Proso-Millet-Starch-Based Edible Films: An Innovative Approach for Food Industries
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Starch Isolation from Proso Millet
2.3. Physical and Chemical Properties of the Starch
2.3.1. Amylose Content, Solubility and Swelling Power
2.3.2. Water Binding Capacity
2.3.3. Light Transmittance
2.3.4. Film Preparation
2.3.5. Film Thickness and Moisture Content
2.3.6. Water Vapor Permeability
2.3.7. Solubility
2.4. X-ray Diffraction (XRD)
2.5. Scanning Electron Microscopy (SEM)
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Antioxidant Properties of Proso Millet starch and Films
2.7.1. Total Phenolic Content (TPC)
2.7.2. DPPH Radical Scavenging Capacity Assay
2.7.3. Total Antioxidant Capacity (TAC)
2.7.4. Condensed Tannin Content (CTC)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Functional Properties of Starch
3.1.1. Amylose Content, Swelling Power and Solubility
3.1.2. Light Transmittance
3.1.3. Water Binding Capacity
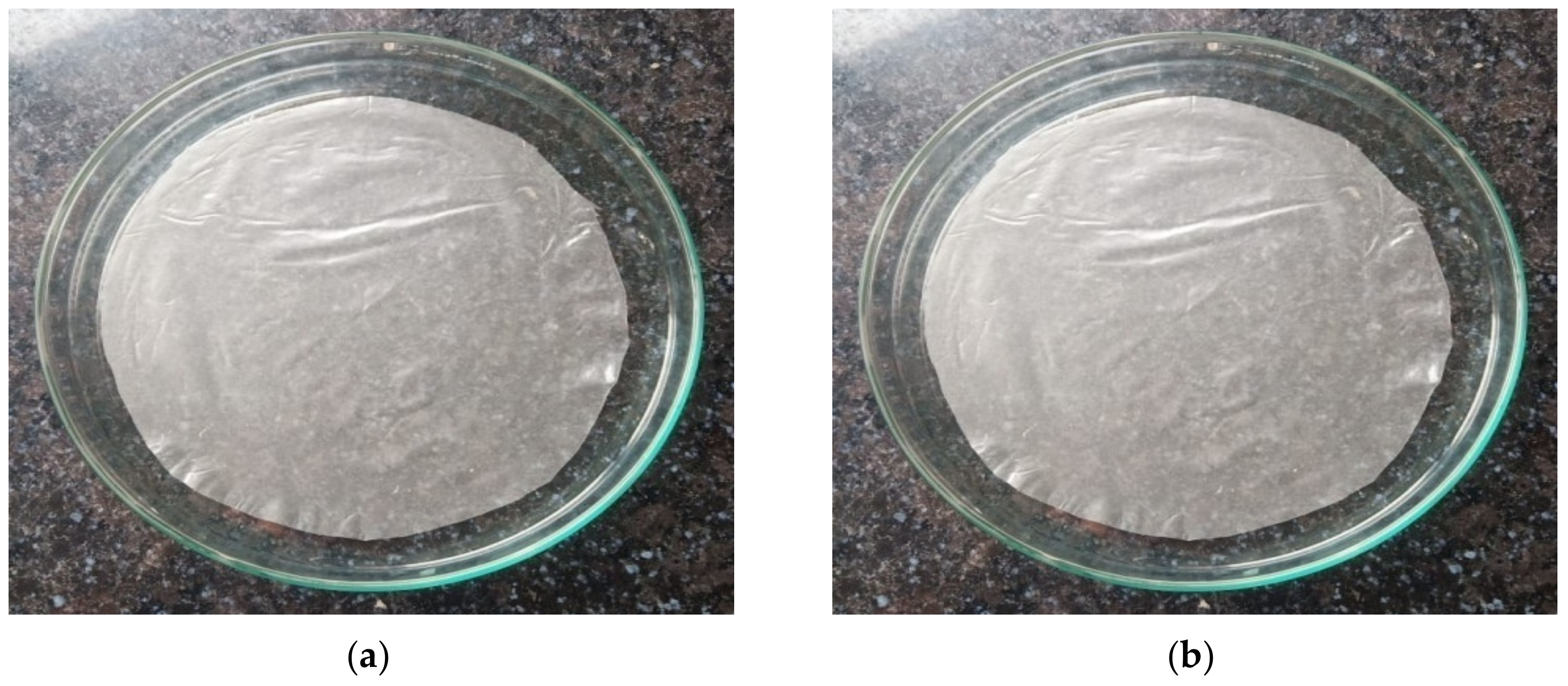
3.2. Starch Film Properties
3.2.1. Film Thickness and Moisture Content
3.2.2. Water Vapor Permeability
3.2.3. Solubility
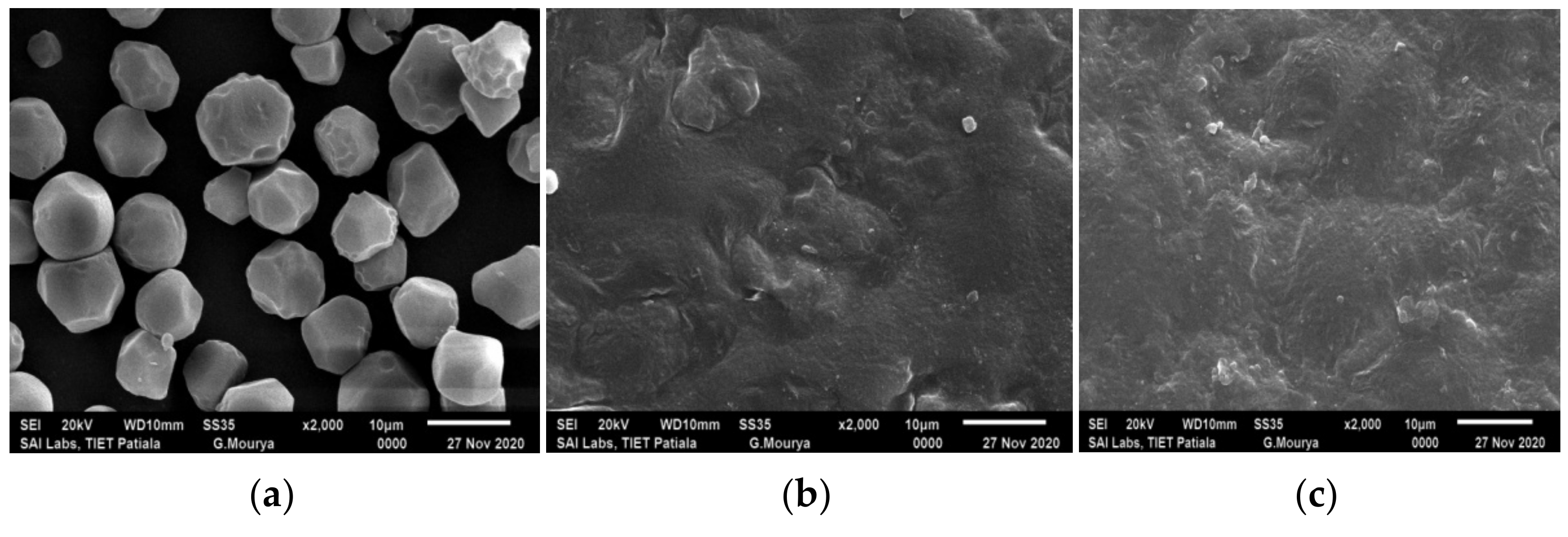
3.3. SEM
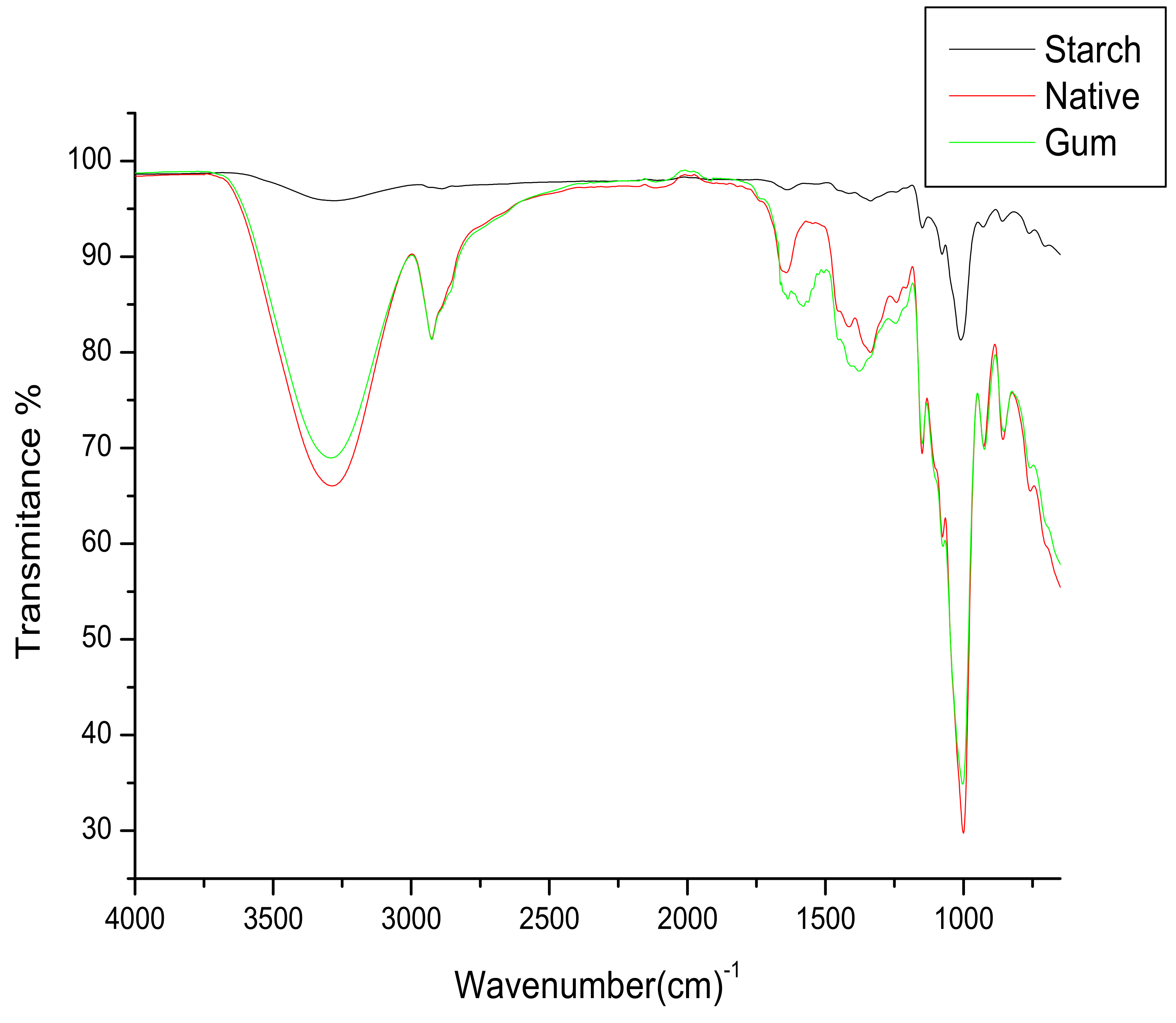
3.4. FTIR
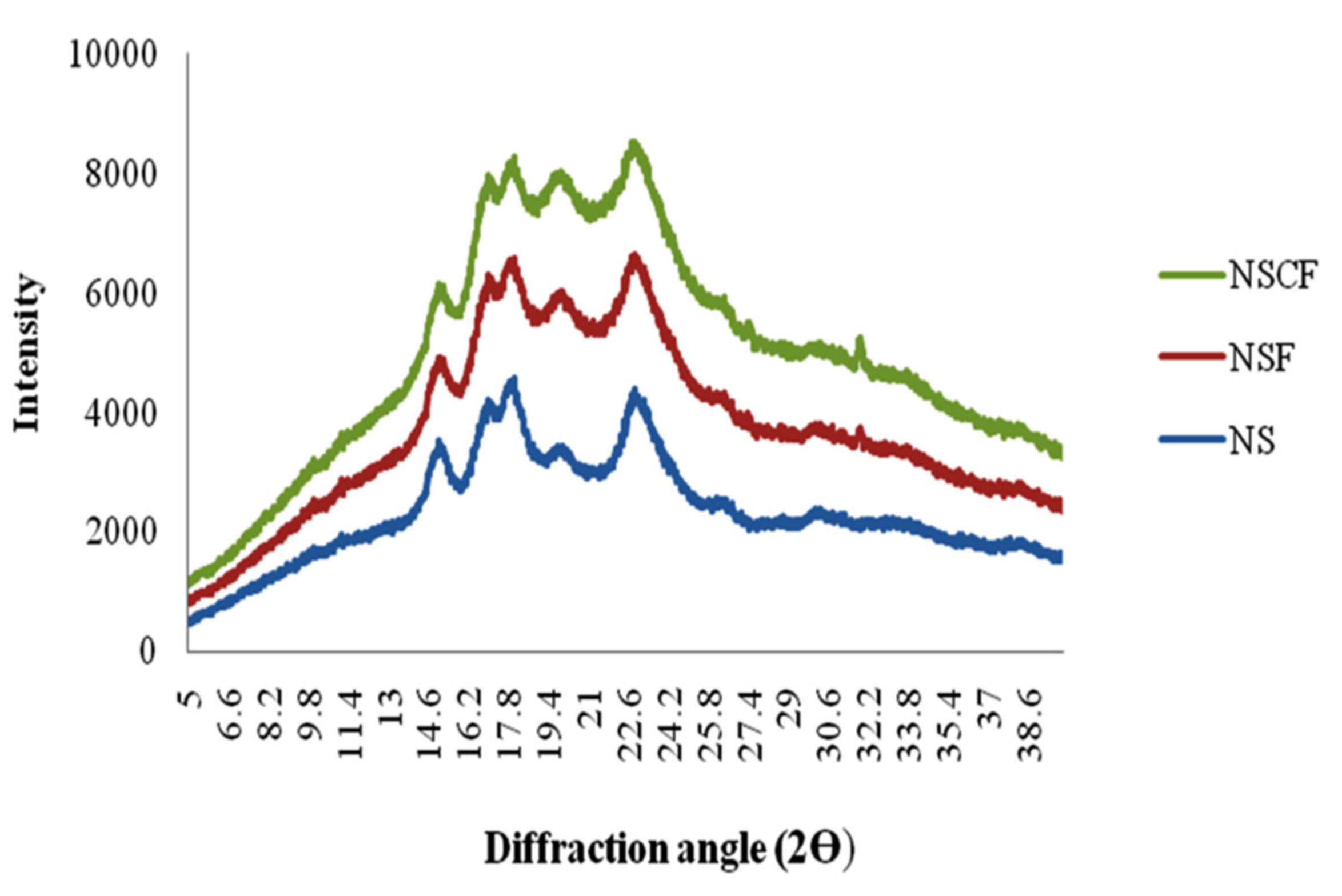
3.5. XRD
3.6. Antioxidant Properties of the Proso Millet Starch and Films
3.6.1. TPC and CTC
3.6.2. DPPH Assay and TAC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dietrich, T.; Velasco, M.V.; Echeverría, P.; Pop, B.; Rusu, A. Crop and plant biomass as valuable material for BBB. Alternatives for valorization of green wastes. In Biotransformation of Agricultural Waste and by-Products: The Food, Feed, Fibre, Fuel (4F) Economy; Elsevier: San Diego, CA, USA, 2016. [Google Scholar]
- Li, K.; Zhang, T.; Narayanamoorthy, S.; Jin, C.; Sui, Z.; Li, Z.; Li, S.; Wu, K.; Liu, G.; Corke, H. Diversity analysis of starch physicochemical properties in 95 proso millet (Panicum miliaceum L.) accessions. Food Chem. 2020, 324, 126863. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, E.; Duizer, L.M.; McSweeney, M.B. Descriptive analysis of a new proso millet product. Int. J. Gastron. Food Sci. 2017, 8, 14–18. [Google Scholar] [CrossRef]
- Gong, X.; Dang, K.; Lv, S.; Zhao, G.; Tian, L.; Luo, Y.; Feng, B. Interspecific root interactions and water-use efficiency of intercropped proso millet and mung bean. Eur. J. Agron. 2020, 115, 126034. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, H.; Zhang, D.; Li, J.; Gong, X.; Feng, B.; Xue, Z.; Yang, P. Effects of ridging and mulching combined practices on proso millet growth and yield in semi-arid regions of China. Field Crop. Res. 2017, 213, 65–74. [Google Scholar] [CrossRef]
- Vianna, T.C.; Marinho, C.O.; Junior, L.M.; Ibrahim, S.A.; Vieira, R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Deng, Y.; Liu, T.; Lu, D. Effect of starch film containing thyme essential oil microcapsules on physicochemical activity of mango. LWT—Food Sci. Technol. 2020, 131, 109700. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Raszkowska-Kaczor, A.; Rogacz, D.; Zarska, S.; Kapusniak, J. From high oleic vegetable oils to hydrophobic starch derivatives: II. Physicochemical, processing and environmental properties. Carbohydr. Polym. 2020, 243, 116499. [Google Scholar] [CrossRef]
- Volpe, V.; De-Feo, G.; De Marco, I.; Pantani, R. Use of sunflower seed fried oil as an ecofriendly plasticizer for starch and application of this thermoplastic starch as a filler for PLA. Ind. Crop. Prod. 2018, 122, 545–552. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef]
- Bangar, S.P.; Siroha, A.K.; Nehra, M.; Trif, M.; Ganwal, V.; Kumar, S. Structural and Film-Forming Properties of Millet Starches: A Comparative Study. Coatings 2021, 11, 954. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Cheng, M. Preparation and Characterization of Potato Starch Film with Various Size of Nano-SiO2. Polymers 2018, 10, 1172. [Google Scholar] [CrossRef]
- Kalam Azad, M.O.; Jeong, D.I.; Adnan, M.; Salitxay, T.; Heo, J.W.; Naznin, M.T.; Lim, J.D.; Cho, D.H.; Park, B.J.; Park, C.H. Effect of Different Processing Methods on the Accumulation of the Phenolic Compounds and Antioxidant Profile of Broomcorn Millet (Panicum miliaceum L.) Flour. Foods 2019, 8, 230. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Naczk, M.; Shahidi, F. Effect of processing on the antioxidant activity of millet grains. Food Chem. 2012, 133, 1–9. [Google Scholar] [CrossRef]
- Farooq, M.; Azadfar, E.; Rusu, A.; Trif, M.; Poushi, M.K.; Wang, Y. Improving the Shelf Life of Peeled Fresh Almond Kernels by Edible Coating with Mastic Gum. Coatings 2021, 11, 618. [Google Scholar] [CrossRef]
- Abdou, E.S.; Sorour, M.A. Preparation and characterization of starch/carrageenan edible films. Int. Food Res. J. 2014, 21, 191–195. [Google Scholar]
- Al-Hashimi, A.G.; Ammar, A.B.; Govindan, L.; Cacciola, F.; Lakhssassi, N. Development of a Millet Starch Edible Film Containing Clove Essential Oil. Foods 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.K.; Smith, R.C. Recent advances in starch-based films toward food packaging applications: Physicochemical, mechanical, and functional properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1–53. [Google Scholar] [CrossRef]
- Rusu, A.V.; Criste, F.L.; Mierliţă, D.; Socol, C.T.; Trif, M. Formulation of Lipoprotein Microencapsulated Beadlets by Ionic Complexes in Algae-Based Carbohydrates. Coatings 2020, 10, 302. [Google Scholar] [CrossRef]
- Davydova, V.N.; Sorokina, I.V.; Volod’ko, A.V.; Sokolova, E.V.; Borisova, M.S.; Yermak, I.M. The Comparative Immunotropic Activity of Carrageenan, Chitosan and Their Complexes. Mar. Drugs 2020, 18, 458. [Google Scholar] [CrossRef]
- Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. On the use of carrageenan matrices for the development of antiviral edible coatings of interest in berries. Food Hydrocoll. 2019, 92, 74–85. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, L.; Calinoiu, L.F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ferenczi, L.J.; Stefanescu, B.E.; Vodnar, D.C. Inhibitory Potential of Lactobacillus Plantarum on Escherichia coli. Bull. UASVM Food Sci. Technol. 2017, 74. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Liu, R.; Niu, W. Phytochemical and antiproliferative activity of proso millet. PLoS ONE 2014, 9, e104058. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.S.; Singh, N. Some properties of corn starches II: Physicochemical, gelatinization, retrogradation, pasting and gel textural properties. Food Chem. 2007, 101, 1499–1507. [Google Scholar] [CrossRef]
- Williams, P.C.; Kuzina, F.D.; Hlynka, I. Rapid colorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 1970, 47, 411–420. [Google Scholar]
- Yamazaki, W.T. An alkaline water retention capacity test for the evaluation of cookies in baking potentialities of soft winter wheat flours. Cereal Chem. 1953, 30, 242–246. [Google Scholar]
- Craig, S.A.S.; Maningat, C.C.; Seib, P.A.; Hoseney, R.C. Starch paste clarity. Cereal Chem. 1989, 66, 173–182. [Google Scholar]
- Romero-Bastida, C.A.; Bello-Pérez, L.A.; García, M.A.; Martino, M.N.; Solorza-Feria, J.; Zaritzky, N.E. Physicochemical and microstructural characterization of films prepared by thermal and cold gelatinization from non-conventional sources of starches. Carbohydr. Polym. 2005, 60, 235–244. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, N.; Sandhu, K.S.; Guraya, H.S. Physicochemical, morphological, thermal and rheological properties of starches separated from kernels of some Indian mango cultivars (Mangifera indica L.). Food Chem. 2004, 85, 131–140. [Google Scholar] [CrossRef]
- Yu, J.; Fleming, S.L.; Williams, B.; Williams, E.V.; Li, Z.; Somma, P.; Rieder, C.L.; Goldberg, M.L. Greatwall kinase: A nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J. Cell Biol. 2004, 16, 487–492. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenumcomplex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Julkunen-Titto, R. Phenolics constituents in the leaves of northern willos: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Singh, M.; Adedeji, A.A. Physicochemical and functional properties of proso millet starch. Starch/Staerke 2016, 61, 1165–1174. [Google Scholar]
- Singh, N.; Kaur, L.; Sandhu, K.S.; Kaur, J.; Nishinari, K. Relationships between physicochemical, morphological, thermal, rheological properties of rice starches. Food Hydrocoll. 2006, 20, 532–542. [Google Scholar] [CrossRef]
- Kasemsuwan, T.; Jane, J. A quantitative method for the survey of starch phosphate derivatives and starch phospholipids by 31P nuclear magnetic resonance spectroscopy. Cereal Chem. 1996, 73, 702–707. [Google Scholar]
- Olayinka, O.O.; Adebowale, K.O.; Olu-Owolabi, B.I. Effect of heat-moisture treatment on physicochemical properties of white sorghum starch. Food Hydrocoll. 2008, 22, 225–230. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using response surface methodology. Int. J. Biol. Macromol. 2020, 143, 704–713. [Google Scholar] [CrossRef]
- Ahmad, M.; Hani, N.M.; Nirmal, N.P.; Fazial, F.F.; Mohtar, N.F.; Romli, S.R. Optical and thermo-mechanical properties of composite films based on fish gelatin/rice flour fabricated by casting technique. Prog. Org. Coat. 2015, 84, 115–127. [Google Scholar] [CrossRef]
- Shaikh, M.; Haider, S.; Ali, T.M.; Hasnain, A. Physical, thermal, mechanical and barrier properties of pearl millet starch films as affected by levels of acetylation and hydroxypropylation. Int. J. Biol. Macromol. 2019, 124, 209–219. [Google Scholar] [CrossRef]
- Sukhija, S.; Singh, S.; Riar, C.S. Analyzing the effect of whey protein concentrate and psyllium husk on various characteristics of biodegradable film from lotus (Nelumbo nucifera) rhizome starch. Food Hydrocoll. 2016, 60, 128–137. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch-CMC-nanoclay biodegradable film. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Cuq, J.L.; Guilbert, S. Rheological model for the mechanical properties of myofibrillar protein-based films. J. Agric. Food Chem. 1996, 44, 1116–1122. [Google Scholar] [CrossRef]
- García, N.L.; Famá, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. A comparison between the physico-chemical properties of tuber and cereal starches. Food Res. Int. 2009, 42, 976–982. [Google Scholar] [CrossRef]
- Shi, A.M.; Wang, L.J.; Li, D.; Benu, A. The effect of annealing and cryoprotectants on the properties of vacuum-freeze dried starch nanoparticles. Carbohydr. Polym. 2012, 88, 1334–1341. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Bai, Y.; Xu, X.; Jin, Z. Structure and properties of maize starch processed with a combination of α-amylase and pullulanase. Int. J. Biol. Macromol. 2013, 52, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Liu, X.; Zhang, Y.; Yao, K. Study of phase behavior on chitosan/viscose rayon blend film. J. Appl. Polym. Sci. 1998, 67, 1965–1972. [Google Scholar] [CrossRef]
- Ramos, O.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Pocas, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured there from. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Toro-Márquez, L.A.; Merino, D.; Mendieta, J.R. Hydrogen-bonding interactions and compostability of bionanocomposite films prepared from corn starch and nano-fillers with and without added Jamaica flower extract. Food Hydrocoll. 2019, 89, 283–293. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, F.; Li, Y.; Shoemaker, C.F.; Xia, W. Preparation and characterization of pulullan-chitosan and pullulan-carboxymethyl chitosan blended films. Food Hydrocoll. 2013, 30, 82–91. [Google Scholar] [CrossRef]
- Baek, S.K.; Song, K.B. Characterization of active biodegradable films based on proso millet starch and curcumin. Starch/Stärke 2018, 71, 3–4. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S. Phenolic content, antioxidant potential and DNA damage protection of pearl millet (Pennisetum glaucum) cultivars of North Indian region. Food Meas. 2017, 11, 126–133. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Bioaccessibility and antioxidant potential of millet grain phenolics as affected by simulated in vitro digestion and microbial fermentation. J. Funct. Food 2012, 4, 226–237. [Google Scholar]
- Kumari, D.; Madhujith, T.; Chandrasekara, A. Comparison of phenolic content and antioxidant activities of millet varieties grown in different locations in Sri Lanka. Food Sci. Nutr. 2017, 5, 474–485. [Google Scholar] [CrossRef]
- Foti, M.; Ruberto, G. Kinetic solvent effects on phenolic antioxidant determined by spectrophotometric measurements. J. Agric. Food Chem. 2001, 49, 342–348. [Google Scholar] [CrossRef]
- Das, S.; Khound, R.; Santra, M.; Santra, D.K. Beyond Bird Feed: Proso Millet for Human Health and Environment. Agriculture 2019, 9, 64. [Google Scholar]
- Dasa, F.; Binh, L.N. A Comparative Study on Rheological, Functional and Color Properties of Improved Millet Varieties and Injera. J. Agric. Sci Food Res. 2019, 10, 1–8. [Google Scholar] [CrossRef]



| Extraction Phase | Sample Type | TPC (mgGAE/g) | CTC (mgCE/100 g) | DPPH (%Inhibition) | TAC (mg AAE/g) |
|---|---|---|---|---|---|
| Water | Starch | 0.68 d ± 0.06 | 0.60 b,c ± 0.04 | 3.68 d ± 0.11 | 0.36 b,c ± 0.05 |
| Native film | 0.54 c ±0.04 | 0.52 b ± 0.09 | 3.14 c ± 0.16 | 0.27 b ± 0.08 | |
| Starch–ĸ-carrageenan film | 0.47 b ± 0.08 | 0.41 a ± 0.02 | 2.95 b ± 0.21 | 0.21 a ± 0.04 | |
| Methanol | Starch | 0.75 e ± 0.03 | 0.69 c ± 0.04 | 3.71 d ± 0.25 | 0.41 c ± 0.07 |
| Native film | 0.46 b ± 0.05 | 0.54 b ± 0.08 | 2.98 b ± 0.19 | 0.29 b ± 0.05 | |
| Starch–ĸ-carrageenan film | 0.39 a ± 0.03 | 0.45 a ± 0.05 | 2.62 a ± 0.14 | 0.24 a ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punia Bangar, S.; Sandhu, K.S.; Rusu, A.V.; Kaur, P.; Purewal, S.S.; Kaur, M.; Kaur, N.; Trif, M. Proso-Millet-Starch-Based Edible Films: An Innovative Approach for Food Industries. Coatings 2021, 11, 1167. https://doi.org/10.3390/coatings11101167
Punia Bangar S, Sandhu KS, Rusu AV, Kaur P, Purewal SS, Kaur M, Kaur N, Trif M. Proso-Millet-Starch-Based Edible Films: An Innovative Approach for Food Industries. Coatings. 2021; 11(10):1167. https://doi.org/10.3390/coatings11101167
Chicago/Turabian StylePunia Bangar, Sneh, Kawaljit Singh Sandhu, Alexandru Vasile Rusu, Pinderpal Kaur, Sukhvinder Singh Purewal, Maninder Kaur, Navneet Kaur, and Monica Trif. 2021. "Proso-Millet-Starch-Based Edible Films: An Innovative Approach for Food Industries" Coatings 11, no. 10: 1167. https://doi.org/10.3390/coatings11101167
APA StylePunia Bangar, S., Sandhu, K. S., Rusu, A. V., Kaur, P., Purewal, S. S., Kaur, M., Kaur, N., & Trif, M. (2021). Proso-Millet-Starch-Based Edible Films: An Innovative Approach for Food Industries. Coatings, 11(10), 1167. https://doi.org/10.3390/coatings11101167









