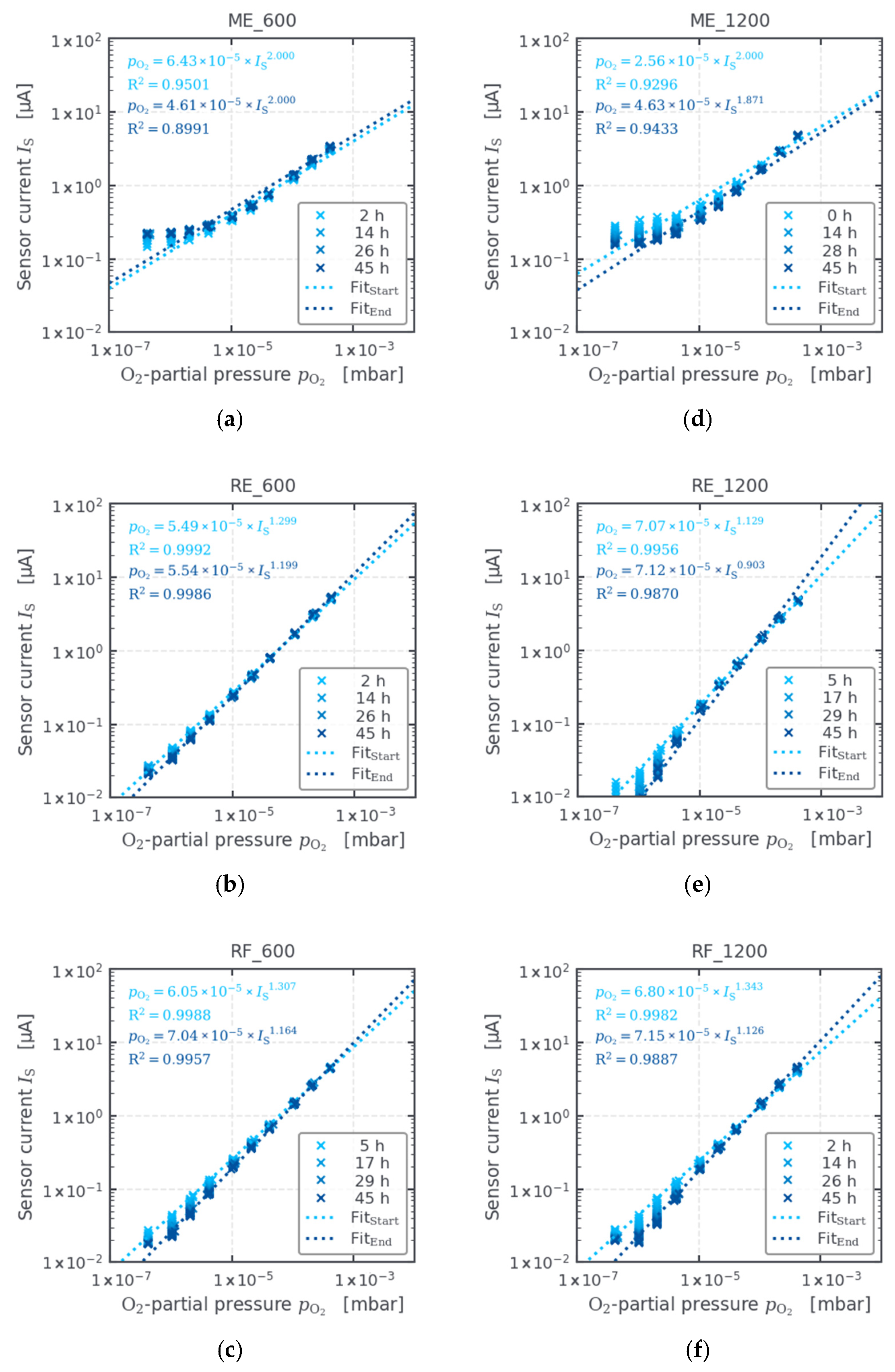
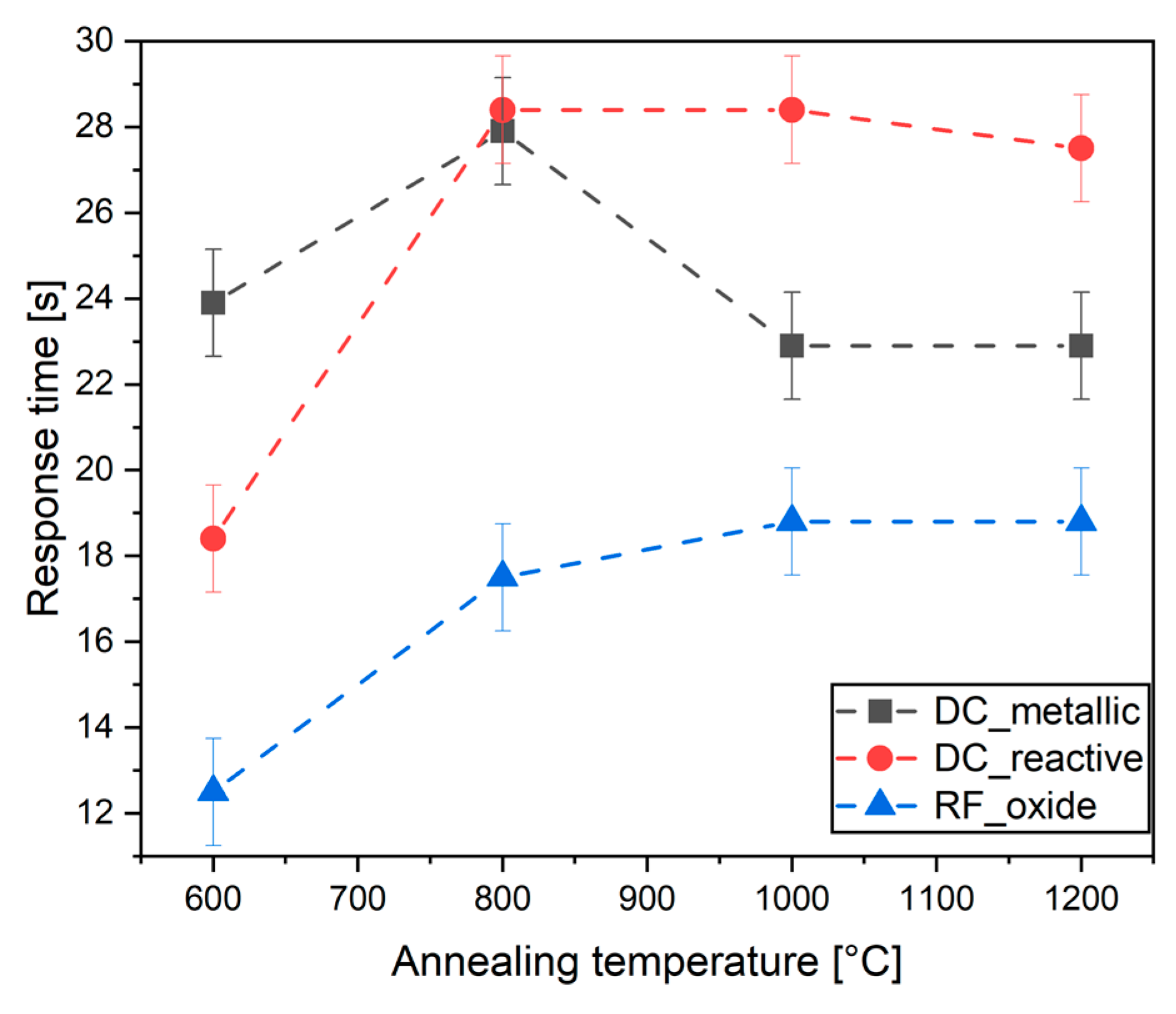
3.4. Oxygen Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, A.; Braun, R.; Schulz, U. PVD thermal barrier coating systems for Mo–Si–B alloys. Mater. High Temp. 2018, 35, 195–203. [Google Scholar] [CrossRef]
- Keyvani, A. Microstructural stability oxidation and hot corrosion resistance of nanostructured Al2O3/YSZ composite compared to conventional YSZ TBC coatings. J. Alloys Compd. 2015, 623, 229–237. [Google Scholar] [CrossRef]
- Budinovskii, S.A.; Chubarov, D.A.; Matveev, P.V.; Smirnov, A.A. Deposition and Properties of the Ceramic TBC Layer Prepared by Magnetron Sputtering. Russ. Metall. 2019, 2019, 1280–1284. [Google Scholar] [CrossRef]
- Jo, S.; Sharma, B.; Park, D.-H.; Myung, J. Materials and nano-structural processes for use in solid oxide fuel cells: A review. J. Korean Ceram. Soc. 2020, 57, 135–151. [Google Scholar] [CrossRef]
- Bae, J.; Chang, I.; Kang, S.; Hong, S.; Cha, S.W.; Kim, Y.B. Post-annealing of thin-film yttria stabilized zirconia electrolytes for anode-supported low-temperature solid oxide fuel cells. J. Nanosci. Nanotechnol. 2014, 14, 9294–9299. [Google Scholar] [CrossRef]
- Jang, D.Y.; Kim, H.K.; Kim, J.W.; Bae, K.; Schlupp, M.V.; Park, S.W.; Prestat, M.; Shim, J.H. Low-temperature performance of yttria-stabilized zirconia prepared by atomic layer deposition. J. Power Sources 2015, 274, 611–618. [Google Scholar] [CrossRef]
- Nurhamizah, A.R.; Ibrahim, Z.; Muhammad, R.; Wahab, Y.; Sakrani, S. Effect of Annealing Temperature on Platinum/YSZ Thin Film Fabricated Using RF and DC Magnetron Sputtering. In Solid State Phenomena; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2017; Volume 268, pp. 229–233. [Google Scholar] [CrossRef]
- Liu, Y.; Parisi, J.; Sun, X.; Lei, Y. Solid-state gas sensors for high temperature applications—A review. J. Mater. Chem. A 2014, 2, 9919–9943. [Google Scholar] [CrossRef]
- Akasaka, S. Thin film YSZ-based limiting current-type oxygen and humidity sensor on thermally oxidized silicon substrates. Sens. Actuators B Chem. 2016, 236, 499–505. [Google Scholar] [CrossRef]
- Xu, L.; Dai, Z.; Duan, G.; Guo, L.; Wang, Y.; Zhou, H.; Liu, Y.; Cai, W.; Wang, Y.; Li, T. Micro/Nano gas sensors: A new strategy towards in-situ wafer-level fabrication of high-performance gas sensing chips. Sci. Rep. 2015, 5, 10507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butz, B.; Störmer, H.; Gerthsen, D.; Bockmeyer, M.; Krüger, R.; Ivers-Tiffée, E.; Luysberg, M. Microstructure of Nanocrystalline Yttria-Doped Zirconia Thin Films Obtained by Sol-Gel Processing. J. Am. Ceram. Soc. 2008, 91, 2281–2289. [Google Scholar] [CrossRef]
- Yamada, T.; Okuda, R.; Hirakoso, H.; Kozuka, H. Sol–gel preparation of yttria-stabilized zirconia thin films and transfer to polycarbonate substrates. J. Sol.-Gel. Sci. Technol. 2019, 92, 554–561. [Google Scholar] [CrossRef]
- Schlupp, M.V.F.; Evans, A.; Martynczuk, J.; Prestat, M. Micro-Solid Oxide Fuel Cell Membranes Prepared by Aerosol-Assisted Chemical Vapor Deposition. Adv. Energy Mater. 2014, 4, 1301383. [Google Scholar] [CrossRef]
- Solovyev, A. Comparison of Sputter-deposited Single and Multilayer Electrolytes based on Gadolinia-doped Ceria and Yttria- stabilized Zirconia for Solid Oxide Fuel Cells. Int. J. Electrochem. Sci. 2020, 231–240. [Google Scholar] [CrossRef]
- Peters, C.; Weber, A.; Butz, B.; Gerthsen, D.; Ivers-Tiffée, E. Grain-Size Effects in YSZ Thin-Film Electrolytes. J. Am. Ceram. Soc. 2009, 92, 2017–2024. [Google Scholar] [CrossRef]
- Mengucci, P.; Barucca, G.; Caricato, A.P.; Di Cristoforo, A.; Leggieri, G.; Luches, A.; Majnia, G. Effects of annealing on the microstructure of yttria-stabilised zirconia thin films deposited by laser ablation. Thin Solid Film. 2005, 478, 125–131. [Google Scholar] [CrossRef]
- Briois, P.; Gourba, E.; Billard, A.; Ringuedé, A.; Cassir, M. Microstructure—Electrical properties relationship of YSZ thin films reactively sputter-deposited at different pressures. Ionics 2001, 11, 301–305. [Google Scholar] [CrossRef]
- Paek, J.Y.; Chang, I.; Park, J.H.; Ji, S.; Cha, S.W. A study on properties of yttrium-stabilized zirconia thin films fabricated by different deposition techniques. Renew. Energy 2014, 65, 202–206. [Google Scholar] [CrossRef]
- López-Gándara, C.; Ramos, F.M.; Cirera, A. YSZ-Based Oxygen Sensors and the Use of Nanomaterials: A Review from Classical Models to Current Trends. J. Sens. 2009, 2009, 1–15. [Google Scholar] [CrossRef]
- Schwarzwaelder, C. Inkjet Method for the Development of Solid State Electrolyte Sensors for Space Applications; University of Stuttgart: Stuttgart, Germany, 2016. [Google Scholar]
- Götsch, T.; Wallisch, W.; Stöger-Pollach, M.; Klötzer, B.; Penner, S. From zirconia to yttria: Sampling the YSZ phase diagram using sputter-deposited thin films. AIP Adv. 2016, 6, 25119. [Google Scholar] [CrossRef] [Green Version]
- Rusli, N.A.; Muhammad, R.; Ghoshal, S.K.; Nur, H.; Nayan, N. Annealing temperature induced improved crystallinity of YSZ thin film. Mater. Res. Express 2020, 7, 56406. [Google Scholar] [CrossRef]
- Rusli, N.A.; Muhammad, R.; Ghoshal, S.K.; Nur, H.; Nayan, N.; Jaafar, S.N. Bias voltage dependent structure and morphology evolution of magnetron sputtered YSZ thin film: A basic insight. Mater. Res. Express 2019, 6, 106414. [Google Scholar] [CrossRef]
- Smeacetto, F.; Salvo, M.; Ajitdoss, L.C.; Perero, S.; Moskalewicz, T.; Boldrini, S.; Doubova, L.; Ferraris, M. Yttria-stabilized zirconia thin film electrolyte produced by RF sputtering for solid oxide fuel cell applications. Mater. Lett. 2010, 64, 2450–2453. [Google Scholar] [CrossRef]
- Kastelik, R. Messsysteme für Sauerstoff-Sensoren auf Basis von Festkörperelektrolyten; University of Stuttgart: Stuttgart, Germany, 2020. [Google Scholar]
- Patel, P.; Hull, T.R.; McCabe, R.W.; Flath, D.; Grasmeder, J.; Percy, M. Mechanism of thermal decomposition of poly(ether ether ketone) (PEEK) from a review of decomposition studies. Polym. Degrad. Stab. 2010, 95, 709–718. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Huang, X.; Zhang, H.; Xue, Q.; Xu, H.; Wang, L.; Feng, Z. The effect of powder grain size on the microstructure and electrical properties of 8 mol% Y2O3 -stabilized ZrO2. RSC Adv. 2017, 7, 39153–39159. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, K.M.; Rajeswara Rao, L.L.; Jampana, N. Investigation of Annealing Induced Yttria Segregation in Sputtered Yttria-Stabilized Zirconia Thin Films. J. Am. Ceram. Soc. 2015, 98, 3389–3397. [Google Scholar] [CrossRef]
- An, J.; Bae, J.; Hong, S.; Koo, B.; Kim, Y.-B.; Gür, T.M.; Prinz, F.B. Grain boundary blocking of ionic conductivity in nanocrystalline yttria-doped ceria thin films. Scr. Mater. 2015, 104, 45–48. [Google Scholar] [CrossRef] [Green Version]
- Vonk, V.; Khorshidi, N.; Stierle, A.; Dosch, H. Atomic structure and composition of the yttria-stabilized zirconia (111) surface. Surf. Sci. 2013, 612, 69–76. [Google Scholar] [CrossRef]
- Ridder, M.D.; Welzenis, R.G.V.; Gon, A.W.D.V.D.; Brongersma, H.H.; Wulff, S.; Chu, W.-F.; Weppner, W. Subsurface segregation of yttria in yttria stabilized zirconia. J. Appl. Phys. 2002, 92, 3056–3064. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; An, J.; Lee, M.H.; Fritz, B.P.; Lee, W. Effects of surface chemistry and microstructure of electrolyte on oxygen reduction kinetics of solid oxide fuel cells. J. Power Sources 2015, 295, 74–78. [Google Scholar] [CrossRef]
- Sridhar, K.; Blanchard, J. Electronic conduction in low oxygen partial pressure measurements using an amperometric zirconia oxygen sensor. Sens. Actuators B 1999, 59, 60–67. [Google Scholar] [CrossRef]
- Durá, O.J.; La López de Torre, M.A.; Vázquez, L.; Chaboy, J.; Boada, R.; Rivera-Calzada, A.; Santamaria, J.; Leon, C. Ionic conductivity of nanocrystalline yttria-stabilized zirconia: Grain boundary and size effects. Phys. Rev. B 2010, 81. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Maier, J. Grain Boundary Blocking Effect in Zirconia: A Schottky Barrier Analysis. J. Electrochem. Soc. 2001, 148, E121–E126. [Google Scholar] [CrossRef]
- Guo, X.; Waser, R. Electrical properties of the grain boundaries of oxygen ion conductors: Acceptor-doped zirconia and ceria. Prog. Mater. Sci. 2006, 51, 151–210. [Google Scholar] [CrossRef]
- Lee, H.B.; Prinz, F.B.; Cai, W. Atomistic simulations of grain boundary segregation in nanocrystalline yttria-stabilized zirconia and gadolinia-doped ceria solid oxide electrolytes. Acta Mater. 2013, 61, 3872–3887. [Google Scholar] [CrossRef]
- Schlupp, M.V.F.; Scherrer, B.; Ma, H.; Grolig, J.G.; Martynczuk, J.; Prestat, M.; Gauckler, L.J. Influence of microstructure on the cross-plane oxygen ion conductivity of yttria stabilized zirconia thin films. Phys. Status Solidi A 2012, 209, 1414–1422. [Google Scholar] [CrossRef]
- Yeh, T.-H.; Lin, R.-D.; Cherng, J.-S. Significantly enhanced ionic conductivity of yttria-stabilized zirconia polycrystalline nano-film by thermal annealing. Thin Solid Film 2013, 544, 148–151. [Google Scholar] [CrossRef]
- Mills, E.M.; Kleine-Boymann, M.; Janek, J.; Yang, H.; Browning, N.D.; Takamura, Y.; Kim, S. YSZ thin films with minimized grain boundary resistivity. Phys. Chem. Chem. Phys. 2016, 18, 10486–10491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Sigle, W.; Maier, J. Blocking Grain Boundaries in Yttria-Doped and Undoped Ceria Ceramics of High Purity. J. Am. Ceram. Soc. 2003, 86, 77–87. [Google Scholar] [CrossRef]
- Bae, J.W.; Park, J.Y.; Hwang, S.W.; Yeom, G.Y.; Kim, K.D.; Cho, Y.A.; Jeon, J.S.; Choi, D. Characterization of Yttria-Stabilized Zirconia Thin Films Prepared by Radio Frequency Magnetron Sputtering for a Combustion Control Oxygen Sensor. J. Electrochem. Soc. 2000, 147, 2380–2384. [Google Scholar] [CrossRef] [Green Version]
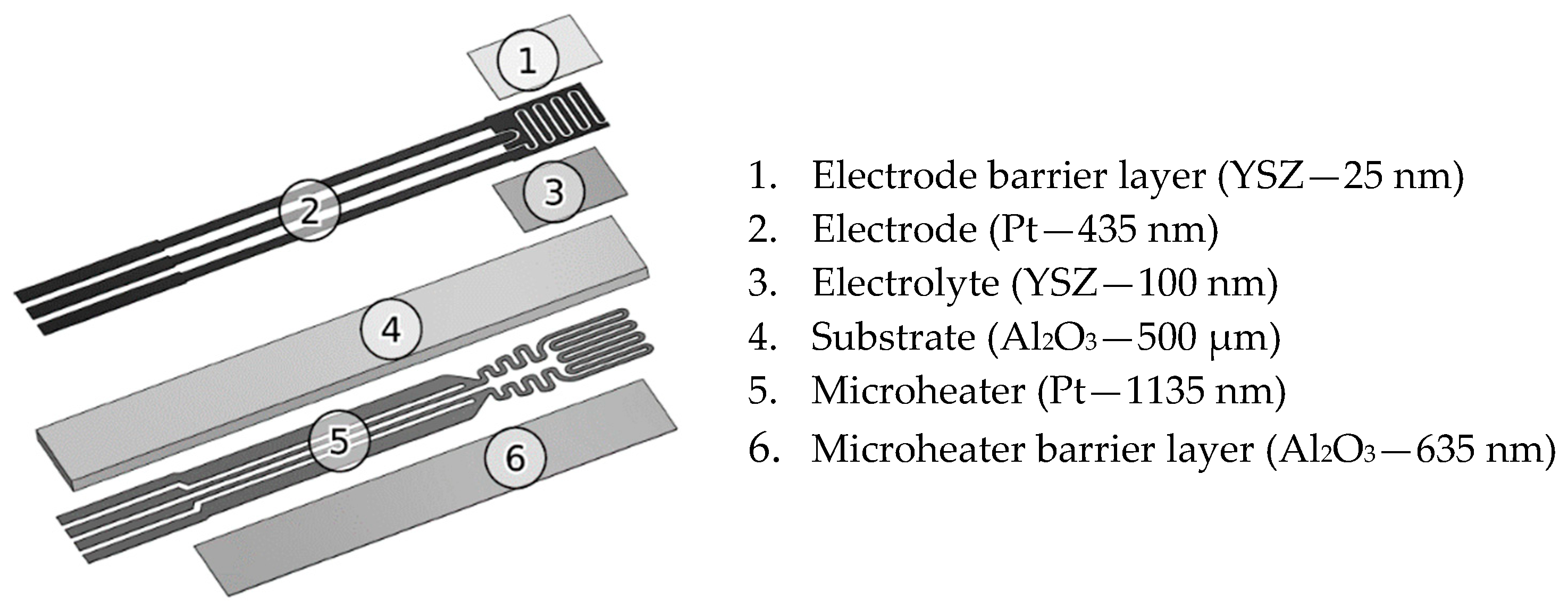



| Power Source | Mode | Sputtering Power Density (w/cm2) | Ar Flow (sscm) | O2 Flow (sscm) |
|---|---|---|---|---|
| direct current | metallic | 1.27 | 58 | 0 |
| direct current | reactive | 2.08 | 58 | 15 |
| radio frequency | oxide | 5.09 | 28 | 0 |
| 600 °C | 800 °C | 1000 °C | 1200 °C | |
|---|---|---|---|---|
| DC-metallic | 11 | 13 | 26 | 32 |
| DC-reactive | 27 | 26 | 29 | 29 |
| RF-oxide | 13 | 18 | 27 | 29 |
| Sample | C | O | Al | Si | Ag | Zr | Y | Y2O3 (mol.%) |
|---|---|---|---|---|---|---|---|---|
| DC-reactive_600 | 19.9 | 54.9 | 0 | 0 | 0 | 20.0 | 5.3 | 11.7 |
| DC-metallic_600 | 22.6 | 52.7 | 0 | 0 | 0.3 | 17.7 | 6.8 | 16.1 |
| RF-oxide_as-sputtered | 26.1 | 51.0 | 0 | 0 | 0 | 19.2 | 3.7 | 8.8 |
| RF-oxide_600 | 22.4 | 52.9 | 0 | 0 | 0 | 20.7 | 3.9 | 8.6 |
| RF-oxide_800 | 38.9 | 42.0 | 0 | 0 | 0 | 15.5 | 3.0 | 8.8 |
| RF-oxide_1000 | 21.9 | 53.9 | 1.1 | 1.8 | 0 | 17.6 | 3.4 | 8.8 |
| RF-oxide_1200 | 19.4 | 54.8 | 1.7 | 4.4 | 0 | 16.4 | 3.3 | 9.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz Alpuche, E.; Gröger, P.; Wang, X.; Kroyer, T.; Fasoulas, S. Influence of the Sputtering Technique and Thermal Annealing on YSZ Thin Films for Oxygen Sensing Applications. Coatings 2021, 11, 1165. https://doi.org/10.3390/coatings11101165
Paz Alpuche E, Gröger P, Wang X, Kroyer T, Fasoulas S. Influence of the Sputtering Technique and Thermal Annealing on YSZ Thin Films for Oxygen Sensing Applications. Coatings. 2021; 11(10):1165. https://doi.org/10.3390/coatings11101165
Chicago/Turabian StylePaz Alpuche, Emilio, Pascal Gröger, Xuetao Wang, Thomas Kroyer, and Stefanos Fasoulas. 2021. "Influence of the Sputtering Technique and Thermal Annealing on YSZ Thin Films for Oxygen Sensing Applications" Coatings 11, no. 10: 1165. https://doi.org/10.3390/coatings11101165
APA StylePaz Alpuche, E., Gröger, P., Wang, X., Kroyer, T., & Fasoulas, S. (2021). Influence of the Sputtering Technique and Thermal Annealing on YSZ Thin Films for Oxygen Sensing Applications. Coatings, 11(10), 1165. https://doi.org/10.3390/coatings11101165






