Imparting Strong Antifouling Properties to the Transparent PVB Coating through the Zwitterionic Compound Condensation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Preparation of Hybrid Zwitterionic Precursor
2.3. Preparation of the Zwitterionic Component Embedded in PVB Coating
2.4. Characteristics of the Physicochemical Properties of the Coatings
2.5. Characteristics of the Mechanical Properties of the Coatings
2.6. Characteristics of Anti-Oil Adhesion Property of Coating
2.7. The Resistance for Protein Settlement of Coating
2.8. The Resistance for Marine Algae of Coating
2.9. The Marine Field Test of Coating
3. Results and Discussion
3.1. The Physicochemical Properties of the Coatings
3.2. The Mechanical Properties of Coating
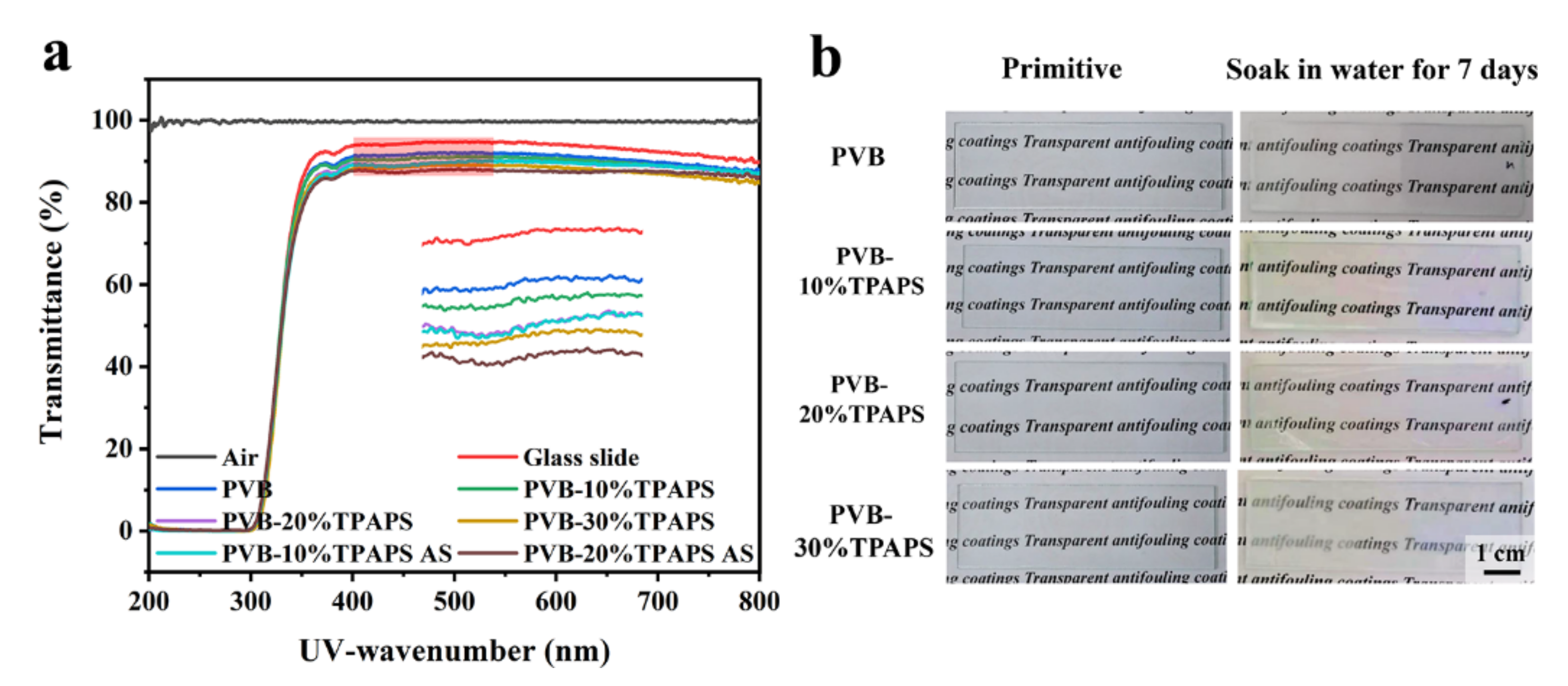
3.3. The Light Transmission Properties of Coating
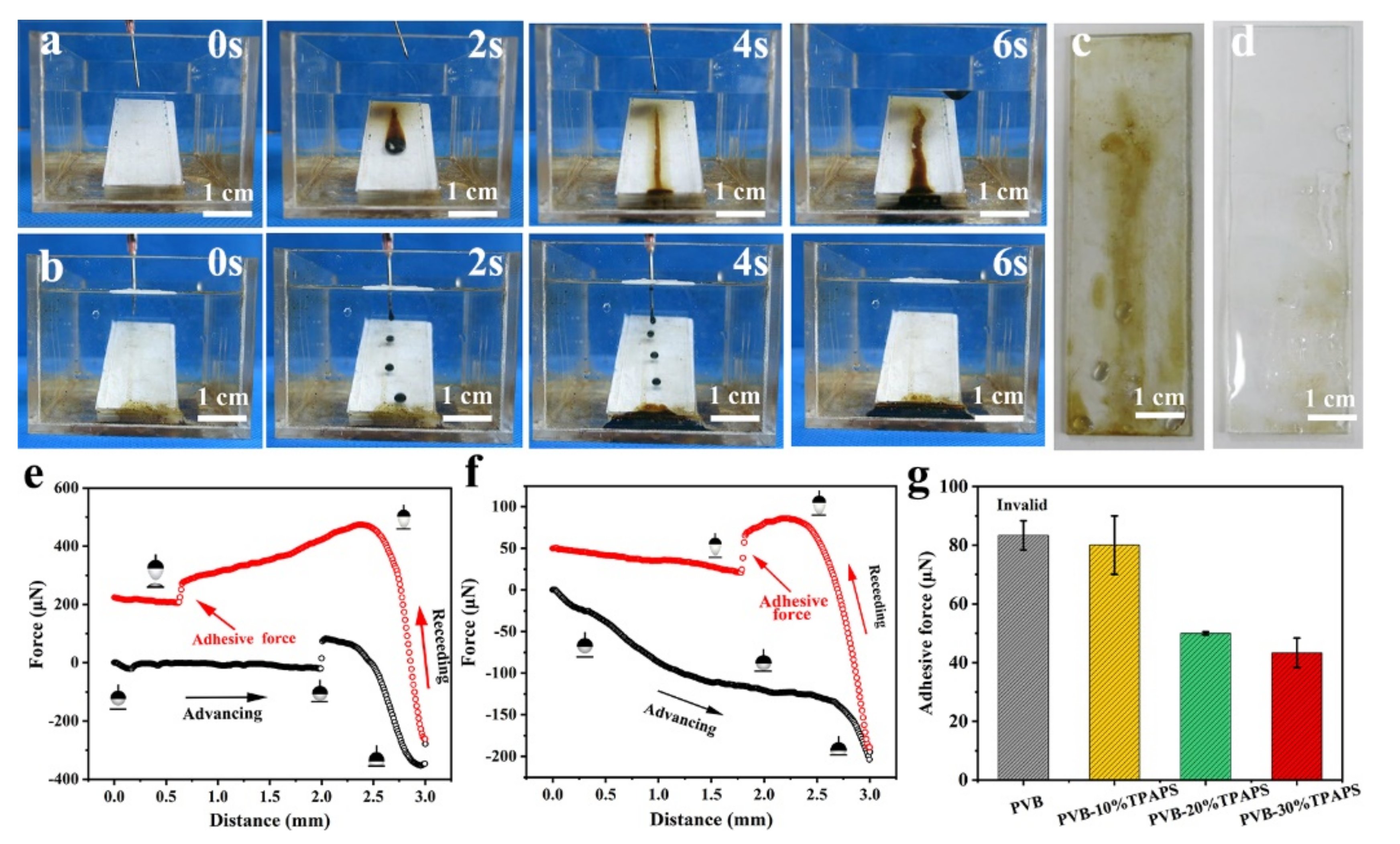
3.4. The Anti-Oil-Adhesion Properties of the Coatings
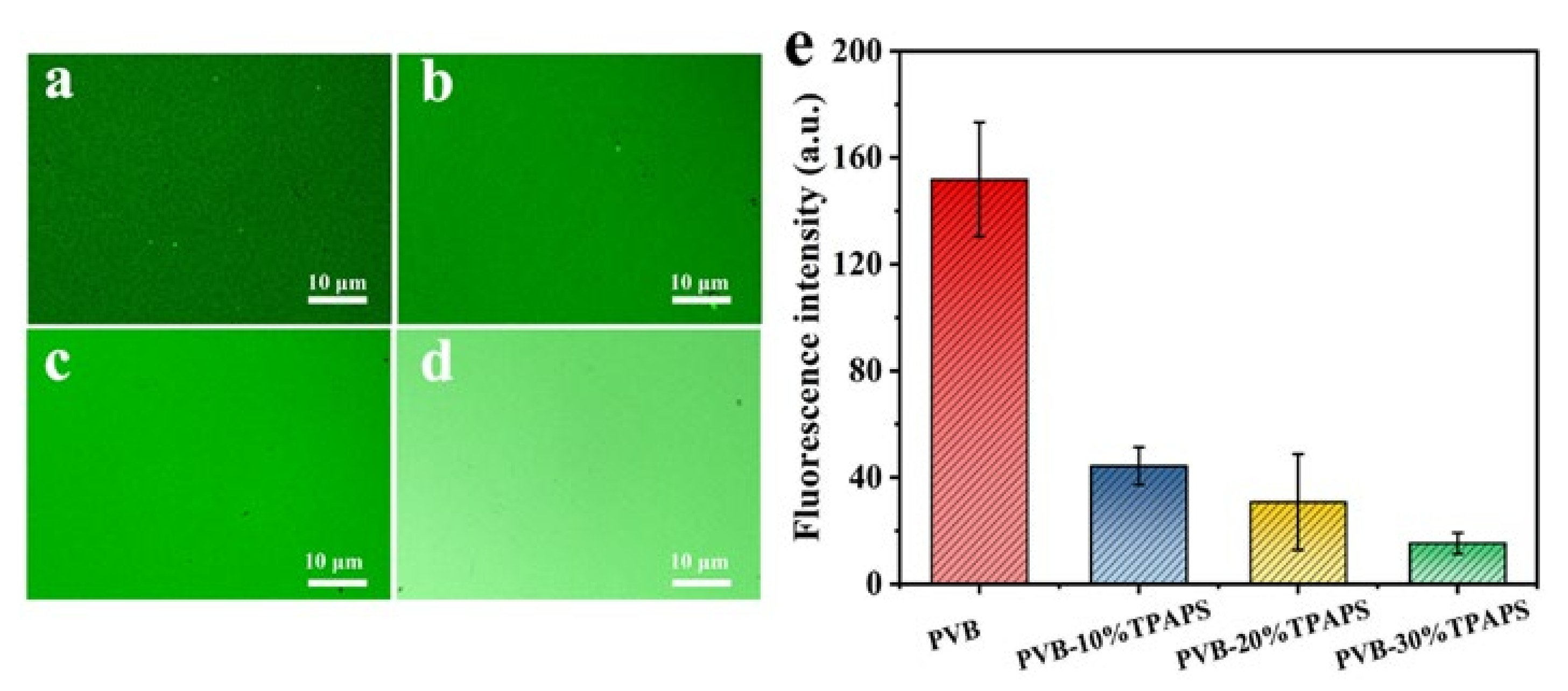
3.5. The Resistance Capacities of the Coatings to Proteins
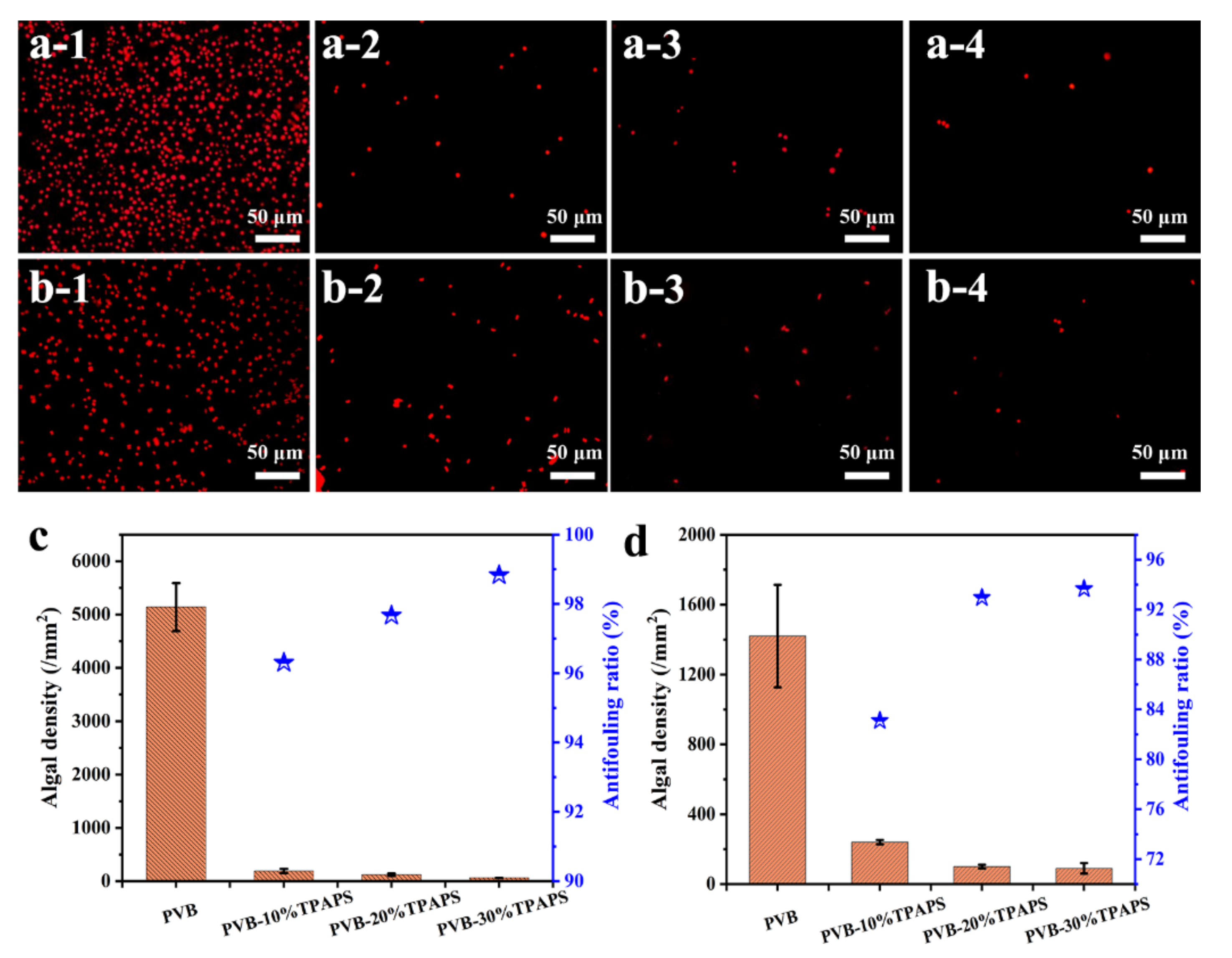
3.6. The Antifouling Capacity for Marine Algae of Coating
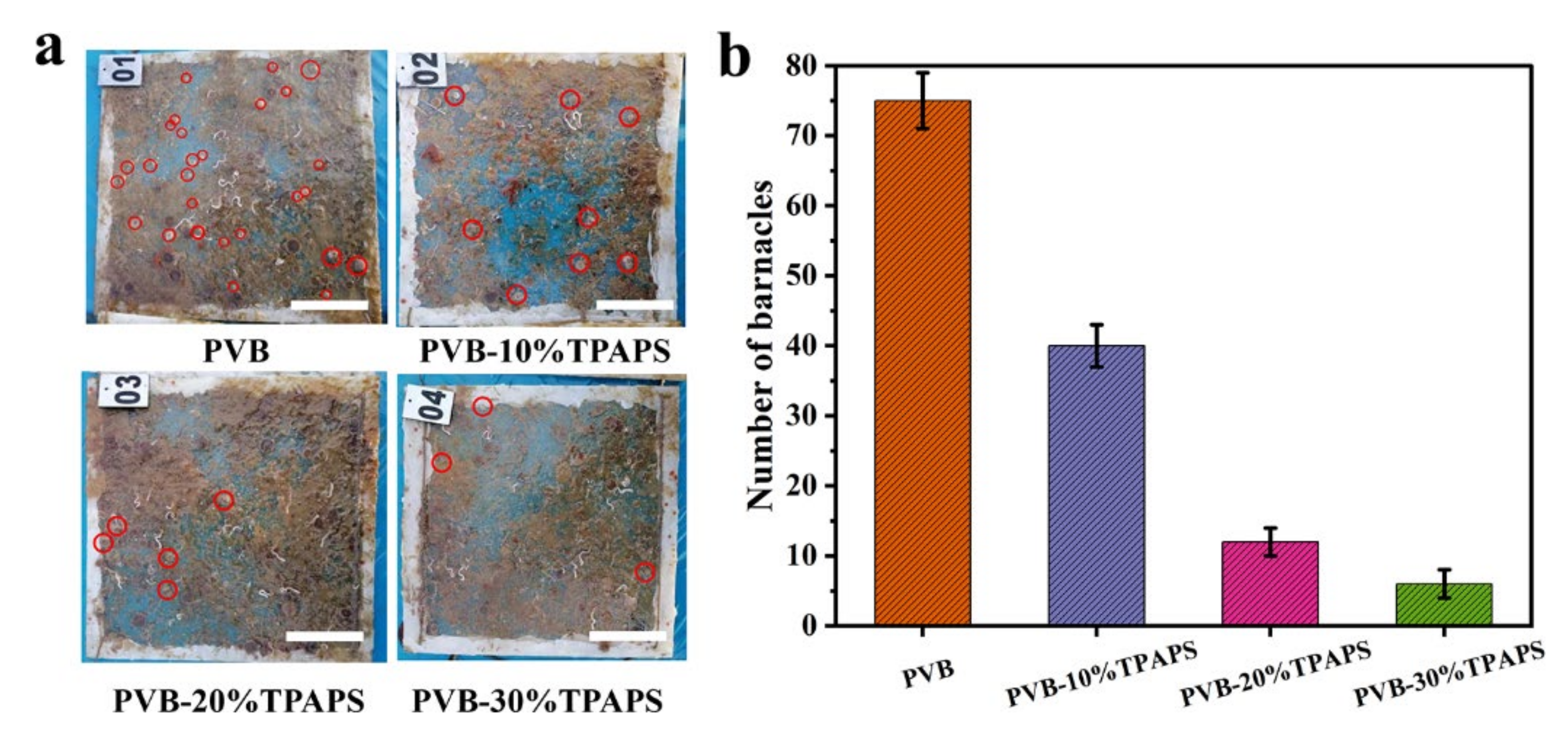
3.7. The Marine Field Test of the Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinagre, P.A.; Simas, T.; Cruz, E.; Pinori, E.; Svenson, J. Marine Biofouling: A European Database for the Marine Renewable Energy Sector. J. Mar. Sci. Eng. 2020, 8, 495. [Google Scholar] [CrossRef]
- Richards, C.; Briciu-Burghina, C.; Jacobs, M.R.; Barrett, A.; Regan, F. Assessment of Antifouling Potential of Novel Transparent Sol Gel Coatings for Application in the Marine Environment. Molecules 2019, 24, 2983. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Liu, X.; Shi, S.; Huang, R.; Su, R.; Qi, W.; He, Z. Design and Mechanisms of Antifouling Materials for Surface Plasmon Resonance Sensors. Acta Biomater. 2016, 40, 100. [Google Scholar] [CrossRef]
- Sunny, S.; Cheng, G.; Daniel, D.; Lo, P.; Ochoa, S.; Howell, C.; Vogel, N.; Majid, A.; Aizenberg, J. Transparent antifouling material for improved operative field visibility in endoscopy. Proc. Natl. Acad. Sci. USA 2016, 113, 11676. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhang, D.; Sun, S.; Li, T.; Sun, Y. Fabrication of Slippery Lubricant-Infused Porous Surface with High Underwater Transparency for the Control of Marine Biofouling. ACS Appl. Mater. Interfaces 2017, 9, 972–982. [Google Scholar] [CrossRef]
- He, X.; Lou, T.; Yang, Z.; Bai, X.; Yuan, C.; Wang, C.; Neville, A. Lubricant-infused titania surfaces with high underwater transparency for antifouling applications: A combined experimental and molecular dynamics study. Appl. Surf. Sci. 2021, 543, 148848. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Zhang, F.; Gao, S.; Wang, A.; Fang, W.; Jin, J. Zwitterionic Nanohydrogel Grafted PVDF Membranes with Comprehensive Antifouling Property and Superior Cycle Stability for Oil-in-Water Emulsion Separation. Adv. Funct. Mater. 2018, 28, 1804121. [Google Scholar] [CrossRef]
- Fenati, R.A.; Quinn, M.S.; Bilinsky, H.C.; Neto, C. Antifouling Properties of Liquid-Infused Riblets Fabricated by Direct Contactless Microfabrication. Adv. Eng. Mater. 2020, 23, 2000905. [Google Scholar] [CrossRef]
- Finnegan, C.; Ryan, D.; Enright, A.-M.; Garcia-Cabellos, G. A review of strategies for the detection and remediation of organotin pollution. Crit. Rev. Environ. Sci. Technol. 2018, 48, 77–118. [Google Scholar] [CrossRef]
- Nwuzor, I.C.; Idumah, C.I.; Nwanonenyi, S.C.; Ezeani, O.E. Emerging trends in self-polishing anti-fouling coatings for marine environment. Saf. Extrem. Environ. 2021, 3, 9–25. [Google Scholar] [CrossRef]
- Silva, E.R.; Ferreira, O.; Ramalho, P.A.; Azevedo, N.F.; Bayón, R.; Igartua, A.; Bordado, J.C.; Calhorda, M.J. Eco-friendly non-biocide-release coatings for marine biofouling prevention. Sci. Total Environ. 2019, 650, 2499–2511. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; de With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef]
- Yang, J.; Xue, B.; Zhou, Y.; Qin, M.; Wang, W.; Cao, Y. Spray-Painted Hydrogel Coating for Marine Antifouling. Adv. Mater. Technol. 2021, 6, 2000911. [Google Scholar] [CrossRef]
- Yang, W.J.; Cai, T.; Neoh, K.G.; Kang, E.T.; Dickinson, G.H.; Teo, S.L.; Rittschof, D. Biomimetic anchors for antifouling and antibacterial polymer brushes on stainless steel. Langmuir 2011, 27, 7065–7076. [Google Scholar] [CrossRef]
- Ye, Q.; He, B.; Zhang, Y.; Zhang, J.; Liu, S.; Zhou, F. Grafting Robust Thick Zwitterionic Polymer Brushes via Subsurface-Initiated Ring-Opening Metathesis Polymerization for Antimicrobial and Anti-Biofouling. ACS Appl. Mater. Interfaces 2019, 11, 39171–39178. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Shimano, K.; Okazaki, H.; Kurokawa, T.; Nakajima, T.; Nonoyama, T.; King, D.R.; Gong, J.P. Tough Particle-Based Double Network Hydrogels for Functional Solid Surface Coatings. Adv. Mater. Interf. 2018, 5, 1801018. [Google Scholar] [CrossRef]
- Yao, X.; Liu, J.; Yang, C.; Yang, X.; Wei, J.; Xia, Y.; Gong, X.; Suo, Z. Hydrogel Paint. Adv. Mater. 2019, 31, e1903062. [Google Scholar] [CrossRef]
- Huang, K.T.; Yeh, S.B.; Huang, C.J. Surface Modification for Superhydrophilicity and Underwater Superoleophobicity: Applications in Antifog, Underwater Self-Cleaning, and Oil-Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 21021–21029. [Google Scholar] [CrossRef] [PubMed]
- Shivapooja, P.; Yu, Q.; Orihuela, B.; Mays, R.; Rittschof, D.; Genzer, J.; Lopez, G.P. Modification of Silicone Elastomer Surfaces with Zwitterionic Polymers: Short-Term Fouling Resistance and Triggered Biofouling Release. ACS Appl. Mater. Interfaces 2015, 7, 25586–25591. [Google Scholar] [CrossRef] [PubMed]
- Shafi, H.Z.; Khan, Z.; Yang, R.; Gleason, K.K. Surface modification of reverse osmosis membranes with zwitterionic coating for improved resistance to fouling. Desalination 2015, 362, 93–103. [Google Scholar] [CrossRef]
- Yeh, S.B.; Chen, C.S.; Chen, W.Y.; Huang, C.J. Modification of silicone elastomer with zwitterionic silane for durable antifouling properties. Langmuir 2014, 30, 11386–11393. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J.; Zheng, Y.-Y. Controlled Silanization Using Functional Silatrane for Thin and Homogeneous Antifouling Coatings. Langmuir 2018, 35, 1662–1671. [Google Scholar] [CrossRef]
- Yang, W.; Lin, P.; Cheng, D.; Zhang, L.; Wu, Y.; Liu, Y.; Pei, X.; Zhou, F. Contribution of Charges in Polyvinyl Alcohol Networks to Marine Antifouling. ACS Appl. Mater. Interfaces 2017, 9, 18295–18304. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mills, S.; Bazaka, K.; Bajema, N.; Atkinson, I.; Jacob, M.V. Biodegradable optically transparent terpinen-4-ol thin films for marine antifouling applications. Surf. Coat. Technol. 2018, 349, 426–433. [Google Scholar] [CrossRef]
- Akuzov, D.; Brummer, F.; Vladkova, T. Some possibilities to reduce the biofilm formation on transparent siloxane coatings. Colloids Surf. B Biointerfaces 2013, 104, 303–310. [Google Scholar] [CrossRef]
- Zheng, S.; Li, J. Inorganic–organic sol gel hybrid coatings for corrosion protection of metals. J. Sol.-Gel Sci. Technol. 2010, 54, 174–187. [Google Scholar] [CrossRef]
- Wang, D.; Xu, J.; Tan, J.; Yang, J.; Zhou, S. In situ generation of amphiphilic coatings based on a self-catalytic zwitterionic precursor and their antifouling performance. Chem. Eng. J. 2021, 422, 130115. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Xie, Q.; Chen, Z.; Ma, C.; Zhang, G. Transparent Polymer-Ceramic Hybrid Antifouling Coating with Superior Mechanical Properties. Adv. Funct. Mater. 2021, 31, 2011145. [Google Scholar] [CrossRef]
- Schottner, G. Hybrid Sol−Gel-Derived Polymers: Applications of Multifunctional Materials. Chem. Mater. 2001, 13, 3422–3435. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, H.; Shi, Y.; Cui, J. The mechanical properties of Polyvinyl Butyral (PVB) at high strain rates. Constr. Build. Mater. 2015, 93, 404–415. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, B.P.; Shahi, V.K. 3-[[3-(Triethoxysilyl)propyl]amino]propane-1-sulfonic acid-poly(vinyl alcohol) cross-linked zwitterionic polymer electrolyte membranes for direct methanol fuel cell applications. ACS Appl. Mater. Interfaces 2009, 1, 1002–1012. [Google Scholar] [CrossRef]
- De, G.; Karmakar, B.; Ganguli, D. Hydrolysis–condensation reactions of TEOS in the presence of acetic acid leading to the generation of glass-like silica microspheres in solution at room temperature. J. Mater. Chem. 2000, 10, 2289–2293. [Google Scholar] [CrossRef]
- Paquet, O.; Brochier Salon, M.-C.; Zeno, E.; Belgacem, M.N. Hydrolysis-condensation kinetics of 3-(2-amino-ethylamino)propyl-trimethoxysilane. Mater. Sci. Eng. C-Mater. 2012, 32, 487–493. [Google Scholar] [CrossRef]
- Brochier Salon, M.-C.; Belgacem, M.N. Competition between hydrolysis and condensation reactions of trialkoxysilanes, as a function of the amount of water and the nature of the organic group. Colloids Surf. A 2010, 366, 147–154. [Google Scholar] [CrossRef]
- Zheng, H.; Pan, M.; Wen, J.; Yuan, J.; Zhu, L.; Yu, H. Robust, Transparent, and Superhydrophobic Coating Fabricated with Waterborne Polyurethane and Inorganic Nanoparticle Composites. Ind. Eng. Chem. Res. 2019, 58, 8050–8060. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Leckband, D.E. Protein Adsorption on Grafted Zwitterionic Polymers Depends on Chain Density and Molecular Weight. Adv. Funct. Mater. 2020, 30, 2000757. [Google Scholar] [CrossRef]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Wang, X.; Zhang, C.; Liu, H.; Yang, W.; Cai, M.; Pei, X.; Zhou, F. Self-polishing emulsion platforms: Eco-friendly surface engineering of coatings toward water borne marine antifouling. Prog. Org. Coat. 2020, 149, 105945. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, H.; Xiao, T.; Wu, Y. Application of micro-FTIR spectroscopy to study molecular association of adsorbed water with lignin. Int. J. Biol. Macromol 2019, 131, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, P.; Li, X.; Wang, X. Protein-resistant surface based on zwitterion-functionalized nanoparticles for marine antifouling applications. New J. Chem. 2020, 44, 2059–2069. [Google Scholar] [CrossRef]
- Lou, T.; Bai, X.; He, X.; Yang, Y.; Yuan, C. Molecular dynamics simulation of peptide attachment on Al-based surfaces. Prog. Org. Coat. 2021, 157, 106310. [Google Scholar] [CrossRef]
- Boinovich, L. DLVO forces in thin liquid films beyond the conventional DLVO theory. Curr. Opin. Colloid Interface Sci. 2010, 15, 297–302. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Modin, E.B.; Aleshkin, A.V.; Emelyanenko, K.A.; Zulkarneev, E.R.; Kiseleva, I.A.; Vasiliev, A.L.; Emelyanenko, A.M. Effective Antibacterial Nanotextured Surfaces Based on Extreme Wettability and Bacteriophage Seeding. ACS Appl. Nano Mater. 2018, 1, 1348–1359. [Google Scholar] [CrossRef]
- Su, X.; Yang, M.; Hao, D.; Guo, X.; Jiang, L. Marine antifouling coatings with surface topographies triggered by phase segregation. J. Colloid Interface Sci. 2021, 598, 104–112. [Google Scholar] [CrossRef]
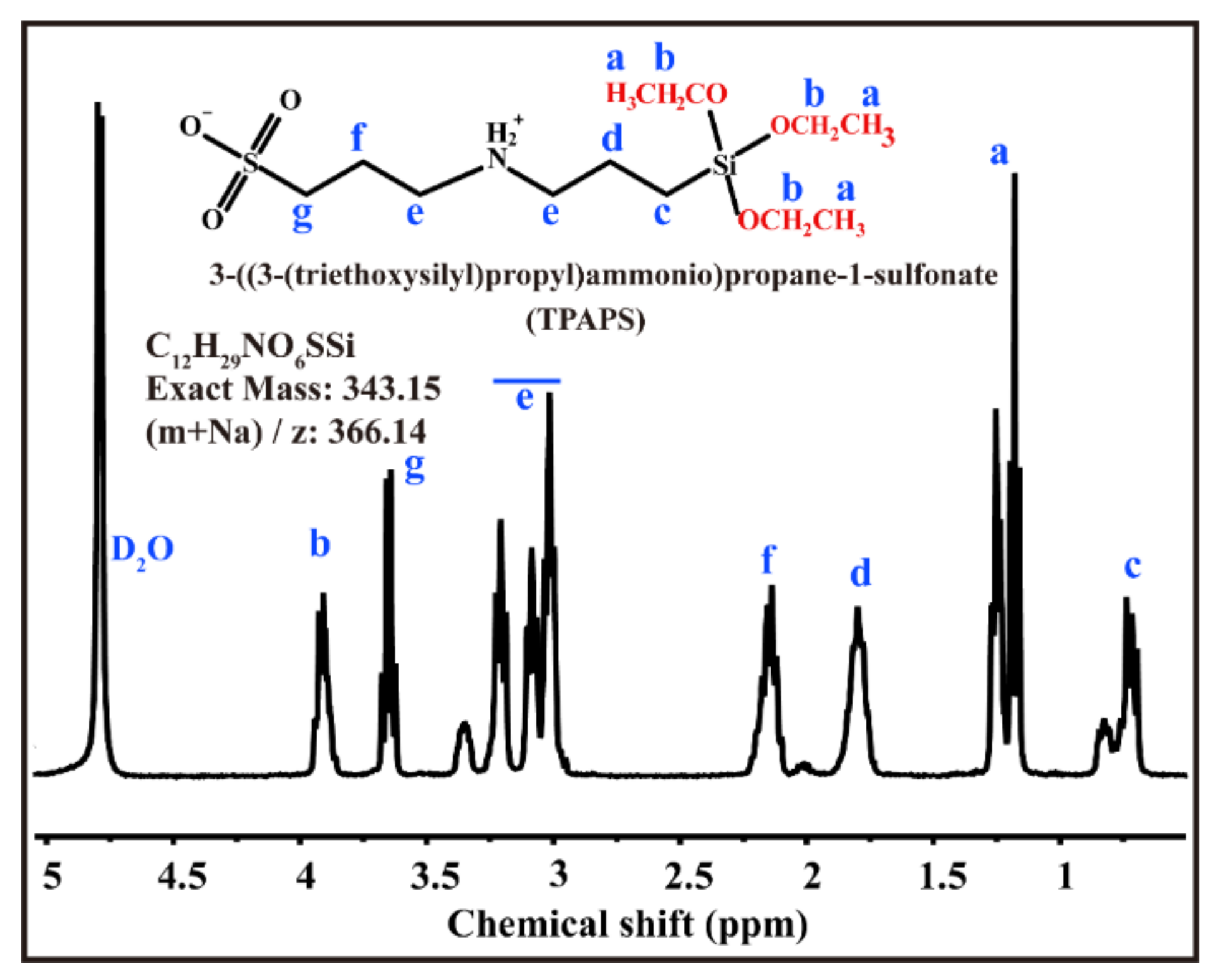
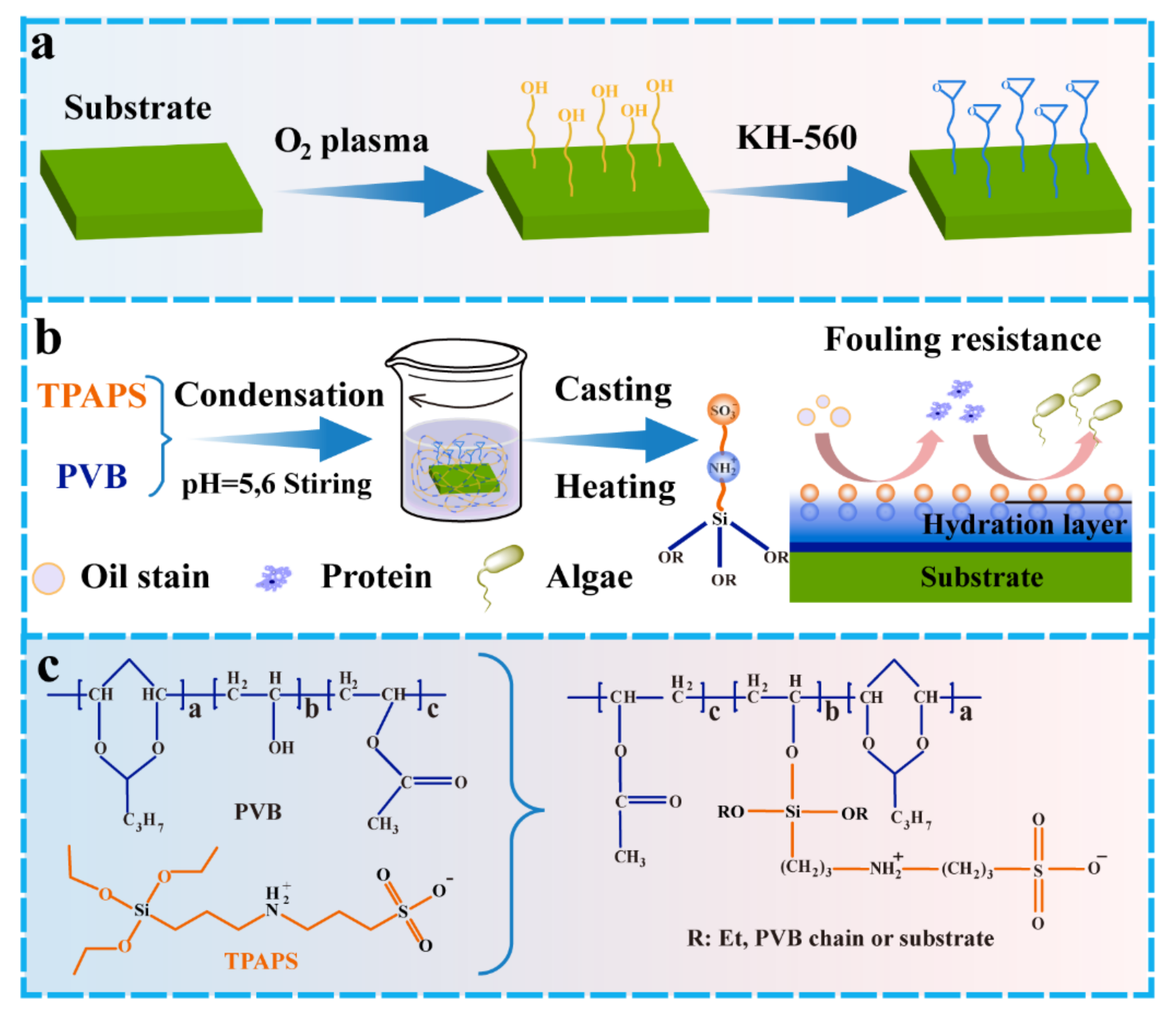
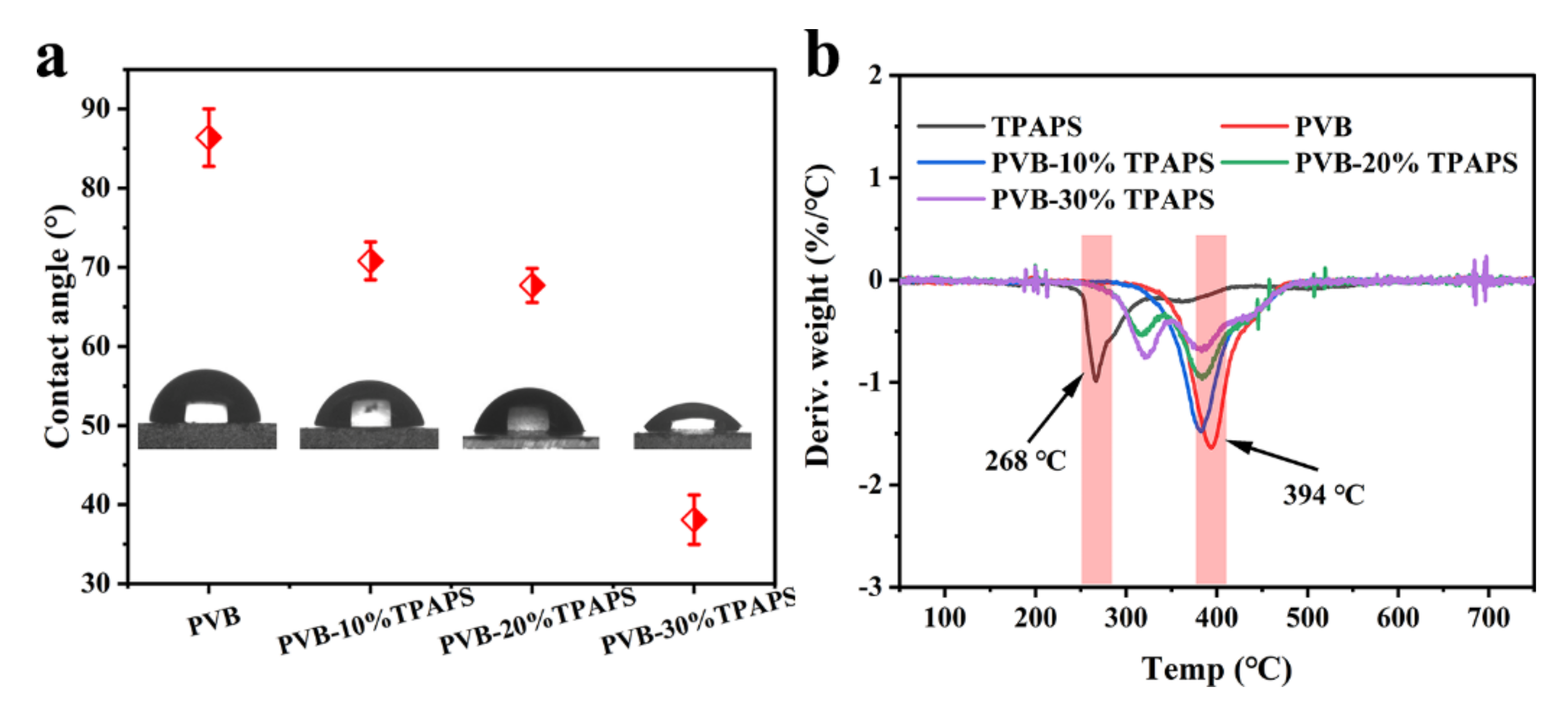


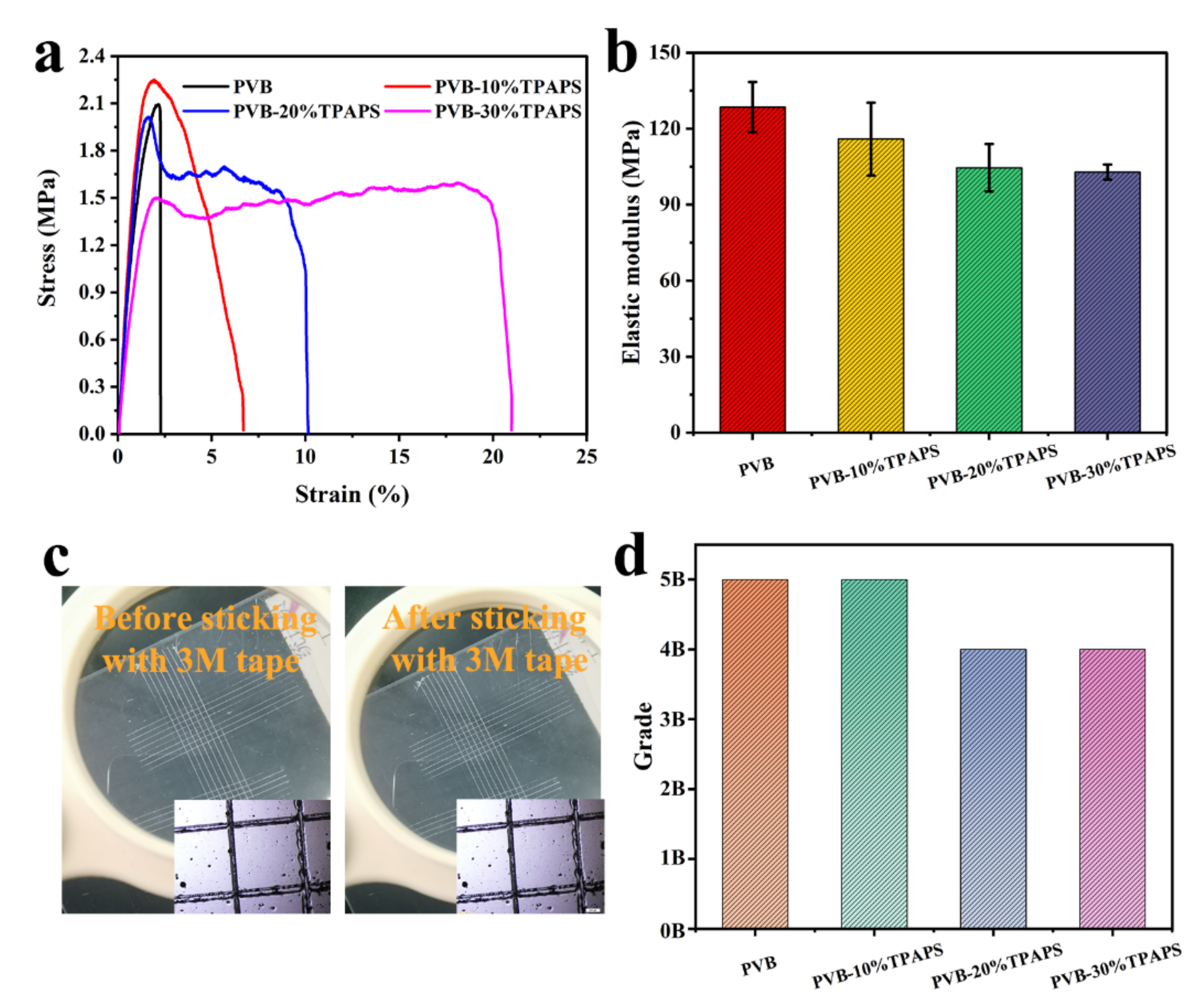
| Sample | Component A/g | Component B/g |
|---|---|---|
| PVB | 10 | N/A |
| PVB-10%TPAPS | 10 | 1 |
| PVB-20%TPAPS | 10 | 2 |
| PVB-30%TPAPS | 10 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Bo, S.; Feng, H.; Yu, B.; Yu, Q.; Yang, W.; Pei, X.; Zhou, F. Imparting Strong Antifouling Properties to the Transparent PVB Coating through the Zwitterionic Compound Condensation. Coatings 2021, 11, 1164. https://doi.org/10.3390/coatings11101164
Zhang J, Bo S, Feng H, Yu B, Yu Q, Yang W, Pei X, Zhou F. Imparting Strong Antifouling Properties to the Transparent PVB Coating through the Zwitterionic Compound Condensation. Coatings. 2021; 11(10):1164. https://doi.org/10.3390/coatings11101164
Chicago/Turabian StyleZhang, Jianbin, Shangshang Bo, Haiyan Feng, Bo Yu, Qiangliang Yu, Wufang Yang, Xiaowei Pei, and Feng Zhou. 2021. "Imparting Strong Antifouling Properties to the Transparent PVB Coating through the Zwitterionic Compound Condensation" Coatings 11, no. 10: 1164. https://doi.org/10.3390/coatings11101164
APA StyleZhang, J., Bo, S., Feng, H., Yu, B., Yu, Q., Yang, W., Pei, X., & Zhou, F. (2021). Imparting Strong Antifouling Properties to the Transparent PVB Coating through the Zwitterionic Compound Condensation. Coatings, 11(10), 1164. https://doi.org/10.3390/coatings11101164








